Summary
Background
Reports of the crude incidence of venous thromboembolism (VTE) in Western countries vary widely. Data regarding risk factors, incidence and recurrence of VTE from deeply-phenotyped community-based cohort studies are needed.
Objectives
To study the incidence, associated mortality, and predisposing factors of VTE in the prospective, longitudinal community-based Framingham Heart Study.
Patients/Methods
The study sample consisted of the Framingham Heart Study Original, Offspring, Third Generation, and Omni cohorts (N=9,754). Incidence rates (IR) were standardized to the 2000 US population. Cox proportional hazards regression models were used to study risk factor associations.
Results
During 1995–2014 (total follow-up time 104,091 person-years [median 9.8 (range 0–20) years]), 297 incident VTE events were observed. Age-adjusted IR of VTE was 20.3/10,000 (95% CI 17.9–22.6). Of the events 120 (40%) were pulmonary embolism (PE) and 177 (60%) were deep venous thrombosis (DVT); 29% were unprovoked, 40% provoked, and 31% cancer-related. Cancer-related VTE was associated with high mortality at 30 days (24.2%), 1 year (66.3%), and 5 years (75.6%). In multivariable models, age and obesity, but no other traditional cardiovascular risk factors, were significantly associated with VTE (hazard ratio [HR] per 10-year increase in age 1.69, 95%CI 1.48–1.92; HR for obesity (BMI ≥30kg/m2) 1.88, 95%CI 1.44–2.45).
Conclusions
We provide data on the epidemiology of VTE. VTE is associated with significant mortality, and prognosis after cancer-related VTE is particularly poor. Traditional cardiovascular risk factors beyond age and obesity are not associated with VTE.
Keywords: Venous Thromboembolism, deep venous thrombosis, pulmonary embolism, epidemiology, incidence, mortality, risk factors
Introduction
Venous thromboembolism (VTE) — comprising pulmonary embolism (PE) and deep venous thrombosis (DVT) — remains a common, preventable disease with high recurrence rate. [1] The incidence of VTE in Western countries has recently been systematically reviewed by a subcommittee of the International Society of Thrombosis and Haemostasis [2]. Unadjusted incidence rates (IR) of VTE range from 8 to 27 per 10, 000 person-years. [3–12] VTE is the cause of major morbidity with an estimated average of 548,000 hospitalizations annually in the US alone. [13] Furthermore, for almost one-quarter of patients experiencing PE, the initial clinical presentation is sudden death. [14] Direct ascertainment of mortality caused by VTE is challenging due to the low rate of autopsies revealing often undiagnosed PE as the immediate cause, but according to one estimate up to over 530,000 annual deaths could be attributed to VTE in the European Union in 2004. [14] VTE events may be associated with predisposing factors (i.e. provoked), or they may be unprovoked, i.e. idiopathic. [15] Pathophysiologic characteristics of VTE include endothelial dysfunction, inflammation and hypercoagulability. [16,17] VTE also has a strong hereditary component. [18,19] Whether traditional cardiovascular risk factors (e.g. smoking, diabetes, hypertension and hyperlipidemia) predispose individuals to VTE remains controversial with accumulating evidence suggesting no or weak association. [20–29]
We describe the epidemiology of incident VTE over two decades of follow-up, and we examine the role of traditional cardiovascular (CV) risk factors measured at baseline, in a community-based prospective cohort, the Framingham Heart Study (FHS).
Methods
Study sample
The study sample consisted of the Framingham Heart Study Original, Offspring, and Third Generation cohorts [30–32] and the Framingham Heart Study Omni cohort. [33] The Original cohort was recruited in 1948; since then, participants have been examined biennially at the FHS Clinic. In 1971 the Offspring cohort was initiated by recruiting offspring (and their spouses) of the Original cohort; participants have been examined roughly every 4 years. The Third Generation cohort, consisting of children of the Offspring cohort, was recruited in 2002 and has returned for one repeat examination. The Omni cohort was added in 1994 and consists of Framingham residents aged 40 to 75 years who self-identified as minority group members. Written informed consent was provided by all participants and the study has been approved by the Boston University Medical Center Institutional Review Board.
VTE has been a systematically adjudicated endpoint since 1994; therefore, we identified January 1, 1995, as the baseline date for follow-up. The total number of eligible participants across the four cohorts was N=9874; 109 participants were excluded: 53 had reported prevalent VTE at inclusion and 56 did not attend the baseline examination for this study. The final study sample was N=9765: 1259 Original cohort, 3914 Offspring cohort, 4089 Third Generation cohort, and 503 Omni cohort. Follow up was from January 1st 1995 to March 24th 2014.
Baseline characteristics
Baseline characteristics were recorded at the following exams that served as the cohort-specific baseline examination for this study: Exam 24 (1995–1998) for the Original cohort, Exam 6 for (1995–1998) for the Offspring cohort, Exam 1 (2002–2005) for the Third Generation cohort, and Exam 1 (1994–1998) for the OMNI cohort. Baseline characteristics were obtained at a preceding exam or the immediate following exam if the participant did not attend the pre-specified baseline exam or had the first incident VTE after January 1st 1995 but before attending the pre-specified baseline examination (n=853). Baseline characteristics were always obtained before the VTE event.
