Virtual reality (VR) environments are a powerful tool for the investigation of brain mechanisms. We introduce the Servoball, a VR treadmill for freely moving rodents. The Servoball is integrated with the animals’ group home cage. Single individuals voluntarily enter using automated access control. Training is highly time efficient, even for cognitively complex VR paradigms.
Keywords: virtual reality, freely moving, 24/7, spatial cognition, operator independent
Abstract
Virtual reality (VR) environments are a powerful tool to investigate brain mechanisms involved in the behavior of animals. With this technique, animals are usually head fixed or secured in a harness, and training for cognitively more complex VR paradigms is time consuming. A VR apparatus allowing free animal movement and the constant operator-independent training of tasks would enable many new applications. Key prospective usages include brain imaging of animal behavior when carrying a miniaturized mobile device such as a fluorescence microscope or an optetrode. Here, we introduce the Servoball, a spherical VR treadmill based on the closed-loop tracking of a freely moving animal and feedback counterrotation of the ball. Furthermore, we present the complete integration of this experimental system with the animals’ group home cage, from which single individuals can voluntarily enter through a tunnel with radio-frequency identification (RFID)-automated access control and commence experiments. This automated animal sorter functions as a mechanical replacement of the experimenter. We automatically trained rats using visual or acoustic cues to solve spatial cognitive tasks and recorded spatially modulated entorhinal cells. When electrophysiological extracellular recordings from awake behaving rats were performed, head fixation can dramatically alter results, so that any complex behavior that requires head movement is impossible to achieve. We circumvented this problem with the use of the Servoball in open-field scenarios, as it allows the combination of open-field behavior with the recording of nerve cells, along with all the flexibility that a virtual environment brings. This integrated home cage with a VR arena experimental system permits highly efficient experimentation for complex cognitive experiments.
NEW & NOTEWORTHY Virtual reality (VR) environments are a powerful tool for the investigation of brain mechanisms. We introduce the Servoball, a VR treadmill for freely moving rodents. The Servoball is integrated with the animals’ group home cage. Single individuals voluntarily enter using automated access control. Training is highly time-efficient, even for cognitively complex VR paradigms.
to understand the role of a specific mechanism in the control of behavior, testing must be performed in a behavioral context. This requires experimental manipulation of the mechanism in ways that meaningfully influence behavior. Virtual reality (VR) experimental environments have become a powerful tool for investigating the neural basis of behavior (Bohil et al. 2011; Dombeck and Reiser 2012; Harvey et al. 2009; Hölscher et al. 2005; Thurley and Ayaz 2016), as they overcome the limitations of experimental physical arenas. VR allows behaviors to be investigated through closed-loop manipulations of the environment and even permit the generation of physically impossible environments (Bohil et al. 2011; Dombeck and Reiser 2012; Thurley and Ayaz 2016). Air-floating trackspheres for rodents permit optical imaging or intracellular recordings of neurons in awake, behaving animals (Domnisoru et al. 2013; Harvey et al. 2009; Harvey et al. 2012; Keller et al. 2012). However, the required head fixation impedes the extent of voluntary behavior, which limits the control of self-motion cues and distorts vestibular inputs.
Mismatch between optic flow and proprioceptive inputs may disrupt hippocampal space representation (Aghajan et al. 2015). It is therefore desirable to have a VR experimental environment that does not require body fixation to allow unperturbed place coding in VR. This is also pertinent in the context of recently developed technologies that permit the brain imaging of freely behaving animals (Anikeeva et al. 2011; Ghosh et al. 2011; Iyer et al. 2014; Sieu et al. 2015). In some systems, rotation around the vertical body axis is possible despite body fixation, leaving vestibular information regarding rotational movement unperturbed (Aronov and Tank 2014; Hölscher et al. 2005; Thurley et al. 2014). This is a first step toward more natural sensory stimulation and behavior in VR, in contrast to that provided by head fixation. Nevertheless, a more comprehensive approach should permit freely moving animals.
Here, we show the results from using such a VR experimental system in which animals can move freely and that uses neither head nor body fixation. In short, the animal is placed in a circular arena, the bottom of which consists of a large sphere that rests on an arrangement of rollers and servomotors. Animal movements are video tracked and the position signal is used for closed-loop position compensation by counterrotating the sphere via the servomotors as the animal attempts to move from its apex, keeping the animal in the center of the arena. This treadmill is surrounded by a circle of monitors that display the visual environment from the animal’s current position in the VR scene. Eight retractable liquid reward devices located at the periphery permit the delivery of food reinforcement at experimentally defined locations. VR scenes may include linear alleys, where compensation is restricted to the long axis so that animals can approach and touch the side walls of the experimental enclosure. In contrast to systems employing head or body fixation (Aghajan et al. 2015; Aronov and Tank 2014; Hölscher et al. 2005; Thurley et al. 2014), the Servoball treadmill stops at the end of an alley and the animal is then able to walk to the reward at the perimeter. In this situation, translational acceleration is available. Thus the freely moving animal on the Servoball can obtain touch information from the physical walls of the arena and receive vestibular information when its body rotates. Regarding translational movement, vestibular encoding will likely be perturbed by the artificial acceleration of the ball when it repositions the rat. Such repositioning may also lead to a mismatch between visual and motor signals.
However, at the end of an alley, where compensation is suspended and VR is stationary, the rat should experience physiological vestibular encoding.
A general difficulty involved in complex behavioral paradigms is the large amount of time required to train the animals. Our VR system also presents a novel solution to this challenge. The VR arena can be accessed directly from the animals’ home cage through a tunnel with an radio-frequency identification (RFID)-controlled gate system. Rats tagged with ID chips voluntarily and individually enter from their group home cage into the VR treadmill experimental arena to perform fully automated experimental sessions. This operator-independent system makes self-determined training sessions possible 24/7, with individuals completing up to 10 self-timed training sessions during each 24-h period.
The rodent VR maze can simulate any environmental scale by adapting the simulation to natural habitat dimensions. We here show that rats in this virtual environment use visual and acoustic cues to reach goal locations. In a series of experiments, we presented rats with visual cues that served as beacons marking a goal location or that served as landmarks defining the angular position of a goal. Furthermore, we tested whether acoustic cues (pure tones differing in frequency or in pulse rate) are sufficient for rat orientation. Our results show that animals learn to use this operator independent system within 3 wk and that rats subsequently solve spatial orientation tasks in this VR environment. Moreover, we successfully combined behavioral testing with recordings from single units in the entorhinal cortex. This makes the VR Servoball attached to the home cage a perfectly controllable experimental environment for freely moving animals that permits highly time-efficient experimentation for a wide range of applications.
METHODS
Animals
Experiments were performed with 12 male and 3 female Long-Evans rats (Rattus norvegicus). Training commenced at an age of 2 mo when animals weighed between 300 and 445 g. Experiments followed national guidelines regarding animal experimentation. The experimental protocol was approved by the local authority Regierung von Oberbayern. We used four groups in succession. In the first group (three females), we established the gating procedure (below). Two groups of four animals passed pretraining and beacon orientation, with one individual per group excluded from the final experimental phase, as these two animals remained passive and barely moved after having been enclosed. By staying within the central area, they did not activate the treadmill function and thus avoided walking on the moving sphere. The four rats in group 3 and one rat in group 2 passed all experiments, including orientation along an acoustic gradient. A group size of four to six rats allowed an adequate balance to be obtained between utilizing experimental capacity and having sufficient individual time slots available for self-determined entering of the Servoball arena for an experimental session (below). For the recording experiments, five male Long-Evans rats (aged 3 mo; 350–500 g) were housed in pairs, maintained on a 12-h light/12-h dark schedule, and tested during the dark phase. During training stages, the rats received water both from the reward system in the experimental arena and during two daily 60-min periods with ad libitum access to water within the home cage.
Servoball VR Home-Cage Environment
The complete experimental system consisted of a home cage, the attached individual sorter that allowed rats to autonomously and individually enter the experimental arena, and the Servoball, a spherical treadmill system for freely moving animals (Fig. 1, A–C). Experiments took place within a 490-mm experimental arena enclosed by a transparent cylinder that limited the movement of the animal to the central part of a 600-mm ball (Fig. 1, A and B). A 100-Hz camera tracked the position and movement of the animal, and this information was used for compensatory movements of the ball and the updating of the virtual environment. The closed-loop feedback control system was driven by two perpendicularly arranged servomotors, with tachometers that counterrotated the sphere underneath the animal to keep it within a circular area close to the apex of the sphere (Fig. 1, A–C). When nearing a wall in the VR environment, the compensation mechanism was suspended and the animal could approach and physically touch the wall of the enclosing cylinder. The position of the animal within the VR scene was the result of its movement in the x and y directions and the compensation distance, which was sensed by two x/y optical encoders directed at the surface of the ball. Visual stimuli and VR were presented as a 360° panorama from an octagon of eight 19-ft TFT monitors (408 × 255 mm, 670-mm internal diameter of the octagon) connected to a graphics card. From the vertical perspective, the field of view spanned 56.5° if viewed from the apex of the sphere; however, this varied between 78.2 and 36.6° depending on the rat’s position within the 490-mm arena. Viewed from the apex, the screens covered 48.8° above and 7.7° below the horizon. Acoustic stimuli were presented from four loudspeakers positioned above the cylinder; all loudspeakers provided the same output. An animal was motivated to explore and exploit the VR environment by the possibility of obtaining water rewards delivered from the eight devices located at the arena’s perimeter. These devices were retractable and could be activated at any experimentally defined locations within the VR environment. On arriving at a feeding location within the VR scene, treadmill compensation was suspended so that the animal could move to the peripheral feeder and obtain a 100-µl liquid reward.
