Abstract
Previous studies have suggested that alterations in excitatory/inhibitory neurotransmitters might play a crucial role in autism spectrum disorder (ASD). Proton magnetic resonance spectroscopy (1H-MRS) can provide valuable information about abnormal brain metabolism and neurotransmitter concentrations. However, few 1H-MRS studies have been published on the imbalance of the two most abundant neurotransmitters in ASD: glutamate (Glu) and gamma-aminobutyric acid (GABA). Moreover, to our knowledge none of these published studies is performed with a study population consisting purely of high-functioning autism (HFA) adolescents. Selecting only individuals with HFA eliminates factors possibly related to intellectual impairment instead of ASD. This study aims to assess Glu and GABA neurotransmitter concentrations in HFA. Occipital concentrations of Glu and GABA plus macromolecules (GABA+) were obtained using 1H-MRS relative to creatine (Cr) in adolescents with HFA (n=15 and n=13 respectively) and a healthy control group (n=17). Multiple linear regression revealed significantly higher Glu/Cr and lower GABA+/Glu concentrations in the HFA group compared to the controls. These results imply that imbalanced neurotransmitter levels of excitation and inhibition are associated with HFA in adolescents.
Keywords: Magnetic resonance spectroscopy, Glutamate, Gamma-aminobutyric acid
1. Introduction
Autism spectrum disorder (ASD) is a pervasive neurodevelopmental disorder, which is characterized by deficits in social interaction, impaired verbal and non-verbal communication, and restricted and repetitive behavior (American Psychiatric Assocition, 1994). The neural mechanisms of ASD are not yet completely understood, although there is evidence that ASD is associated with abnormal brain development (Barendse et al., 2013; Baruth et al., 2013; Rump et al., 2009; Schaefer and Lutz, 2006). Furthermore, several previous post-mortem studies (Fatemi et al., 2009; Zikopoulos and Barbas, 2013) and electroencephalography (EEG) studies (Orekhova et al., 2008; Rubenstein and Merzenich, 2003; Snijders et al., 2013) have suggested impairments in the regulation of the excitatory/inhibitory (E/I) system of individuals with ASD. To study the E/I system a magnetic resonance technique can be used that quantifies the primary excitatory and inhibitory neurotransmitters.
Proton magnetic resonance spectroscopy (1H-MRS) allows for a non-invasive in vivo measurement of metabolite concentrations in a specific part of the brain. Using 1H-MRS, the radio-frequency signals that arise from nuclear spins can be detected. The resonant frequencies of these signals depend on their chemical environment and are referred to as chemical shift in parts per million of the proton frequency (ppm) (Puts and Edden, 2012). Since the corresponding chemical shifts for most metabolites are known, the 1H-MRS spectrum can be analyzed to yield metabolite concentrations. Therefore, it is possible to study potential abnormalities in brain neurochemistry using 1H-MRS. In this study, the 1H-MRS is used to quantify the concentrations of neurotransmitters glutamate (Glu), the primary excitatory neurotransmitter (Simpkins and Simpkins, 2013) and gamma-aminobutyric acid (GABA), the primary inhibitory neurotransmitter (Lainhart et al., 2013). Glu can be quantified using the point resolved-spectroscopy (PRESS) method, however, the GABA peaks in this 1H-MRS spectrum are relatively small and overlap with more intense signals from other metabolites. Therefore, to determine the GABA concentration, the J-difference editing method called Mescher-Garwood point-resolved spectroscopy (MEGA-PRESS) is used (Mescher et al., 1998). This method exploits the fact that the GABA peak at 3.0 ppm is coupled to a signal at a different chemical shift (1.9 ppm). Now, a frequency-selective pulse (i.e. editing pulse) at this chemical shift will have an indirect effect on the GABA signal. Obtaining two spectra, with and without the editing pulse, and subtracting them will yield a spectrum which only contains those signals affected by the editing pulse.