VTE diagnosis and classification
At each examination, participants underwent a medical history and physical examination. Medical records and imaging reports were obtained for each reported physician visit (out-patient) or hospital admission (in-patient) by a participant with symptoms suggestive of VTE. Diagnosis of VTE was based on clinical symptoms and signs suggestive of VTE coupled with objective imaging data obtained from physician office records, hospital records, or autopsy reports. PE was diagnosed using either ventilation perfusion (V/Q) scan or computed tomography angiography, or at autopsy. Isolated subsegmental PE was included. DVT was diagnosed using a venogram, Doppler ultrasound, impedance plethysmography (IPG) (N=0), 125I fibrinogen leg scan (N=1), or a combination of the IPG and leg scan (N=0). DVT located outside the lower or upper extremities, as well as in the superficial veins, or restricted to the calf only, was not included. The final VTE diagnosis was confirmed by a panel of three Framingham Heart Study physicians. Information on predisposing factors was obtained from medical records.
Predisposing factors to VTE
A supplemental manual review of the Framingham chart and medical records obtained for confirmation of the VTE diagnosis was performed by an experienced physician to abstract information on predisposing factors for VTE in participants with an incident event. Any of the following factors were considered predisposing factors if present within 3 months prior to VTE: Surgery defined as any surgical operation; any fracture/trauma requiring medical attention; hospitalization defined as any admission to a hospital ward; immobilization defined as bed rest ≥2 days; self-reported use of hormone replacement therapy or oral contraceptives. Self-reported travel >4 hours within 1 month prior to VTE was counted as a predisposing factor. Pregnancy was defined as a predisposing factor if VTE occurred any time during pregnancy or within 3 months after delivery (puerperium) or miscarriage.
A VTE event was considered provoked if at least one predisposing factor (surgery, fracture, hospitalization, immobilization, HRT/OC use, travel >4h, pregnancy/puerperium) other than cancer was present; a VTE event was considered cancer-related if there was a diagnosis of active cancer (excluding only non-melanoma skin cancer). Active cancer was defined as new cancer diagnosis within six months prior to event or three months after VTE, any treatment for cancer within the six months prior to event, recurrent cancer, or the presence of metastasis. In the absence of a documented provoking factor or cancer, a VTE event was considered unprovoked. These three VTE groups were mutually exclusive. We further categorized participants with VTE events into the DVT group and the PE group. The DVT group included only deep venous thrombosis events, but the PE group included individuals with PE with or without concomitant DVT.
A recurrent VTE event was defined as a first occurrence of thrombosis in a previously uninvolved lower/upper extremity venous (DVT) or pulmonary (PE) segment. A recurrent event in the same location would be considered only if objective imaging in the interim showed complete resolution of the clot. All recurrences were adjudicated by a panel of three physicians to verify a new event clinically clearly independent of the first event..
Cardiovascular risk factor definitions
CV risk factors were recorded at baseline exam. Diabetes mellitus was defined as fasting plasma glucose ≥ 126 mg/dl (7.0 mmol/L), non-fasting plasma glucose ≥ 200 mg/dl (11.1 mmol/L) or treatment with hypoglycemic agents. [34] Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or current use of antihypertensive treatment. Hypercholesterolemia was defined as total cholesterol ≥200 mg/dl (5.2 mmol/L) or LDL cholesterol ≥130 mg/dl (3.4 mmol/L) or treatment with lipid-lowering medication. Prevalent CVD was defined by an existing diagnosis of myocardial infarction, stroke or peripheral arterial disease (either a history of intermittent claudication or documented lower extremity revascularization). Body mass index was calculated as weight in kilograms divided by height in meters squared. Current cigarette smoking was defined by self-reported regular smoking of cigarettes within 1 year of the examination. Aspirin use was defined as self-reported use of aspirin ≥81 mg at least three pills per week.
Mortality outcome
All cohorts remain under continuous surveillance and all deaths that occurred prior to January 1, 2014 were included in this study. Deaths were identified using multiple strategies including routine participant contact for research examinations or health history updates, surveillance at the local hospital, search of obituaries in the local newspaper, and if needed through use of the National Death Index. Death certificates were routinely obtained and all hospital and nursing home records prior to death and autopsy reports (if performed) were requested. In addition, if there was insufficient information to determine a cause of death, the next of kin were interviewed by a senior investigator. All records pertinent to the death were reviewed by a panel comprised of three Framingham Heart Study physicians. [35]
Statistical methods
We calculated person- years (PYS) of follow-up as VTE date (for incidence) or death date (for mortality) minus the baseline examination date, with censoring at date of last contact. For each event type and overall we estimated the crude incidence rates (IR) per 10,000 person-years as [(number of events)/[(event follow-up time])*10,000. Confidence intervals were estimated using the Poisson distribution. To account for confounding effects of age and also the differential age distribution as a result of combining four cohorts, IRs were directly standardized to the 2000 US population (sex pooled) using 2000 census data [36] First, for each 5-year age stratum between 20 and 95, a population weight was calculated as the proportion of the US population in each age group. Each age stratum-specific IR (#events/person-years) was multiplied by the corresponding weight and the products was summed to obtain an overall age-standardized IR. Sex-specific IRs were similarly calculated.
We used predisposing factors to categorize VTE events as “unprovoked”, “provoked”, and “cancer-related”. When analyzing factors related to VTE incidence (overall and by sub-type), we used Cox proportional hazards regression models to estimate hazards ratios (HRs) and 95% CIs. We entered in multivariable-adjusted Cox regression model those risk factors that attained statistical significance (p < 0.05) in univariate models.
Due to limited number of recurrent events, we present recurrence rate as a simple percentage. For survival analysis, we fitted Cox regression models in which VTE was analyzed as a time dependent covariate. We also generated Kaplan-Meier plots for each VTE sub-type for survival after VTE.