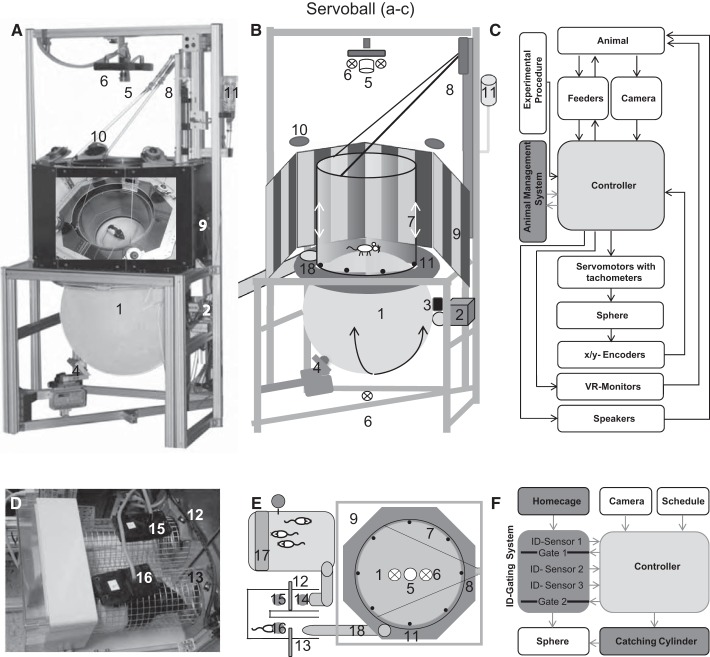
Fig. 1.
Experimental apparatus and procedure. Photograph (A), schematic illustration (B), and functional diagram (C) of the Servoball setup. A 60-cm sphere (treadmill, 1) rests on a multidirectional roller system (2 and 4). The sphere is rotated by two orthogonal feedback-controlled position servos (2 and 3). The 2-dimensional position of the freely moving animal is sensed by infrared (6) high-speed video (5). Animal movement is restricted by a Perspex glass cylinder (7), which is moved up and down by a servomotor (8). VR is presented on eight peripheral TFT monitors (9). Four loudspeakers (10) are located above the cylinder. Retractable liquid feeders (11) are integrated beneath the cylinder. D–F: animal management system: individual sorter (D), home cage with food supply (E, 17), sorter, Servoball arrangement (E) with functional diagram (F). 14–16: ID sensors; 12 and 13: gates; 18: tunnel giving access to the Servoball. See methods for a description of the individual gating procedure. A: the photograph placed on the backside of the monitor ring shows a view into the system with a rat. VR, virtual reality.
The Animal Management System, with Sorting and Shutting-In Procedures
The home cage was connected to the Servoball via an individual separation and gating system, the sorter (Fig. 1, D–F), which was equipped with two electronic guillotine gates (Winter and Schaefers 2011). A rat entering the sorter was identified at ID sensor 1. If the Servoball or sorter were unoccupied and the rat’s individual schedule permitted the next experimental session to commence, gate 1 opened for the rat to enter the gated area. On detection at ID sensor 2, gate 1 was closed. On being detected at ID sensor 3, and if no other animal was detected at ID sensors 2 or 3 during a 30-s waiting interval, gate 2 opened and the rat could proceed to the experimental arena. The rat entered from below onto a circular platform located between monitors and the ball while the acrylic glass cylinder was raised by 8–10 cm. When the rat was within the central region of the ball, the shut-in mechanism commenced, whereby the cylinder slowly descended to 8 mm above the ball’s surface. Movement of the rat during the shut-in procedure reversed cylinder movement, and this process repeated until the rat was shut in or returned to the home cage.
Servoball, Optical Tracking, and Movement Compensation
The 60-cm ball was composed of 6-mm polyethylene weighing 6,500 g and had a roundness precision of ± 4 mm. The color was translucent white and transmitted infrared illumination from below (Fig. 1 A and B). To increase surface friction, the ball was sandblasted before its first use. The ball rested on a multidirectional roller system. The animal’s movement on the sphere was restricted by a transparent acrylic glass cylinder (outer diameter/inner diameter: 500/486 mm, height: 500 mm, Plexiglas XT) suspended from a motorized linear slide at ~8 mm above the ball’s surface. The cylinder provided tactile information regarding boundaries and prevented a rat from leaving the Servoball during a session.
The horizontal position of the freely moving animal was detected by a camera (Basler 602f, 100 frames/s; Basler, Ahrensburg, Germany) with a 6-mm lens (F = 1.2) and firewire connection to the PC. Long-Evans rats, with their sharp color transition from a dark hood to white body fur, are well suited to tracking. We used the optical center of mass in the black areas of fur to determine two-dimensional coordinates. Before each experiment, a background image was taken and later subtracted from the images taken during a session. This increased the contrast between the animal and the ball surface. The image was cropped to the area within the cylinder and then segmented, resulting in one or several objects. An erosion mechanism eliminated very small objects and smoothed edges. A dilatation mechanism filled holes in the segmented object. Normally, only one object remained, but for multiple objects, the largest was selected. Its center of gravity was taken as the position of the animal. Detection was performed at 100 Hz using the Halcon machine vision library (MVTec). The deviation of the animal’s positional signal to the sphere’s top center was used to generate feedback signals that drove the motors to compensate displacement. The speed of the motors was proportional to the deviation between the center and the position of the animal. Two optical sensors (ADNS-3080, 100 Hz; Agilent Technologies) placed above the servomotors sensed the ball’s rotation.
In contrast to air-cushioned systems, where a treadmill is moved by the animal’s own power (Hölscher et al. 2005), our system used an active compensating mechanism via tracking of the animal’s movement, and activating motors that counterrotated the Servoball so that the animal stayed within the top central region of the treadmill. The technical complexity is higher than in the air-cushioned systems, causing technical challenges such as systematic errors in tracking (e.g., small movements of a rat sitting on the top of the ball produced a random walk-like drift of the tracked position). Such systematic errors accumulated over the duration of a trial but could be removed online or post hoc from tracking data. The mechanical powering of the system resulted in slight vibrations of the Servoball. This may be a reason for the overly cautious behavior of some animals. Optical sensors tracked the length of travel paths during the compensation mode. Animal movement within the arena while no compensation occurred was tracked by the camera.
The sphere was rotated by two feedback-controlled position servos arranged at right angles and controlled by four-quadrant controllers driven from the PC using an analog voltage. Integrated motor tachometers provided velocity feedback for damping acceleration as a component of controller integrated velocity control. The motors transmitted force via nitrile butadiene rubber rollers (NBR/SBR 80° ± 5 Shore A, r = 15 mm). The drive rollers provided two of the three supports on which the ball rested, and were located slightly below its equator. The orthogonal position of the servos permitted pitch and roll rotation. A ball bearing supported the ball at a counterpart position to the rubber rollers, forming a triangular support at angles of 90°/135°/135°, 190 mm below the level of the motors’ drive rollers.
Calculation of Position Compensation
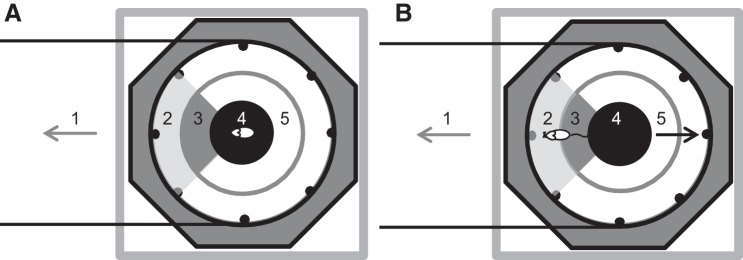
Compensation was here defined as the adaptation of the ball’s rotary movement in response to the animal’s position in the circular arena. The experimental arena was subdivided into three circular areas. Within the 144-mm diameter apex area, no compensation occurred (Fig. 2, A and B, area 4). Within the ring between the 72- and 215-mm radii, the rotational speed was a linear function of the distance to the apex (Fig. 2, A and B, area 2) with a maximum acceleration of 0.78 m/s2. With the animal within the ring between the 215-mm and 243-mm radii, the system could accelerate to the maximum rotational speed of 0.31 m/s (Fig. 2, A and B, area 3). Values for maximal acceleration and velocity were set to lower values at the beginning of habituation. The conversion of camera pixels to metric used the 486-mm inner diameter of the cylinder as a reference.
