Abstract
Previous studies have shown that the traditional herbal complex Gosha-jinki-gan (GJG) improves diabetic neuropathy and insulin resistance. The present study was undertaken to elucidate the molecular mechanisms related with the long-term effects of GJG administration on insulin action in vivo and the early steps of insulin signaling in skeletal muscle in streptozotocin (STZ) diabetes. Rats were randomized into five subgroups: (1) saline treated control, (2) GJG treated control, (3) 2-unit insulin + saline treated diabetic, (4) saline + GJG treated diabetic and (5) 2-unit insulin + GJG treated diabetic groups. After seven days of treatment, euglycemic clamp experiment at an insulin infusion rate of 6 mU/kg/min was performed in overnight fasted rats. Despite the 2-unit insulin treatment, the metabolic clearance rates of glucose (MCR, ml/kg/min) in diabetic rats were significantly lower compared with the controls (11.4 ± 1.0 vs 44.1 ± 1.5; P < 0.001), and were significantly improved by insulin combined with GJG or GJG alone (26 ± 3.2 and 24.6 ± 2.2, P < 0.01, respectively). The increased insulin receptor (IR)-β protein content in skeletal muscle of diabetic rats was not affected by insulin combined with GJG administration. However, the decreased insulin receptor substrate-1 (IRS-1) protein content was significantly improved by treatment with GJG. Additionally, the increased tyrosine phosphorylation levels of IR-β and IRS-1 were significantly inhibited in insulin combined with GJG treated diabetes. The present results suggest that the improvement of the impaired insulin sensitivity in STZ-diabetic rats by administration of GJG may be due, at least in part, to correction in the abnormal early steps of insulin signaling in skeletal muscle.
Keywords: Gosha-jinki-gan (GJG), insulin sensitivity, IRS-1, tyrosine-phosphorylation
Introduction
Recently, traditional Chinese medicine (called Kampo medicine in Japan) has been recognized once again for its efficacy and there has been a noticeable increase in its popularity around the world (1) because of its supposedly less frequent side effects (2). One of the main characteristics of traditional medicine is that it has been developed through experience passed on from one generation to another. In other words, traditional medicine emphasizes subjective symptoms, while Western medicine emphasizes objective findings (i.e., practitioners of traditional medicine do not easily lend themselves to randomized, double-blind, placebo-controlled trials). Consequently, despite a long history of application of traditional medicine, scientific evidences about its effects have been superficial. On the other hand, the requirement for scientific validation of the ancient wisdom (the Kampo medicine) from the clinical front has increased markedly.
Streptozotocin (STZ)-induced diabetic rats are thought to be good models of type 1 diabetes mellitus with insulin deficiency and insulin resistance as metabolic characteristics. However, in this model of diabetes, the impaired insulin-stimulated skeletal muscle glucose metabolism and transporter translocation cannot be restored to normal levels despite insulin therapy (3), and this is concordant with human type 1 diabetes (4,5). It is considered that insulin therapy cannot correct the altered insulin signaling mechanism in diabetic skeletal muscle and consequently, cannot normalize the glucose transport system (6). Furthermore, the precise mechanism may involve abnormalities in the content and/or tyrosine phosphorylation of insulin signaling proteins in STZ-diabetic skeletal muscle (7,8), expressing a decreased efficiency of glucose metabolism (8,9). In any case, alterations in the early steps of insulin signaling have been recognized as an important component in several insulin-resistant states.
Gosha-jinki-gan (GJG), a traditional herbal combined prescription composed of 10 crude herbs (Table 1), has been described as being useful in the treatment of many subjective symptoms, including fatigability, cold in the extremities, leg numbness and pain, copious urine with thirst, cloudy vision in old age and lumbago (10). GJG is considered to be a useful approach for the improvement of subjective symptoms such as numbness (11–13), sensation of cold (11,12) and pain in the extremities (13) associated with diabetic neuropathy. Some evidences suggest that GJG administration has a vasodilating (14,15) and antinociceptive effect (16), improving the insulin resistance (17) in STZ-diabetes as a result of increased nitric oxide (NO) production. However, the molecular mechanisms associated with these GJG effects are still not clarified adequately. Therefore, the present study was undertaken firstly to determine the long-term effects of insulin injection combined with GJG administration on the insulin sensitivity in STZ-induced diabetic rats. Our second objective was to investigate whether it is true that GJG treatment potentiates the insulin action, by means of an insulin tolerance test. We further studied the molecular effect of combined GJG administration on the early steps of the insulin-signaling pathway in skeletal muscle.
Table 1.
Composition of GJG
| Component | % |
|---|---|
| Rehmanniae Radix | 17.9 |
| Achyranthis Radix | 10.7 |
| Coprni Fructus | 10.7 |
| Dioscoreae Rhizoma | 10.7 |
| Plantaginis Semen | 10.7 |
| Alismatis Rhizoma | 10.7 |
| Hoclen | 10.7 |
| Moutan Cortex | 10.7 |
| Cinnamomi Cortex | 3.6 |
| Aconiti Tuber | 3.6 |
The GJG used in this study was an unprepared bulk powder.