Several invasive studies have already demonstrated different levels of Glu and GABA in the autistic brain. In these studies increased levels of GABA (Dhossche et al., 2002; El-Ansary and Al-Ayadhi, 2014) and Glu (Aldred et al., 2003; El-Ansary and Al-Ayadhi, 2014; Tu et al., 2012) and decreased levels of the Glu/GABA ratio (El-Ansary and Al-Ayadhi, 2014) were reported in blood plasma. Moreover, previous non-invasive 1H-MRS studies reported increased cerebral concentrations of Glu in Heschl’s gyrus and the anterior cingulate cortex (Brown et al., 2013; Joshi et al., 2013). Moreover, decreased concentrations of GABA in anterior cingulate cortex, motor and auditory cortex, frontal lobe and Heschl’s gyrus (Cochran et al., 2015; Gaetz et al., 2014; Harada et al., 2011; Kubas et al., 2012; Rojas et al., 2014) are reported, supporting that an imbalanced E/I system exists in individuals with ASD (Brix et al., 2015).
In the current study, occipital neurotransmitter concentrations are determined in adolescents with high-functioning autism (HFA) and healthy controls (HC), excluding individuals with autism who have intellectual deficits. This way, factors possibly related to intellectual impairment instead of ASD, are eliminated. Moreover, the motivation to investigate adolescents specifically follows from the relevance of social stress. Although the social impairments are independent of age and developmental level, the related psychosocial stress may disproportionally increase during adolescence (Nicpon et al., 2010; Pellicano, 2010). During this developmental period, opinions and evaluations of peers become increasingly salient and many adolescents with ASD begin to notice how they differ from their peers (Burnett et al., 2009; Crone and Dahl, 2012; Steinberg, 2005). This is especially noticeable in HFA with ASD as they, more than their low-functioning counterparts, seek and initiate social interaction with peers (Bauminger et al., 2003; Hauck et al., 1995). Of the aforementioned previous 1H-MRS studies, only one was performed with a study population consisting purely of high-functioning adolescents (Joshi et al., 2013). However, in this particular study only measures of Glu concentrations were obtained. Therefore, the current study is the first to explore whether the concentrations of both Glu and GABA are altered in HFA adolescents compared to HC possibly indicative of an imbalance of the E/I system. Finally, the occipital lobe was chosen as it yields favorable spectral quality, moreover, it has previously been shown to be involved in atypical visual processing in ASD (Samson et al., 2012).
2. Methods
2.1. Participants
Thirty-three adolescents were included in this study, of which 15 with ASD and 18 HC. The individuals with ASD were recruited from special secondary education school ‘de Berkenschutse’ (located in Heeze, the Netherlands), and all the HC attended regular secondary school. All the ASD participants were clinically diagnosed according to Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV-TR) (American Psychiatric Assocition, 1994) and based on individual assessment, using the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2001). To assess intelligence, the full-scale IQ (FSIQ) was measured using the Wechsler Intelligence Scale for Children third edition (WISC-III) (Wechsler, 1991). In this particular study, all the adolescents with ASD had a FSIQ over 100, therefore meeting the commonly used criteria of high-functioning autism (clinically diagnosed ASD accompanied with a FSIQ of >75). Finally, to assess behavioral problems the parents/caregivers of each subject filled out the Child Behavior Checklist (CBCL/6–18) (Achenbach and Rescorla, 2001) yielding three scores: the total score and scores for internalizing and externalizing problems. Four adolescents with ASD used medication: 3 adolescents used methylphenidate, but discontinued medication at least 20-h before scanning allowing for complete washout. Methylphenidate is often used in treatment of attention deficit hyperactivity disorder (ADHD), however in this case it was used against impaired executive functioning, which is not restricted to ADHD (Geurts et al., 2004). One adolescent with ASD used pipamperon (antipsychotic) and did not discontinue medication. None of the HC adolescents used medication. None of the adolescents with ASD had a diagnosis for other co-morbid psychological disorders or psychiatric diseases (including ADHD) as formulated in the DSM-IV-TR. However, the DSM-IV-TR criteria did not yet allow a diagnosis of co-occurring ASD and ADHD, possibly leading towards an incorrect diagnosis of ADHD. Furthermore, it has been shown that around two thirds of the individuals with ASD also show symptoms of ADHD (Leitner, 2014), suggesting that co-occurrence of ADHD could be present in the individuals included in this study.