Fig. 2.
Schematic of compensation. Zoning of the experimental arena as used for the compensation algorithm. Top view of the setup with the octagonal structure of the TFT-screens, positions of feeders (black dots), and the cylinder surrounding the experimental arena. The animal is located at the right end of a virtual corridor that extends to the left (1). A: the rat is in the center of the inner circle (4) and the compensation mechanism is inactive. B: the rat has left the inner circle (4), crossed the acceleration zone (3), and entered the outer circle (2), where counterrotation of the sphere is set to the maximum of 0.31 m/s. Directions of movement are indicated by arrows representing the animal (gray arrow) and the ball (black arrow). Note that in the example given, the animal is located at the right end of a virtual corridor. Therefore, compensation is only activated when the animal enters the left quarter zone (marked in gray). In the remaining three quarters of the arena (5), compensation is inactivated, so that the animal can walk up to and touch the surrounding wall without initiating movement of the sphere. Black horizontal lines indicate the accessible zone of the path.
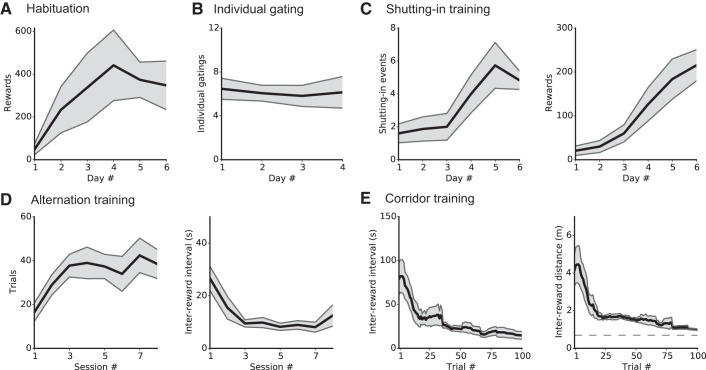
Fig. 4.
Duration of pretraining phases. A–E: different functions of the Servoball home-cage system are successively activated. A: habituation of group-living rats to entering the Servoball VR arena and using the liquid feeders. Initially, all ID-sorter gates are open and the cylinder is permanently raised, giving all animals simultaneous access to the eight activated liquid reward feeders. Data show the number of rewards collected per individual during 24 h during the 6-day habituation phase. B: learning individual admission to the Servoball arena. After the ID-sorter function is activated, only single animals can access the Servoball arena from the home cage. Data show the number of passages through the ID-sorter and to the Servoball per individual and per 24-h period during the four days of this phase. C: habituation to becoming enclosed by the cylinder before receiving rewards. In this phase, feeders only become active after the cylinder has been lowered to shut in the rat. The cylinder moves downwards when the rat is in the center of the sphere. Left: individual shutting-in events; right: individual rewards per 24 h. D: alternation training. This training occurred within the stationary 49-cm Servoball arena without compensation. Data show successful individual trials per 10-min session (left) and time intervals between rewards (right). E: treadmill training with locomotion compensation activated. Alternation between the 2 ends of a corridor. Lines to the left and right show moving averages from 15 trials, and the gray area shows SE; n = 15 for A, B, and C; n = 8 for D and E. For calculation of the minimum distance in E, see the appendix.
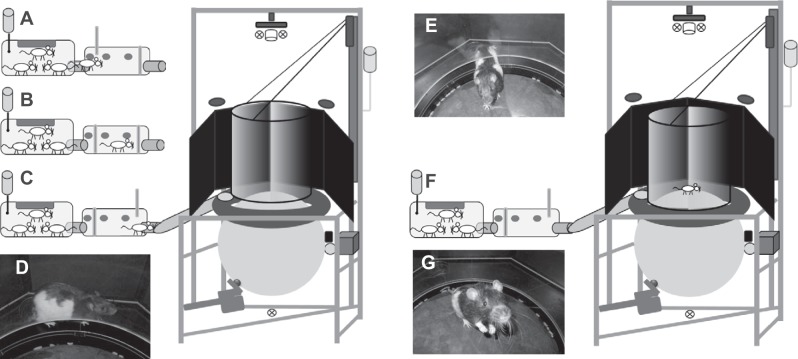
Fig. 3.
Sequence of the start of a typical experimental session. A: the group home cage with a single rat that has already passed reader 1 and gate 1 and entered the sorter. B: sorter gates are closed and the software ensures that only a single animal has entered. C: gate 2 has opened and the rat enters the tube to the Servoball arena. From the platform surrounding the sphere (D), the rat can enter the experimental arena (E) through the lifted cylinder. F: staying in the center of the experimental arena, the rat initializes the lowering process of the cylinder. G: rat shut in the arena with the cylinder moved downwards. For the description of a sequence of a typical experimental session, see text.
Calculation of Minimal Distances
To reach the target feeder, a rat was minimally required to move from the inner circle (radius: 72 mm) to the cylinder wall (radius: 245 mm). The rat’s tracking point, however, never quite reached the wall, as this was ~60 mm behind the tip of the snout. Thus, when the compensation mode was deactivated, the minimal distance was 0.11 m. This distance increased during the compensation mode by the length of an arm or corridor, e.g., 1.11 m for a 1.00-m arm (see Figs. 4E and 6C, broken line).
Fig. 6.
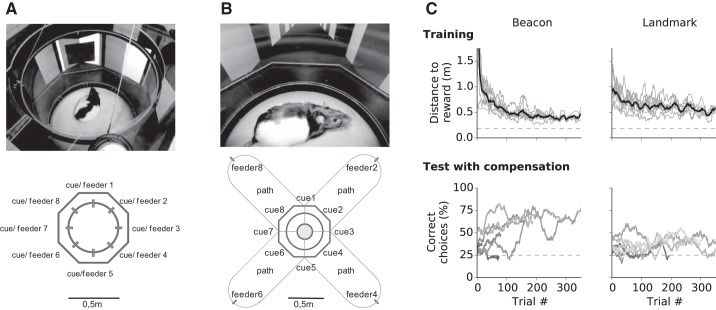
Navigation with visual cues. Experimental design during training initially performed within the 49-cm arena without compensation (A) and during tests in a 4-arm radial maze (B) with 1-m arms and active compensation. C: during beacon experiments (left), the cue “hovered” in mid-air above the entry of an alley (cues 2, 4, 6, and 8); during landmark experiments, the landmark was located on the wall to the left of the correct alley (cues 1, 3, 5, and 7). The results from the beacon (left) and landmark (right) experiments show the progressive reduction in locomotion distance to reward during task acquisition (top) in the 49-cm arena and the performance during tests in treadmill mode in the four-arm radial maze (bottom). Individual data are in gray (a 50-trial moving average) and group means are in black. Note advanced liquid dispensers in A. Top left: n = 8; bottom left: n = 7; top right: n = 9; bottom right: n = 7. Dashed lines in C show minimum distance (top) and level for chance performance (bottom).
Simulation of the Virtual Environments
The simulation of the virtual environment was implemented using DirectX. The texture of the virtual walls consisted of dark and light gray vertical stripes. The floor was textured with a square pattern and the sky/ceiling was colored black. Monitors presented the views of eight virtual cameras positioned on the top of the ball at a level of 144 mm (corresponding to the lower edge of the screens). Bezel compensation was used to respect the frames of the monitors and displayed lines in a straight fashion. Animals could physically sense the virtual walls of the corridor touching the cylinder wall. Landmarks and cues were presented at a height of 35 mm in the VR, so that an animal could pass in the space underneath. Monitors operated at 60 Hz.
Training Procedures
During habituation, gates were open and rats could move freely through the tunnel between the home cage and the Servoball, where all eight feeders were activated. Rats were identified on passing the ID sensors. Thereafter, the gating system was activated and the rats could only pass individually.
In the next training stage, rats learned to remain in the center of the Servoball arena. This triggered the lowering of the arena’s acrylic glass cylinder. However, if a rat prematurely left the central area, the cylinder again moved upwards. This back and forth process lasted until the rat was finally enclosed or returned to the home cage. The retractable feeders became available only after the arena was enclosed. The next phase involved alternation training, during which a rat was required to shuttle back and forth between two feeders located opposite to each other to obtain water rewards. During this phase, the compensation mechanism remained deactivated. After the acquisition of alternation, we activated the servo-driven locomotion compensation for the first time, which turned the ball into a treadmill. In this mode, the treadmill was used to compensate for rat movement such that the animal remained physically in place but could still move through virtual space. The compensating distance was gradually increased from 0.1 to 4.0 m.
