Abstract
This report describes a systematic literature review of voucher and related monetary-based contingency management (CM) interventions for substance use disorders (SUDs) over 5.2 years (November 2009 through December 2014). Reports were identified using the search engine PubMed, expert consultations, and published bibliographies. For inclusion, reports had to (a) involve monetary-based CM; (b) appear in a peer-reviewed journal; (c) include an experimental comparison condition; (d) describe an original study; (e) assess efficacy using inferential statistics; (f) use a research design allowing treatment effects to be attributed to CM. Sixty-nine reports met inclusion criteria and were categorized into 7 research trends: (1) extending CM to special populations, (2) parametric studies, (3) extending CM to community clinics, (4) combining CM with pharmacotherapies, (5) incorporating technology into CM, (6) investigating longer-term outcomes, (7) using CM as a research tool. The vast majority (59/69, 86%) of studies reported significant (p < 0.05) during-treatment effects. Twenty-eight (28/59, 47%) of those studies included at least one follow-up visit after CM was discontinued, with eight (8/28, 29%) reporting significant (p < 0.05) effects. Average effect size (Cohen’s d) during treatment was 0.62 (95% CI: 0.54, 0.70) and post-treatment it was 0.26 (95% CI: 0.11, 0.41). Overall, the literature on voucher-based CM over the past 5 years documents sustained growth, high treatment efficacy, moderate to large effect sizes during treatment that weaken but remain evident following treatment termination, and breadth across a diverse set of SUDs, populations, and settings consistent with and extending results from prior reviews.
Keywords: Contingency management, Financial incentives, Substance use disorders, Psychosocial interventions, Behavioral interventions, Behavioral economics, Behavior change
1. Introduction
Substance use disorders (SUDs) are highly prevalent in the U.S. as they are in other developed countries. These disorders undermine health and longevity and are tremendously costly economically. In the U.S. population, for example, approximately 20% of adults report past month tobacco use, 25% report past month risky alcohol use (e.g., binge drinking), and 10% report past month illicit drug use (SAMSHA, 2014). Excessive use of tobacco, alcohol, and illicit drugs in the U.S. each year is estimated to result in N600 thousand premature deaths, 166 billion dollars in U.S. annual healthcare costs, and 700 billion dollars in overall annual costs related to crime, lost work productivity, and healthcare combined (U.S. Department of Health and Human Services, 2014; CDC, 2014; NDIC, 2011). Similar patterns of use and adverse consequences are well documented internationally (UNODC, 2015). Given these enormous adverse impacts, the development of more effective treatments for SUDs is a critically important public health priority.
Contingency management (CM) interventions wherein financial incentives are provided contingent on objective evidence of behavior change have shown impressive levels of efficacy across a wide range of SUDs (Higgins et al., 2008). Early iterations of this general treatment model were first reported in the late 1960s (Elliott and Tighe, 1968) and further refined in the 1970s and 1980s, typically among opioid-dependent populations enrolled in methadone-based substitution therapy (Stitzer and Higgins, 1995). Voucher-based CM wherein individuals receive vouchers exchangeable for retail items or other financial incentives was introduced in the early 1990s as part of efforts to develop efficacious outpatient treatments for cocaine dependence (Higgins et al., 1991, 1993). The success of that model in promoting sustained periods of cocaine abstinence was associated with an expansion of CM research to other SUDs and an acceleration of research on this treatment strategy (Fig. 1).
Fig. 1.
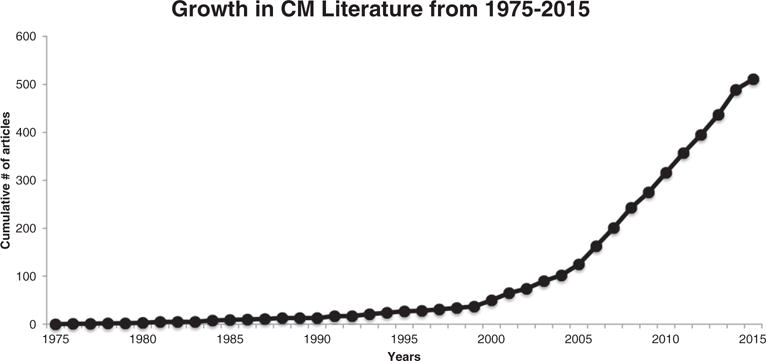
Cumulative plot of the number of citations identified in a PubMed search of the term ‘contingency management’ involving substance use disorders (SUDs). The search included all citations through May of 2015.
Along with this growth in CM research came the need for periodic literature reviews. Our group previously published two comprehensive literature reviews on this topic (Higgins et al., 2011; Lussier et al., 2006), with the present report representing the third in what is intended to be a systematic series that covers the time period from the introduction of the voucher model in 1991 to the present. The initial review was a meta-analysis comprising 40 controlled studies published in peer-reviewed journals between January 1991 and March 2004 (13.25 years). The review focused exclusively on treatment effects during the intervention period. There was overwhelming evidence of efficacy of this treatment approach for increasing abstinence from drug use and retention in treatment across a wide range of different types of SUDs, with overall effect sizes in the moderate range (Lussier et al., 2006). That review also examined potential moderators of treatment efficacy identifying two that significantly increased effect size (i.e., higher incentive monetary values and shorter delays in delivering incentives).
The second review in this series was a narrative review that again examined controlled studies published in peer-reviewed journals (Higgins et al., 2011). The time period of interest was from April 2004 (i.e., the end date in the prior review) to October 2009 (5.5 years). Sixty-seven reports met inclusion criteria, a more than three-fold increase in publications per year over the earlier review. The results were consistent with the earlier review in providing overwhelming evidence of efficacy, with 59 of the 67 (88%) reports noting significant treatment effects. Importantly, this second review also organized the growth in the CM literature into seven trends, a practice that is continued in the current review.
Important to mention is that there have been other reviews of the CM literature. One focused on CM involving a wide range of different types of incentives (Prendergast et al., 2006), another was limited to only CM studies that used a probabilistic schedule of incentive delivery (Benishek et al., 2014), still another on CM interventions implemented in outpatient methadone maintenance clinics (Griffith et al., 2000), and one that examined only CM studies that included a cost-effectiveness analysis (Shearer et al., 2015). However, none of those reviews duplicate the focus of the present systematic review on contributing to a series exclusively focused on the use of vouchers and related financial incentives among those with SUDs.
In the two prior reports in this series, several gaps in the CM literature were identified. Lussier et al. (2006) called for additional studies on the influence of incentive value/magnitude and CM duration on treatment efficacy. Higgins et al. (2011) recommended that future studies address technical obstacles that, at the time the review was written, had precluded the use of CM with certain SUDs (e.g., alcohol) or to individuals residing in geographically remote areas. Both reports recommended that future research on this topic should evaluate the maintenance of treatment gains after the incentives were discontinued.
The aims of the present review include (1) characterizing the foci and outcomes of CM studies published in the 5.2 year period between November 2009 through 2014, (2) characterizing research trends across this most recent period compared to the prior reviews, and (3) evaluating whether previously identified gaps in the CM literature have been addressed.
2. Methods
The methods employed in the current review are based on those used in Higgins et al. (2011). More specifically, the literature search was conducted using Pubmed, the search engine of the U.S. National Library of Medicine. Pubmed was searched using the term “vouchers OR contingency management,” targeting articles published between November 1, 2009 through December 31, 2014. Reference sections of review papers retrieved by this search were also searched. All articles were reviewed for inclusion by at least two of the authors and discrepancies resolved through discussion.
For inclusion, reports had to (a) involve a monetary-based CM intervention; (b) appear in a peer-reviewed journal; (c) include an experimental comparison condition; (d) report during-treatment results from an original, prospective experimental study; (e) examine treatment effects using inferential statistics; and (f) use a research design allowing attribution of treatment effects to CM. These criteria differ from the inclusion criteria used in the initial review where they also required that studies focus on individuals enrolled in formal treatment and have a sample size ≥10 individuals (Lussier et al., 2006), but match those used in the immediately preceding review (Higgins et al., 2011).
The reports included in the current review were characterized in terms of the behavior change targeted by the CM intervention, whether CM produced statistically significant (p < 0.05) treatment effects, whether the study examined post-incentive treatment effects, the size (Cohen’s d) of treatment effects reported, and discernible trends in the CM literature. Effect sizes were not quantified in the 2011 review. A study was categorized as having a significant treatment effect if effects of the CM intervention on at least one primary outcome during treatment or at treatment termination assessment were significant (p < 0.05). Similarly, a study was categorized as having a significant effect at follow-up if significant treatment effects on one or more primary outcomes remained significant following discontinuation of incentives (p < 0.05). As such, only those studies reporting significant during-treatment effects were evaluated for post-incentive treatment effects, with the focus on examining the sustainability of treatment effects once incentives were discontinued. In contrast to the prior review wherein articles were categorized into only a single primary trend, articles in the current review were also assigned to secondary trends when appropriate (see Tables 1–4). Identifying additional trends in the literature was not part of the initial review. Authors worked in pairs in identifying trends with any discrepancies resolved through discussion.
Table 1.
