Abstract
Ozone therapy is currently being used in the treatment of ischemic disorders, but the underlying mechanisms that result in successful treatment are not well known. This study assesses the effect of ozone therapy on the blood flow in the middle cerebral and common carotid arteries. Seven subjects were recruited for the therapy that was performed by transfusing ozone-enriched autologous blood on 3 alternate days over 1 week. Blood flow quantification in the common carotid artery (n = 14) was performed using color Doppler. Systolic and diastolic velocities in the middle cerebral artery (n = 14) were estimated using transcranial Doppler. Ultrasound assessments were conducted at the following three time points: 1) basal (before ozone therapy), 2) after session #3 and 3) 1 week after session #3. The common carotid blood flow had increased by 75% in relation to the baseline after session #3 (P < 0.001) and by 29% 1 week later (P = 0.039). In the middle cerebral artery, the systolic velocity had increased by 22% after session #3 (P = 0.001) and by 15% 1 week later (P = 0.035), whereas the diastolic velocity had increased by 33% after session #3 (P < 0.001) and by 18% 1 week later (P = 0.023). This preliminary Doppler study supports the clinical experience of achieving improvement by using ozone therapy in peripheral ischemic syndromes. Its potential use as a complementary treatment in cerebral low perfusion syndromes merits further clinical evaluation.
Keywords: color Doppler, ischemia, low perfusion, transcranial Doppler
Introduction
Cerebral low perfusion syndromes have significant clinical and social repercussions. An important field in neurological research includes the search for more effective drugs and other methods in order to ameliorate this problem. Ozone therapy is a non-conventional therapy that has been used for several years in the treatment of ischemic disorders, particularly of the lower limbs (1–3). However, to date, very few studies have systematically evaluated blood flow changes resulting from ozone therapy.
With regard to this, the effect of ozone therapy on the blood flow in the middle cerebral artery (MCA) and the common carotid artery (CCA) was investigated in the current study.
Subjects and Methods
Patients
In this study, the blood flow in 28 arteries (14 MCA and 14 CCA) was evaluated in 7 subjects—5 patients and 2 healthy volunteers. The subjects were from our university hospital. The patients who underwent elective ozone therapy, which was unrelated to the treatment of their cerebral vascular diseases, were from the Radiation Oncology department. Their scheduled medication was not modified during the study period. The volunteers were members of the clinical staff of the departments involved in the investigation. The study included 5 males and 2 females with a mean age of 58 years (range, 34–78). Informed consent was obtained from all the participants prior to inclusion in the study. The study was approved by the Institutional Ethical Committee. Table 1 summarizes the details of the subjects that participated in this study.
Table 1.
Patients and control subjects included in the study
| Patient | Age (years) | Characteristics |
|---|---|---|
| #1 | 67 | aComplementary treatment during radiochemotherapy for advanced carcinoma of hypopharynx. |
| bArterial hypertension under drug treatment and hyperglycemia under dietary treatment. | ||
| #2 | 74 | aComplementary treatment during radiochemotherapy for advanced carcinoma of base of tongue. |
| bChronic obstructive bronchitis (COB) under treatment with bronchodilator inhalers. | ||
| #3 | 63 | aComplementary treatment during radiochemotherapy for advanced carcinoma of supraglottis. |
| bHyperuricemia treated with allopurinol. Multiple sclerosis treated with baclofen. | ||
| #4 | 51 | aRadiation-induced necrosis of thyroid cartilage (radiotherapy was administered for carcinoma of glottis several years ago). |
| bTreatment with corticosteroids. | ||
| #5 | 78 | aChronic ulceration with calcaneous exposure and transplant failure. |
| bInsulin-dependent diabetes, arterial hypertension under drug treatment. Stroke 1 year ago. Duodenal ulcers. | ||
| #6 | 43 | Healthy subject. |
| #7 | 34 | Healthy subject. |
aReason for ozone therapy.