For experiments that tested spatial cognition with visual cues, a rat was placed in an octagonal arena and a 12 × 12 cm black square was displayed above the target feeder. During initial training for a new task, the treadmill function was inactivated. Each trial began with the presenting of a black square; after a delay of 5 s, the target feeder was advanced for the rat to collect a reward, while the other seven feeders remained retracted. Next, after the cue (black square) was presented, all eight feeders were advanced. A feeder visit was detected from the nose poke into a photo-gate sensor of a feeder. A trial ended with the first choice made by the rat. After an incorrect choice, a 30-s timeout was given that commenced once the rat returned to the center.
Subsequently, the compensation mode was activated. Initial arm length (compensation distance) was 0.1 m, which then increased to 0.5 m on the second day of task acquisition and to 1.0 m during testing. In the experiment involving compensation, the cues were presented in the central crossing of the four arms above the entrance to the reward arm. On choosing the incorrect, unbaited arm, the trial was terminated after a compensation distance of 110 mm, which corresponded to a movement into an arm by one body length. Next, the rat was teleported back to the central crossing.
After a correct choice and reward collection, feeders were retracted and the VR scene disappeared as the monitors turned black. The following trial commenced after the rat had returned to the starting position at the center of the ball.
During subsequent spatial orientation experiments, the visual cue served as a landmark instead of as a beacon. To accomplish this, a black square was presented at a position that was offset by 45° from the side of the reward feeder. Again, during initial training, only the baited feeder was advanced, while during testing all eight feeders were advanced. To help animals maintain performance, correction trials were performed after incorrect choices. For this, the wrongly chosen feeder was retracted and the animal was again required to choose among the remaining feeders. This procedure was repeated until the correct feeder was located. During the compensation mode and after an incorrect choice, the correction trials were given by teleporting an animal back to the maze’s center and removing the incorrectly entered arm from the VR scene, thus reducing the available options. This was repeated until the correct arm was entered.
Orientation with Acoustic Cues
To test a rat’s ability to use acoustic cues for orienting within VR, a linear corridor (4 m) was coupled to a gradient of acoustic cues that changed along the corridor in either pulse rate or frequency from a high value at one end to a low value at the other end. Rats began a trial at one of two starting positions (0.25 or 0.75 of the track’s length) and could hear the sound cue corresponding to that location. As the animal moved, the cue changed with position along the VR corridor. This change provided the additional polarity information. From the position and VR orientation, a rat could identify the end of the corridor closest to its current position. A reward was available at this near end.
Sequence of a Typical Experimental Session
Figure 3 explains the sequence of a typical experimental session. An individual rat living in the home cage (Fig. 3A) voluntarily approached the gating system where it was sensed by the first ID sensor while still in the tube connected to the home cage. If the arena was unoccupied and the animal’s individual experimental schedule permitted, the first (Fig. 3B, left) gate opened and the animal entered the gating system (Fig. 3B). The left gate closed and the sorting process proceeded as described above. Through a tube (Fig. 3C), the rat entered the circle of monitors to the platform surrounding the treadmill. The cylinder was raised to the waiting position, leaving an 8- to 10-cm gap through which the rat could access the treadmill (Fig. 3, D and E). The ball was locked into position by the servomotors and the VR was turned off. The rat’s position was tracked by video, and as soon as it arrived at the center of the arena on top of the ball (Fig. 3, F and G), the surrounding cylinder was lowered. The rat was now enclosed in the area for a session of typically 20 min in duration. The rat could walk in any direction within the arena. If this was an experiment containing compensation, rotary compensation was activated when the rat moved in the direction of the current alley, but only after it had crossed the boundary of a virtual central circle (Fig. 2, A and B). Within the central circle, the rat could turn and move slightly without activating the compensation mechanism. When the rat moved in a direction perpendicular to the current linear alley, compensation was not activated and the rat could walk toward and touch the cylinder wall. Water rewards were given at the end of the alleys at certain locations within the virtual maze. As the rat approached the end of an alley, the compensation mechanism ceased when the distance to the end had decreased to 25 cm (half the arena diameter). For this final section, the rat physically moved to the feeder in the arena’s wall. When the sphere stopped shortly before the end of an alley, the respective, previously retracted feeder was advanced into the arena. A trial ended when the animal made nose-poke contact with the feeder. The monitors displaying the VR scene turned off. To start the next trial, the rat was required to return to the sphere’s apex. After an incorrect choice, a 30-s timeout delayed the commencement of the following trial. An experimental session ended after a fixed time interval, and trials were not limited by number (Supplemental Video S1; Supplemental Material for this article is available online at the Journal website). When a session ended, the trial procedure immediately stopped, compensation was deactivated, and the cylinder moved upwards. The rat could then leave the sphere and walk through the connecting tube and through the open gate (right) into the gating system. On detection by an ID sensor, the right gate closed, the left gate opened and the rat returned to the home cage. In the home cage, the rat could either return to the gating system for the following session or remain in the home cage.
Electrophysiological Recordings
Behavioral training in an open-field virtual environment.
During the first stage (2-3 days), the rats were trained to associate feeders with water rewards. All feeders were in the advanced position, and the servo-driven locomotion compensation was turned off. The rats were required to explore the 49-mm arena for 20 min. During this time, the rats learned to poke their noses into the nose poke sensors and collect the rewards. During the second stage (3 wk), rats were trained to associate a black beacon with a reward feeder. During the first week, the rats could collect rewards from only one feeder marked by the beacon on the wall behind it, and every 5 min the position of the beacon and the reward randomly changed. This procedure was repeated for 2 wk, during which the duration of the beacon’s position was reduced to 3 min and then 1 min. In the third stage (4–6 days), the rats could collect a reward from only one feeder, which was marked by the beacon on the wall and the position of the beacon, and the reward position was randomly changed subsequent to the rat collecting a reward. During the fourth stage (4 wk), the environment was gradually enlarged and the servo-driven locomotion compensation was activated. Rats learned to adapt to the compensation of the ball. Each time the environment was enlarged, the rat was required to reach distances further from the beacon to collect the reward. After 9 wk, the rats were trained to move freely in the 1.2 × 1.2 m virtual environment and to collect rewards each time from one of the eight randomly appearing beacons.
Electrode implantation and surgery.
Tetrodes were constructed from four twisted 17-µm diameter HM-L coated platinum-iridium wires (90\/10%; California Fine Wire) and mounted in groups of four into a microdrive with a single turning screw and no separation between tetrodes. The electrode tips were plated with platinum to reduce electrode impedances to between 200 and 300 kV at 1 kHz. Anesthesia was induced by placing the animal in an induction cage with isoflurane 5% vapor. Following this, the rats were rapidly moved into a digital stereotaxic frame (Kopff), which had a mask connected to an isoflurane pump. Air flow was maintained at 1.2 l/min with 0.5–3.5% isoflurane, as determined by toe pinch reflexes and by constantly monitoring the rat respiratory rate. The body temperature of the rats was maintained at approximately 36°C using a closed-loop temperature controller (FHC) connected to a rectal temperature probe and a heating-pad placed under the rat. Local anesthetic (Lidocain) was applied to the skin before making the incision. A hole was drilled into the dorsal skull anterior to the transverse sinus to reach the entorhinal cortex. The coordinates for the entorhinal implant were 4.5 mm medilateral relative to the lambda and 0.3 mm anterior to the border of the transverse sinus. The tetrodes were slightly angled in the sagittal plane (10°, pointing in the anterior direction). A bone screw (shaft diameter: 1.17 mm; length: 4.70 mm) with a soldered wire was fixed to the skull in the frontal plate and served as a ground screw. An additional set of three to five bone screws (shaft diameter: 0.85 mm; length: 4.00 mm) were fixed to the skull and served as anchor screws for the mechanical stability of the implanted microdrive. The screws were then covered with dental acrylic that was used to secure the microdrive to the screws and to the skull.
Data collection.
During data collection, each rat was connected to the recording equipment (Neuralynx) via a unity-gain preamplifier (HS-16 or HS-18; Neuralynx) that was attached on one side to a connector on top of a custom-built microdrive and on the other side to a cable that allowed the animal to move freely within the VR cylinder space. The recorded signal was amplified (1,400–5,000 times) and band-pass filtered (6,00–6,000 Hz, Lynx-8; Neuralynx). A voltage threshold (70 μV) was used for collecting 1-ms spike waveforms, which were sampled at 30.3 kHz (0.25 ms before the peak of the spike and 0.75 ms after; Neuralynx Cheetah).