Materials and Methods
STZ was purchased from Sigma (St. Louis, MO, USA) and neutral insulin from Novo Nordisk (Copenhagen, Denmark). Anti-insulin receptor (IR)-β, anti-insulin receptor substrate (IRS)-1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Hyperfilm was purchased from Amersham (Little Chalfont, Buckinghamshire, UK). All other reagents were of biochemical grade. Herbal extract of GJG was kindly provided by Tsumura Co. (Tokyo, Japan).
Experimental Animals, Diabetes Induction
Male Wistar rats (n = 42) aged 8 weeks (220–230 g) were purchased form CLEA (Shizuoka, Japan). The rats were housed in a room maintained at constant humidity (60 ± 5%), temperature (23 ± 1 °C), and a 12-h light/dark cycle (lighting from 8:00 to 20:00). The standard diet obtained from Oriental Yeast Co. Ltd., (Chiba, Japan) and tap water were available ad libitum throughout the study. All experimental procedures were approved by and carried out in accordance with the guidelines of Nagoya University for the care and use of laboratory animals (detailed procedures are shown in Fig. 1). After a one-week acclimatization period, the rats were divided into two groups and one group was rendered diabetic with a single STZ injection from the tail vein as previously described (18); control rats were only administered citrate buffer. Animals with postprandial blood glucose levels greater than 300 mg/dl 3 days after STZ injection were considered diabetic. After 1 day of recovery from STZ treatment, 2 units of insulin, enough to prevent ketosis but not enough to normalize hyperglycemia (19), were subcutaneously injected between 9:00 h∼10:00 h daily in the diabetic rats. Thereafter, the rats were further randomized into five subgroups: (1) saline treated control, (2) GJG treated control, (3) insulin + saline treated diabetic, (4) saline + GJG treated diabetic and (5) insulin + GJG treated diabetic groups.
Figure 1.
Timetable for the experimental procedures. The number of rats is shown in parentheses. Surgical procedures (#) were performed 3 days after STZ treatment.
Surgical Procedures
Three days after STZ treatment, the animals were anesthetized with sodium pentobarbital (50 mg/kg) and the surgical procedures were performed as described in our previous reports (18,20). During the clamp study, jugular and femoral catheters were used for blood sampling and insulin and/or glucose infusion, respectively.
Exogenous Insulin and GJG Administration
The medicine administration was started one night after surgery. In the insulin treated groups, the dose of ultralente insulin was not changed. The GJG, dissolved in 5 ml/kg saline, was injected through a gastric tube once a day, and the dose of GJG was 800 mg/kg BW, according to a previous study (17); the remaining rats were administered only saline. In the saline + GJG treated diabetic group, insulin injection was replaced by saline. The rats were administered these treatments for 1 week and then subjected to the euglycemic clamp study after overnight fasting.
Euglycemic Clamp Study
The clamp studies were undertaken in the morning after an overnight fast (about 12 h). The euglycemic clamp was performed as previously described (18). Briefly, 6.0 mU/kg/min insulin was continuously infused, the blood glucose concentrations of diabetic rats were lowered to 140 mg/dl, and then clamped at this concentration by periodic adjustment of the glucose infusion rate. The blood glucose concentrations were measured every 10 min and the euglycemia of diabetic and normal rats was maintained at around 139 ± 7 and 70 ± 4 mg/dl, respectively. The glucose infusion rate (GIR) was calculated every 10 min during the clamp study. The metabolic clearance rate for glucose (MCR, ml/kg/min) was then obtained from GIR by dividing it with the corresponding blood glucose concentration. The MCRs from 60 to 90 min for the euglycemic clamp were regarded as indices of insulin action in peripheral tissues, since a plateau in the glucose infusion rate was achieved during this period, as reported previously (18). After the euglycemic clamp study, the rats were anesthetized and the gastrocnemius muscles were excised, immediately freeze-clamped at the liquid nitrogen temperature and stored at −80°C until analyses.
Insulin Tolerance Test
An insulin tolerance test was performed on half of the insulin treated and insulin + GJG treated diabetic rats (six rats in each group). Food was withdrawn at 9:00 h∼10:00 h, following which the diabetic rats were injected with 2 units of insulin; blood samples were drawn from the tail vein at −10, 0, 30, 60 and 120 min for glucose concentration measurements.