bConcomitant diseases or treatments (no changes were made in the medications during the period of Doppler evaluation). The study was planned with three ozone therapy sessions to evaluate the initial effects under the same conditions. Patient #1, #2 and #3 (cancer patients) were required to commence their scheduled radiochemotherapy after session #3; therefore, ethical considerations precluded delay in the cancer treatment. Ozone therapy was continued during the radiochemotherapy; however, radiotherapy of the cervical and carotid areas altered the subsequent Doppler evaluations. Patient #4 suffered from hemorrhage of the larynx. Ozone therapy was stopped after session #3 to enable the patient to undergo surgery. The usual complications associated with the surgical treatment of this radiation-induced necrosis were absent. Patient #5 who suffered from several vascular diseases was treated with systemic and local ozone therapy for a chronic wound. Patient #6 and #7 (healthy subjects recruited from among the hospital staff) also received 3 sessions of ozone therapy to evaluate the Doppler Effect. Further sessions were neither scheduled nor administered.
Ozone Therapy
Ozone therapy was administered by autologous blood transfusion on 3 alternate days over 1 week. The procedure involved the collection of 200 ml venous blood into a blood bag containing heparin (25 IU/ml) and CaCl2 (5 mM). The O3/O2 gas mixture was prepared from clinical-grade O2 using the OZON 2000 medical device (Zotzmann + Stahl GmbH, Plüderhausen, Germany). The blood was mixed with 200 ml of O3/O2 gas mixture at a concentration of 60 μg/ml in a sterile single-use 300 ml container. Subsequently, the blood was slowly re-introduced into the patient via the antecubital vein, after being passed through a sterile 0.20 μm filter. The blood remained outside the body for approximately 15–30 min, and no adverse reactions were observed.
Doppler studies were conducted on the following three occasions: 1) before session #1; 2) after session #3; and 3) 1 week after session #3.
Transcranial Doppler Velocimetry
Systolic and diastolic velocities (in cm/s) were measured in the MCA by the transtemporal approach using a transcranial Doppler (TCD) with a 2 MHz probe from an Angiodine-2 Fluo-Link 300® device. The patient was alert, relaxed and seated when the absence of stenoses was confirmed. The Doppler insonation angle was <60°.
Common Carotid Blood Flow Quantification
Blood flow quantification of CCA was performed using a color Doppler, Philips Ultrasound P-800 unit®, with time-domain processing. This technique simultaneously evaluates the velocity and the vessel diameter, and the data is presented in terms of ml/min. The usefulness and validity of this technique has been previously described (4,5). The patient was alert, relaxed and in the supine position when the absence of significant stenoses in the extracranial carotid arteries was confirmed. A 7.5 MHz linear high-definition probe with a Doppler insonation angle of <60° was used. We obtained information regarding the volume of blood flow (in ml/min) in both CCAs at ≥2 cm prior to the carotid bifurcation.
All ultrasound studies were performed bilaterally by the same radiologist in order to minimize interobserver variability (6). When an optimal image of stable blood flow was obtained, recordings over at least three cardiac cycles were made. This was repeated at least three times in order to preclude operator-induced or technical inaccuracies. The median values that were obtained were used in the statistical analyses.
Neither blood pressure nor hemoglobin levels were measured.
Statistical Analysis
The SPSS 7.0 for Windows software package (SPSS-Ibérica, Madrid, Spain) was used throughout the study. The normality of distribution of data was assessed by the Kolmogorov–Smirnov test. Two-sided tests were applied. The data are expressed as mean ± SD. The paired t-test was used to compare differences between the baseline and the two time-point measurements following the ozone therapy. Linear correlation was assessed by the Pearson's r test. The differences were considered to be significant when P < 0.05.
Results
Transcranial Doppler Velocimetry
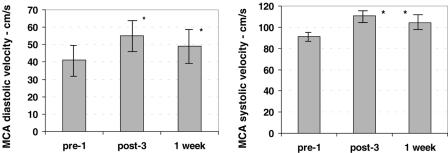
The baseline systolic velocity in MCA was 90.9 ± 6.1 cm/s. After session #3, it increased to 111 ± 7.3 cm/s (increase 22%, P = 0.001), and 1 week later, it was 104.3 ± 8 cm/s (increase 15%, P = 0.035). The baseline diastolic velocity in MCA was 41.1 ± 4.4 cm/s. After session #3, it increased to 54.6 ± 4.6 cm/s (increase 33%, P < 0.001), and 1 week later, it was 48.6 ± 5 cm/s (increase 18%, P = 0.023) (Fig. 1).