Extending the intervention to special populations.
| Study | Primary trend | Additional trends | n | CM duration (weeks) | Maximum earnings | Statistically significant treatment effect | Included follow up? | Statistically significant effects at follow-up | Drug of abuse | CM target |
|---|---|---|---|---|---|---|---|---|---|---|
| Alessi and Petry (2014) | Special populations | Community clinics | 45 | 4 | CNBD | Yes | Yes | No | Nicotine | Abstinence |
| Businelle et al. (2014) | Special populations | N/A | 68 | 5 | $150 | Yes | No | N/A | Nicotine | Abstinence |
| Drummond et al. (2014) | Special populations | N/A | 100 | 24 | $225 | Yes | No | N/A | Nicotine | Abstinence |
| Dunn et al. (2010) | Special populations | Pharmacotherapy | 40 | 2 | $362.50 | Yes | Yes | No | Nicotine | Abstinence |
| García-Fernández et al. (2013) | Special populations | N/A | 108 | 24 | $2196.00 | Yes | No | N/A | Cocaine | Abstinence |
| Hagedorn et al. (2013) | Special populations | Community clinics | 332 | 8 | CNBD | Yes | Yes | No | Polydrug | Abstinence |
| Hertzberg et al. (2013) | Special populations | Technology | 22 | 4 | $690 (530 for CM) | No | N/A | N/A | Nicotine | Abstinence |
| Higgins et al. (2014) | Special populations | Parametric questions, longer-term outcomes | 130 | 52 | $1180 | Yes | Yes | No | Nicotine | Abstinence |
| Holtyn et al. (2014) | Special populations | N/A | 98 | 30 | $6000 | Yes | Yes | No | Cocaine/Opioids | Abstinence + other therapeutic goal |
| Kaminer et al. (2014) | Special populations | N/A | 79 | 7 | CNBD | No | N/A | N/A | Marijuana | Abstinence |
| Kelly et al. (2014) | Special populations | Community clinics | 160 | 6 | CNBD | Yes | No | N/A | Polydrug | Other therapeutic goal |
| Kendzor et al. (2014) | Special populations | Pharmacotherapy | 146 | 4 | $150 | Yes | Yes | Yes | Nicotine | Abstinence |
| Kidorf et al. (2013) | Special populations | Community clinics | 125 | 12 | $300.00 | Yes | No | N/A | Opioids | Other therapeutic goal |
| Krishnan-Sarin et al. (2013) | Special populations | N/A | 157 | 4 | $262 | Yes | Yes | No | Nicotine | Abstinence |
| McDonell et al. (2013) | Special populations | Community clinics | 176 | 12 | CNBD | Yes | Yes | Yes | Polydrug | Abstinence |
| Menza et al. (2010) | Special populations | N/A | 127 | 12 | $453.75 | No | N/A | N/A | Methamphetamine | Abstinence |
| Ondersma et al. (2012) | Special populations | Community clinics, parametric, technology | 110 | 10 | $50.00 | No | N/A | N/A | Nicotine | Abstinence |
| Petry et al. (2010) | Special populations | Community clinics, parametric | 170 | 24 | CNBD | Yes | Yes | No | Cocaine/opioids | Other therapeutic goal |
| Petry et al. (2013) | Special populations | Community clinics | 19 | 8 | CNBD | Yes | No | N/A | Cocaine | Abstinence |
| Reback et al. (2010) | Special populations | Community clinics | 131 | 24 | CNBD | Yes | Yes | Yes | Polydrug | Abstinence + other therapeutic goal |
| Schottenfeld et al. (2011) | Special populations | Longer-term outcomes | 145 | 24 | $935 | Yes | Yes | Yes | Cocaine | Abstinence |
| Secades-Villa et al. (2013) | Special populations | Community clinics | 118 | 24 | $2196.00 | Yes | No | N/A | Cocaine | Abstinence |
| Stanger et al. (2009) | Special populations | N/A | 69 | 12 | $570 | Yes | Yes | No | Marijuana | Abstinence |
Note: primary trend refers to main trend authors assigned study to; additional trends refers to additional trends study was assigned to aside from the primary trend; n refers to sample size (across all groups); CM duration refers to the number of weeks during which contingent incentives could be earned (CNBD = duration could not be determined); Maximum earnings refers to the maximum amount that could be earned in the intervention (CNBD = maximum earnings could not be determined); Statistically significant treatment effects and effects at follow-up defined in all studies as outcomes significant at p < 0.05; Follow up effects were only evaluated if during treatment effects were statistically significant; drug of abuse refers to drug targeted by CM intervention; CM target refers to the behavior on which incentives were contingent.
Table 4.
Investigating longer-term outcomes and trends using CM as a research tool.
| Study | Primary trend | Additional trends | n | CM duration (weeks) | Maximum earnings | Statistically significant treatment effect | Included follow up? | Statistically significant effects at follow-up | Drug of abuse | CM target |
|---|---|---|---|---|---|---|---|---|---|---|
| Carroll et al. (2012) | Longer-term outcomes | Special populations, community clinics | 127 | 12 | $250 | Yes | Yes | No | Marijuana | Abstinence + other therapeutic goals |
| DeFulio and Silverman (2011) | Longer-term outcomes | N/A | 51 | 52 | CNBD | Yes | Yes | No | Cocaine | Abstinence |
| McKay et al. (2010) | Longer-term outcomes | N/A | 100 | 12 | $1150.00 | Yes | Yes | Yes | Cocaine | Abstinence |
| Secades-Villa et al. (2011) | Longer-term outcomes | N/A | 64 | 52 | $1111.58 | Yes | Yes | Yes | Cocaine | Abstinence |
| Wang et al. (2014) | Longer-term outcomes | Community clinics | 2662 | 24 | CNBD | No | N/A | N/A | Opioids | Abstinence + other therapeutic goals |
| Bradstreet et al. (2014) | As a research tool | N/A | 34 | 2 | $507.50 | Yes | No | N/A | Nicotine | Abstinence |
| Kurti and Dallery (2014) | As a research tool | N/A | 20 | 4 | CNBD | Yes | No | N/A | Nicotine | Abstinence |
Note: primary trend refers to main trend authors assigned study to; Additional trends refers to additional trends study was assigned to aside from the primary trend; n refers to sample size (across all groups); CM duration refers to the number of weeks during which contingent incentives could be earned (CNBD = duration could not be determined); Maximum earnings refers to the maximum amount that could be earned in the intervention (CNBD = maximum earnings could not be determined); Statistically significant treatment effects and effects at follow-up defined in all studies as outcomes significant at p < 0.05; Follow up effects were only evaluated if during treatment effects were statistically significant; drug of abuse refers to drug targeted by CM intervention; CM target refers to the behavior on which incentives were contingent.
Cohen’s d was used as the measure of effect size and was calculated for each study, except for ten reports that did not have enough information available to calculate an effect size (Killeen et al., 2012; Krishnan-Sarin et al., 2013; Kurti and Dallery, 2014; Meredith et al., 2011; Ondersma et al., 2012; Reback et al., 2010; Tuten et al., 2012; Walker et al., 2010; Winstanley et al., 2011; Wang et al., 2014). For continuous outcomes, study effect sizes were computed based on the reported test statistic. If an appropriate test statistic was not available, effect sizes were computed based on means and standard deviations (or standard errors) presented in the text, tables or figures. For dichotomous outcomes, study effect sizes were computed based on the odds ratio. If an odds ratio was not reported, a 2 × 2 table was constructed from the reported percentages and the odds ratio was calculated. Odds ratios were then converted to Cohen's d. For studies involving multiple incentive-based treatment conditions, a single effect size was obtained by combining the incentive-based treatment conditions and calculating the effect size for the combined treatment conditions versus control condition or by calculating a weighted average of the individual effect sizes for each incentive-based treatment condition relative to the control condition. Random effects meta-analysis models were used to calculate the estimated average effect sizes and the corresponding 95% confidence intervals across studies and within the seven trends. These models were analyzed using Comprehensive Meta-Analysis 3.0 (Biostat Inc., Englewood, NJ).
3. Results
3.1. Overall search results
The search identified 801 reports for review, of which 69 (9%) met inclusion criteria. Of the 732 studies excluded, 589 (80.5%) did not involve monetary-based CM, 114 (15.6%) did not report results from an original, experimental study, 23 (3.1%) did not use a research design allowing treatment effects to be attributed to CM, and 6 (1%) did not include an experimental comparison condition.
Sixty-nine articles across 5.2 years represent an annual publication rate of 13.3 reports. Most of these studies focused exclusively on increasing abstinence from drug use (51 studies or 73.9%). Ten studies (14.4%) exclusively targeted another therapeutic goal, and 8 (11.5%) targeted both abstinence and another therapeutic goal.
CM was highly efficacious across the three different targets, with 59 of 69 studies (86%) reporting significant treatment effects. Among studies exclusively targeting abstinence, 43 of 51 studies (84%) reported significant treatment effects; 9 of 9 studies (100%) exclusively targeting other therapeutic goals reported significant treatment effects, as did 7 of 9 studies (78%) targeting both abstinence and other therapeutic goals. Average effect size among studies reporting significant during-treatment effects was 0.66 (95% CI: 0.58, 0.75). Average effect size across all studies examining during-treatment effects was 0.62 (95% CI: 0.54, 0.70).
Among the 59 studies reporting significant treatment effects, 28 (47%) included at least one follow-up assessment after the incentives intervention was discontinued, with eight of these 28 studies (29%) reporting significant treatment effects at one or more follow-up visits. Average effect size across studies reporting significant post-treatment effects was 0.43 (95% CI: 0.24, 0.62). Average effect size across all studies examining post-treatment effects was 0.26 (95% CI: 0.11, 0.41).
3.2. Trends in the literature
The 69 reports meeting inclusion criteria were categorized into seven trends, six of which were consistent with those in the immediately prior review (Higgins et al., 2011). Trends represented in both reviews include (1) extending CM to special populations, (2) conducting parametric studies, (3) extending CM to community clinics,(4) combining CM with pharmacotherapies, (5) investigating longer-term outcomes, and (6) using CM as a research tool. The present review also includes an additional trend of (7) integrating novel technologies (e.g., Smartphones) into CM. In the prior review an additional trend identified was extending CM to new SUDs, but that was not a primary aim in any of the studies in the current review. Below we comment on the above trends starting with those involving the largest number of reports and working to the least, while giving priority to primary trends.
3.2.1. Extending CM to special populations
Extending CM to special populations (e.g., adolescents, pregnant women) is the trend under which the most studies were categorized (23/69, 33%) (Table 1). Eighteen of these 23 studies (78%) targeted abstinence, 2 (9%) targeted abstinence and another outcome, and 3 (13%) targeted another outcome only. Nineteen of the 23 studies (83%) reported significant treatment effects. Effect sizes could be calculated for 17 of those 19 studies, the average of which was 0.67 (95% CI: 0.53, 0.82). Effect sizes could be calculated for 20 of the total 23 studies categorized under this trend, the average of which was 0.64 (95% CI: 0.49, 0.78). No systematic differences in efficacy by CM target or population were noted.
Twelve studies with significant during-treatment effects also reported outcomes following discontinuation of the incentives, with four (33%) of those studies reporting significant treatment effects at one or more follow-up assessments. Effect sizes could be calculated for three of those four studies, the average of which was 0.27 (95% CI: 0.10, 0.43).