Analysis of the Total Protein Amount of IR-β and IRS-1
The expression of the insulin-signaling proteins in skeletal muscle was measured using the Western blotting method, as previously described (18, 21). Briefly, frozen samples were homogenized using a Polytron homogenizer, following which the homogenates were maintained at the ice temperature and centrifuged at 38 000 rpm at 4°C for 1 h. Supernatant proteins (40 mg) of each sample were size-fractionated by SDS-PAGE and then transferred to PVDF membranes. The membranes were then incubated overnight with anti-IRβ and anti-IRS-1 antibodies at 4°C. Bound antibodies were detected by incubation with Goat Anti-Rabbit IgG for 1 h at room temperature. After washing, blotted proteins were visualized using the enhanced chemiluminescence detection system (ECL Plus, Amersham). Quantification of the band intensity on the Hyperfilm was performed using the public domain NIH image software.
Immunoprecipitation and Western Blotting Analysis of IR-β and IRS-1 Tyrosine Phosphorylation
The supernatants containing equal amounts of protein (1 mg/ml for each tube) were incubated overnight at 4°C with anti-IR-β (5 mg/ml) or anti-IRS-1 (5 mg/ml), and then with 20 μl of protein A agarose beads at 4°C for 4 h. The immune complexes were washed according to the procedure described by others (22). Samples were re-suspended in treatment buffer with β-mercaptoethanol and boiled for 5 min. Phosphorylated proteins were separated by SDS-PAGE and the membranes containing bound proteins were incubated with anti-phosphotyrosine antibody. Phosphorylated proteins were visualized by the same method described above.
Blood Assays
Blood glucose concentration was determined using a glucose analyzer (model 2300; Yellow Springs Instrument, OH, USA). Plasma insulin was assayed with a radioimmunoassay kit (Phadesepa Insulin RIA, Pharmacia AB, Sweden).
Statistical Analysis
All values are expressed as means ± SE. Data were analyzed by the one-way analysis of variance. When a significant difference was found (P < 0.05), the results were further compared with the Fisher's PLSD test. The StatView 5.0 software (SAS Institute Inc., Cary, NC) was used for statistical analysis.
Results
Body Weight, Blood Glucose and Plasma Insulin Levels
The body weight, blood glucose and plasma insulin levels before and immediately after the euglycemic clamp are shown in Table 2. Body weight and basic plasma insulin levels were significantly lower in diabetic rats compared with the controls. Seven days of treatment with GJG did not affect the body weight and insulin levels throughout the experimental period. The blood glucose levels in the 7 days GJG-treated diabetes tended to be lower than those of saline-treated diabetes, but not significantly. During the clamp studies, steady state plasma insulin concentrations were not significantly different among all experimental groups.
Table 2.
Body weight and concentrations of blood glucose and plasma insulin before and after the euglycemic clamp procedure at 6.0 mU/kg/min insulin infusion
| Group | Body wt (g) | Glucose (mg/dl) | Insulin (mU/ml) | ||
|---|---|---|---|---|---|
| Basic | During clamp (90 min) | Basic | During clamp (90 min) | ||
| Non-diabetic | |||||
| Saline (6) | 223 ± 5 | 67 ± 1 | 69 ± 2 | 8.1 ± 0.3 | 103 ± 5 |
| GJG (6) | 230 ± 7 | 70 ± 2 | 71 ± 3 | 7.9 ± 0.4 | 100 ± 4 |
| Diabetic | |||||
| Insulin + saline (6) | 200 ± 5 | 190 ± 18** | 137 ± 3 | 4.0 ± 0.1* | 99 ± 6 |
| Saline + GJG (6) | 204 ± 6 | 185 ± 20** | 135 ± 4 | 4.1 ± 0.2* | 102 ± 10 |
| GJG + Insulin (6) | 203 ± 7 | 178 ± 23** | 131 ± 5 | 4.2 ± 0.2* | 106 ± 9 |
Values are means ± SE.
*P < 0.05 vs normal control;
**P < 0.001 vs normal control.
Effect of 7 Days Administration of Insulin Combined with GJG on MCRs
During the clamp studies, the MCRs of diabetic rats that were administered insulin + saline were significantly lower (11.4 ± 1.5 ml/kg/min) compared with control animals (44.1 ± 1.0 ml/kg/min; P < 0.001). In contrast, diabetic rats subjected to 7 days of GJG administration with and without insulin injection showed significantly higher MCRs (26.1 ± 3.2 ml/kg/min and 23.1 ± 2.0 ml/kg/min, respectively, as shown in Fig. 2). The MCR in the only GJG-treated diabetic rats did not reach the levels of the insulin + GJG-treated diabetic rats; however, there was no significant difference between these groups. No effects of GJG treatment were seen on the MCRs of normal rats.
Figure 2.

MCRs during the euglycemic clamp procedure in normal and diabetic rats (insulin + saline; insulin + GJG; saline + GJG). Data are expressed as the means ± SE for six rats in each group. *P < 0.01 vs insulin + GJG-treated and saline + GJG-treated diabetic. **P < 0.001 vs all diabetic groups.