Figure 1.
Transcranial Doppler during ozone therapy. Left. Diastolic velocity (in cm/s) in the middle cerebral artery (MCA) increased by 33% at the end of session #3 (P < 0.001), and an 18% increase persisted for 1 week after session #3 (P = 0.023). Right. Systolic velocity in MCA increased by 22% at the end of session #3 (P = 0.001), and a 15% increase persisted for 1 week after session #3 (P = 0.035). The error bars are the 95% confidence intervals. Significant differences (P < 0.05) are indicated with an asterisk (*).
Common Carotid Blood Flow Quantification
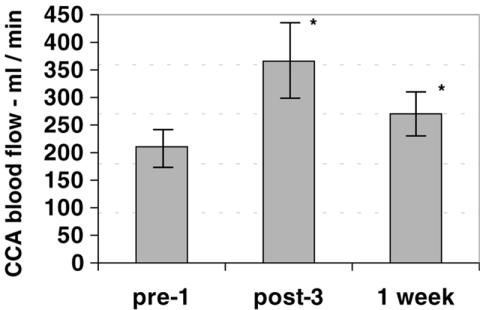
The baseline CCA blood flow was 233 ± 19 ml/min. After session #3, it increased to 407 ± 38 ml/min (increase 75%, P < 0.001), and 1 week later, it was 301 ± 22 ml/min (increase 29%, P = 0.039) (Fig. 2).
Figure 2.
Carotid blood flow during ozone therapy. Blood flow quantification (in ml/min) in the common carotid artery (CCA) increased by 75% at the end of session #3 (P < 0.001), and a 29% increase persisted for 1 week after session #3 (P = 0.039). The error bars are the 95% confidence intervals. Significant differences (P < 0.05) are indicated with an asterisk (*).
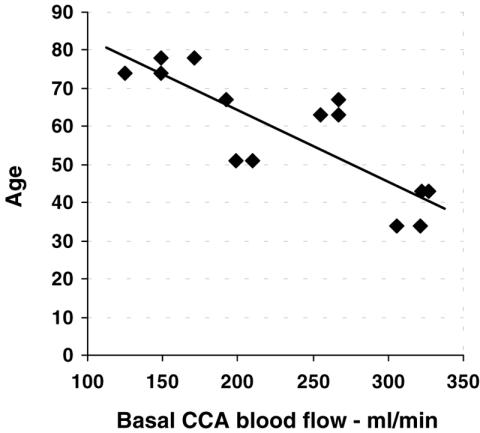
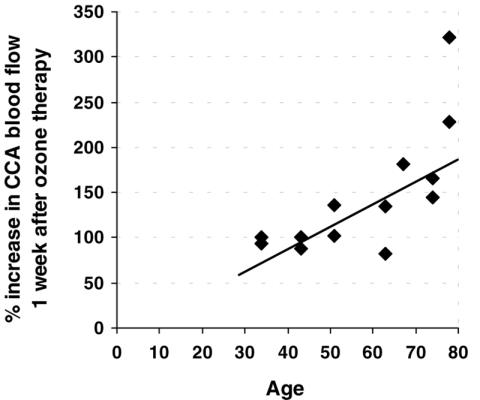
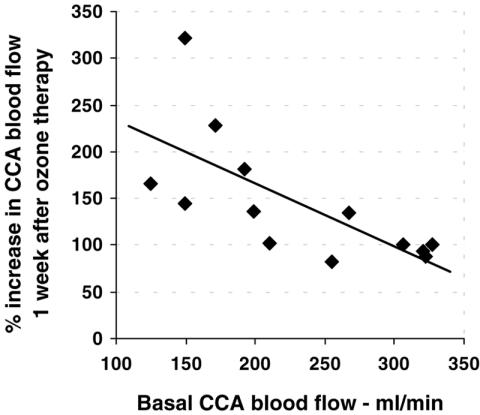
The baseline CCA blood flow directly correlated with the MCA diastolic velocity (r = 0.557; P = 0.039) and inversely correlated with age (r = 0.825; P < 0.001) (Fig. 3). The percentage increase in CCA blood flow 1 week after session #3 was directly correlated with age (r = 0.735; P = 0.004) (Fig. 4) and inversely correlated with the initial values of the CCA blood flow (r = 0.691; P = 0.009). In older patients, the increase in CCA blood flow was higher and that in basal perfusion was lower (Fig. 5) (Note: in Figs 4 and 5, the Doppler data for the left arteries of one patient 1 week after session #3 were not available due to technical reasons).