We present descriptive statistics using mean and standard deviation (SD) for continuous variables or n (%) for categorical variables. We performed all statistical analyses using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Baseline characteristics
Baseline characteristics are shown in Table 1 and Supplementary Tables S1 and S2. Due to our study design in which we pooled four different cohorts, age at baseline differed across the cohorts. The overall mean (SD) age in years was 54 (±17); by each cohort, the mean age was 83 (±6) for the Original, 59 (±10) for the Offspring, 40 (±9) for the Third Generation, and 52 (±9) for the Omni cohort. The majority of our study population is White (94.2%). There were 119 (1.2%) individuals of Asian origin, 183 (1.9%) African-Americans, 203 (2.1%) Hispanics, 3 (0.03%) Native Americans, and 61 (0.6%) identified as other.
Table 1.
Baseline characteristics
| Variable | Non-cases n=9468 | Any VTE n=297 | Unprovoked n=81 | Provoked n=112 | Cancer-related n=86 |
|---|---|---|---|---|---|
| Age, years | 53 (17) | 64 (13) | 62 (14) | 64 (14) | 64 (11) |
| Male sex | 4253 (45) | 139 (47) | 48 (59) | 44 (39) | 41 (48) |
| Smoking | 1394 (15) | 39 (13) | 9 (11) | 15 (13) | 14 (16) |
| Total cholesterol (mg/dL) | 197 (39) | 205 (38) | 203 (49) | 203 (30) | 208 (35) |
| BMI | 27.2 (5.3) | 29.6 (6.4) | 31.1 (6.9) | 28.5 (5.8) | 29.1 (5.6) |
| Diabetes | 683 (7) | 36 (12) | 8 (10) | 13 (12) | 14 (16) |
| Prevalent CVD | 1086 (12) | 40 (14) | 10 (12) | 18 (16) | 8 (9) |
| Hypertension | 3364 (36) | 151 (51) | 41 (51) | 58 (52) | 43 (50) |
| Aspirin use | 2053 (22) | 98 (34) | 23 (29) | 41 (37) | 27 (33) |
Data presented as mean (SD) for continuous variables and as n (%) for categorical variables.
Abbreviations: VTE=venous thromboembolism; BMI=body mass index; CVD=cardiovascular disease.
Incidence
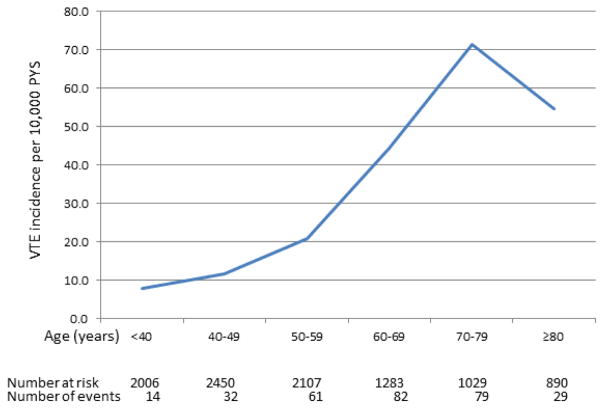
During total follow-up time of 104,091 PYS 297 incident VTE events occurred in the 9,765 participants analyzed. The unadjusted IR of VTE was 26.8 (95% CI 23.8–30.1)/10,000 PYS. The incident VTE events consisted of 120 (40%) PE and 177 (60%) DVT. There were 8 cases of upper extremity DVT. Of the 277 incident VTE events for which sufficient clinical information was available to allow subcategorization, 81 (29%) were unprovoked, 112 (40%) were provoked, and 86 (31%) were cancer-related, yielding crude IRs of 7.8/10,000 (95% CI 6.2–9.7), 10.8/10,000 (95% CI 8.9–12.9), and 8.3/10,000 (95% CI 6.6–10.2), respectively. The distribution of unprovoked, provoked, and cancer-related VTE across 10-year age-groups is shown in Supplementary Table 3. Incidence rate increased sharply with increasing baseline age-group (Figure 1). The overall age-adjusted IR for VTE was 20.3/10,000 (95% CI 17.9–22.6). Age-adjusted IR was higher in men than women [23.5/10,000 (95% CI 19.7–27.4) vs. 17.5/10,000 (95% CI 14.7–20.4), respectively; p<0.05]. This difference was driven by the higher age-adjusted IR of DVT in men [IR 15.5/10,000 (95% CI 12.6–18.4) vs. 9.6/10,000 (95% CI 7.6–11.6), respectively; p<0.05]; no difference was seen in PE incidence. Table 2 shows event frequencies and age-adjusted IRs (pooled and stratified by PE or DVT in both sexes).
Figure 1. Crude IR according to age group.
Crude incidence rate (per 10,000 person-years) of VTE according to age groups. Vertical bars represent 95% confidence intervals of incidence rates.
Table 2.