Among the 15 studies categorized under this trend that included one or more follow-up assessments, effect sizes could be calculated for 10, with an average effect size of 0.23 (95% CI: −0.01, 0.47).
Regarding illicit drug use disorders, studies were reported on CM reducing marijuana use (Kaminer et al., 2014; Stanger et al., 2009) among adolescents, and psychomotor stimulant and poly-drug use among those with co-morbid mental illness (García-Fernández et al., 2013; Kelly et al., 2014; McDonell et al., 2013; Petry et al., 2013), socioeconomic disadvantage (Secades-Villa et al., 2013), pregnant women and mothers of young children (Schottenfeld et al., 2011), sexual minorities (specifically men who have sex with men, Menza et al., 2010; Reback et al., 2010), those with HIV infection (Petry et al., 2010), and military veterans (Hagedorn et al., 2013).
Cigarette smoking is highly prevalent in the special populations listed above and nine of the 24 reports in this trend (38%) focused on smoking cessation, including studies with socioeconomically disadvantaged pregnant (Higgins et al., 2014; Ondersma et al., 2012) and non-pregnant adults (Kendzor et al., 2014), adolescents (Krishnan-Sarin et al., 2013), homeless individuals (Businelle et al., 2014), and individuals with co-morbid SUDs (Alessi and Petry, 2014; Drummond et al., 2014; Dunn et al., 2010) or mental illness (Hertzberg et al., 2013). This application of CM is still in the initial efficacy-testing stages of development for most of these populations except for pregnant women where research is now focused on late-stage efficacy and cost-effectiveness testing (see Higgins and Solomon, 2016).
3.2.2. Investigating parametric questions
Investigating questions about how altering CM parameters impacts treatment outcomes is obviously an important area of inquiry (Table 2). In the current review, 11 out of 69 studies (16%) were categorized under this trend. Ten of the 11 studies (91%) targeted abstinence, and one (9%) targeted both abstinence and another therapeutic goal. Ten studies (91%) reported significant during-treatment effects with an average effect size of 0.63 (95% CI: 0.44, 0.82). The one study that failed to produce a significant during-treatment effect also failed to include sufficient information to calculate an effect size. Thus, the overall effect size is the same as the one reported above for studies producing significant effects.
Table 2.
Investigating parametric questions and extending the intervention into community clinics.
| Study | Primary trend | Additional trends | n | CM duration (weeks) | Maximum earnings | Statistically significant treatment effect | Included follow up? | Statistically significant effects at follow-up | Drug of abuse | CM target |
|---|---|---|---|---|---|---|---|---|---|---|
| Festinger et al. (2014) | Parametric | N/A | 222 | 12 | CNBD | Yes | No | N/A | Cocaine | Abstinence |
| Kirby et al. (2013) | Parametric | N/A | 130 | 36 | CNBD | Yes | No | N/A | Cocaine | Abstinence |
| Lamb et al. (2010) | Parametric | N/A | 146 | 12 | $1157.50 | Yes | No | N/A | Nicotine | Abstinence |
| Ledgerwood et al. (2014) | Parametric | N/A | 81 | 3 | CNBD | Yes | Yes | No | Nicotine | Abstinence |
| Packer et al. (2012) | Parametric | N/A | 103 | 1 | $207.50 | Yes | No | N/A | Nicotine | Abstinence |
| Petry et al. (2012c) | Parametric | Community clinics | 442 | 12 | $560 | Yes | Yes | No | Cocaine | Abstinence + other therapeutic goals |
| Petry et al. (2014a) | Parametric | Community clinics | 240 | 12 | $900 | Yes | Yes | No | Cocaine | Abstinence |
| Tuten et al. (2012) | Parametric | Special populations | 133 | 13 | $1364 | No | N/A | N/A | Cocaine/Opioids | Abstinence |
| Roll et al. (2013) | Parametric | N/A | 118 | 16 | $500.00 | Yes | Yes | Yes | Methamphetamine | Abstinence |
| Romanowich and Lamb (2010) | Parametric | N/A | 57 | 3 | $100.00 | Yes | Yes | No | Nicotine | Abstinence |
| Romanowich and Lamb (2013) | Parametric | N/A | 30 | 1 | $375.00 | Yes | Yes | No | Nicotine | Abstinence |
| Branson et al. (2012) | Community clinics | Special populations | 52 | 3 | CNBD | Yes | No | N/A | Polydrug | Other therapeutic goals |
| Chen et al. (2013) | Community clinics | N/A | 246 | 12 | CNBD | Yes | No | N/A | Opioids | Abstinence + other therapeutic goal |
| García-Fernández et al. (2011) | Community clinics | N/A | 68 | 24 | $2196.00 | Yes | No | N/A | Cocaine | Abstinence |
| Hser et al. (2011) | Community clinics | N/A | 319 | 12 | CNBD | Yes | No | N/A | Opioids | Abstinence + other therapeutic goal |
| Jiang et al. (2012) | Community clinics | N/A | 160 | 12 | $435.80 | No | N/A | N/A | Opioids | Abstinence + Other Therapeutic Goal |
| Killeen et al. (2012) | Community clinics | Special populations | 31 | 10 | CNBD | No | N/A | N/A | Marijuana | Abstinence |
| Petitjean et al. (2014) | Community clinics | N/A | 60 | 4 | CNBD | Yes | Yes | No | Cocaine | Abstinence |
| Petry et al. (2011) | Community clinics | N/A | 239 | 12 | CNBD | Yes | Yes | No | Polydrug | Abstinence + other therapeutic goals |
| Petry et al. (2012a) | Community clinics | N/A | 130 | 12 | CNBD | Yes | Yes | No | Cocaine | Abstinence |
| Petry et al. (2012b) | Community clinics | N/A | 43 | 12 | CNBD | Yes | No | N/A | Polydrug | Abstinence |
| Secades-Villa et al. (2014) | Community clinics | N/A | 92 | 6 | $412 | Yes | Yes | Yes | Nicotine | Abstinence |
| Walker et al. (2010) | Community clinics | N/A | 90 | 10,14 | CNBD | Yes | No | N/A | Polydrug | Other therapeutic goals |
Note: primary trend refers to main trend authors assigned study to; Additional trends refers to additional trends study was assigned to aside from the primary trend; n refers to sample size (across all groups); CM duration refers to the number of weeks during which contingent incentives could be earned (CNBD = duration could not be determined); Maximum earnings refers to the maximum amount that could be earned in the intervention (CNBD = maximum earnings could not be determined); Statistically significant treatment effects and effects at follow-up defined in all studies as outcomes significant at p < 0.05; Follow up effects were only evaluated if during treatment effects were statistically significant; drug of abuse refers to drug targeted by CM intervention; CM target refers to the behavior on which incentives were contingent.
Six studies with significant treatment effects also reported outcomes following discontinuation of incentives, with one study (17%) reporting significant treatment effects at follow-up and an effect size of 0.43 (95% CI: −0.004, 0.86).A total of seven studies within this trend included one or more follow-up assessments. An effect size could be calculated for three of those seven studies, with an average of 0.33 (95% CI: −0.20, 0.86).
Parameters investigated included durations of the incentives intervention (Kirby et al., 2013; Roll et al., 2013), effect of delay in incentive delivery (Packer et al., 2012), cash versus voucher incentive type (Festinger et al., 2014), incentive monetary value (Packer et al., 2012; Petry et al., 2012c; Petry et al., 2014a; Romanowich and Lamb, 2010), incentive schedule (Ledgerwood et al., 2014; Romanowich and Lamb, 2013; Tuten et al., 2012; Lamb et al., 2010), and efficacy of CM in combination with a novel treatment component (Kurti and Dallery, 2014). Results from these studies indicated that increased incentive duration and value generally improves outcomes (e.g., Kirby et al., 2013; Roll et al., 2013) and delays between verification of abstinence and incentive delivery diminishes outcomes (Packer et al., 2012). A study comparing cash and voucher incentives noted that both were efficacious although the former may be more cost-effective (Festinger et al., 2012). The majority of studies evaluating incentive magnitude reported that higher magnitude incentives were more efficacious (Packer et al., 2012; Petry et al., 2012c, Romanowich and Lamb, 2010), although one did not (Petry et al., 2014a). Parametric studies evaluating schedule of incentives varied greatly. Two studies illustrated that higher values of incentives at the beginning of a schedule do not improve outcomes (Ledgerwood et al., 2010; Romanowich and Lamb, 2013—see also Higgins et al., 2014 categorized under special populations); another study reported no difference between a fixed and escalating schedule of incentives, although authors attributed lack of differences to delay in incentive delivery (Tuten et al., 2012). Another study evaluating incentive schedule among hard-to-treat (HTT) versus easy-to-treat (ETT) smokers using schedules with or without shaping reported that ETT smokers responded to either schedule, whereas HTT smokers had better outcomes with shaping (Lamb et al., 2010).
3.2.3. Extending the intervention into community clinics
Extending CM into community clinics remained a clear trend in the current review (Table 2). Twelve of the 69 studies (17%) were categorized under this trend, with six (50%) targeting abstinence, four (33%) targeting abstinence and another outcome, and two (17%) targeting another outcome only. Ten studies (83%) reported significant during-treatment effects. An effect size could be calculated for nine of the studies reporting significant during-treatment effects, with an average of 0.53 (95% CI: 0.34, 0.73). An effect size could be calculated for 10 of the 12 studies categorized under this trend, with an average of 0.50 (95% CI: 0.31, 0.70).
Four of the studies with significant treatment effects also reported outcomes following the discontinuation of incentives with one of the four (25%) reporting significant effects at follow-up and an effect size of 0.53 (95% CI: 0.05, 1.02). A total of six studies within this trend included one or more follow-up assessments. An effect size could be calculated for four of those six studies, with an average of 0.27 (95% CI: −0.09, 0.63).
Across the twelve studies in this trend, the approaches to implementing CM in community clinics included training clinicians to deliver the treatment (Petry et al., 2012a; Petry et al., 2012b), including CM in group therapy sessions (Branson et al., 2012; Killeen et al., 2012; Petry et al., 2011; Walker et al., 2010), and disseminating the treatment to community clinics internationally (Chen et al., 2013; García-Fernández et al., 2011; Hser et al., 2011; Jiang et al., 2012; Petitjean et al., 2014; Secades-Villa et al., 2014). Both studies that focused on training community clinicians to deliver CM reported significant treatment effects (Petry et al., 2012a; Petry et al., 2012b). Among the four studies where CM was added to group counseling, three reported significant effects on attendance (Branson et al., 2012) or both attendance and abstinence outcomes (Petry et al., 2012a; Petry et al., 2012b), whereas one study reported no effect of CM on abstinence (Killeen et al., 2012). The lack of treatment effects in this latter study was attributed to using very low magnitude incentives with a difficult-to-treat population (i.e., marijuana dependent adolescents).