Figure 3.
Relationship between baseline blood flow and age. Baseline values of the common carotid artery (CCA) blood flow were inversely correlated with the age of the patients (r = 0.825; P < 0.001). A lower blood flow was observed in older patients.
Figure 4.
Relationship between age and blood flow increase post-ozone therapy. The percentage increase in CCA blood flow 1 week after session #3 was directly correlated with age (r = 0.735; P = 0.004). A higher increase was observed after ozone therapy in older patients.
Figure 5.
Relationship between the baseline blood flow and its increase post ozone therapy. The correlation in CCA blood flow between baseline values and the percentage increase 1 week after session #3 was highly significant (r = 0.691; P = 0.009), i.e., there is a higher percentage increase in CCA corresponding to a lower initial blood flow. Note: the percentages under 100% indicate a decrease in blood flow at this time point.
Discussion
Although biomedical applications of ozone therapy can be traced back to the end of the 19th century, numerous aspects of the effects of the therapy remain unexplored.
The airways are precluded in this therapy, which uses ozone-enriched autologous blood transfusion; therefore, lung toxicity resulting from oxidative stress is avoided. Ozone, per se, does not enter the organism; the effects that are observed are mediated by the rapid oxidation of certain substances in the blood in the transfusion recipient. In appropriate concentrations, this can up-regulate the synthesis of antioxidants in blood (7). This property has been very actively investigated with respect to the protection against free radical damage associated with heart (8), kidney (9) and liver (10) disorders. The mechanisms proposed to explain the vascular effects include the liberation of vasoactive substance as well as the improvement in erythrocyte flexibility and blood rheology (1,11,12).
Several studies that included control subjects have indicated that when ozone-free oxygen is used, the beneficial biochemical (7,10) and rheological (1) responses are not observed. The changes in the MCA and/or CCA blood flow occurring during ozone therapy were assessed in the present study that did not include non-ozonized blood transfusion and each patient was his/her own control.
As indicated by the CCA measurements, the increase in diastolic velocity in the MCA is compatible with a decrease in vascular resistance, a rheological improvement (1,12) and an overall increase in blood flow. The inverse correlation between the percentage increase in CCA blood flow and the initial values is compatible with a microvascular redistribution resulting in better oxygenation in tissues with poor blood supply. This was tentatively demonstrated in our previous studies by the direct measurement of muscle and tumor oxygenation using polarographic electrodes (13,14).
These rheological and vascular effects suggest that coadjuvant ozone therapy could decrease the vasoconstriction that is secondary to hyperoxia. Techniques such as carbogen breathing or hyperbaric chambers are used to increase the amount of O2 dissolved in arterial blood. However, when prolonged for >15–30 min, these therapies can lead to an increase in peripheral vascular resistance along with a generalized vasoconstriction in most organs (15). Decreased cerebral blood flow secondary to hyperoxia has indeed been documented in humans by transcranial Doppler (16) and magnetic resonance (17) studies.
The above-mentioned effects of ozone therapy and data from the present study, especially the potentially greater effect in older patients or in those with lower initial blood flow, augur well for its use in cerebral low perfusion syndromes and stroke. This is further supported by the clinical experience gained in a study that assessed 150 patients with ischemic cerebrovascular disease treated with prolonged ozone therapy (18).