Age-adjusted incidence of VTE presented by sex and VTE type (PE or DVT).
| Any VTE | PE | DVT | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| All (103,559 PYS) | Events (n) | Incidence rate (95% CI) | Events (n) | Incidence rate (95% CI) | Events (n) | Incidence rate (95% CI) |
| Any VTE | 297 | 20.3 (17.9–22.6) | 120 | 8.0 (6.4–9.6) | 177 | 12.3 (10.6–14.0) |
| Men (45,661 PYS) | ||||||
| Any VTE | 139 | 23.5 (19.7–27.4) | 52 | 8.0 (6.2–9.9) | 87 | 15.5 (12.6–18.4) |
| Women (57,898 PYS) | ||||||
| Any VTE | 158 | 17.5 (14.7–20.4) | 68 | 8.0 (5.9–10.0) | 90 | 9.6 (7.6–11.6) |
Abbreviations: VTE=venous thromboembolism; PYS= person years of follow-up; PE= pulmonary embolism, includes all individuals with PE with or without concomitant DVT; DVT= deep venous thrombosis only, no PE at incident event; CI= confidence interval
Predisposing factors
In participants with provoked VTE (n=112), 31% had one, 30% two and 38% three or more predisposing factors (surgery, fracture/trauma, hospitalization, immobilization, HRT/OC use, travel). In cancer-related VTE, one additional predisposing factor was identified in 20% of cases, two in 21% and three or more factors in 13%, respectively, whereas in 47% cancer was the only provoking factor. The median number of predisposing factors for provoked VTE was 2 (range 1–4), and in cancer-related VTE the median was 1 (range 0–4). Hospitalization was the most common predisposing factor, present in 78% of provoked VTE and 51% of cancer-related VTE.
Mortality
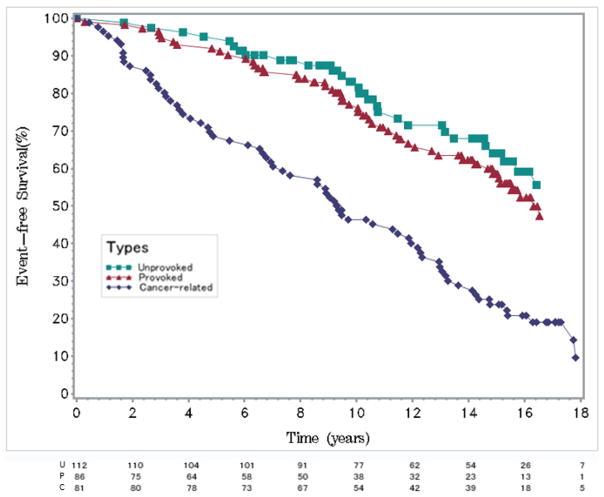
Over the study period of 104,669 PYS, 152 of 297 participants with VTE and 2045 of the 9,468 participants without VTE died. Crude all-cause mortality rate in persons with any VTE was 145 (95% CI 123–170)/1,000 PYS, whereas the mortality rate in those without VTE was 20 (95% CI 19–21)/1,000 PYS. Overall, the age- and sex-adjusted log-hazards for mortality were four-fold higher in participants with VTE, HR=4.02 (95% CI 3.40–4.74), compared to those without, with the HR higher for PE [HR=2.55 (95% CI 2.08–3.13)] than DVT [HR=1.45 (95% CI 1.11–1.89), (p<0.05)]. Table 3 shows age- and sex-adjusted HRs for mortality according to VTE type. The HRs for death after an unprovoked or provoked VTE were similar [2.38 (95% CI 1.62–3.48) and 2.37 (95% CI 1.78–3.16), respectively]. However, cancer-related VTE was associated with very high risk of all-cause mortality with HR 16.43 (95% CI 12.88–20.94) (Figure 2). There was no statistically significant difference between HRs for mortality in men versus women (p=0.40).
Table 3.
Age- and sex-adjusted Cox proportional hazard ratios for all-cause mortality in participants with VTE in comparison to those without VTE events.
| Any VTE | PE | DVT | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| All | Deaths/PYS | HR (95% CI) | Deaths/PYS | HR (95% CI) | Deaths/PYS | HR(95% CI) |
| Any VTE | 152/1051 | 4.02 (3.40–4.74) | 55/471 | 2.55 (2.08–3.13) | 97/580 | 1.45 (1.11–1.89) |
| Unprovoked | 27/332 | 2.38 (1.62–3.48) | 10/146 | 1.49 (0.92–2.40) | 17/186 | 0.88 (0.47–1.64) |
| Provoked | 48/536 | 2.37 (1.78–3.16) | 18/265 | 1.48 (1.03–2.12) | 30/271 | 0.89 (0.56–1.41) |
| Cancer-related | 69/128 | 16.43 (12.88–20.94) | 25/56 | 10.45 (7.74–14.13) | 44/72 | 5.92 (3.99–8.80) |
|
| ||||||
| MEN | ||||||
|
| ||||||
| Any VTE | 68/561 | 3.60 (2.81–4.62) | 22/250 | 2.44 (1.81–3.28) | 46/310 | 1.16 (0.76–1.77) |
|
| ||||||
| WOMEN | ||||||
|
| ||||||
| Any VTE | 84/490 | 4.41 (3.52–5.52) | 22/221 | 2.65 (2.00–3.52) | 51/270 | 1.73 (1.22–2.45) |
Abbreviations: VTE= venous thromboembolism; PE= pulmonary embolism, with or without concomitant DVT; DVT= deep venous thrombosis; CI= confidence interval; HR= hazard ratio; PYS=person-years
Figure 2. Mortality according to VTE subgroup.
Mortality according to VTE subgroup [unprovoked (U), provoked (P), cancer-related (C)]. Event-free survival probability is presented on the Y-axis and time in years on the X-axis. Number of persons at risk per group across time is presented underneath the figure.