Another set of studies in this category investigated implementation of CM in community clinics internationally. For example, CM was implemented in two studies conducted in community clinics in Spain, where it was effective in reducing cocaine use (García-Fernández et al., 2011) and cigarette smoking (Secades-Villa et al., 2014). A Swiss group evaluated the relative efficacy of CM + Cognitive Behavior Therapy (CBT) vs. CBT alone in treating cocaine dependence in a community clinic (Petitjean et al., 2014). CM + CBT produced greater during-treatment but not post-treatment cocaine abstinence. Lastly, three CM studies were conducted in methadone maintenance clinics in China to promote both attendance and abstinence. CM significantly increased attendance and abstinence in two of those studies (Chen et al., 2013; Hser et al., 2011) but not the third (Jiang et al., 2012).
Collectively, the above studies provide sound support for the efficacy of CM when implemented in community clinics in the U.S. and abroad.
3.2.4. Incorporating new technologies into CM
CM interventions often entail frequent objective monitoring of target behaviors, which can be cumbersome on clinical staff and patients alike and also limit the reach of CM. Investigators are increasingly utilizing remote behavior-monitoring technologies to surmount this problem (see Kurti et al., 2016, for a review). That and other novel technological advances described below led us to include this additional trend (Table 3). Eight of the 69 studies (11.5%) were categorized under this trend, with seven (87.5%) targeting abstinence from cigarette smoking (three studies) and alcohol use (four studies) and the eighth (12.5%) targeting adherence to remote monitoring of cocaine craving. All eight studies (100%) reported significant treatment effects. An effect size could be calculated for seven of those 8 studies, with an average of 0.70 (95% CI: 0.42, 0.98). Only one of these studies evaluated post-incentives maintenance of treatment effects, which were not significant; the effect size was −0.05 (95% CI: −0.85, 0.76) (Dallery et al., 2013).
Table 3.
Incorporating new technologies in CM AND combining pharmacotherapies with CM.
| Study | Primary trend | Additional trends | n | CM duration (weeks) | Maximum earnings | Statistically significant treatment effect | Included follow up? | Statistically significant effects at follow-up | Drug of abuse | CM target |
|---|---|---|---|---|---|---|---|---|---|---|
| Alessi and Petry (2013) | Technology | N/A | 30 | 4 | $340 | Yes | No | N/A | Alcohol | Abstinence |
| Barnett et al. (2011) | Technology | N/A | 13 | 2 | $154 | Yes | No | N/A | Alcohol | Abstinence |
| Dallery et al. (2013) | Technology | N/A | 77 | 7 | $530 | Yes | Yes | No | Nicotine | Abstinence |
| Dougherty et al. (2014) | Technology | N/A | 26 | 8 | $300 | Yes | No | N/A | Alcohol | Abstinence |
| Lindsay et al. (2014) | Technology | N/A | 57 | 2 | CNBD | Yes | No | N/A | Cocaine | Other therapeutic goal |
| McDonell et al. (2012) | Technology | N/A | 15 | 4 | $96 | Yes | No | N/A | Alcohol | Abstinence |
| Meredith and Dallery (2013) | Technology | N/A | 32 | 2 | $56.25 | Yes | No | N/A | Nicotine | Abstinence |
| Meredith et al. (2011) | Technology | N/A | 13 | 2 | $161.50 | Yes | No | N/A | Nicotine | Abstinence |
| Defulio et al. (2012) | Pharmacotherapy | N/A | 38 | 24 | CNBD | Yes | No | N/A | Opioids | Other therapeutic goal |
| Dunn et al. (2013) | Pharmacotherapy | Special populations | 67 | 26 | CNBD | Yes | No | N/A | Opioids | Other therapeutic goal |
| Everly et al. (2011) | Pharmacotherapy | N/A | 35 | 26 | CNBD | Yes | No | N/A | Opioids | Other therapeutic goal |
| Gray et al. (2011) | Pharmacotherapy | Special populations | 134 | 6 | $275 | Yes | Yes | No | Nicotine | Abstinence |
| Ling et al. (2013) | Pharmacotherapy | N/A | 202 | 16 | $1460.00 | No | N/A | N/A | Opioids | Abstinence |
| Tidey et al. (2011) | Pharmacotherapy | Special populations | 57 | 3 | $350 | Yes | No | N/A | Nicotine | Abstinence |
| Umbricht et al. (2014) | Pharmacotherapy | N/A | 171 | 12 | $1155.00 | No | No | N/A | Cocaine | Abstinence |
| Winstanley et al. (2011) | Pharmacotherapy | N/A | 145 | 12 | $1155.00 | Yes | No | N/A | Cocaine | Abstinence |
Note: primary trend refers to main trend authors assigned study to; Additional trends refers to additional trends study was assigned to aside from the primary trend; n refers to sample size (across all groups); CM duration refers to the number of weeks during which contingent incentives could be earned (CNBD = duration could not be determined); Maximum earnings refers to the maximum amount that could be earned in the intervention (CNBD = maximum earnings could not be determined); Statistically significant treatment effects and effects at follow-up defined in all studies as outcomes significant at p < 0.05; Follow up effects were only evaluated if during treatment effects were statistically significant; drug of abuse refers to drug targeted by CM intervention; CM target refers to the behavior on which incentives were contingent.
The three studies targeting cigarette smoking monitored smoking status by having participants use a web camera to submit time-stamped videos of breath carbon monoxide (CO) testing over a study website (Dallery et al., 2013; Meredith et al., 2011; Meredith and Dallery, 2013). Two of those studies also included group contingencies wherein teams of participants communicated with each other over an online support forum (Meredith et al., 2011; Meredith and Dallery, 2013).
Two of the four studies targeting alcohol monitored use with the Secure Continuous Remote Alcohol Monitor (SCRAM) device, a real-time, continuous, transdermal monitor (Barnett et al., 2011; Dougherty et al., 2014). In the third study, alcohol abstinence was increased while monitoring use through time-stamped videos of participants blowing into a breathalyzer using a cellphone (Alessi and Petry, 2013). The fourth study targeting alcohol increased abstinence while monitoring alcohol intake by analyzing twice-weekly urine samples for ethyl glucuronide (EtG), an alcohol metabolite with a 3-day detection period (McDonell et al., 2012).
The final study in this trend was a feasibility study demonstrating that CM was effective at increasing adherence to a schedule of regular reporting of cocaine cravings/use using an interactive voice response (IVR) system (Lindsay et al., 2014).
3.2.5. Combining CM with pharmacotherapies
Combining CM with pharmacotherapies is the fourth largest trend in the review (Table 3). Eight of the 69 included studies (11.5%) were categorized under this trend, with five (62.5%) targeting abstinence, and three (37.5%) targeting another therapeutic goal. Six studies (75%) reported significant during-treatment effects. An effect size could be calculated for five of those six studies, with an average of 1.03 (95% CI: 0.69, 1.36). Average during-treatment effect size for seven of the eight studies categorized under this trend was 0.67 (95% CI: 0.41, 0.92).
Only one of the six studies reporting significant during-treatment effect assessed outcomes following discontinuation of an efficacious during-treatment incentives intervention, and treatment effects were not maintained; the effect size was 0.41 (95% CI: −0.53, 1.35) (Gray et al., 2011). There were a total of two studies within this trend that included one or more follow-up assessments, with an average effect size of 0.21 (95% CI: −0.35, 0.76).
The directions taken by studies included in this trend were: (1) evaluating effects of CM and pharmacotherapy alone versus in combination (Gray et al., 2011; Tidey et al., 2011; Winstanley et al., 2011; Umbricht et al., 2014), (2) assessing the addition of CM to a pharmacotherapy to sustain treatment effects (Ling et al., 2013), and (3) reinforcing pharmacotherapy adherence using CM (DeFulio et al., 2012; Everly et al., 2011; Dunn et al., 2013).
Two of the four studies that evaluated the independent versus combined effects of pharmacotherapy plus CM targeted cigarette smoking. In Gray et al. (2011), the combination of bupropion plus CM more effectively reduced adolescent cigarette smoking than bupropion or CM alone. In contrast, combining bupropion with CM was no more effective than CM alone in smokers with schizophrenia (Tidey et al., 2011). The other two studies of this type targeted cocaine use among opiate-dependent participants. In Winstanley et al. (2011), CM alone was more effective in reducing cocaine use relative to fluoxetine alone or CM plus fluoxetine together, whereas in Umbricht et al. (2014) neither the combination of CM plus topiramate nor either of these treatment components alone reduced cocaine use. Thus, with the exception of adolescent smokers, combining CM with pharmacotherapy did not improve outcomes in these studies, which is not encouraging but also not surprising in that in at least two of the studies there was little evidence that the pharmacotherapy alone was efficacious for the SUD investigated (Umbricht et al., 2014; Winstanley et al., 2011).
To further examine CM in opiate-dependent patients, Ling et al. (2013) compared outcomes among those receiving buprenorphine in combination with standard drug abuse counseling only or in combination with CM, CBT, or CM and CBT. Outcomes across the four treatment conditions did not differ significantly, which is inconsistent with an extensive literature demonstrating that CM improves outcomes above opioid substitution therapy and drug abuse counseling only (e.g., Silverman et al., 1996a; Silverman et al., 1996b).
The remaining three studies demonstrated that employment-based reinforcement promotes adherence to extended-release (Defulio et al., 2012; Dunn et al., 2013; Everly et al., 2011) and oral naltrexone (Dunn et al., 2013), an opioid antagonist that can decrease opiate use but for which adherence is typically poor.