Effect of Administration of Insulin Combined with GJG During the Insulin Tolerance Test
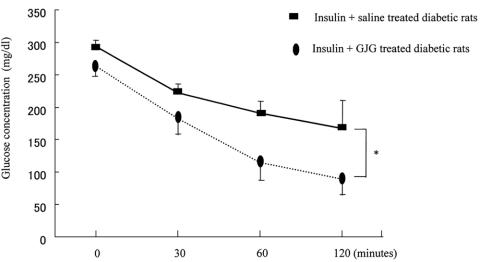
At the 120 min time point of the insulin tolerance test, the average blood glucose of the 2-unit insulin + GJG-treated group was significantly lower than the 2-unit insulin injection only group (90 ± 24 vs 167 ± 43 mg/dl; Fig. 3). The results of the insulin tolerance test suggested that GJG administration significantly potentiates the impaired insulin action, decreasing the hyperglycemia of diabetic rats. The results agreed with the above data in which GJG administration improved the insulin-regulated glucose uptake in STZ-diabetes.
Figure 3.
Insulin tolerance test: blood glucose levels of the insulin + GJG-treated diabetic group tended to be lower than insulin-treated diabetic group at the 0 min time point, but not significantly. At the 120 min time point, the average blood glucose of the insulin + GJG-treated group was significantly lower than the only insulin injection group. Data are expressed as the means ± SE for six rats in each group. *P < 0.05.
Effects of Administration of Insulin Combined with GJG on IR-β and IRS-1 Protein Contents in Skeletal Muscle
The total amount of skeletal muscle IR-β protein (Fig. 4A) was significantly increased in diabetic rats compared with control (128% of control, P < 0.05). GJG administration did not correct the abnormal protein content of IR-β. In contrast, the IRS-1 protein (Fig. 5A) was significantly decreased in insulin-treated diabetes compared with control (47% of control, P < 0.001). However, it was increased to 65% of the level observed in control by insulin injection + GJG administration.
Figure 4.
IR-β protein content (A) and tyrosine phosphorylation levels (B) in the gastrocnemius muscle. IP, Immunoprecipitation; IB, Immunoblotting; Ty, Phosphotyrosine. Data are expressed as the means ± SE for five rats in each group. *P < 0.05 vs insulin + saline-treated and insulin + GJG-treated diabetic. **P < 0.05 vs controls and insulin + GJG-treated diabetic.
Figure 5.
IRS-1 protein content (A) and tyrosine phosphorylation levels (B) in gastrocnemius muscle. IP, Immunoprecipitation; IB, Immunoblotting; Ty, Phosphotyrosine. Data are expressed as the means ± SE for five rats in each group. *P < 0.05 vs insulin + GJG-treated diabetic. **P < 0.001 vs all diabetic groups.
Effects of Administration of Insulin Combined with GJG on the Tyrosine Phosphorylation of IR-β and IRS-1 in Skeletal Muscle
The tyrosine phosphorylation level of IR-β was determined by immunoblotting the IR-β antibody immunoprecipitates with the phosphotyrosine antibody. As shown in Fig. 4B, the tyrosine phosphorylation level of IR-β in skeletal muscle of STZ-diabetes was significantly increased when compared with control (140% of control, P < 0.05). The overexpressed tyrosine phosphorylation of IR-β was corrected by GJG treatment. The same trend was found in IRS-1 tyrosine phosphorylation. As shown in Fig. 5B, the abnormal increases in IRS-1 tyrosine phosphorylation induced by STZ (137% of control, P < 0.05) was just about reversed by GJG treatment.
Discussion
In the present study, we investigated the long-term effects of GJG combined with a small dose of exogenous insulin on the impaired insulin action of STZ-diabetes. The results of the euglycemic clamp study suggested that the insulin action was significantly impaired by STZ treatment, despite the injection of 2 units of insulin. However, the impaired insulin sensitivity was significantly improved by 7 days administration of insulin combined with GJG or GJG alone. The results were in agreement with the insulin tolerance test, i.e., GJG administration potentiates the action of exogenously administered insulin, which results in decreased hyperglycemia in STZ-diabetes.
Although GJG administration was shown to significantly potentiate the impaired insulin action, 7 days of GJG administration did not significantly decrease the fasting blood glucose. The possible explanation for this dissonant observation may be that, after mild STZ treatment, the diabetic rats were injected with a minimum dose of exogenous insulin. Despite the fact that no measurement of urine glucose was performed in the current study, it has been reported that this type of treatment results in a significant urinary glucose loss and polyurea (19,23). Furthermore, loss of glucose through the urine may result in hyperphagia, with the diabetic rats consuming food in excess to compensate for these losses (24). Therefore, in order to precisely determine the effects of GJG administration on blood glucose levels in STZ-diabetes, mild food restriction would be required in future studies. Incidentally, diet restriction and exercise therapies are useful for improving insulin action (25,26), in order to enhance the clinical benefits brought about by the treatment with GJG; thus, self-control is very important for the diabetic patient.