Two different methods of 1H-MRS, point resolved spectroscopy (PRESS) and Mescher-Garwood point-resolved spectroscopy (MEGA-PRESS) (Mescher et al., 1998), were used in all subjects. Creatine (Cr) and Glu were quantified by the PRESS method and GABA was quantified by the MEGA-PRESS spectral editing method. Since some contribution from co-editing macromolecules was expected using this technique, GABA plus macromolecules will be labeled as GABA+. One of the PRESS and five of the MEGA-PRESS acquisitions were excluded due to bad spectral quality after visual inspection. Therefore, the total of included subjects per group (PRESS and a MEGA-PRESS) is slightly different (32 vs 28).
For the PRESS group, which contained a total of 32 participants, the HFA group consisted of 15 adolescents (1 female) between 14 and 18 years old with a FSIQ ranging from 107 to 124. The PRESS HC group consisted of 17 healthy adolescents (1 female) between 12 and 17 years old with a FSIQ ranging from 105 to 135. Significant differences between the HFA and HC group were assessed using independent samples t-tests. No significant differences between the two groups were found in age, intelligence and gender. The groups did show significant differences in all the CBCL scores (total, internalizing and externalizing). An overview of these subject characteristics is shown in Table 1. The MEGA-PRESS group contains 28 subjects (13 HFA, 15 HC), and displayed highly similar characteristics with significant differences between the HC and HFA individuals in the three CBCL scores (data not shown).
Table 1.
Subject characteristics of healthy controls (HC) and individuals with high-functioning autism (HFA). Means and SD are given, as well as the p-value for assessing significant differences between the groups. FSIQ, full-scale IQ; CBCL, Child Behavior Checklist.
| HC group | HFA group | t-Score | p-Value | |
|---|---|---|---|---|
| # | 17 | 15 | – | – |
| Age (years) | 15.3±1.4 | 16.2±1.4 | −1.991 | .056 |
| Gender (M/F) | (16/1) | (14/1) | .089 | .930 |
| Intelligence (FSIQ) | 112±7 | 117±5 | −1.968 | .058 |
| CBCL total | 10±8 | 44±24 | −5.232 | .001 |
| CBCL internalizing | 3±3 | 13±9 | −4.099 | .001 |
| CBCL externalizing | 2±2 | 9±6 | −4.162 | .001 |
2.2. MRI and 1H-MRS acquisition
Magnetic resonance spectroscopy was performed on a 3.0 T scanner (Philips Achieva, Best, The Netherlands). For anatomic reference and segmentation, T1-weighted three-dimensional (3D) turbo field echo (TFE) images were acquired with the following parameters: Repetition time (TR) 8.2 ms, echo time (TE) 3.7 ms, flip angle 8°, matrix 240×240, field of view (FOV) 256×256×180 mm3, 1 mm adjacent coronal slices.
1H-MRS spectra were acquired from a single 3×3×3 cm3 voxel located in the occipital lobe, (Fig. 1). The occipital lobe was chosen as it provides the best spectral quality (Near et al., 2014). PRESS (TR/TE=2000/35 ms, 128 averages, VAPOR water suppression) and MEGA-PRESS (TR/TE=2000/68 ms, 320 averages, editing pulses at 1.9 (ON) and 7.46 ppm (OFF) interleaved in 40 blocks, MOIST water suppression, and 10:40 min acquisition time) sequences were used. Additionally, spectra without water suppression were acquired directly after the PRESS (TR/TE=2000/35 ms, 16 averages) and MEGA-PRESS (TR/TE=2000/68 ms, 8 averages) sequences.
Fig. 1.
Placement of the proton magnetic resonance spectroscopy (1H-MRS) voxel in the occipital region.
2.3. Analysis
The acquired PRESS data (Fig. 2a) of the included subjects was analyzed using LCModel (Version 6.2-2B), which analyzes spectra as a linear combination of basis spectra using a simulated basis set and the acquired MEGA-PRESS spectral editing data (Fig. 2b) was analyzed using Gannet (Version 2.0) (Edden et al., 2014).
Fig. 2.