Tracking the position of the animal was performed using the Servoball optical tracking. The position of the rat in the 49-mm diameter cylinder was detected by a camera located above the cylinder (Xcam, Ycam), and the ball’s rotation as an indication of rat movement was detected by the two optical sensors placed above the servomotors (Xcomp, Ycomp); see Servoball, Optical Tracking, and Movement Compensation).
The position of the rat in the virtual environment (in units of real world mm) was computed by the following equations:
where αx = 0.557, αy = 0.543 were used to map the camera units into real world millimeter units, G is the gain between the optic flow and the ball rotation, and βx = 111, βy = 124 were used to map the ball rotation units into real world millimeters. αx, αy, βx, And βy were measured in a calibration session.
The two types of data (neuronal spikes and rat position) were time-stamped using different clocks. To synchronize the two clocks, the Servoball system sent 0-V/5-V-TTL signals to the Neuralynx system each 10 s with a jitter of 0–1 s. Synchronization was performed offline by matching between the two reports of the TTL trains in the two systems.
Spike sorting.
Spike sorting was manually performed offline using graphical cluster-cutting software (SpikeSort3D; Neuralynx). Each spike was graphically represented as a point in a two- or three-dimensional parameter space consisting of the energy or the height (peak to trough distance) of the spike on two or three of the tetrode’s four channels. To ensure that the well-separated clusters indeed contained spikes of a single unit, we checked that a refractory period (2 ms) was present in the interspike interval histogram of the cluster spikes (see Fig. 8E).
Fig. 8.
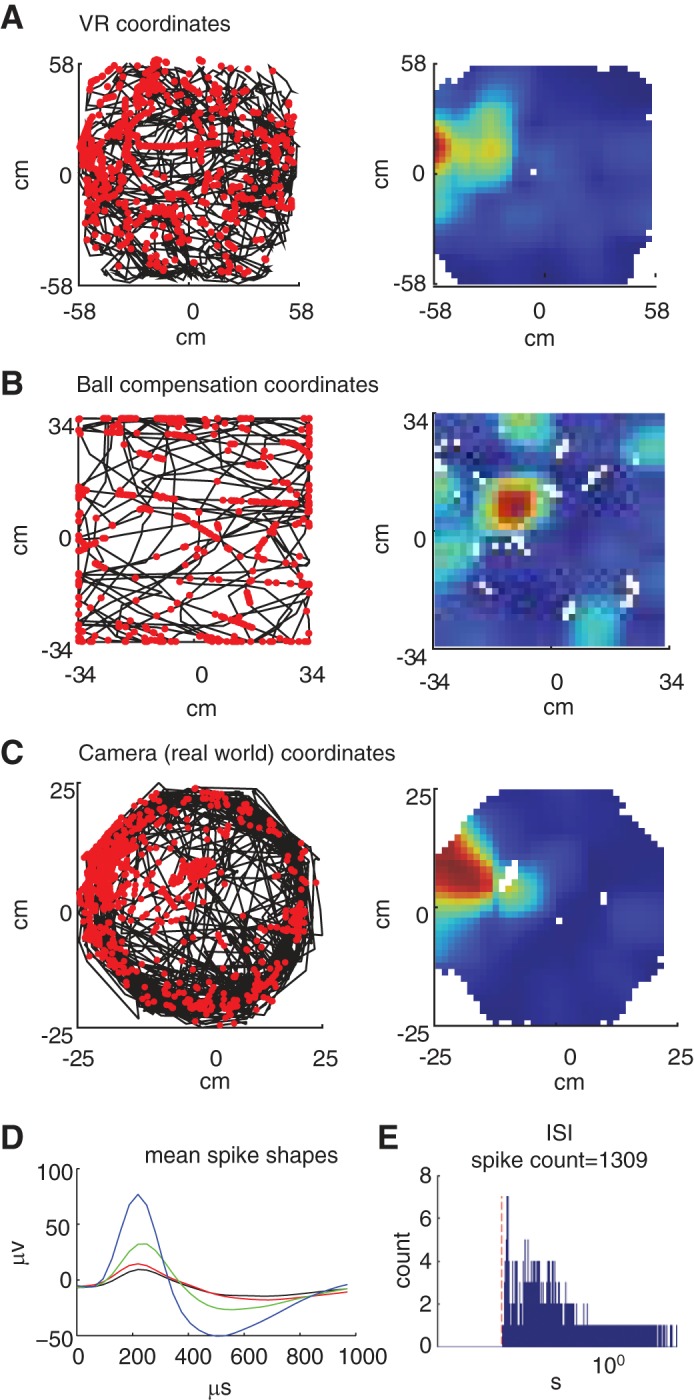
Recording of neural activity in a cell of the entorhinal cortex. One 30-min trial in which the rat was running freely in the environment and collecting water rewards from one of the eight possible reward sites in the virtual environment. A, left: trajectory of the rat and spikes in the virtual environment; the black line represents the trajectory of the rat in the virtual environment. Each red dot marks the location of the rat when the cell fired a spike. A, right: rate maps of the trajectory and spikes. B, left: trajectory and spikes according to the compensation of the ball; the black line represents the rotation of the ball during the trial (Xcomp, Ycomp). Each red dot marks the degree of rotation of the ball when the cell fired a spike. Right: Rate maps of the trajectory and spikes. C, left: trajectory and spikes according to the 50-cm diameter cylinder (Xcam, Ycam). The black line represents the trajectory of the rat according to the cylinder (Xcam, Ycam). Each red dot marks the locations of the rat when the cell fired a spike. C, right: rate maps of the trajectory and spikes. D: mMean spike shape: The mean spike shapes of each electrode in recording tetrode (red, green, blue, and black). E: interspike interval (ISI) histogram: Histogram of the ISI of the spike train. The red dashed line marks the 2-ms interval (refractory period). The x (time)-axis is in logarithmic scale.
Data analysis.
spatial rate map.
The firing rate map of the cell was computed by partitioning the arena into 35 equally spaced bins and dividing the number of spikes fired in each bin by the total amount of time spent in that bin. The rate map was then smoothed using a Gaussian kernel with an SD of 1.5 bins.
RESULTS
Individual Gating to the VR Arena
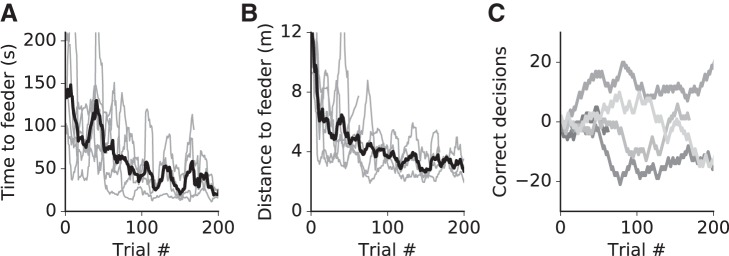
When with the group, even cautious individuals explored quickly. Within 4–6 days, the overall number of individual visits to feeders reached 374 ± 143 (means ± SE, day 5) per day (Fig. 4A, n = 4 groups with 3–4 individuals per group). After individual gating was switched to the VR arena, the animals passed the sorter without additional training (Fig. 4B). Each animal visited the Servoball arena 7 ± 1 times (means ± SE, n = 15) for drinking sessions per day. After activating the “shutting-in” procedure, rats required 5 days to adapt and reach a level of approximately six self-activated sessions per 24 h (Fig. 4C). Rats required two sessions (less than a day) to learn alternation (Fig. 4D) and then completed 38 ± 7 trials (means ± SE) per session with interreward intervals of 9.5 ± 2 s (means ± SE) (n = 8 subjects from sessions 3 to 8).
Treadmill Training
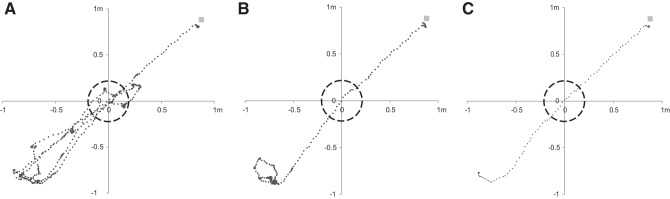
In a first series of experiments, compensation extended the locomotion distance between two opposing feeders. Rats required ~100 trials (1-3 days) to adapt to the treadmill function. This included becoming accustomed to the compensation mechanism and learning to walk on the moving substrate. Performance during training on a 62-cm corridor is shown in Fig. 4E. In subsequent sessions, the length of the corridor was gradually increased to 4.0 m (data not shown). Figure 5 shows a training example of a rat that adapted within the first 10 trials of a session to the extension of the virtual path from a length of 1.4 to 2.0 m.
Fig. 5.