STZ-diabetes leads to the development of insulin resistance in hepatic and peripheral tissues (27,28). The hepatic glucose production was reported to be inhibited by insulin in a dose-dependent fashion (29) and was suppressed at 100 μU/ml insulin in the plasma (30). During the 6.0 mU/g/min insulin infusion rate, insulin levels of about 100 μU/ml were achieved. Consequently, in our experiments, insulin-stimulated glucose disposal under these conditions is mainly due to the reduced glucose uptake in skeletal muscle. In other words, the results of the present study suggest that GJG administration would improve the insulin sensitivity in peripheral tissues (i.e., skeletal muscle). Previous studies have indicated that the vasodilating (14,15) and antinociceptive (16) effects of GJG, as well as improvement in the platelet aggregation (31) and insulin sensitivity (17), are connected with NO production. Moreover, NO is an important endogenous vasodilator that mediates various physiological functions, which may be associated with increased rates of glucose metabolism in skeletal muscle (32–34), and has an aldose reductase inhibition effect (35). Additionally, decreased nitric oxide synthase (NOS) activation could contribute to the genesis of many pathological conditions such as diabetic vasculopathy and neuropathy (36,37). Based on these evidences, we speculated that the pharmacological mechanism of GJG might be related to the NO pathway, although we did not directly measure the effects of GJG administration on the production of NO. Recent studies have provided direct evidence for a complete biochemical pathway involving the IR-β, IRS-1 and PI 3-kinase that can account for the important physiological actions of insulin in stimulating the production of NO (32,33,38). Additionally, cinnamon extract (a component herb of GJG) has been reported to potentiate the in vivo insulin-regulated glucose utilization (20) and to prevent the insulin resistance induced by a high-fructose diet by enhancing insulin signaling (39). Therefore, we clarified the effects of GJG administration on the initial steps of the insulin-signaling cascade by analyzing skeletal muscle of STZ-diabetes.
Previous studies have suggested that IRS-1 plays a central role in the glucose transport in skeletal muscle and that decreased expression of the IRS-1 protein is involved in the development of insulin resistance (40–43). STZ-diabetes is associated with large increases in insulin-stimulated IRS-1 tyrosine phosphorylation (8,9,18), despite the decreased levels of total IRS-1 protein in skeletal muscle (8,18). Our results showed that insulin combined with GJG treatment significantly increases the amount of the IRS-1 protein (Fig. 5A) and inhibits its increased tyrosine phosphorylation level more than insulin alone. However, these levels did not reach those of the controls (Fig. 5B). We also determined the upstream of IRS-1 in the insulin-signaling pathway: the protein content of IR-β in the diabetic rats was significantly increased compared with controls, and GJG administration did not affect the IR-β protein content. In contrast, the overexpressed tyrosine phosphorylation levels of IR-β in STZ-diabetes were corrected by insulin combined with GJG administration. The activation of PI 3-kinase is required for insulin to stimulate glucose transport (44,45). Although we have not assessed the effect on the activation of PI 3-kinase, it was previously described that PI 3-kinase activation closely correlates with IRS-1 phosphorylation in skeletal muscle of STZ-diabetes (46). The present data revealed that insulin + GJG treatment inhibits the increased IRS-1 tyrosine phosphorylation levels (Fig. 5B); therefore, these improvements are thought to improve the abnormal activation of PI 3-kinase (8,9) resulting in the correction of decreased efficiency of glucose metabolization.
One of the characteristics of the Kampo medicine prescription is that it is usually a mixture of individual crude herbs. Although the main constituents and pharmacological properties (biological activities, toxicological aspects, etc.) of each component herb of GJG have been detailed in previous reports (2,20,39,47,48), the synergistic or synthetic effects between individual herbs have not been fully understood. Some evidences suggested that GJG could have multiple effects like vasodilatation (14,15), aldose reductase inhibition (49), antioxidant effect (50) and anti-nociceptive effect (16) owing to the interactions between individual herbal components, and may provide a wide range of therapeutic potential and utility (2). This view is in agreement with our previous research (10–12, 17) and studies conducted by others (13,15). In summary, based on these evidences, GJG may be a useful synthetic traditional medicine prescription for the treatment of diabetic neuropathy with multiple pathogenetic mechanisms.
The two noteworthy findings of this study were that, firstly, GJG with or without a small dose of exogenous insulin produced a significant improvement in the insulin sensitivity in STZ-diabetes and, secondly, the molecular effect of GJG probably occurred via correction of the abnormal early steps of the insulin signaling pathway. Thus, correction of the abnormal early step of insulin signaling in skeletal muscle of GJG-treated STZ-diabetic rats may play an important role in improving insulin sensitivity in vivo. In conclusion, the current study suggests that long-term GJG administration would ameliorate the insulin sensitivity, at least in part, by correcting the abnormal insulin signaling in STZ-diabetes. However, further investigation on other molecular details regarding GJG is needed.