3.2.6. Investigating longer-term outcomes
Although 28 of the studies with significant treatment effects in the current review conducted follow-up assessments to evaluate post-treatment outcomes, the five studies (7.2%) categorized under this trend were those explicitly focused on investigating longer-term outcomes as a primary aim (Table 4). Three of these five studies (60%) targeted abstinence and two (40%) targeted abstinence and another therapeutic outcome. Four studies (80%) reported significant during-treatment effects with an average effect size of 0.52 (95% CI: 0.19, 0.86). The one study that failed to produce a significant during-treatment effect also failed to include sufficient information to calculate an effect size. Thus, the overall effect size is the same as the one reported above for studies producing significant effects.
All four studies that produced significant treatment effects also measured outcomes following discontinuation of the incentives, with two (50%) of those studies maintaining significant effects at follow-up assessments with an average effect size of 0.79 (95% CI: 0.35, 1.22). Three of those four studies (two that produced significant follow-up effects and one that did not) included sufficient information to calculate a post-treatment effect size with an overall average of 0.49 (95% CI: 0.02, 0.97).
Studies in this trend took one of two directions. The first direction involved comparing outcomes when CM was administered alone versus combined with another treatment that might be expected to increase during-treatment abstinence. Wang et al. (2014) reported that supplementing methadone maintenance therapy (MMT) with CM failed to reduce heroin use and incidences of HIV infection relative to MMT alone (Wangetal., 2014). A second study reported that combining CM with the community reinforcement approach (CRA) improved post-treatment cocaine abstinence rates and general psychosocial functioning relative to standard care (Secades-Villa et al., 2011). The other two studies evaluated CM combined with CBT. In one of those studies, combining CM with CBT produced better cocaine use outcomes than standard care or CBT alone (McKay et al., 2010), whereas in the other study combining CM with CBT failed to decrease longer-term cannabis use significantly more than CBT or CM alone (Carroll et al., 2012). These discrepant findings with respect to outcomes when combining CM and CBT are difficult to reconcile as the targeted drugs (cocaine vs. marijuana) and CM schedule arrangements differed considerably. However, it does merit mention that the negative findings reported by Carroll et al. regarding the combined effects of CM and CBT compared to CM delivered alone is consistent with other research on this particular treatment combination (e.g., Petitjean et al., 2014; Rawson et al., 2002; Rawson et al., 2006).
The second direction taken involved only one study and used an arrangement wherein opioid-dependent individuals earned access to paid employment contingent on cocaine abstinence. This therapeutic workplace sustained cocaine abstinence throughout one year of abstinence-contingent employment, but treatment effects were not maintained following discontinuation of the abstinence contingency (DeFulio and Silverman, 2011).
3.2.7. Using CM as a research tool
Using CM as a research tool is one application that currently is quite underutilized (Table 4). This refers to using CM to experimentally manipulate drug use or other targets in order to answer other research questions (e.g., how a period of initial abstinence influences the probability of longer-term abstinence, or how regular use of a novel pharmacotherapy alters illicit drug use). Only two of the 69 studies (2.8%) in the current review were categorized under this trend (Bradstreet et al., 2014; Kurti and Dallery, 2014). Both reported significant treatment effects. An effect size could be calculated for one of the studies, which was 3.27 (95% CI: 2.1, 4.45). Neither examined post-treatment outcomes.
Bradstreet et al. (2014) used CM to promote differential levels of recent abstinence from cigarette smoking in order to experimentally examine impacts on cue-induced craving and response inhibition in a Go/No-Go task. Abstinence across a two-week period produced statistically significant and robust decreases in generalized craving relative to 1–2 days of abstinence, although no differences in cue-induced craving or response inhibition were noted. Kurti and Dallery (2014) administered CM in a laboratory setting to examine whether a laboratory-based model of this treatment combined with physical exercise had greater impacts on craving and smoking relative to exercise or CM alone. No significant differences were noted between combined CM and exercise versus CM alone.
Future research that uses CM as a research tool has the potential to contribute new knowledge about numerous important aspects of SUDs, including drug use impacts on epigenetic profiles, brain function and structure, immune function, and other health outcomes impacted by SUDs.
4. Discussion
Over the past 5.2 years, voucher-based CM studies have continued to appear in the literature at a healthy pace of 13.3 studies per year, which is directly comparable to the 14.4 studies per year across the five years covered by the immediately prior review (Higgins et al., 2011). Considered together, the number of CM studies published per year over the past decade represents a substantial increase beyond the rate of 3.0 studies per year (4.2 per year if studies that would have met inclusion criteria in the present review are included) reported in Lussier et al. (2006), which covered the years 1991–2004. In addition to consistency in the pace with which CM studies have been published over the past ten years, the efficacy of CM treatments have remained consistent as well, with the current and prior reviews reporting an 86% and 88% efficacy rate, respectively. Collapsing across the three reviews, this represents 176 controlled studies on the use of voucher-based CM with substance users, with 151 (86%) of those studies supporting efficacy. For studies reporting significant treatment effects while the incentives were in place, average effect size was moderate to large, and for those studies where significant treatment effects remained significant at one or more follow-ups following discontinuation of the incentives intervention average effect size was small. The same is true for effect sizes based on all studies assessing during-treatment effects and all studies assessing post-treatment effects. By any standard for evaluating development of treatments for SUDs that we are familiar with, that is a striking level of empirical support for efficacy. Indeed, this empirical support has led the National Institute on Clinical Excellence to recommend nationwide adoption of CM for intensive outpatient treatments for illicit drug use disorders in the U.K. (Pilling et al., 2007) and more recently a similar action within the U.S. Veteran Administration hospital system (Petry et al., 2014b).
Also consistent across the present and immediately prior reviews are the trends into which studies can be categorized, with two exceptions: (1) the trend extending the intervention to additional SUDs was removed in the current review as no new SUDs were targeted in this review period, and (2) the addition of the trend incorporating new technology into CM was new in the current review. There was also consistency across the current and immediately prior reviews with regards to substantial focus on use of CM with special populations. This appears to be where CM is clearly finding a niche, perhaps because CM has been demonstrated to be among the most effective treatments at promoting abstinence in these populations. In comprehensive meta-analyses on smoking-cessation interventions for pregnant women, for example, CM produces larger treatment effects than any intervention tested in controlled trials going back to 1985 (see Higgins and Solomon, 2016).
In the two prior reviews by our group, we identified several priorities for future CM research. In Lussier et al. (2006), we called for increased evaluation of intervention duration and voucher incentive value. Studies in the current review that evaluated these parameters support earlier findings that longer duration of treatment and higher value incentives moderate treatment efficacy. In the immediately prior review (Higgins et al., 2011), we called for the development of novel monitoring technologies to facilitate frequent and accurate monitoring of alcohol intake so that CM could be used with this highly prevalent SUD and also for extending CM to individuals residing in geographically remote areas. The development of remote monitoring devices such as the SCRAM bracelet and internet and smartphone arrangements for monitoring breath alcohol and carbon monoxide levels within the past 5.2 years has contributed to the substantial progress evident in this review (also see Kurti et al., 2016). The use of urine EtG monitoring also holds promise for extending CM to alcohol use disorders (McDonell et al., 2012).
Another priority identified in both of the prior reviews was further examination of CM effects on longer-term abstinence and other post-treatment outcomes. The proportion of studies that included ≥ one follow-up assessment in the present review (47%) increased slightly above the proportion that included follow-ups in the prior review (41%). In addition, the proportion of studies supporting the maintenance of treatment effects following the discontinuation of incentives also appears to have increased between reviews, from 21% in the immediately prior review to 29% in the current review. Clearly there is a weakening in the magnitude of treatment effect size when comparing overall effects while incentives are in place compared to overall effects after their discontinuation, with generally moderate to large effect sizes observed during the former (i.e., 0.62 (95% CI: 0.54, 0.70)) and small effect sizes in the latter (i.e., 0.26 (95% CI: 0.11, 0.41)). Others have noted this trend toward more CM studies examining the sustainability of post-intervention treatment effects as well (McKay et al., 2010), but more needs to be done in terms of moving in the direction of all CM studies including follow-up assessments and increases in the quality of these efforts. As CM studies increasingly focus on longer-term behavior change, it will be important for researchers to consider carefully how to program for the maintenance of treatment effects using naturalistic or more contrived reinforcers for healthy living. Note that in the small handful of studies that were explicitly focused on promoting longer-term outcomes (Table 4), average overall effect size was 0.49 (95% CI: 0.02, 0.97). Combined treatment interventions that increase during-treatment abstinence and provide skills for sustaining abstinence may extend the duration of abstinence post-treatment (McKay et al., 2010; Higgins et al., 2000), as might employment-based workplace contingencies or other comparable arrangements that are designed to keep programmed contingencies for reinforcing abstinence in place long-term or chronically (Silverman et al., 2012). Important to underscore is that this need for greater focus on promoting and sustaining longer-term behavior change is an important priority and challenge for CM. Also important to note, however, is that the need for greater focus on sustaining behavior change is not unique to CM and extends to all behavioral, psychosocial, and pharmacological interventions for SUDs and other chronic conditions (e.g., obesity) where behavior is a proximal cause.
As development of CM continues, it will be important to remain sensitive to the importance of parameters that impact efficacy and effect size (e.g., short delays between engaging in the target behavior and earning reinforcement, larger incentive values, and longer duration interventions). Implementing lower-cost CM and/or less intensive CM interventions (e.g., shorter intervention durations, less frequent behavior monitoring) may increase the likelihood of adoption by community clinics with fewer resources, etc., but such modifications can be expected to reduce treatment effect size as well. To simultaneously maintain the efficacy of CM interventions while promoting greater dissemination, it will be necessary for future research to integrate the findings from existing research (e.g., adherence to the parameters revealed to be critical to treatment efficacy in experimental evaluations) with new developments (e.g., incorporating technology in CM). Most importantly, cost-effectiveness should be the eventual arbiter in such matters, but is an area where CM and other treatment development research for SUDs is lacking. In a recent review on cost-effectiveness studies on use of CM with illicit drug use disorders, for example, only nine studies were identified (Shearer et al., 2015). While results were generally supportive, they were also deemed inconclusive. We consider greater attention to that gap to be a high priority in future CM research along with continued attention to longer-term outcomes discussed above, especially studies wherein preparation for maintenance of treatment effects was part of the during-treatment intervention or programmed contingencies of reinforcement for healthy behavior change are sustained longer-term.