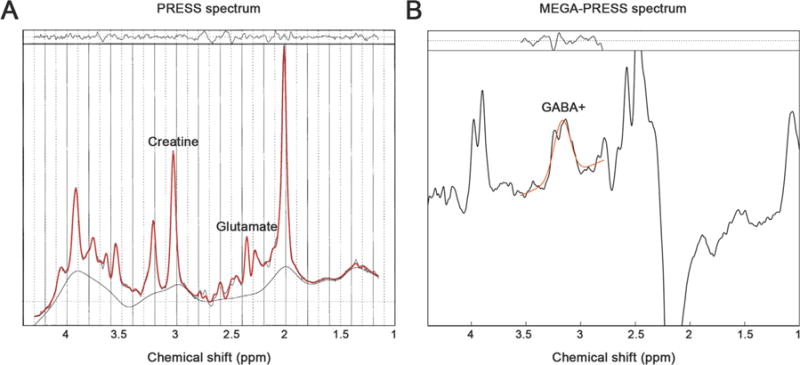
Plots of the (a) Point resolved spectroscopy (PRESS) spectrum, where the Cr and Glu peaks are indicated and (b) Mescher-Garwood point resolved spectroscopy (MEGA-PRESS) edited difference spectrum, where the GABA+ peak is indicated. In both cases the red line is a fit of the data and the residuals of this fit are shown in the top of both plots. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In this study the metabolite concentrations Glu and GABA+ (in institutional units) are expressed as a ratio to a reference concentration, Cr, obtained from the PRESS spectrum and the MEGA-PRESS OFF spectrum, respectively. The reference concentration is expected to be stable among the subjects. Both water and Cr are considered relatively stable (Soares and Law, 2009), however they can change in severe or chronic disorders (de Graaf, 2012). Cr itself is determined relative to water. Furthermore, to examine the imbalance of the E/I system the ratio GABA+/Glu is determined.
Concentrations of specific metabolites can vary in different tissues (Bhattacharyya et al., 2011). Therefore, the measurements of the metabolite concentrations can be highly influenced by the composition of the 27 ml 1H-MRS voxel. To account for potential differences in voxel composition, the brain was segmented (Ashburner and Friston, 2005) into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) using Statistical Parametric Mapping v12 (SPM12). Thereafter, the metabolite concentrations are corrected for the fraction of CSF (1), which is assumed to contain negligible amounts of metabolic concentrations. Subsequently, to account for differences in the GM and WM fractions, these fractions are included as covariates in the regression analysis.
| (1) |
Multiple linear regression was performed on the acquired data using the Statistical Package for the Social Sciences (IBM SPSS, v22). As dependent variables the Glu/Cr, GABA+/Cr and GABA+/Glu concentrations were taken. The independent variable was the diagnostic group of the participants (HC vs HFA). Moreover, to account for voxel composition, age, intelligence and CBCL score differences between the groups, GM and WM fractions, age, FSIQ and CBCL total scores, respectively, were added as covariates to the regression model.
3. Results
The multiple regression analysis (Table 2) of Glu/Cr (F(6, 25) = 3.264, p=.016, R2=.439) revealed a significant higher Glu/Cr for individuals with HFA compared to HC (p=.026). Furthermore, the multiple regression analysis of the GABA+/Glu ratio (F(6,20) = 3.418, p = .017, R2=.506) showed a significant lower concentration for individuals with HFA compared to HC (p=.006). Finally, the multiple regression analysis of GABA+/Cr concentration (F(6,21) = 1.717, p =.166, R2=.329) did not yield significant differences between the HFA and the HC (p = .067).
Table 2.
Results of the multiple linear regression analysis for Glu/Cr, GABA+/Glu and GABA+/Cr (*p<.05, **p<.01). Glu, glutamate; GABA+, gamma-aminobutyric acid plus macromolecules; Cr, creatine; B, regression coefficient; β, relative influence of regression coefficients; 95% CI, 95% confidence interval; LB, lower bound; UB, upper bound.
| Glu/Cr
|
GABA+/Glu
|
GABA+/Cr
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | β | 95% CI
|
B | β | 95% CI
|
B | β | 95% CI
|
||||
| LB | UB | LB | UB | LB | UB | |||||||
| Diagnostic group | .148 | .581* | .019 | .277 | .019 | −.753** | −.032 | −.006 | −.014 | −.526 | −.028 | .001 |
When females were excluded from the analysis, the GABA+/Glu ratio remained significantly lower for individuals with HFA compared to HC (p=.014), and Glu/Cr was higher, although not significantly but with a trend (p = .081).