Acknowledgments
This work was supported in part by research grants from Projects on Aging and Health from the Ministry of Health, Labor and Welfare, Japan (H13–009) and from the Kampo Medicine Institute, Japan.
Glossary
Abbreviations
- STZ
streptozotocin
- GJG
Gosha-jinki-gan
- IR-β
insulin receptor-β
- IRS-1
insulin receptor substrate-1
- PI 3-kinase
phosphatidylinositol 3-kinase
- MCR
metabolic clearance rate for glucose
References
- 1.Borchers A, Sakai S, Henderson G, et al. Shosaiko-to and other Kampo (Japanese herbal) medicines: a review of their immunomodulatory activities. J Ethnopharmacol. 2000;73:1–13. doi: 10.1016/s0378-8741(00)00334-2. [DOI] [PubMed] [Google Scholar]
- 2.Nishizawa M, Sutherland W, Nukada H. Gosha-jinki-gan (herbal medicine) in streptozocin-induced diabetic neuropathy. J Neurol Sci. 1995;132:177–181. doi: 10.1016/0022-510x(95)00141-n. [DOI] [PubMed] [Google Scholar]
- 3.Barnard RJ, Youngren JF, Kartel DS, Martin DA. Effects of streptozotocin-induced diabetes on glucose transport in skeletal muscle. Endocrinology. 1990;126:1921–1926. doi: 10.1210/endo-126-4-1921. [DOI] [PubMed] [Google Scholar]
- 4.Yki-Jarvinen H, Koivisto VA. Continuous subcutaneous insulin infusion therapy decreases insulin resistance in type 1 diabetes. J Clin Endocrinol Metab. 1984;58:659–666. doi: 10.1210/jcem-58-4-659. [DOI] [PubMed] [Google Scholar]
- 5.Simonson DC, Tamborlane WV, Sherwin RS, Smith JD, DeFronzo RA. Improved insulin sensitivity in patients with type I diabetes mellitus after CSII. Diabetes. 1985;34(Suppl 3):80–86. doi: 10.2337/diab.34.3.s80. [DOI] [PubMed] [Google Scholar]
- 6.Giorgino F, Logoluso F, Davalli AM, et al. Islet transplantation restores normal levels of insulin receptor and substrate tyrosine phosphorylation and phosphatidylinositol 3-kinase activity in skeletal muscle and myocardium of streptozotocin-induced diabetic rats. Diabetes. 1999;48:801–812. doi: 10.2337/diabetes.48.4.801. [DOI] [PubMed] [Google Scholar]
- 7.Giorgino F, Chen JH, Smith RJ. Changes in tyrosine phosphorylation of insulin receptors and a 170,000 molecular weight nonreceptor protein in vivo in skeletal muscle of streptozotocin-induced diabetic rats: effects of insulin and glucose. Endocrinology. 1992;130:1433–1444. doi: 10.1210/endo.130.3.1531627. [DOI] [PubMed] [Google Scholar]
- 8.Saad MJ, Araki E, Miralpeix M, Rothenberg PL, White ML, Kahn CR. Regulation of insulin receptor substrate-1 in liver and muscle of animal models of insulin resistance. J Clin Invest. 1992;90:1839–1849. doi: 10.1172/JCI116060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giorgino F, Chen JH, Smith RJ. Changes in tyrosine phosphorylation of insulin receptors and a 170,000 molecular weight nonreceptor protein in vivo in skeletal muscle of streptozotocin-induced diabetic rats: effects of insulin and glucose. Endocrinology. 1992;130:1433–1444. doi: 10.1210/endo.130.3.1531627. [DOI] [PubMed] [Google Scholar]
- 10.Qin B, Sato Y. Effectiveness of the traditional Chinese (Kampo) medicine in diabetic peripheral neuropathy. Nagoya Journal of Health, Physical Fitness and Sports. 2004;27(1):55–61. [Google Scholar]
- 11.Sato Y, Sakamoto N. Recent Advances in Traditional Medicine in East Asia. Amsterdam: Excerpta Medica; 1985. Treatment of diabetic neuropathy with Niu-Che-Sen-Qi-Wan; pp. 376–383. [Google Scholar]
- 12.Sakamoto N, Sato Y, Goto Y, et al. Treatment of diabetic neuropathy with traditional Oriental medicine-comparison between Goshajinkigan and Mecobalamin treatment. J Japan Diab Soc. 1987;30:729–737. [Google Scholar]
- 13.Tawata M, Kurihara A, Nitta K, Iwase E, Gan N, Onaya T. The effects of goshajinkigan, a herbal medicine, on subjective symptoms and vibratory threshold in patients with diabetic neuropathy. Diabetes Res Clin Pract. 1994;26:121–128. doi: 10.1016/0168-8227(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki Y, Goto K, Ishige Y, Komatsu Y, Kamei J. Effects of Gosha-jinki-gan, a Kampo medicine, on peripheral tissue blood flow in streptozotocin-induced diabetic rats. Methods Find Exp Clin Pharmacol. 1998;20:321–328. doi: 10.1358/mf.1998.20.4.485687. [DOI] [PubMed] [Google Scholar]