In sum, CM continues to be a highly efficacious intervention that produces large to medium effects during treatment and follow-up, respectively, and across a wide variety of SUDs, populations, and settings. Further dissemination of CM into the public and private sector has substantial promise for improving public health by promoting abstinence and related therapeutic changes among those struggling with SUDs. Further development of this intervention model will be enhanced by greater attention to promoting and sustaining longer-term change, strategies to increase treatment reach through remote monitoring, and careful examination of impacts on health, quality-of-life, and societal (e.g., crime, economic productivity) outcomes and cost-effectiveness.
Acknowledgments
Funding
This project was supported in part by Research Grants R01HD075669 and R01HD078332 from the National Institute of Child Health and Human Development, Center of Biomedical Research Excellence award P20GM103644 from the National Institute of General Medical Sciences, and Institutional Training Award T32DA07242 from the National Institute on Drug Abuse. The funding sources had no other role in this project other than financial support.
Footnotes
Competing interests
The authors have no conflicts of interest to report.
Transparency document
The Transparency document associated with this article can be found, in online version.
References
* Designates studies that were included for analysis in the review.
- *.Alessi SM, Petry NM. A randomized study of cellphone technology to reinforcement abstinence in the natural environment. Addiction. 2013;108:900–909. doi: 10.1111/add.12093. http://dx.doi.org/10.1111/add.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Alessi SM, Petry NM. Smoking reductions and increased self-efficacy in a randomized controlled trial of smoking abstinence-contingent incentives in residential substance abuse treatment patients. Nicotine Tob Res. 2014;16(11):1436–1445. doi: 10.1093/ntr/ntu095. http://dx.doi.org/10.1093/ntr/ntu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Barnett NP, Tidey J, Murphy JG, Swift R, Coldby SM. Contingency management for alcohol use reduction: a pilot study using a transdermal alcohol sensor. Drug Alcohol Depend. 2011;118:391–399. doi: 10.1016/j.drugalcdep.2011.04.023. http://dx.doi.org/10.1016/j.drugalcdep.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL, Festinger DS. Prize-based contingency management for the treatment of substance abusers: a meta analysis. Addiction. 2014;109:1426–1436. doi: 10.1111/add.12589. http://dx.doi.org/10.1111/add.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Branson CE, Barbuti AM, Clemmey P, Herman L, Bhutia P. A pilot study of low-cost contingency management to increase attendance in an adolescent substance abuse program. Am J Addict. 2012;21:126–129. doi: 10.1111/j.1521-0391.2011.00204.x. http://dx.doi.org/10.1111/j.1521-0391.2011.00204.x. [DOI] [PubMed] [Google Scholar]
- *.Bradstreet MP, Higgins ST, McClernon FJ, Kozink RV, Skelly JM, Washio Y, Lopez AA, Parry MA. Examining the effects of initial smoking abstinence on response to smoking-related stimuli and response inhibition in a human laboratory model. Psychopharmacology. 2014;231(10):2145–2158. doi: 10.1007/s00213-013-3360-x. http://dx.doi.org/10.1007/s00213-013-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Businelle MS, Kendzor DE, Kesh A, Cuate EL, Poonawalla IB, Reitzel LR, Okuyemi KS, Wetter DW. Small financial incentives increase smoking cessation in homeless smokers: a pilot study. Addict Behav. 2014;39:717–720. doi: 10.1016/j.addbeh.2013.11.017. http://dx.doi.org/10.1016/j.addbeh,.2013.11.017. [DOI] [PubMed] [Google Scholar]
- *.Carroll KM, Nich C, LaPaglia DM, Peters EN, Easton CJ, Petry NM. Combining cognitive behavioral therapy and contingency management to enhance their effects in treating cannabis dependence: less can be more, more or less. Addiction. 2012;107:1650–1659. doi: 10.1111/j.1360-0443.2012.03877.x. http://dx.doi.org/10.1111/j.1360-0443.2012.03877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Excessive Drinking Costs U.S. $223.5 Billion. 2014 www.cdc.gov/features/alcoholconsumption/ (Accessed March 9, 2015)
- *.Chen W, Hong Y, Zou X, McLaughlin MM, Xia Y, Ling L. Effectiveness of prize-based contingency management in a methadone maintenance program in China. Drug Alcohol Depend. 2013;133:270–274. doi: 10.1016/j.drugalcdep.2013.05.028. http://dx.doi.org/10.1016/j.drugalcdep.2013.05.028. [DOI] [PubMed] [Google Scholar]
- *.Dallery J, Raiff BR, Grabinski MJ. Internet-based contingency management to promote smoking cessation: a randomized controlled study. J Appl Behav Anal. 2013;46:750–764. doi: 10.1002/jaba.89. http://dx.doi.org/10.1002/jaba.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.DeFulio A, Everly JJ, Leoutsakos JMS, Umbricht A, Fingerhood M, Bigelow GE, Silverman K. Employment-based reinforcement of adherence to an FDA approved extended release formulation of naltrexone in opioid-dependent adults: a randomized controlled trial. Drug Alcohol Depend. 2012;120(1–3):48–54. doi: 10.1016/j.drugalcdep.2011.06.023. http://dx.doi.org/10.1016/j.drugalcdep.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.DeFulio A, Silverman K. Employment-based abstinence reinforcement as a maintenance intervention for the treatment of cocaine dependence: post intervention outcomes. Addiction. 2011;106(5):960–967. doi: 10.1111/j.1360-0443.2011.03364.x. http://dx.doi.org/10.1111/j.1360-0443.2-11.-3364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Dougherty DM, Hill-Kapturczak N, Liang Y, Karns TE, Cates SE, Lake SL, Mullen J, Roache JD. Use of continuous transdermal alcohol monitoring during a contingency management procedure to reduce excessive alcohol use. Drug Alcohol Depend. 2014;142:301–306. doi: 10.1016/j.drugalcdep.2014.06.039. http://dx.doi.org/10.1016/j.drugalcdep.2014.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Drummond MB, Astermborski J, Lambert AA, Goldberg S, Stitzer ML, Merlo CA, Rand CS, Wise RA, Kirk GD. A randomized study of contingency management and spirometric lung age for motivating smoking cessation among injection drug users. BMC Public Health. 2014;14(761) doi: 10.1186/1471-2458-14-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Dunn KE, Sigmon SC, Reimann EF, Badger GJ, Heil SH, Higgins ST. A contingency-management intervention to promote initial smoking cessation among opioid-maintained patients. Exp Clin Psychopharmacol. 2010;18(1):37–50. doi: 10.1037/a0018649. http://dx.doi.org/10.1037/a0018649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Dunn K, Defulio A, Everly JJ, Donlin WD, Aklin WM, Nuzzo PA, Leoutsakis JMS, Umbricht A, Fingerhood M, et al. Employment-based reinforcement of adherence to oral naltrexone treatment in unemployed injection drug users. Exp Clin Psychopharmacol. 2013;21(1):74–83. doi: 10.1037/a0030743. http://dx.doi.org/10.1037/a0030743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Tighe TJ. A technique for controlling behavior in natural life settings. J Appl Behav Anal. 1968;1(3):263–266. doi: 10.1901/jaba.1968.1-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Everly JJ, DeFulio A, Koffarnus MN, Leoutsakos JMS, Donlin WD, Aklin WM, Umbricht A, Fingerhood M, Bigelow GE, et al. Employment-based reinforcement of adherence to depot naltrexone in unemployed opioid-dependent adults: a randomized controlled trial. Addiction. 2011;106(7):1309–1318. doi: 10.1111/j.1360-0443.2011.03400.x. http://dx.doi.org/10.1111/j.1360-0443.2011.03400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Festinger DS, Dugosh KL, Kirby KC, Seymour BL. Contingency management for cocaine treatment: cash vs. vouchers. J Subst Abus Treat. 2014;47:168–174. doi: 10.1016/j.jsat.2014.03.001. http://dx.doi.org/10.1016/j.jsat.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.García-Fernández G, Secades-Villa R, García-Rodríguez O, Sánchez-Hervás E, Fernández-Hermida JR, Higgins ST. Adding voucher-based incentives to community reinforcement approach improves outcomes during treatment for cocaine dependence. Am J Addict. 2011;20:456–461. doi: 10.1111/j.1521-0391.2011.00154.x. http://dx.doi.org/10.1111/j.1521-0391.2011.00154.x. [DOI] [PubMed] [Google Scholar]
- *.García-Fernández G, Secades-Villa R, García-Rodríguez O, Pena-Suarez E, Sánchez-Hervás E. Contingency management improves outcomes in cocaine-dependent outpatients with depressive symptoms. Exp Clin Psychopharmacol. 2013;21(6):482–489. doi: 10.1037/a0033995. http://dx.doi.org/10.1037/a0033995. [DOI] [PubMed] [Google Scholar]
- Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: a meta-analysis. Drug Alcohol Depend. 2000;58:55–66. doi: 10.1016/s0376-8716(99)00068-x. [DOI] [PubMed] [Google Scholar]
- *.Gray KM, Carpenter MJ, Baker NL, Hartwell KJ, Lewis AL, Hiott W, Deas D, Upadhyaya HP. Bupropion SR and contingency management for adolescent smoking cessation. J Subst Abus Treat. 2011;40:77–86. doi: 10.1016/j.jsat.2010.08.010. http://dx.doi.org/10.1016/j.jsat.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hagedorn HJ, Noorbaloochi S, Baines Simon A, Bangerter A, Stitzer ML, Stetler CB, Kivlahan D. Rewarding early abstinence in veterans health administration addiction clinic. J Subst Abus Treat. 2013;45:109–117. doi: 10.1016/j.jsat.2013.01.006. http://dx.doi.org/10.1016/j.jsat.2013.01.006. [DOI] [PubMed] [Google Scholar]
- *.Hertzberg JS, Carpenter VL, Kirby AC, Calhoun PS, Moore SD, Dennis MF, Dennis PA, Derdert EA, Beckham JC. Mobile contingency management as an adjunctive smoking cessation treatment for smokers with posttraumatic stress disorder. Nicotine Tob Res. 2013;15(11):1934–1938. doi: 10.1093/ntr/ntt060. http://dx.doi.org/10.1093/ntr/ntt060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991;148(9):1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Hughes JR, Foerg F, Badger G. Achieving cocaine abstinence with a behavioral approach. Am J Psychiatry. 1993;150(5):763–769. doi: 10.1176/ajp.150.5.763. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Ogden DE, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J Consult Clin Psychol. 2000;68(1):64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Silverman K, Heil SH. Contingency Management in Substance Abuse Treatment. The Guilford Press; New York, NY: 2008. [Google Scholar]