An independent samples t-test showed that the CSF-corrected Cr concentrations did not significantly differ among the HFA group (mean ± SD, 6.03 ± .43) and the HC (mean ± SD, 5.91 ± .52), p = .525.
4. Discussion
The aim of this study was to investigate whether adolescents with HFA have altered concentrations of excitatory and inhibitory neurotransmitters compared to HC.
The multiple linear regression analysis revealed two significant differences in metabolite concentrations of the HFA group compared to the HC; an increase of Glu/Cr concentration and a decrease of GABA+/Glu concentration. As we observed these changes in participants without cognitive decline, it is safe to conclude that the observed alterations are not due to differences in cognitive performance. In this study, no significant differences were found in Cr between the HFA group and HC, which indicates that the elevation of Glu/Cr was not found due to differences in Cr. Furthermore, since the analysis accounts for CSF, WM and GM fractions, the findings of Glu/Cr and GABA+/Glu are not driven by differences in voxel composition between the two groups.
The Glu/Cr concentration in HFA adolescents is shown to be significantly higher compared to HC. Increased Glu concentration in individuals with ASD has been reported in previous 1H-MRS studies (Brown et al., 2013; Joshi et al., 2013). Also, increased Glu levels in blood plasma has been reported (Aldred et al., 2003; Tu et al., 2012), however it is unclear whether there is an unambiguous relation between these levels and the concentration measured in the brain, as Glu is unable to cross the blood-brain barrier (Tirouvanziam et al., 2012). Glu is the most abundant ex-citatory neurotransmitter in the brain, inducing activation by binding to post-synaptic receptors. Therefore, the higher levels of Glu in individuals with HFA with respect to HC found in this study suggest over-activity in the occipital region. A previous functional MRI, study suggested that over-activity in the occipital lobe in ASD is compensatory (Soulières et al., 2009). Furthermore, it has been previously speculated that higher levels of Glu in ASD can be responsible for excitotoxicity, the process of damaging neurons due to the over-activity (Evers and Hollander, 2008). However, purely based on the findings of this study, such a conclusion cannot be drawn. Moreover, N-Acetylaspartate plus N-Acet-ylaspartylglutamate (NAAG) measurements indicate that the neurons in individuals with HFA are not severely affected, as there are no significant differences between groups (post hoc t-test analysis, p=.351). Therefore, there is no evidence for excitotoxicity in this study, and the reported over-activity is more likely to be compensatory.
In this study the concentration of GABA+/Glu is significantly lower in the HFA group, which suggests that GABAergic activity is decreased in the HFA group while glutamatergic activity is increased compared to the HC. Previously, it has been suggested that an imbalance of the E/I system would be located especially in the frontal lobe (Harada et al., 2011). However, in this study it is shown that this effect is also observable in the occipital region. From these results we might hypothesize that the imbalance of excitation and inhibition is widespread across the brain. To test this hypotheses a future study should quantify the levels of GABA+/Glu in ASD and HC across multiple locations in the brain. Furthermore, previous functional MRI studies have reported altered activity in the occipital lobe in ASD (Samson et al., 2012; Soulières et al., 2009). The occipital lobe plays a crucial role in visual processing, therefore our results provide supporting evidence for altered visual processing in HFA compared to HC. As mentioned previously, this might be a compensatory effect.
Decreases of GABAergic activity has been reported in several previous 1H-MRS ASD studies (Gaetz et al., 2014; Harada et al., 2011; Kubas et al., 2012; Rojas et al., 2014). Also, elevated plasma GABA levels in ASD have been previously reported (Dhossche et al., 2002; El-Ansary and Al-Ayadhi, 2014). However, as GABA is also unable to cross the blood-brain barrier, a straightforward relationship between the plasma levels and the measured neurotransmitter concentrations is complicated. In this study, a considerable trend towards significant decrease of GABA+ in HFA compared to HC is reported. GABA is the major inhibitory neurotransmitter in the brain, thus preventing the formation of action potentials. A reduction of occipital GABAergic inhibitory activity could lead to hyper-excitability of occipital cortical minicolumnar circuits; vertical columns of functionally related neurons which are important for the flow of E/I information in the cortex (Mountcastle, 1997). The aforementioned hyper-excitability causes increased ‘noise’ in cortical systems (Rubenstein and Merzenich, 2003), which in turn could lead to atypical development of the occipital region. A possibly related finding is that the cortical minicolums are shown to be narrower in individuals with ASD (Casanova et al., 2003).