- 15.Shikano M, Niwa T. Effect of Chinese medicine in diabetic patients. Biomedical Thermography. 1987;7:176–179. [Google Scholar]
- 16.Suzuki Y, Goto K, Ishige Y, Komatsu Y, Kamei J. Antinociceptive mechanism of Gosha-jinki-gan in streptozotocin-induced diabetic animals: role of nitric oxide in the periphery. Jpn J Pharmacol. 1999;79:387–391. doi: 10.1254/jjp.79.387. [DOI] [PubMed] [Google Scholar]
- 17.Hu X, Sato J, Oshida Y, Xu M, Bajotto G, Sato Y. Effect of Gosha-jinki-gan (Chinese herbal medicine: Niu-Che-Sen-Qi-Wan) on insulin resistance in streptozotocin-induced diabetic rats. Diabetes Res Clin Pract. 2003;59:103–111. doi: 10.1016/s0168-8227(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 18.Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Effects of keishi-ka-jutsubu-to (traditional herbal medicine: Gui-zhi-jia-shu-fu-tang) on in vivo insulin action in streptozotocin-induced diabetic rats. Life Sci. 2003;73:2687–2701. doi: 10.1016/s0024-3205(03)00640-4. [DOI] [PubMed] [Google Scholar]
- 19.Tominaga M, Kimura M, Sugiyama K, et al. Effects of seishin-renshi-in and Gymnema sylvestre on insulin resistance in streptozotocin-induced diabetic rats. Diabetes Res Clin Pract. 1995;29:11–17. doi: 10.1016/0168-8227(95)01116-u. [DOI] [PubMed] [Google Scholar]
- 20.Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res Clin Pract. 2003;62:139–148. doi: 10.1016/s0168-8227(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 21.Nagasaki M, Nakai N, Oshida Y, Sato Y, et al. Exercise training prevents maturation-induced decreases in insulin receptor substrate-1 and phosphatidylinositol 3-kinase in rat skeletal muscle. Metabolism. 2000;49:954–959. doi: 10.1053/meta.2000.6758. [DOI] [PubMed] [Google Scholar]
- 22.Goodyear LJ, Giorgino F, Sherman LA, Carey J, Smith RJ, Dohm GL. Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J Clin Invest. 1995;95:2195–2204. doi: 10.1172/JCI117909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katovich MJ, Meldrum MJ, Vasselli JR. Beneficial effects of dietary acarbose in the streptozotocin-induced diabetic rat. Metabolism. 1991;40:1275–1282. doi: 10.1016/0026-0495(91)90028-u. [DOI] [PubMed] [Google Scholar]
- 24.Cameron NE, Cotter MA. Metabolic and vascular factors in the pathogenesis of diabetic neuropathy. Diabetes. 1997;46(Suppl 2):S31–S37. doi: 10.2337/diab.46.2.s31. [DOI] [PubMed] [Google Scholar]
- 25.Nakai N, Shimomura Y, Sato Y, et al. Exercise training prevents maturation-induced decrease in insulin sensitivity. J Appl Physiol. 1996;80:1963–1967. doi: 10.1152/jappl.1996.80.6.1963. [DOI] [PubMed] [Google Scholar]
- 26.Cameron-Smith D, Habito R, Barnett M, Collier GR. Dietary guar gum improves insulin sensitivity in streptozotocin-induced diabetic rats. J Nutr. 1997;127:359–364. doi: 10.1093/jn/127.2.359. [DOI] [PubMed] [Google Scholar]
- 27.Youn JH, Kim JK, Buchanan TA. Time courses of changes in hepatic and skeletal muscle insulin action and GLUT4 protein in skeletal muscle after STZ injection. Diabetes. 1994;43:564–571. doi: 10.2337/diab.43.4.564. [DOI] [PubMed] [Google Scholar]
- 28.Bevilacqua S, Barrett EJ, Smith D, et al. Hepatic and peripheral insulin resistance following streptozotocin-induced insulin deficiency in the dog. Metabolism. 1985;34:817–825. doi: 10.1016/0026-0495(85)90105-2. [DOI] [PubMed] [Google Scholar]
- 29.Smith D, Rossetti L, Ferrannini E, et al. In vivo glucose metabolism in the awake rat: tracer and insulin clamp studies. Metabolism. 1987;36:1167–1174. doi: 10.1016/0026-0495(87)90244-7. [DOI] [PubMed] [Google Scholar]
- 30.DeFronzo R, Bonadonna R, Ferrannini E. Pathogenesis of NIDDM: a balanced overview. Diabetes Care. 1992;15:318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki Y, Goto K, Ishige A, Komatsu Y, Kamei J. Effect of Gosha-jinki-gan, a Kampo medicine, on enhanced platelet aggregation in streptozotocin-induced diabetic rats. Jpn J Pharmacol. 1998;78:87–91. doi: 10.1254/jjp.78.87. [DOI] [PubMed] [Google Scholar]