- Higgins ST, Sigmon SC, Heil SH. Contingency management in the treatment of substance use disorders: trends in the literature. In: Ruiz P, Strain E, editors. Lowinson and Ruiz's Substance Abuse: a Comprehensive Textbook. Lippincott Williams & Wilkins; Philadelphia, PA: 2011. pp. 603–621. [Google Scholar]
- *.Higgins ST, Washio Y, Lopez AA, Heil SH, Solomon LJ, Lynch ME, Hanson JD, Higgins TM, Skelly JM, Redner R. Examining two different schedules of financial incentives for smoking cessation among pregnant women. Prev Med. 2014;68:51–57. doi: 10.1016/j.ypmed.2014.03.024. http://dx.doi.org/10.1016/j.ypmed.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Solomon LJ. Some recent developments on financial incentives for smoking cessation among pregnant and newly postpartum women. Curr Addict Rep. 2016;3(1):9–18. doi: 10.1007/s40429-016-0092-0. http://dx.doi.org/10.1007/s40429-016-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Holtyn AF, Koffarnus MN, Defulio A, Sigurdsson SO, Strain EC, Schwartz RP, Leoutsakos JMS, Silverman K. The therapeutic workplace to promote treatment engagement and drug abstinence in out-of-treatment injection drug users: A randomized controlled trial. Prev Med. 2014;68:62–70. doi: 10.1016/j.ypmed.2014.02.021. http://dx.doi.org/10.106/j.ypmed.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hser Y, Li J, Jiang H, Zhang R, Du J, Zhang C, Zhang B, Evans E, Wu F. Effects of a randomized contingency management intervention on opiate abstinence and retention in methadone maintenance treatment in China. Addiction. 2011;106(10):1801–1809. doi: 10.1111/j.1360-0443.2011.03490.x. http://dx.doi.org/10.1111/j.1360-0443.2011.03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Jiang H, Du J, Wu F, Wang Z, Fan S, Li Z, Hser Y, Zhao M. Efficacy of contingency management in improving retention and compliance to methadone maintenance treatment: a random controlled study. Shanghai Arch Psychiatry. 2012;24(1):11–19. doi: 10.3969/j.issn.1002-0829.2012.01.002. http://dx.doi.org/10.3969/j.issn.1002-0829.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kaminer Y, Burleson JA, Burke R, Litt MD. The efficacy of contingency management for adolescent cannabis use disorder: a controlled study. Subst Abus. 2014;35(4):391–398. doi: 10.1080/08897077.2014.933724. http://dx.doi.org/10.1080/08897077.2014.933724. [DOI] [PubMed] [Google Scholar]
- *.Kelly TM, Daley DC, Douaihy AB. Contingency management for patients with dual disorders in intensive outpatient treatment for addiction. J Dual Diagn. 2014;10(3):108–117. doi: 10.1080/15504263.2014.924772. http://dx.doi.org/10.1080/15504263.2014.924772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kendzor DE, Businelle MS, Poonawalla IB, Cuate EL, Kesh A, Rios DM, Ma P, Balis DS. Financial incentives for abstinence among socioeconomically disadvantaged individuals in smoking cessation treatment. Am J Public Health. 2014;105(6):1198–1205. doi: 10.2105/AJPH.2014.302102. http://dx.doi.org/10.2105/AJPH.2014.302.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kidorf M, Brooner RK, Gandotra N, Antoine D, King VL, Peirce J, Ghazarian S. Reinforcing integrated psychiatric service attendance in an opioid-agonist program: A randomized and controlled trial. Drug Alcohol Depend. 2013;133:30–36. doi: 10.1016/j.drugalcdep.2013.06.005. http://dx.doi.org/10.1016/j.drugalcdep.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Killeen TK, McRae-Clar AL, Waldrop AE, Upadhyaya H, Brady KT. Contingency management in community programs treating adolescent substance abuse: a feasibility study. J Child Adolesc Psychiatr Nurs. 2012;25:33–41. doi: 10.1111/j.1744-6171.2011.00313.x. http://dx.doi.org/10.1111/j.1744-6171.2011.00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kirby KC, Carpenedo CM, Dugosh KL, Rosenwasser BJ, Benishek LA, Janik A, Keashen R, Bresani E, Silverman K. Randomized clinical trial examining duration of voucher-based reinforcement therapy for cocaine abstinence. Drug Alcohol Depend. 2013;132(3):639–645. doi: 10.1016/j.drugalcdep.2013.04.015. http://dx.doi.org/10.1016/j.drugalcdep.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Krishnan-Sarin S, Cavallo DA, Cooney JL, Schepis TS, Kong G, Liss TB, McMahon TJ, Nich C. An exploratory randomized controlled trial of a novel highschool-based smoking cessation intervention for adolescent smokers using abstinence-contingent incentives and cognitive behavioral therapy. Drug Alcohol Depend. 2013;132(1–2):346–351. doi: 10.1016/j.drugalcdep.2013.03.002. http://dx.doi.org/10.1016/j.drugalcdep.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kurti AN, Dallery J. A laboratory-based evaluation of exercise plus contingency management for reducing cigarette smoking. Drug Alcohol Depend. 2014;144:201–209. doi: 10.1016/j.drugalcdep.2014.09.012. http://dx.doi.org/10.1016/j.drugalcdep.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Kurti AN, Davis D, Redner R, Jarvis B, Zvorsky I, Keith DR, Higgins ST. Incorporating technology into incentive-based interventions to promote health-related behavior change: a systematic literature review, 2004–2015. Trans Issues Psychol Sci. 2016;2(2):128–152. doi: 10.1037/tps0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lamb RJ, Kirby KC, Morral AR, Galbicka G, Iguchi MY. Shaping smoking cessation in hard-to-treat smokers. J Consult Clin Psychol. 2010;78(1):62–71. doi: 10.1037/a0018323. http://dx.doi.org/10.1037/a0018323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Ledgerwood DM, Arfken CL, Petry NM, Alessi SM. Prize contingency management for smoking cessation: a randomized trial. Drug Alcohol Depend. 2014;140:208–212. doi: 10.1016/j.drugalcdep.2014.03.032. http://dx.doi.org/10.1016/j.drugalcdep.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lindsay JA, Minard CG, Hudson S, Green CE, Schmitz JM. Using prize-based incentives to enhance daily interactive voice response (IVR) compliance: a feasibility study. J Subst Abus Treat. 2014;46(1) doi: 10.1016/j.jsat.2013.08.003. http://dx.doi.org/10.1016/j.jsat.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. http://dx.doi.org/10.1111/j.1360-0443.2006.01311. [DOI] [PubMed] [Google Scholar]
- *.Ling W, Hillhouse M, Ang A, Jenkins J, Fahey J. Comparison of behavioral treatment conditions in buprenorphine maintenance. Addiction. 2013;108:1788–1798. doi: 10.1111/add.12266. http://dx.doi.org/10.1111/add.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.McDonell MG, Howell DN, McPherson S, Cameron JM, Srebnik D, Roll JM, Ries RK. Voucher-based reinforcement for alcohol abstinence using the ethyl-glucuronide alcohol biomarker. J Appl Behav Anal. 2012;45:161–165. doi: 10.1901/jaba.2012.45-161. http://dx.doi.org/10.1091/jaba.2012.45-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.McDonell MG, Srebnik D, Angelo F, McPherson S, Lowe JM, Sugar A, Short RA, Roll JM, Ries RK. A randomized controlled trial of contingency management for psycho-stimulant use in community mental health outpatients with co-occurring serious mental illness. Am J Psychiatry. 2013;170(1):94–101. doi: 10.1176/appi.ajp.2012.11121831. http://dx.doi.org/10.1176/appi.ajp.2012.11121831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.McKay JR, Lynch KG, Coviello D, Morrison R, Cary MS, Skalina L, Plebani J. Randomized trial of continuing care enhancements for cocaine dependent patients following initial engagement. J Consult Clin Psychol. 2010;78(1):111–120. doi: 10.1037/a0018139. http://dx.doi.org/10.1037/a0018139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Menza TW, Jameson DR, Hughes JP, Colfax GN, Shoptaw S, Golden MR. Contingency management to reduce methamphetamine use and sexual risk among men who have sex with men: a randomized controlled trial. BMC Public Health. 2010;10(774) doi: 10.1186/1471-2458-10-774. http://dx.doi.org/10.1186/1471-2458-10-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Meredith SE, Grabinski MJ, Dallery J. Internet-based group contingency management to promote abstinence from cigarette smoking: a feasibility study. Drug Alcohol Depend. 2011;118(1):23–30. doi: 10.1016/j.drugalcdep.2011.02.012. http://dx.doi.org/10.1016/j.drugalcdep.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Meredith SE, Dallery J. Investigating group contingencies to promote brief abstinence from cigarette smoking. Exp Clin Psychopharmacol. 2013;21(2):144–154. doi: 10.1037/a0031707. http://dx.doi.org/10.1037/a0031707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NDIC. National Drug Threat Assessment. United States Department of Justice; Washington, DC: 2011. www.justice.gov/archive/ndic/pubs44/44849/44849p.pdf. [Google Scholar]
- *.Ondersma SJ, Svikis DS, Lam PK, Connors-Burge VS, Ledgerwood DM, Hopper JA. A randomized trial of computer-delivered brief intervention and low-intensity contingency management for smoking during pregnancy. Nicotine Tob Res. 2012;14(3):351–360. doi: 10.1093/ntr/ntr221. http://dx.doi.org/10.1093/ntr/ntr221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Packer RP, Howell DN, McPherson S, Roll JM. Investigating reinforcer magnitude and reinforcer delay: a contingency management analog study. Exp Clin Psychopharmacol. 2012;20(4):287–292. doi: 10.1037/a0027802. http://dx.doi.org/10.1037/a0027802. [DOI] [PubMed] [Google Scholar]
- *.Petitjean SA, Dürsteler-MacFarland KM, Krokar MC, Strasser J, Mueller SE, Degen B, Trombini MV, Vogel M, Walter M. A randomized, controlled trial of combined cognitive-behavioral therapy plus prize-based contingency management for cocaine dependence. Drug Alcohol Depend. 2014;145:94–100. doi: 10.1016/j.drugalcdep.2014.09.785. http://dx.doi.org/10.1016/j.drugalcdep.2014.09.785. [DOI] [PubMed] [Google Scholar]