The current study has a few limitations. First, the included population was relatively small, possibly limiting the general applicability of the outcomes. Second, there is a possibility that the individuals with ASD included in this study could have co-occurring ADHD, however since the DSM-IV-TR criteria were used this could not be confirmed. Furthermore, the effect of medication cannot be ruled out completely, but we expect it to be negligible. Third, the GABA concentration is measured with the contribution of co-editing macromolecules. Therefore, differences in macromolecules across participants can affect the results. However, the macromolecules contribution is presumably constant in all the participants (Bogner et al., 2010). Fourth, using the 1H-MRS technique, tissue levels of neurotransmitter concentrations are obtained rather than the amount or activity of neurotransmitter receptors which are responsible for the E/I regulation. Fifth, neurotransmitter concentrations are only measured in a single voxel, positioned in the occipital lobe, while no data on visual processing was available for evaluation of, and association with, occipital cognitive functions. Furthermore, there is no evidence on the global nature of the observed neurotransmitter effects, since we only acquired data form a single voxel. Last, when females were excluded from analysis, Glu/Cr was not significantly different anymore, however it still showed a trend, whereas GABA+/Glu was still significant, which indicates that the results are highly similar to the complete analysis.
5. Conclusion
In the present study, changes in the concentrations of Glu/Cr, GABA+/Glu and GABA+/Cr in adolescents with HFA compared to HC were observed. Increased Glu/Cr and decreased GABA+/Glu was found in the occipital region of individuals with HFA compared to the HC, suggesting that changes in the E/I system may contribute to the atypical brain development in autism-spectrum disorder.
Acknowledgments
This study applies tools developed under National Institute of Health (NIH) R01 EB016089 and P41 EB015909; RAEE and NAJP also receive salary support from these grants.
We would like to acknowledge Marc Geerlings and Jos Slenter (Radiology & Nuclear Medicine, Maastricht University Medical Center, Maastricht, the Netherlands) for continuous hardware and software support. We also would like to acknowledge Remco Berting for assistance with image acquisition.
Footnotes
Contributors
GSD and JFAJ researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. EMB, APA, TVV, NAJP, RAEE, SZ, MPHH, RPCK contributed to discussion, critically reviewed the manuscript. The authors report no potential conflicts of interest for this manuscript.
Conflict of interest
The authors report no potential conflicts of interest for this manuscript.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington: 2001. [Google Scholar]
- Aldred S, Moore KM, Fitzgerald M, Waring RH. Plasma amino acid levels in children with autism and their families. J Autism Dev Disord. 2003;33:93–97. doi: 10.1023/a:1022238706604. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Assocition. Diagnostic and Statistical Manual of Mental Disorders. fourth. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. http://dx.doi.org/10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Barendse EM, Hendriks MP, Jansen JF, Backes WH, Hofman PA, Thoonen G, Kessels RP, Aldenkamp AP. Working memory deficits in high-functioning adolescents with autism spectrum disorders: neuropsychological and neuroimaging correlates. J Neurodev Disord. 2013;5:14. doi: 10.1186/1866-1955-5-14. http://dx.doi.org/10.1186/1866-1955-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruth JM, Wall CA, Patterson MC, Port JD. Proton magnetic resonance spectroscopy as a probe into the pathophysiology of autism spectrum disorders (ASD): a review. Autism Res. 2013;6:119–133. doi: 10.1002/aur.1273. http://dx.doi.org/10.1002/aur.1273. [DOI] [PubMed] [Google Scholar]