Thirty-day, 1-year, and 5-year all-cause mortality among participants with any VTE was 11.8%, 29.6%, and 43.1%, respectively (Table 4). Acute 30-day mortality was similar for PE (13.3%) and DVT (10.7%), p=0.50. There was no statistically significant difference in long-term mortality at 5-years for DVT (45.8%) versus PE (39.2%), p=0.26. Cancer-related VTE was associated with significant short- and long-term mortality: mortality at 30-days was 24.2%; at 1-year 66.3%; and at 5-years 75.6%.
Table 4.
All-cause mortality at 30-days, one year and five years.
| 30-day mortality | Any VTE | PE | DVT |
|---|---|---|---|
| All VTE | 35/297 (11.8%) | 16/120 (13.3%) | 19/177 (10.7%) |
| Unprovoked | 3/81 (3.7%) | 2/36 (5.6%) | 1/45 (2.2%) |
| Provoked | 10/112 (8.9%) | 5/47 (10.6%) | 5/65 (7.7%) |
| Cancer-related | 21/86 (24.2%) | 9/34 (26.5%) | 12/52 (23.1%) |
| Referent | 38/9468 (0.4%) | ||
|
| |||
| One-year mortality | |||
|
| |||
| All VTE | 88/297 (29.6%) | 30/120 (25.0%) | 58/177 (32.8%) |
| Unprovoked | 8/81 (9.9%) | 2/32 (6.3%) | 6/45 (13.3%) |
| Provoked | 19/112 (17.0%) | 7/47 (14.9%) | 12/65 (18.5%) |
| Cancer-related | 57/86 (66.3%) | 20/34 (58.8%) | 37/52 (71.2%) |
| Referent | 231/9468 (2.4%) | ||
|
| |||
| Five-year mortality | |||
|
| |||
| All VTE | 128/297 (43.1%) | 47/120 (39.2%) | 81/177 (45.8%) |
| Unprovoked | 18/81 (22.2%) | 5/36 (13.9%) | 13/45 (28.9%) |
| Provoked | 39/112 (34.8%) | 16/47 (30.4%) | 23/65 (35.4%) |
| Cancer-related | 65/86 (75.6%) | 24/34 (70.6%) | 41/52 (78.8%) |
| Referent | 874/9468 (9.2%) | ||
Data presented as N of deaths/N of individuals (%). Abbreviations: VTE= venous thromboembolism; PE= pulmonary embolism, with or without concomitant DVT; DVT= deep venous thrombosis
Recurrence
We analyzed the incidence of a first recurrence of a VTE event among 297 participants who experienced at least one VTE. The follow-up was 1,081 PYS (median 2.1; range 0–17.9 years). Forty-one ( 13.8%) participants had at least one recurrent event. The percentage of participants with a recurrent event after the index unprovoked, provoked and cancer-related VTE event was 12.3%, 12.5% and 19.8%, respectively. Recurrence was more frequent after PE (23.3%) than after DVT (7.3%), p<0.0001.
CV risk factors
The association of traditional cardiovascular disease (CVD) risk factors was studied in univariate models using 10-year age intervals, sex, obesity (defined as BMI ≥30 kg/m2), diabetes, prevalent CVD, hypertension, hypercholesterolemia, aspirin use, and smoking as variables. Increasing age, sex, obesity, diabetes, hypertension, hypercholesterolemia and aspirin use, but neither smoking nor prevalent CVD, were statistically significantly associated with incident VTE in univariate models (Table S4). However, in the multivariable model (Table 5) adjusting for factors that attained statistical significance in the univariate Cox regression models, only age and obesity were statistically significantly associated with incident VTE. For every 10-year increase in age, the hazard for VTE increased by almost 70% [HR 1.69 ((95%CI) 1.48–1.92)]. The HR for obesity (BMI ≥30kg/m2) was 1.88 (95%CI 1.44–2.45)]. Female sex was protective compared to men in the subgroup of unprovoked VTE.
Table 5.
Multivariable hazard ratios for incident VTE events
| Any VTE | Unprovoked | Provoked | Cancer-related | |
|---|---|---|---|---|
| Factor | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Age ( per 10 years) | 1.69 (1.48–1.92)* | 1.58 (1.32–1.90)* | 1.58 (1.36–1.84)* | 1.74 (1.46–2.08)* |
| Female sex | 0.78 (0.60–1.02) | 0.42 (0.26–0.68)* | 1.13 (0.76–1.67) | 0.78 (0.50–1.22) |
| Obese (BMI ≥30) | 1.88 (1.44–2.45)* | 2.74 (1.75–4.30)* | 1.15 (0.75–1.76) | 1.97 (1.25–3.10)* |
| Diabetes | 1.13 (0.75–0.70) | 0.77 (0.35–1.71) | 1.23 (0.66–2.28) | 1.72 (0.93–3.19) |
| Aspirin use | 1.09 (0.81–1.45) | 0.77 (0.46–1.27) | 1.18 (0.78–1.78) | 0.88 (0.54–1.42) |
| Hypertension | 1.03 (0.78–1.37) | 1.14 (0.70–1.85) | 1.07 (0.71–1.62) | 0.82 (0.51–1.32) |
| Hypercholesterolemia | 1.01 (0.76–1.34) | 0.97 (0.62–1.53) | 1.20 (0.81–1.78) | 1.17 (0.74–1.84) |
p<0.05.