The present Doppler study was planned with only three ozone therapy sessions for several reasons. Firstly, we wanted to evaluate the effect of ozone therapy and to observe whether the effect could be maintained for a prolonged period, which has been suggested by the clinical experience gained from its use in sessions widely separated over several days. Hence, we decided to perform the third session approximately 1 week later without any intervening sessions. Secondly, we wanted to administer the same number of sessions to all the patients in the study. However, some of them were cancer patients who needed to commence their scheduled radiochemotherapy. Therefore, in order to avoid interference with the scheduled radiochemotherapy, the present ozone study was performed during the period when oncologic staging and planning of the radiotherapy were carried out. Hence, the number of ozone therapy sessions for Doppler evaluation was less than that considered necessary for a full-fledged ozone therapy, which usually lasts for several weeks or even months. The Doppler Effect after several additional sessions could indeed be higher than that currently observed. Data on the optimal separation between the ozone therapy sessions are not currently available. Further, the schedule could vary depending on the desired clinical effect (antioxidant, enhancing the immune or vascular system, etc.). Nevertheless, the current study supports the clinical experience gained in the treatment of vascular disorders, employing widely separated sessions over extended periods (2,3). Two or three applications per week appear to be sufficient in providing significant vascular improvement. However, changes observed over a mere 1 or 2 weeks are usually not sufficient to improve chronic clinical conditions. The current findings regarding a residual effect, which is still significantly elevated over baseline 1 week after the last session, support our postulation that one or two additional sessions per week can be effective during the initial maintenance period. The mode and timing of administration of additional sessions over a period of months need to be explored for the optimization of the sessions.
In the course of this study, our hospital facilities were transferred to a different location in our city, and we were unable to conduct further Doppler studies using the same equipment. Therefore, we decided to increase the study sample by including two healthy subjects from our hospital. We could not evaluate the differences between patients and healthy subjects due to the scarcity of patients. Only patient #5 had suffered a CVA/stroke that may modify the Doppler evaluation in the carotid and middle cerebral arteries. However, patients with localized tumors do not appear to have a systemic vascular alteration or an altered vascular response. Therefore, we assumed that the effect observed in these arteries is a general effect, which does not differ from that observed in the healthy subjects or the patients that were studied.
Further studies, which include new technologies such as interstitial multichannel laser Doppler used to quantify fluctuations in microvascular perfusion during ozone therapy, are in progress in order to ascertain some of the remaining doubts regarding the efficacy of ozone therapy.
In conclusion, this preliminary Doppler study demonstrates, albeit in a small number of subjects, that ozone therapy increases blood flow in CCA and MCA with a prolonged effect such that it can be very easily assessed by TCD and carotid ultrasound. These data support the clinical experience of achieving improvement using ozone therapy in peripheral ischemic syndromes. Its potential use as a complementary treatment in cerebral low perfusion syndromes warrants further clinical investigation.
Conflict of Interest
The study was supported in part by a grant (FUNCIS 98–31) from the Health and Research Foundation of the Autonomous Government of the Canary Islands, Spain.
Acknowledgments
The study was supported in part by a grant (FUNCIS 98–31) from the Health and Research Foundation of the Autonomous Government of the Canary Islands, Spain.
We wish to thank Dr R. Reyes (Department of Interventional and Vascular Radiology) and Dr G. Rovira-Dupláa (Ozone therapy Unit of the Quirón Clinic, Barcelona, Spain) for their valuable advice in conducting this study. We also thank R. Martin-Oliva (Head of Department of Medical Physics) and Dr M. A. Hernández (Head of Department of Radiation Oncology) for their administrative and clinical support with the equipment. Editorial assistance was provided by Dr Peter R. Turner, t-SciMed, Reus, Spain.