- *.Petry NM, Weinstock J, Alessi SM, Lewis MW, Dieckhaus K. Group-based randomized trial of contingencies for health and abstinence in HIV patients. J Consult Clin Psychol. 2010;78(1):89–97. doi: 10.1037/a0016778. http://dx.doi.org/10.1037/a0016778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Petry NM, Weinstock J, Alessi SM. A randomized trial of contingency management delivered in the context of group counseling. J Consult Clin Psychol. 2011;79(5):686–696. doi: 10.1037/a0024813. http://dx.doi.org/10.1037/a0024813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Petry NM, Alessi SM, Ledgerwood DM. A randomized trial of contingency management delivered by community therapists. J Consult Clin Psychol. 2012a;80(2):286–298. doi: 10.1037/a0026826. http://dx.doi.org/10.1037/a0026826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Petry NM, Alessi SM, Ledgerwood DM. Contingency management delivered by community therapists in outpatient settings. Drug Alcohol Depend. 2012b;122(1–2):86–92. doi: 10.1016/j.drugalcdep.2011.09.015. http://dx.doi.org/10.1016/j.drugalcdep.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Petry NM, Barry D, Alessi SM, Rounsaville CCK. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. J Consult Clin Psychol. 2012c;80(2):276–285. doi: 10.1037/a0026883. http://dx.doi.org/10.1037/a0026883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Petry NM, Alessi SM, Rash CJ. A randomized study of contingency management in cocaine-dependent patients with severe and persistent mental health disorders. Drug Alcohol Depend. 2013;130(0):234–237. doi: 10.1016/j.drugalcdep.2012.10.017. http://dx.doi.org/10.1016/jdrugalcdep.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Petry NM, Alessi SM, Barry D, Carroll KM. Standard magnitude prize reinforcers can be as efficacious as larger magnitude reinforcers in cocaine-dependent methadone patients. J Consult Clin Psychol. 2014a doi: 10.1037/a0037888. http://dx.doi.org/10.1037/a0037888 (Advance online publication) [DOI] [PMC free article] [PubMed]
- Petry NM, DePhilippis D, Rash CJ, Drapkin M, McKay JR. Nationwide dissemination of contingency management: the Veterans Administration initiative. Am J Addict. 2014b;23(3):205–210. doi: 10.1111/j.1521-0391.2014.12092.x. http://dx.doi.org/10.1111/j.1521-0391.2014.12092.x (2014 May–Jun) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling S, Strang J, Gerada C, NICE Psychosocial interventions and opioid detoxification for drug misuse: summary of NICE guidance. BMJ. 2007;335(7612):203–205. doi: 10.1136/bmj.39265.639641.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta analysis. Addiction. 2006;101(11):1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. http://dx.doi.org/10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Huber A, McCann M, Shoptaw S, Farabee D, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Arch Gen Psychiatry. 2002;59(9):817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101(2):267–274. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- *.Reback CJ, Peck JA, Dierst-Davies R, Nuno M, Kamien JB, Amass L. Contingency management among homeless out-of-treatment men who have sex with men. J Subst Abus Treat. 2010;39(3):255–263. doi: 10.1016/j.jsat.2010.06.007. http://dx.doi.org/10.1016/j.jsat.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Roll JM, Chudzynski J, Cameron JM, Howell DN, McPherson S. Duration effects in contingency management treatment of methamphetamine disorders. Addict Behav. 2013;38(9):2455–2462. doi: 10.1016/j.addbeh.2013.03.018. http://dx.doi.org/10.1016/j.addbeh.2013.0.3.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Romanowich P, Lamb RJ. Effects of escalating and descending schedules of incentives on cigarette smoking in smokers without plans to quit. J Appl Behav Anal. 2010;43:357–367. doi: 10.1901/jaba.2010.43-357. http://dx.doi.org/10.1901/jaba.2010.43–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Romanowich P, Lamb RJ. The effect of framing incentives as either losses or gains with contingency management for smoking cessation. Addict Behav. 2013;38(4):2084–2088. doi: 10.1016/j.addbeh.2013.01.007. http://dx.doi.org/10.1016/j.addbeh.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Schottenfeld RS, Moore B, Pantalon MV. Contingency management with community reinforcement approach or twelve-step facilitation drug counseling for cocaine dependent pregnant women or women with young children. Drug Alcohol Depend. 2011;118:48–55. doi: 10.1016/j.drugalcdep.2011.02.019. http://dx.doi.org/10.1016/j.alcdep.2011.02.019. [DOI] [PubMed] [Google Scholar]
- *.Secades-Villa, García-Rodríguez O, García-Fernández G, Sánchez-Hervás E, Fernández-Hermida JR, Higgins ST. Community reinforcement approach plus vouchers among cocaine dependent outpatients: twelve-month outcomes. Psychol Addict Behav. 2011;25(1):174–179. doi: 10.1037/a0021451. http://dx.doi.org/10.1037/a0021451. [DOI] [PubMed] [Google Scholar]
- *.Secades-Villa R, García-Fernández G, Peña-Suárez E, García-Rodríguez O, Sánchez-Hervás E, Fernández-Hermida JR. Contingency management is effective across cocaine-dependent outpatients with different socioeconomic status. J Subst Abus Treat. 2013;44:349–354. doi: 10.1016/j.jsat.2012.08.018. http://dx.doi.org/10.1016/j.jsat.2012.08.018. [DOI] [PubMed] [Google Scholar]
- *.Secades-Villa R, García-Rodríguez O, López-Núñez C, Alonso-Pérez F, Fernández-Hermida JR. Contingency management for smoking cessation among treatment-seeking patients in a community setting. Drug Alcohol Depend. 2014;140:63–68. doi: 10.1016/j.drugalcdep.2014.03.030. http://dx.doi.org/10.1016/j.drugalcdep.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Shearer J, Tie H, Byford S. Economic evaluations of contingency management in illicit misuse programmes: a systematic review. Drug Alcohol Rev. 2015 doi: 10.1111/dar.12240. http://dx.doi.org/10.1111/da. [DOI] [PubMed]
- Silverman K, Higgins ST, Brooner RK, Montoya ID, Cone EJ, Schuster CR, Preston KL. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Arch Gen Psychiatry. 1996a;53(5):409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Higgins ST, Brooner RK, Montoya ID, Contoreggi C, Umbricht-Schneiter A, Schuster CR, Preston KL. Increasing opiate abstinence through voucher-based reinforcement therapy. Drug Alcohol Depend. 1996b;41(2):157–165. doi: 10.1016/0376-8716(96)01246-x. [DOI] [PubMed] [Google Scholar]
- Silverman K, DeFulio A, Sigurdsson SO. Maintenance of reinforcement to address the chronic nature of drug addiction. Prev Med. 2012;55:S46–S53. doi: 10.1016/j.ypmed.2012.03.013. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Stanger C, Budney AJ, Kamon JL, Thostensen J. A randomized trial of contingency management for adolescent marijuana abuse and dependence. Drug Alcohol Depend. 2009;105(3):240–247. doi: 10.1016/j.drugalcdep.2009.07.009. http://dx.doi.org/10.1016/j.drugalcdep.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer ML, Higgins ST. Behavioral treatment of drug and alcohol abuse. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the Fourth Generation of Progress. Raven Press; New York: 1995. pp. 1807–1819. [Google Scholar]
- SAMSHA. National Survey on Drug Use and Health, 2013. 2014 http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf (Accessed October 17, 2015)
- *.Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Reid N. Effects of contingency management and bupropion on cigarette smoking in smokers with schizophrenia. Psychopharmacology. 2011;217(2):279–287. doi: 10.1007/s00213-011-2282-8. http://dx.doi.org/10.1007/s00213-011-2282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Tuten M, Svikis DS, Keyser-Marcus L, O’Grady KE, Jones HE. Lessons learned from a randomized trial of fixed and escalating contingency management schedules in opioid-dependent pregnant women. Am J Drug Alcohol Abuse. 2012;38(4):286–292. doi: 10.3109/00952990.2011.643977. http://dx.doi.org/10.3109/00952990.2011.643977. [DOI] [PubMed] [Google Scholar]
- *.Umbricht A, DeFulio A, Winstanley EL, Tompkins DA, Peirce J, Mintzer MZ, Strain EC, Bigelow GE. Toprimate for cocaine dependence during methadone maintenance treatment: a randomized controlled trial. Drug Alcohol Depend. 2014;140:92–100. doi: 10.1016/j.drugalcdep.2014.03.033. http://dx.doi.org/10.1016/j.drugalcdep.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC. United Nations Office on Drugs and Crime 2015 World Drug Report. 2015 https://www.unodc.org/unodc/en/frontpage/2015/June/2015-world-drug-report-finds-drug-use-stable–access-to-drug-and-hiv-treatment-still-low.html (Accessed October 17, 2015)
- U.S. Department of Health and Human Services. The health consequences of smoking—50 years of progress. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. (A Report of the Surgeon General). [Google Scholar]
- *.Walker R, Rosvall T, Field CA, Allen S, McDonald D, Salim Z, Ridley N, Adinoff B. Disseminating contingency management to increase attendance in two community substance abuse treatment centers: lessons learned. J Subst Abus Treat. 2010;39(3):202–209. doi: 10.1016/j.jsat.2010.05.010. http://dx.doi.org/10.1016/j.jsat2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Wang L, Wei X, Wang X, Li J, Li H, Jia W. Long-term effects of methadone maintenance treatment with different psychosocial intervention models. PLoS One. 2014;9(2):e87931. doi: 10.1371/journal.pone.0087931. http://dx.doi.org/10.1371/journal.pone.0087931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Winstanley EL, Bigelow GE, Silverman K, Johnson RE, Strain EC. A randomized controlled trial of fluoxetine in the treatment of cocaine dependence among methadone-maintained patients. J Subst Abus Treat. 2011;40(3):255–264. doi: 10.1016/j.jsat.2010.11.010. http://dx.doi.org/10.1016/j.jsat.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