Multivariable-adjusted Cox models were adjusted for age, sex and for variables that were significant in univariate models. The referent category is FHS participants without VTE events. Abbreviations: BMI= body mass index. CI= confidence interval; HR= hazard ratio
Discussion
Here we report the epidemiology of VTE in the Framingham Heart Study, a large community-based study with comprehensive follow-up of almost two decades up to 2014. The age-adjusted incidence rate of VTE is 20.3/10 000 person-years. Incidence was highly dependent on age, as previously reported. [9,11,13,37] The crude and age-adjusted IRs are higher than in most previous reports. [2–12] The difference could largely be explained by age of the study cohort, in addition to differences in study inclusion and exclusion criteria, method of follow-up, and diagnostic methods. Although individual covariates, including obesity, diabetes, hypertension, hypercholesterolemia and aspirin use, were statistically significantly associated with incident VTE in univariate models, only increasing age and obesity remained associated with incident VTE after multivariable-adjustment. This observation supports the current understanding of a minor role of traditional CV risk factors as predictors of VTE.
Our study confirms and extends to a recent time period the evidence for significant excess mortality associated with VTE [37–39], with risk of death 4-times higher than the reference group without VTE. 30-day mortality after a VTE is 11.8%, and long-term mortality at 5-years 43.1%. Cancer-related mortality accounts for a large proportion of these numbers. Nevertheless, 30-day all-cause mortality after a provoked VTE is almost 9%. Surprisingly, we did not observe a difference in mortality between PE and DVT. This might suggest improved outcomes from advances in the treatment of acute PE in the last decades. It seems unlikely that the difference in observed mortality after incident PE compared to DVT could reflect missed PE discovered at autopsy, since there was a slight increase in autopsy rate in our study cohort from 7.8% during 1995–1999 to 10.0% during 2010–2014.
Hospitalization for any cause was present in almost 80% of provoked VTE in our sample. This could be partially explained with the inherent bias of the review process and greater likelihood of obtaining hospital records than doctor’s office notes. However, many of the VTE-related deaths could potentially be prevented by prophylactic treatment in high-risk situations, even though direct causality between mortality and VTE cannot be assessed in our study cohort. Accordingly, various risk prediction models have been implemented in hospitalized persons. [40]
In our study unprovoked VTE was associated with 2.4-fold increase in risk of all-cause mortality, with 4% mortality at 30-days and 22% at 5-years. At present, instruments for predicting a first unprovoked VTE are lacking, and further research is warranted to improve prediction and understanding the underlying pathogenesis. Indeed, in the overall cohort, when we examined for predictors of VTE, only increasing age and obesity were significant predictors. Although other previously reported VTE risk factors, including cigarette smoking and other well measured cardiovascular risk factors, predicted future VTE in univariate analysis, the associations were not significant in multivariable-adjusted models. Aspirin use has been reported to reduce VTE [41,42], but we did not observe any protective effect in multivariable models; however, our study may be statistically underpowered due to relatively small numbers of VTE events and limited numbers of aspirin users. The absence of strong, specific predictors of first VTE justifies large-scale collaborative epidemiologic consortium studies using multiple biomarkers or genomewide genomic studies to identify predictors of both provoked and unprovoked VTE.
We chose to analyze patients with cancer-related VTE separately instead of pooling cancer in general as one of the predisposing factors for provoked VTE. The pathophysiology of cancer-related thrombosis differs markedly from other forms of VTE. Not only can tumors affect blood flow mechanically, but they also secrete agents causing a prothrombotic state. [43] Our results show that cancer-related VTE has a dismal prognosis and is associated with extremely high all-cause mortality with only 24% 5-year survival rate. This figure compares to 5-year survival rate of lung cancer (18%). [44] The risk of death after a cancer-related VTE is 16-times higher than in those without VTE, which is in line with previous reports. [45,46] In our study sample it is not possible to evaluate the direct causality of VTE with increased mortality, but clearly cancer patients with VTE events are at very high risk. However, VTE could be a mere sign of advanced cancer, and whether prophylactic measures to prevent VTE in cancer patients could modify the unfavorable prognosis warrants further research.
VTE is a chronic disease with significant recurrence rates. Prandoni et al. have reported 40% recurrence after 8 years of follow-up in patients after the cessation of anticoagulants. [1] In our community-based cohort recurrence was less frequent, only 13% during a shorter median follow-up of 2.1 years. However, using stringent criteria for recurrent events might lead to an underestimation of recurrence rate after first VTE. Comparing the rates is compromised by the lack of information on use of anticoagulation after the incident event. Interestingly, in our study recurrence was more frequent after a PE (21%) than a DVT (7%). Findings similar to ours were reported in a previous study. [47] In contrast, Prandoni et al. report recurrence being 50% more likely after a DVT than PE. [1]
The strength of our study lies in the community-based approach with in-depth follow-up over two decades. Family studies provide unbiased point estimates for cardiovascular outcomes provided that families were approximately randomly selected. That was the case with the first generation, the Original cohort. Furthermore, the cardiovascular risk profile of the Third Generation at intake exam was consistent with the general US population and any changes were consistent with nationwide trends. Therefore, despite the family structure of our cohort, we do not expect the incidence rates to differ significantly from the unrelated population. All VTE events were extensively verified by objective imaging data and review of the medical records. Data on VTE events limited to the calf is not available, but inclusion of distal DVT would likely have further increased the incidence in our cohort. The major limitations are lack of information of anticoagulant use hampering the evaluation of recurrence. Furthermore, we did not have information of possible prophylactic treatment at high-risk situations. Consistently collected information on the use of central venous catheters or chemotherapy was not available thus limiting the analysis of the role of additional provoking factors in cancer-related VTE. Since VTE events were systematically collected beginning in 1995 we do not have reliable data on possible prevalent events before that time, and are unable to assess the family history of VTE. No thrombophilia testing was performed thus preventing analysis of familial predisposition. Also, information on predisposing factors is only available for persons with VTE thus prohibiting more comprehesive analysis of the role of single risk-factors. Due to the majority of the study subjects being middle-aged or older, we were unable to provide a reliable estimate of pregnancy-related VTE. FHS is comprised of mainly European ancestry, and the limited sample size of non-European participants makes it difficult to extrapolate to other ethnicities.