References
- 1.Giunta R, Coppola A, Luongo C, Sammartino A, Guastafierro S, Grassia A, et al. Ozonized autohemotransfusion improves hemorheological parameters and oxygen delivery to tissues in patients with peripheral occlusive arterial disease. Ann Hematol. 2001;80:745–748. doi: 10.1007/s002770100377. [DOI] [PubMed] [Google Scholar]
- 2.Romero A, Menéndez S, Gómez M, Ley J. La Ozonoterapia en los estadios avanzados de la aterosclerosis obliterante. Angiología. 1993;45:146–148. [PubMed] [Google Scholar]
- 3.Rovira G, Galindo N. La ozonoterapia en el tratamiento de las úlceras crónicas de las extremidades inferiores. Angiología. 1991;2:47–50. [PubMed] [Google Scholar]
- 4.Maulik D, Kadado T, Downing G, Phillips C. In vitro and in vivo validation of time domain velocity and flow measurement technique. J Ultrasound Med. 1995;14:939–947. doi: 10.7863/jum.1995.14.12.939. [DOI] [PubMed] [Google Scholar]
- 5.Westra SJ, Levy DJ, Chaloupka JC, Hill JA, Robert JM, Sayre JW, et al. Carotid artery volume flow: in vivo measurement with time-domain processing US. Radiology. 1997;202:725–729. doi: 10.1148/radiology.202.3.9051025. [DOI] [PubMed] [Google Scholar]
- 6.Schoning M, Scheel P. Color duplex measurement of cerebral blood flow volume: intra- and interobserver reproducibility and habituation to serial measurements in normal subjects. J Cereb Blood Flow Metab. 1996;16:523–531. doi: 10.1097/00004647-199605000-00020. [DOI] [PubMed] [Google Scholar]
- 7.León OS, Menéndez S, Merino N, Castillo R, Sam S, Pérez L, et al. Ozone oxidative preconditioning: a protection against cellular damage by free radicals. Mediators Inflamm. 1998;7:289–294. doi: 10.1080/09629359890983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernández F, Menéndez S, Wong R. Decrease of blood cholesterol and stimulation of antioxidative response in cardiopathy patients treated with endovenous ozone therapy. Free Radical Biol Med. 1995;19:115–119. doi: 10.1016/0891-5849(94)00201-t. [DOI] [PubMed] [Google Scholar]
- 9.Barber E, Menendez S, Leon OS, Barber MO, Merino N, Calunga JL, et al. Prevention of renal injury after induction of ozone tolerance in rats submitted to warm ischemia. Mediators Inflamm. 1999;8:37–41. doi: 10.1080/09629359990702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peralta C, León OS, Xaus C, Prats N, Jalil EC, Sala-Planell E, et al. Protective effect of ozone treatment on the injury associated with hepatic ischemia reperfusion: antioxidant-pro-oxidant balance. Free Rad Res. 1999;31:191–196. doi: 10.1080/10715769900300741. [DOI] [PubMed] [Google Scholar]
- 11.Bocci V. Autohaemotherapy after treatment of blood with ozone: a reappraisal. J Int Med Res. 1994;22:131–143. doi: 10.1177/030006059402200301. [DOI] [PubMed] [Google Scholar]
- 12.Verrazzo G, Coppola L, Luongo C, Sammartino A, Giunta R, Grassia A, et al. Hyperbaric oxygen, oxygen-ozone therapy, and rheologic parameters of blood in patients with peripheral occlusive arterial disease. Undersea Hyperb Med. 1995;22:17–22. [PubMed] [Google Scholar]
- 13.Clavo B, Pérez JL, Catalá L, López L, Suárez G, Lloret M, et al. Effect of ozone therapy on muscle oxygenation. J Altern Complem Med. 2003;9:251–256. doi: 10.1089/10755530360623365. [DOI] [PubMed] [Google Scholar]
- 14.Clavo B, Perez JL, Lopez L, Suarez G, Lloret M, Rodriguez V, et al. Ozone therapy for tumor oxygenation: a pilot study. Evid Based Complement Alternat Med. 2004;1:93–98. doi: 10.1093/ecam/neh009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergo GW, Tyssebotn I. Cardiovascular effects of hyperbaric oxygen with and without addition of carbon dioxide. Eur J Appl Physiol. 1999;80:264–275. doi: 10.1007/s004210050592. [DOI] [PubMed] [Google Scholar]
- 16.Omae T, Ibayashi S, Kusuda K, Nakamura H, Yagi H, Fujishima M. Effects of high atmospheric pressure and oxygen on middle cerebral blood flow velocity in humans measured by transcranial Doppler. Stroke. 1998;29:94–97. doi: 10.1161/01.str.29.1.94. [DOI] [PubMed] [Google Scholar]
- 17.Watson NA, Beards SC, Altaf N, Kassner A, Jackson A. The effect of hyperoxia on cerebral blood flow: a study in healthy volunteers using magnetic resonance phase-contrast angiography. Eur J Anaesthesiol. 2000;17:152–159. doi: 10.1046/j.1365-2346.2000.00640.x. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez MM, Garcia JR, Menéndez S, Devesa E, Valverde S. Ozonoterapia en la enfermedad cerebrovascular isquémica. Revista Cenic Ciencias Biológicas. 1998;29:145–148. [Google Scholar]