Exemplary tracking paths. Exemplary tracking paths from a single session of one individual moving between the ends of a 2.0-m alley. The lines in A–C are plotted in the VR coordinate system and show data from trials 1, 5, and 10. In the sessions before the trials shown here, the rat was exposed to a 1.4 m alley. The actual distances moved and durations of the trials were 10.1 m and t = 71 s (T1, A), 3.8 m and 25.5 s (T5, B), and 2.6 m and 10.7 s (T10, C). The dashed circle indicates the diameter of the Servoball arena (49 cm) in relation to the dimensions of the alley. During training, the length of the virtual alleys was successively extended (from 1.4 to 2.0 m) and the rats adapted to this quickly, usually during the first session after the implementation of a change. Gray square top right: target feeder.
Beacon Orientation
Animals had passed all phases of pretraining within 3 wk. The following experiments tested the use of visual cues for navigation in VR (Fig. 6, A and B). In the first experiment, we investigated beacon-based orientation. Rats required 100–150 trials to learn the task (Fig. 6C, top left). After 100 trials, the locomotion distance traveled by an animal to reach the reward feeder had dropped from an initial 1.0 ± 0.6 m (means ± SE) to <0.5 m. During subsequent tests, all eight feeders were activated. All animals acquired the task (binomial test with 12.5% chance level, P < 0.001, n = 12, see Fig. A1). As a final step, the treadmill function was activated. During this function, we only presented a four-arm radial maze (Fig. 6B, bottom) to reduce complexity. Entering a maze arm by one body length was designated a decision. Six of 10 animals learned the task within 50 trials (binomial test, chance level 25%, P < 0.05, n = 6; Fig. 6C, bottom left).
Landmark Orientation
During training, only the feeder at the target location was presented. Within 100 trials, rats had reduced the locomotion distance to reward from 0.8 ± 0.21 m (means ± SE) to less than 0.5 m (Fig. 6C, top right). In subsequent tests, all feeders were presented, and rats chose the correct feeder location at a level above that of chance alone (binomial test with chance level 12.5%, P < 0.001, n = 10). After activating compensation mode with a four-arm maze, four rats successfully learned to enter the correct arm by using the landmark information (binomial test with chance level 25%, P < 0.001, n = 4, see Fig. A2).
Orientation Along an Acoustic Gradient
In natural environments, acoustic cues emanating from stationary sources or from fixed directions can also provide spatial information useful for orientation. We examined whether rats were able to use acoustic cues (Fig. 7A) instead of visual cues for orienting and locating a goal in VR. Preliminary data are shown in Fig. 7B. The rats learned the task and their behavior became increasingly more directed (see Fig. A3, A and B). After ~100 trials, they reached asymptotic performance for time or distance needed to arrive at a feeder position. At the end of training with a frequency gradient, one rat was able to use the pitch signal to identify its position along the path and orient toward the nearest reward feeder (binomial test, P = 0.04, see Fig. A3C). In the pulse rate version of the experiment, four individuals learned to use the pulse rate signal to identify their start location along the 4-m track and choose the correct direction toward the rewarding feeder and two of the four reached significance level (binomial test, P < 0.05, Fig. 7B).
Fig. 7.
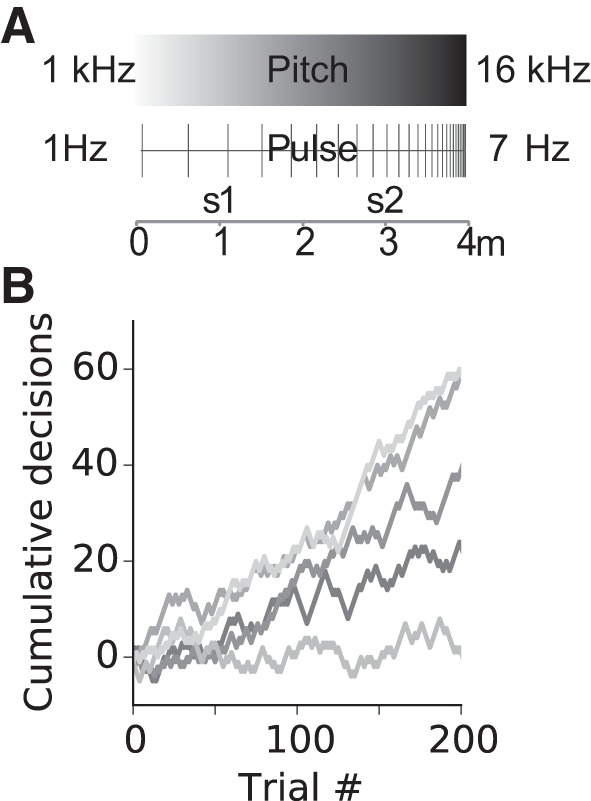
Navigation with acoustic cues. A: schematics of acoustic signal characteristics as they changed along a 4-m virtual corridor. In different experiments, either a frequency gradient or a pulse rate gradient was used. Trials commenced at the 1- or 3-m corridor location (s1 and s2). The visible virtual corridor was seemingly endless and absent visual cues indicating position. Positional information was contained in the frequency or pulse rate of the acoustic signal. From its starting location, the animal was required to move to the end of the closest corridor to obtain a reward. B: performance during pulse trials shown as cumulative correct (+1) or incorrect (−1) decisions for n = 5 animals. Line segments with positive slopes indicate phases with predominantly correct choices. Data are 50-trial moving averages. The four loudspeakers presented acoustic signals all with the same output.
Recordings of Neural Activity
As a final step, we aimed to provide evidence of the feasibility of recording neural activity during spatial behavior in Servoball VR. We implanted tetrodes into the medial entorhinal cortex (MEC). After pretraining, rats were connected to the recording equipment and moved freely in a virtual open field measuring 1.2 × 1.2 m. One of the eight feeders was randomly marked by a beacon, indicating the position of a reward. The trajectory of the rat in the virtual world and the positions where the single unit fired are shown in Fig. 8.
DISCUSSION
Behavioral experiments with rodents in VR seem to allow for the perfect control of sensory cues while investigating neural mechanisms (Dombeck and Reiser 2012; Thurley and Ayaz 2016). However, stimulation within most VR systems is limited to the visual system to which vestibular, tactile and proprioceptive inputs may be incongruent. Fixing the head or body unavoidably leads to mismatch, and this may perturb neural processing, e.g., altered hippocampal place cell activity in VR as compared with real world situations (Aghajan et al. 2015; Ravassard et al. 2013). A major challenge for rodent VR systems is controlling for the presence of experimentally intended sensory stimuli and the absence of unintended sensory stimuli and their consistent interplay. In the Servoball VR system, rodents move freely, and stimulation is therefore closer to natural conditions regarding the touch sensation of the walls and rotational vestibular encoding. However, while the treadmill is in the rotary compensation mode, the translational movement itself is only virtual. Furthermore, the artificial acceleration of the ball when it repositions the rat may lead to a mismatch between visual and motor signals when feedback correction is imperfect. With our 100-Hz camera, we had a 10-ms latency for detecting movement and an additional 10- to 25-ms latency for the servomotors to accelerate, adding to a minimum delay of 35 ms before initiating compensatory movement in reaction to animal displacement. Therefore, we do not consider this system suitable for compensating for small movements; however, it is well able to adapt to the steady pace of an animal moving down a corridor (see Supplementarl Video S1). Thus the unique possibilities of VR, such as convenient, controllable sensory cues, environments of arbitrary size or physically impossible environments, and closed-loop interactions with the animal, are all possible.
The great advantage of our system is that the rat is not head fixed. This may be considered a flaw in a number of applications, such as those involved in calcium imaging. However, the usual head fix implemented in common VR setups dramatically restricts behavior, so that any complex behavior, which surely requires head movement, is impossible to achieve. Moreover, the head fix is stressful for rats, and from our experience, unlike mice, it is dangerous to head fix rats without their consent, as they are much stronger than mice. When performing electrophysiological extracellular recordings from awake, behaving rats, there are cases in which head fixation dramatically alters the results. The most prominent example is perhaps the recording of place cells and grid cells, which clearly differ when the rat is running on a linear track. This can be simulated in the head-fixed VR setup vs. running in the open field, which cannot be simulated in the head-fixed case without creating significant changes to the properties of the cells (compare Aronov et al. 2014, in which the head is not fixed, to other studies with head or body fixation, such as Ravassard et al. 2013). Thus the use of the Servoball in open-field scenarios is of great value for allowing the combination of open-field behavior with recordings of nerve cells, while providing all the flexibility that a virtual environment can give.