Conclusion
In conclusion, our study offers objective information on the epidemiology of VTE. Significant excess mortality is associated with VTE and prognosis after cancer-related VTE is dismal. In multivariable-adjusted models, we could not confirm that traditional CV risk factors beyond age and obesity are associated with VTE. Therefore, our data emphasize the need for a search to identify imaging, biomarker and genomic measures individually and combined, beyond the currently available common factors measured in clinical practice, to achieve more accurate risk prediction of first and recurrent VTE and to identify methods of prevention. Further research to determine the pathophysiologic mechanisms of unprovoked VTE is needed.
Supplementary Material
Highlights.
VTE is the third leading cause of CV morbidity after myocardial infarction and stroke.
We report VTE incidence in a longitudinal community-based cohort.
Traditional CV risk factors beyond age and obesity are not associated with VTE.
Cancer-related VTE is associated with high mortality in the community.
Acknowledgments
Funding sources
This work was supported by the NHLBI contracts NO1-HL 25195 and HHSN268201500001I
We thank the FHS participants for their ongoing participation and dedication to the study making this work possible. The authors wish to thank the data management staff at the Framingham Heart Study for their invaluable help.
Footnotes
Diclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prandoni P, Noventa F, Ghirarduzzi A, et al. Haematologica. 2007;92:199–205. doi: 10.3324/haematol.10516. [DOI] [PubMed] [Google Scholar]
- 2.ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to the global disease burden. J Thromb Haemost. 2014;12:1580–90. doi: 10.1111/jth.12698. [DOI] [PubMed] [Google Scholar]
- 3.Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Martinez C, Cohen AT, Bamber L, et al. Epidemiology of first and recurrent venous thromboembolism: a population-based cohort study in patients without active cancer. Thromb Haemost. 2014;112:255–263. doi: 10.1160/TH13-09-0793. [DOI] [PubMed] [Google Scholar]
- 5.Braekkan SK, Mathiesen EB, Njølstad I, et al. Family history of myocardial infarction is an independent risk factor for venous thromboembolism: the Tromsø study. J Thromb Haemost. 2008;6:1851–1857. doi: 10.1111/j.1538-7836.2008.03102.x. [DOI] [PubMed] [Google Scholar]
- 6.Holst AG, Jensen G, Prescott E. Risk factors for venous thromboembolism: results from the Copenhagen City Heart Study. Circulation. 2010;121:1896–1903. doi: 10.1161/CIRCULATIONAHA.109.921460. [DOI] [PubMed] [Google Scholar]
- 7.Spencer FA, Emery C, Joffe SW, et al. Incidence rates, clinical profile, and outcomes of patients with venous thromboembolism. The Worcester VTE study. J Thromb Thrombolysis. 2009;28:401–409. doi: 10.1007/s11239-009-0378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370–372. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naess IA, Christiansen SC, Romundstad P, et al. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. 2007;5:692–699. doi: 10.1111/j.1538-7836.2007.02450.x. [DOI] [PubMed] [Google Scholar]
- 10.Johansson M, Johansson L, Lind M. Incidence of venous thromboembolism in northern Sweden (VEINS): a population-based study. Thrombosis Journal. 2014;12:6. doi: 10.1186/1477-9560-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oger E. Incidence of venous thromboembolism: a community-based study in Western France. EPI-GETBP Study Group. Groupe d’Etude de la Thrombose de Bretagne Occidentale. Thromb Haemost. 2000;83:657–660. [PubMed] [Google Scholar]
- 12.Ho WK, Hankey GJ, Eikelboom JW. The incidence of venous thromboembolism: a prospective, community-based study in Perth, Western Australia. Med J Aust. 2008;189:144–147. doi: 10.5694/j.1326-5377.2008.tb01947.x. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) Venous thromboembolism in adult hospitalizations—United States, 2007–2009. Morb Mortal Wkly Rep. 2012;61:401–404. [PubMed] [Google Scholar]
- 14.Cohen AT, Agnelli G, Anderson FA, et al. VTE Impact Assessment Group in Europe (VITAE). Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–64. doi: 10.1160/TH07-03-0212. [DOI] [PubMed] [Google Scholar]
- 15.Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12:464–474. doi: 10.1038/nrcardio.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gresele P, Momi S, Migliacci R. Endothelium, venous thromboembolism and ischaemic cardiovascular events. Thromb Haemost. 2010;103:56–61. doi: 10.1160/TH09-08-0562. [DOI] [PubMed] [Google Scholar]
- 17.Riva N, Donadini MP, Ageno W. Epidemiology and pathophysiology of venous thromboembolism: similarities with atherothrombosis and the role of inflammation. Thromb Haemost. 2015;113:1176–1183. doi: 10.1160/TH14-06-0563. [DOI] [PubMed] [Google Scholar]
- 18.Heit JA, Phelps MA, Ward SA, et al. Familial segregation of venous thromboembolism. J Thromb Haemost. 2004;2:731–736. doi: 10.1111/j.1538-7933.2004.00660.x. [DOI] [PubMed] [Google Scholar]
- 19.Zöller B, Li X, Sundquist J, Sundquist K. Age- and gender-specific familial risks for venous thromboembolism: a nationwide epidemiological study based on hospitalizations in Sweden. Circulation. 2011;124:1012–1020. doi: 10.1161/CIRCULATIONAHA.110.965020. [DOI] [PubMed] [Google Scholar]