- 32.Young M, Radda G, Leighton B. Nitric oxide stimulates glucose transport and metabolism in rat skeletal muscle in vitro. Biochem J. 1997;322(Pt 1):223–228. doi: 10.1042/bj3220223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balon TW, Nadler J. Evidence that nitric oxide increases glucose transport in skeletal muscle. J Appl Physiol. 1997;82:359–363. doi: 10.1152/jappl.1997.82.1.359. [DOI] [PubMed] [Google Scholar]
- 34.Roy D, Perreault M, Marette A. Insulin stimulation of glucose uptake in skeletal muscles and adipose tissues in vivo is NO dependent. Am J Physio. 1998;274(4 Pt 1):E692–E699. doi: 10.1152/ajpendo.1998.274.4.E692. [DOI] [PubMed] [Google Scholar]
- 35.Chandra D, Jackson E, Ramana K, Kelley R, Srivastava S, Bhatnagar A. Nitric oxide prevents aldose reductase activation and sorbitol accumulation during diabetes. Diabetes. 2002;51:3095–3101. doi: 10.2337/diabetes.51.10.3095. [DOI] [PubMed] [Google Scholar]
- 36.Chan N, Vallance H, Colhoun M. Nitric oxide and vascular responses in type I diabetes. Diabetologia. 2000;43:137–147. doi: 10.1007/s001250050022. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki T, Yasuda H, Maeda K, Kikkawa R. Hyperalgesia and decreased neuronal nitric oxide synthase in diabetic rats. Neuroreport. 1998;9:243–247. doi: 10.1097/00001756-199801260-00013. [DOI] [PubMed] [Google Scholar]
- 38.Montagnani M, Ravichandran L, Chen H, Esposito D, Quon M. Insulin receptor substrate-1 and phosphoinositide-dependent kinase-1 are required for insulin-stimulated production of nitric oxide in endothelial cells. Mol Endocrinol. 2002;16:1931–1942. doi: 10.1210/me.2002-0074. [DOI] [PubMed] [Google Scholar]
- 39.Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract prevents the insulin resistance induced by a high-fructose diet. Horm Metab Res. 2004;36:199–205. doi: 10.1055/s-2004-814223. [DOI] [PubMed] [Google Scholar]
- 40.Anai M, Funaki M, Ogihara T, et al. Altered expression levels and impaired steps in the pathway to phosphatidylinositol 3-kinase activation via insulin receptor substrates 1 and 2 in Zucker fatty rats. Diabetes. 1998;47:13–23. doi: 10.2337/diab.47.1.13. [DOI] [PubMed] [Google Scholar]
- 41.Araki E, Lipes MA, Patti ME, et al. Alternative pathway of insulin signaling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 42.Tamemoto H, Kadowaki T, Tobe K, et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182–186. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- 43.Yamauchi T, Tobe K, Tamemoto H, et al. Insulin signaling and insulin actions in the muscles and livers of insulin-resistant, insulin receptor substrate 1-deficient mice. Mol Cell Biol. 1996;16:3074–3084. doi: 10.1128/mcb.16.6.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheatham B, Vlahos C, Cheatham L, Wang L, Blenis J, Kahn C. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes: studies with a selective inhibitor wortmannin. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 46.Folli F, Saad M, Backer J, Kahn C. Regulation of phosphatidylinositol 3-kinase activity in liver and muscle of animal models of insulin-resistant and insulin-deficient diabetes mellitus. J Clin Invest. 1993;92:1787–1794. doi: 10.1172/JCI116768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang W, Eisebrand G. Chinese Drugs of Plant Origin. Berlin: Springer-Verlag; 1992. [Google Scholar]
- 48.Huang K. The Pharmacology of Chinese Herbs. Boca Raton FL: CRC Press; 1993. [Google Scholar]
- 49.Aida K, Tawata M, Shindo H, Onya, et al. Isoliquiritigenin: a new aldose reductase inhibitor from glycyrrhize radix. Planta Med. 1990;56:254–258. doi: 10.1055/s-2006-960950. [DOI] [PubMed] [Google Scholar]
- 50.Niwa Y, Miyachi Y. Antioxidant action of natural health products and Chinese herbs. Inflammation. 1986;10:79–91. doi: 10.1007/BF00916043. [DOI] [PubMed] [Google Scholar]