Rats learned to use the automated experimental setup and VR within a few weeks, with 8-wk-old rats learning faster than those 6 wk of age. Most demanding for the animals to learn was the shutting-in process using the downward-closing cylinder. All rats learned to alternate between the ends of a linear track on the actively rotating sphere, comparable to alternating on a linear treadmill with one-dimensional compensation. Approximately 25% of animals showed cautious behavior during the compensation mode. Cautious rats did not leave the central circle for prolonged periods of time, thus avoiding a moving substrate. This was overcome by repeatedly and gradually introducing compensation. As an additional feature, we connected the VR arena to the animal’s home cage and obtained a fully automated, operator-independent behavioral training and operant experimental system that functioned 24 h per day. This was made possible by inserting an automated animal sorter that functioned as the mechanical replacement of the experimenter. For a similar approach with a touch screen setup, automation reduced daily experimental time for training rats by up to 80% (Rivalan et al. 2017). This versatile VR platform is well prepared to be combined with recordings of neural activity for investigating spatial navigation (Hölscher et al. 2005; Thurley et al. 2014; Youngstrom and Strowbridge 2012) and underlying neural mechanisms (Aronov and Tank 2014; Domnisoru et al. 2013; Harvey et al. 2009; Heys et al. 2014; Schmidt-Hieber and Häusser 2013), as well as perceptual decision making (Garbers et al. 2015; Harvey et al. 2012; Kautzky and Thurley 2016). We have included our preliminary results from electrophysiological extracellular recordings as proof of principle to demonstrate that despite the proximity of strong servomotors, such recordings are possible. While the automated access mechanism cannot be used with animals connected to a cable, even these animals can initially proceed through fully automated pretraining while carrying an unconnected (or wireless) head stage. In this case, multiple cages containing single housed animals may need to be connected to a multiple gate system.
The closed-loop manipulations of our VR environment enable multisensory control of multimodal behavior for a freely moving animal. In comparison to VR systems with head (Ravassard et al. 2013; Taube et al. 2013) or body (Hölscher et al. 2005; Thurley et al. 2014) fixation, our system, which permits free movement, provides a wider range of motion cues. The vestibular system and self-motion perception are stimulated by walking and turning on the treadmill. The monitors present a moving background pattern for optic flow and can display spatial cues indicating the locations of hidden rewards. Since an animal can change both its distance and angular point of view from the monitors, it obtains more natural visual information. This is relevant in the context of using more complex two-dimensional virtual space instead of simple straight paths in VR (Aghajan et al. 2015; Aronov and Tank 2014; Harvey et al. 2012; Thurley et al. 2014). Beyond vision, rats can receive tactile information from the surrounding transparent cylinder representing the virtual walls by using direct touch. This is possible at the ends of maze arms and within linear sections of maze arms if the animal moves sideways toward the wall. With its additional acoustic cues, the system allows for the investigation of multisensory control of multimodal behavior during food searches (Cushman et al. 2013).
The results from the virtual radial-maze learning tasks with visual beacon orientation and landmark orientation demonstrated that rats were able to perform allothetic spatial orientation in Servoball VR. Rats learned that a cue indicated the entrance to the VR goal arm and walked down that arm to collect rewards. Rats demonstrated their ability for allothetic orientation by using the angular relationship to a landmark to locate a goal. In VR setups with air-cushioned treadmills, boundaries cannot be physically implemented. Thus, an animal will reach a goal even if its vector of translation occasionally touches or even crosses a virtual wall. In contrast, rats within Servoball VR are confronted with a physical barrier when they reach the boundary of an open area.
Rats also oriented successfully using acoustic gradient cues where a cue was specific to a location in VR and changed either in pulse rate or pitch. Beyond directional acoustic cues (Cushman et al. 2013) that are comparable to a visual beacon, rats are expected to use both qualitative and quantitative components of acoustic cues to derive spatial information in VR. Our experiments show how this type of acoustic stimulation can be integrated into VR. Building on this by using two-dimensional stimulation or free field acoustics would strongly enhance the spectrum of VR system applications.
The electrophysiological data that we obtained provide a proof-of-concept that in this environment with its high-power active servomotors it is possible to perform electrophysiological recordings. Of course, some advantages of our home-cage environment are lost if investigators need to manually tether. However, automated training performed before tethering is still possible with rats carrying a head stage but yet without cable. Even if instrumented rats have to be singly housed, multiple cages with individual gates can be connected to the access tube for an automated sequencing of individual training.
As a tool, the Servoball VR with 24/7 automated training can be combined for cellular and physiological research with optogenetic techniques (Boyden et al. 2005; Zhang et al. 2006) or miniature microscopic applications (Ghosh et al. 2011; Ziv et al. 2013). This would open up new avenues of research using freely moving animals. As an animal does not require physical strength to move the servo-powered ball, the system would also be suited for smaller species, such as mice (see Supplemental Video S2) and gerbils, small primates such as mouse lemurs, or small cursorial birds such as quails, or even insects as done since the 1970s on the predecessor of the Servoball (Kramer 1975; 1976).
GRANTS
This work was supported by the Deutsche Forschungsgemeinschaft (Exc 257, NeuroCure, and Exc 277, Cognitive Interaction Technology).
DISCLOSURES
The Servoball experimental system is now made available through Humboldt University in collaboration with PhenoSys. Y. Winter owns private equity in PhenoSys. The other authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
U.K., K.F., G.T., and S.R. performed experiments; U.K., K.T., G.T., S.R., and D.D. analyzed data; U.K., D.D., and Y.W. interpreted results of experiments; U.K. and K.T. prepared figures; U.K. and Y.W. drafted manuscript; U.K., K.T., D.D., and Y.W. edited and revised manuscript; U.K., K.T., K.F., F.B., A.S., G.T., S.R., D.D., and Y.W. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank physicist Dr. Hans-Ulrich Kleindienst from the Max-Planck Institute for Behavioural Physiology in Seewiesen for dedicated support while developing this technology from 1970s precursors invented at that very Institute.
Appendix
Further Information on Experimental Procedures Using Acoustic Cues
The rat commenced at one of two points (at 0.25 or 0.75 of the track’s length, designated as s1 or s2 in Fig. 7, respectively) selected at random. The animal commenced a trial by entering the central region of the ball (the “inner circle”). The monitors showed the landscape with striped walls along the corridor, and the compensation mode was activated. Compensation occurred for movement along the direction of the corridor until an invisible wall within the visually endless corridor was reached. The sound associated with each location along the corridor was repeatedly emitted. As the rat moved along the corridor, the direction of the change in pitch or pulse rate allowed the rat to determine the orientation of the VR corridor.
The task was to visit the end closest to the starting location, where the rat could obtain a water reward. On collecting the reward and after a 3-s intertrial-interval (ITI) with monitors turned off, a new trial was initiated as soon as the rat had returned to the center position. On selecting an incorrect feeder, an additional timeout (15 s of monitors off) preceded the ITI. A trial started with teleportation to a randomly selected location (s1 or s2). In the pitch experiment, the frequency changed from 1,000 to 16,000 Hz between the two ends of the corridor, and was presented as pulses with a duty cycle of 100 ms on and 233 ms off. At the experimental start locations, the frequencies were 4,750 Hz (s1, Fig. 7) and 12,250 Hz (s2, Fig. 7). In the pulse experiment, the frequency was constant at 10 kHz and was presented as a pulse with a changing duty cycle of 100 ms on and between 50 and 1,000 ms off. At the experimental start locations, the pulse off times were 288 ms (s1, Fig. 7) and 763 ms (s2, Fig. 7).
Figure A1 shows beacon-based navigation. Figure A2 shows landmark-based navigation. Figure A3 shows navigation with acoustic cues.
VR and Experimental Control Software
The last software version used before changing to PhenoSoft Control (PhenoSoft) is openly available on Github, at https://github.com/Servoball.
Fig. A1.
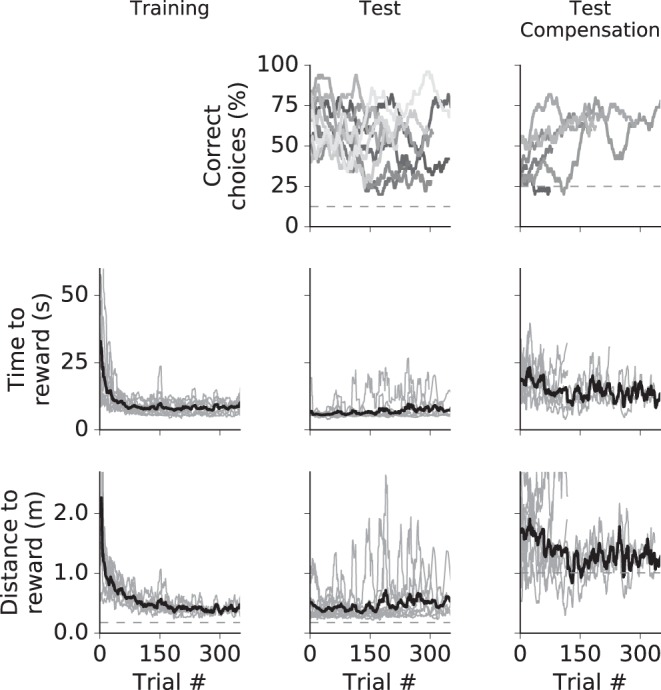
Beacon-based navigation. The columns show data for training, test phase, and test with compensation. Top: cumulative correct decisions for all animals (different animals may have performed different numbers of trials). Dashed line shows level of random choice. Middle: time to reward for all animals (light gray lines) and average (black line). Data from single animals are gliding averages with a 50-trial window. Bottom: distance to reward. Dashed line shows the minimum distance.