- 20.Prandoni P. Venous and arterial thrombosis: two aspects of the same disease? Eur J Intern Med. 2009;20:660–661. doi: 10.1016/j.ejim.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Franchinia M, Mannucci P. Association between venous and arterial thrombosis: Clinical implications. Eur J Intern Med. 2012;23:333–337. doi: 10.1016/j.ejim.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- 23.Cheng YJ, Liu ZH, Yao FJ, et al. Current and former smoking and risk for venous thromboembolism: a systematic review and meta-analysis. PLoS Med. 2013;10:e1001515. doi: 10.1371/journal.pmed.1001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wattanakit K, Lutsey PL, Bell EJ, et al. Association between cardiovascular disease risk factors and occurrence of venous thromboembolism. A time-dependent analysis. Thromb Haemost. 2012;108:508–15. doi: 10.1160/TH11-10-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai AW, Cushman M, Rosamond WD, et al. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med. 2002;162:1182–9. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 26.Holst AG, Jensen G, Prescott E. Risk factors for venous thromboembolism: results from the Copenhagen City Heart Study. Circulation. 2010;121:1896–903. doi: 10.1161/CIRCULATIONAHA.109.921460. [DOI] [PubMed] [Google Scholar]
- 27.Braekkan SK, Mathiesen EB, Njolstad I, et al. Family history of myocardial infarction is an independent risk factor for venous thromboembolism: the Tromsø study. J Thromb Haemost. 2008;6:1851–7. doi: 10.1111/j.1538-7836.2008.03102.x. [DOI] [PubMed] [Google Scholar]
- 28.Gariani K, Mavrakanas T, Combescure C, et al. Is diabetes mellitus a risk factor for venous thromboembolism? A systematic review and meta-analysis of case-control and cohort studies. Eur J Intern Med. 2015 doi: 10.1016/j.ejim.2015.10.001. pii: S0953-6205(15)00330-1. [DOI] [PubMed] [Google Scholar]
- 29.Bai J, Ding X, Du X, et al. Diabetes is associated with increased risk of venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2015;135:90–95. doi: 10.1016/j.thromres.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Dawber TR, Meadors GF, Moore FEJ. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health. 1951;41:279–286. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kannel WB, Feinleib M, McNamara PM, et al. An investigation of coronary heart disease in families: the Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 32.Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 33.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 34.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 35.Lunetta KL, D’Agostino RB, Sr, Karasik D, et al. Genetic correlates of longevity and selected age-related phenotypes: a genome-wide association study in the Framingham Study. BMC Med Genet. 2007;8(Suppl 1):S13. doi: 10.1186/1471-2350-8-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. [accessed Jan 20, 2016]; http://www.census.gov/main/www/cen2000.html.
- 37.Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. 2013;126:832, e13–21. doi: 10.1016/j.amjmed.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 38.Søgaard KK, Schmidt M, Pedersen L, et al. 30-year mortality after venous thromboembolism: a population-based cohort study. Circulation. 2014;130:829–836. doi: 10.1161/CIRCULATIONAHA.114.009107. [DOI] [PubMed] [Google Scholar]
- 39.Flinterman LE, van Hylckama Vlieg A, Cannegieter SC, et al. Long-term survival in a large cohort of patients with venous thrombosis: incidence and predictors. PLoS Med. 2012;9:e1001155. doi: 10.1371/journal.pmed.1001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kahn SR, Lim W, Dunn AS, et al. American College of Chest Physicians. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e195S–226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becattini C, Agnelli G, Schenone A, et al. WARFASA Investigators. Aspirin for preventing the recurrence of venous thromboembolism. NEJM. 2012;366:1959–1967. doi: 10.1056/NEJMoa1114238. [DOI] [PubMed] [Google Scholar]
- 42.Undas A, Brummel-Ziedins K, Mann KG. Why does aspirin decrease the risk of venous thromboembolism? On old and novel antithrombotic effects of acetylic salicylic acid. J Thromb Haemost. 2014;12:1776–1787. doi: 10.1111/jth.12728. [DOI] [PubMed] [Google Scholar]
- 43.Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost. 2013;11:223–233. doi: 10.1111/jth.12075. [DOI] [PubMed] [Google Scholar]
- 44.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 45.Amer MH. Cancer-associated thrombosis: clinical presentation and survival. Cancer Manag Res. 2013;5:165–178. doi: 10.2147/CMAR.S47094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prestidge T, Lee S, Harper P, et al. Survival in patients with malignancy and venous thromboembolism by tumour subtype and thrombus location. Intern Med J. 2012;42:71–74. doi: 10.1111/j.1445-5994.2010.02401.x. [DOI] [PubMed] [Google Scholar]
- 47.Eichinger S, Weltermann A, Minar E, et al. Symptomatic pulmonary embolism and the risk of recurrent venous thromboembolism. Arch Intern Med. 2004;164:92–96. doi: 10.1001/archinte.164.1.92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.