Fig. A2.
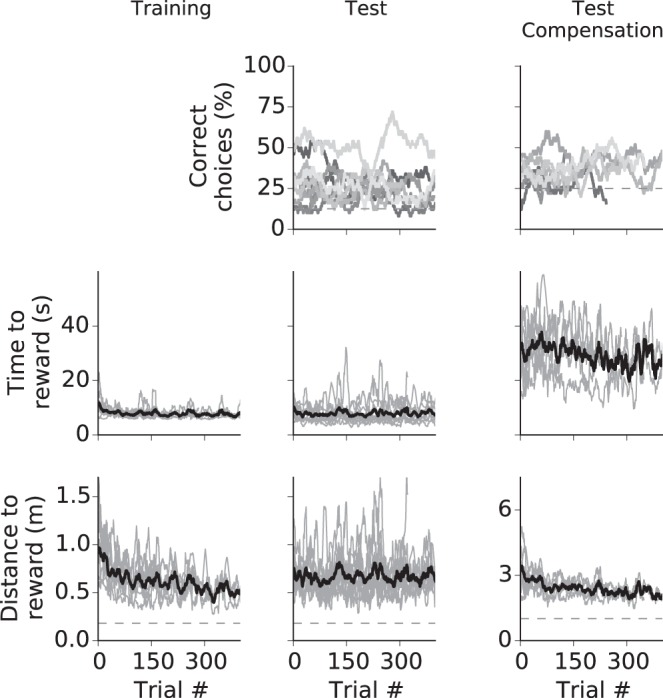
Landmark-based navigation. The columns show data for training, test phase and test with compensation. Top: cumulative correct decisions for all animals (different animals may have performed different numbers of trials). The dashed line shows the level of random choice. Middle: time to reward for all animals (light gray lines) and average (black line). Data from single animals are gliding averages with a 50-trial width. Bottom: distance to reward. Dashed line shows the minimum distance.
Fig. A3.
Navigation with acoustic cues (pitch experiment). Gray lines are time to feeder (A) and distance (B) to feeder for n = 5 animals, and a corresponding mean (black line) and standard error (gray area). Behavior became more directed within the first 100 trials, leading to a reduction of total locomotion distance and time to reach a feeder. Data from single animals are gliding averages with a 50-trial window. Different animals completed different numbers of trials. C: performance in (cumulative decisions, coded with + or −1, respectively) shows positive learning behavior for 1 individual.
REFERENCES
- Aghajan ZM, Acharya L, Moore JJ, Cushman JD, Vuong C, Mehta MR. Impaired spatial selectivity and intact phase precession in two-dimensional virtual reality. Nat Neurosci 18: 121–128, 2015. doi: 10.1038/nn.3884. [DOI] [PubMed] [Google Scholar]
- Anikeeva P, Andalman AS, Witten I, Warden M, Goshen I, Grosenick L, Gunaydin LA, Frank LM, Deisseroth K. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat Neurosci 15: 163–170, 2011. doi: 10.1038/nn.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov D, Tank DW. Engagement of neural circuits underlying 2D spatial navigation in a rodent virtual reality system. Neuron 84: 442–456, 2014. doi: 10.1016/j.neuron.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohil CJ, Alicea B, Biocca FA. Virtual reality in neuroscience research and therapy. Nat Rev Neurosci 12: 752–762, 2011. doi: 10.1038/nrn3122. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8: 1263–1268, 2005. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Cushman JD, Aharoni DB, Willers B, Ravassard P, Kees A, Vuong C, Popeney B, Arisaka K, Mehta MR. Multisensory control of multimodal behavior: do the legs know what the tongue is doing? PLoS One 8: e80465, 2013. doi: 10.1371/journal.pone.0080465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombeck DA, Reiser MB. Real neuroscience in virtual worlds. Curr Opin Neurobiol 22: 3–10, 2012. doi: 10.1016/j.conb.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Domnisoru C, Kinkhabwala AA, Tank DW. Membrane potential dynamics of grid cells. Nature 495: 199–204, 2013. doi: 10.1038/nature11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers C, Henke J, Leibold C, Wachtler T, Thurley K. Contextual processing of brightness and color in Mongolian gerbils. J Vis 15: 13, 2015. doi: 10.1167/15.1.13. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, Schnitzer MJ. Miniaturized integration of a fluorescence microscope. Nat Methods 8: 871–878, 2011. doi: 10.1038/nmeth.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Coen P, Tank DW. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature 484: 62–68, 2012. doi: 10.1038/nature10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Collman F, Dombeck DA, Tank DW. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461: 941–946, 2009. doi: 10.1038/nature08499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heys JG, Rangarajan KV, Dombeck DA. The functional micro-organization of grid cells revealed by cellular-resolution imaging. Neuron 84: 1079–1090, 2014. doi: 10.1016/j.neuron.2014.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölscher C, Schnee A, Dahmen H, Setia L, Mallot HA. Rats are able to navigate in virtual environments. J Exp Biol 208: 561–569, 2005. doi: 10.1242/jeb.01371. [DOI] [PubMed] [Google Scholar]
- Iyer SM, Montgomery KL, Towne C, Lee SY, Ramakrishnan C, Deisseroth K, Delp SL. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat Biotechnol 32: 274–278, 2014. doi: 10.1038/nbt.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautzky M, Thurley K. Estimation of self-motion duration and distance in rodents. R Soc Open Sci 3: 160118, 2016. doi: 10.1098/rsos.160118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller GB, Bonhoeffer T, Hübener M. Sensorimotor mismatch signals in primary visual cortex of the behaving mouse. Neuron 74: 809–815, 2012. doi: 10.1016/j.neuron.2012.03.040. [DOI] [PubMed] [Google Scholar]
- Kramer E. Orientation of the male silkmoth to the sex attractant bombykol. Olfact Taste 5: 329–335, 1975. [Google Scholar]
- Kramer E. The orientation of walking honeybees in odour fields with small concentration gradients. Physiol Entomol 1: 27–37, 1976. doi: 10.1111/j.1365-3032.1976.tb00883.x. [DOI] [Google Scholar]
- Ravassard P, Kees A, Willers B, Ho D, Aharoni D, Cushman J, Aghajan ZM, Mehta MR. Multisensory control of hippocampal spatiotemporal selectivity. Science 340: 1342–1346, 2013. doi: 10.1126/science.1232655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivalan M, Munawar H, Fuchs A, Winter Y. An automated, experimenter-free method for the standardised, operant cognitive testing of rats. PLoS One 12: e0169476, 2017. doi: 10.1371/journal.pone.0169476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Häusser M. Cellular mechanisms of spatial navigation in the medial entorhinal cortex. Nat Neurosci 16: 325–331, 2013. doi: 10.1038/nn.3340. [DOI] [PubMed] [Google Scholar]
- Sieu LA, Bergel A, Tiran E, Deffieux T, Pernot M, Gennisson JL, Tanter M, Cohen I. EEG and functional ultrasound imaging in mobile rats. Nat Methods 12: 831–834, 2015. doi: 10.1038/nmeth.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Valerio S, Yoder RM. Is navigation in virtual reality with FMRI really navigation? J Cogn Neurosci 25: 1008–1019, 2013. doi: 10.1162/jocn_a_00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurley K, Ayaz A. Virtual reality systems for rodents. Curr Zool 63: 109–119, 2016. doi: 10.1093/cz/zow070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurley K, Henke J, Hermann J, Ludwig B, Tatarau C, Wätzig A, Herz AVM, Grothe B, Leibold C. Mongolian gerbils learn to navigate in complex virtual spaces. Behav Brain Res 266: 161–168, 2014. doi: 10.1016/j.bbr.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Winter Y, Schaefers AT. A sorting system with automated gates permits individual operant experiments with mice from a social home cage. J Neurosci Methods 196: 276–280, 2011. doi: 10.1016/j.jneumeth.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Youngstrom IA, Strowbridge BW. Visual landmarks facilitate rodent spatial navigation in virtual reality environments. Learn Mem 19: 84–90, 2012. doi: 10.1101/lm.023523.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods 3: 785–792, 2006. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ. Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci 16: 264–266, 2013. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.