- Bauminger N, Shulman C, Agam G. Peer interaction and loneliness in high functioning children with autism. J Autism Dev Disord. 2003;33:489–507. doi: 10.1023/a:1025827427901. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya PK, Phillips MD, Stone LA, Lowe MJ. In vivo magnetic resonance spectroscopy measurement of gray-matter and white-matter gamma-aminobutyric acid concentration in sensorimotor cortex using a motion-controlled MEGA point-resolved spectroscopy sequence. Magn Reson Imaging. 2011;29:374–379. doi: 10.1016/j.mri.2010.10.009. http://dx.doi.org/10.1016/j.mri.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogner W, Gruber S, Doelken M, Stadlbauer A, Ganslandt O, Boettcher U, Trattnig S, Doerfler A, Stefan H, Hammen T. In vivo quantification of intracerebral GABA by single-voxel 1H-MRS-How reproducible are the results? Eur J Radiol. 2010;73:526–531. doi: 10.1016/j.ejrad.2009.01.014. http://dx.doi.org/10.1016/j.ejrad.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Brix MK, Ersland L, Hugdahl K, Grüner R, Posserud M-B, Hammar Å, Craven AR, Noeske R, Evans CJ, Walker HB, Midtvedt T, Beyer MK. Brain MR spectroscopy in autism spectrum disorder—the GABA excitatory/inhibitory imbalance theory revisited. Front Hum Neurosci. 2015;9 doi: 10.3389/fnhum.2015.00365. http://dx.doi.org/10.3389/fnhum.2015.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Singel D, Hepburn S, Rojas DC. Increased glutamate concentration in the auditory cortex of persons with autism and first-degree relatives: a 1H-MRS study. Autism Res. 2013;6:1–10. doi: 10.1002/aur.1260. http://dx.doi.org/10.1002/aur.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Bird G, Moll J, Frith C, Blakemore SJ. Development during adolescence of the neural processing of social emotion. J Cogn Neurosci. 2009;21:1736–1750. doi: 10.1162/jocn.2009.21121. http://dx.doi.org/10.1162/jocn.2009.21121.Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden D, Gomez J. Disruption in the inhibitory architecture of the cell minicolumn: implications for autism. Neurosci. 2003;9:496–507. doi: 10.1177/1073858403253552. http://dx.doi.org/10.1177/1073858403253552. [DOI] [PubMed] [Google Scholar]
- Cochran DM, Sikoglu EM, Hodge SM, Edden RAE, Foley A, Kennedy DN, Moore CM, Frazier JA. Relationship among glutamine, γ-aminobutyric acid, and social cognition in autism spectrum disorders. J Child Adolesc Psychopharmacol. 2015;25:314–322. doi: 10.1089/cap.2014.0112. http://dx.doi.org/10.1089/cap.2014.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social – affective engagement and goal flexibility. Nature. 2012;13:636–650. doi: 10.1038/nrn3313. http://dx.doi.org/10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- de Graaf RA. Principles of 1H NMR spectroscopy in vivo. In: Choi I-Y, Gruetter R, editors. Neural Metabolism In Vivo, Advances in Neurobiology. Springer US; Boston, MA: 2012. pp. 133–148. http://dx.doi.org/10.1007/978-1-4614-1788-0. [Google Scholar]
- Dhossche D, Applegate H, Abraham A, Maertens P, Bland L, Bencsath A, Martinez J. Elevated plasma gamma-aminobutyric acid (GABA) levels in autistic youngsters: stimulus for a GABA hypothesis of autism. Med Sci Monit. 2002;8:1–6. [PubMed] [Google Scholar]
- Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40:1445–1452. doi: 10.1002/jmri.24478. http://dx.doi.org/10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ansary A, Al-Ayadhi L. GABAergic/glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. J Neuroinflamm. 2014;11 doi: 10.1186/s12974-014-0189-0. http://dx.doi.org/10.1186/s12974-014-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers M, Hollander E. Excitotoxicity in autism. In: Zimmerman AW, editor. Autism. Humana Press; Totowa, NJ: 2008. pp. 133–145. http://dx.doi.org/10.1007/978-1-60327-489-0_6. [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABAA receptor downregulation in brains of subjects with autism. J Autism Dev Disord. 2009;39:223–230. doi: 10.1007/s10803-008-0646-7. http://dx.doi.org/10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Bloy L, Wang DJ, Port RG, Blaskey L, Levy SE, Roberts TPL. GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage. 2014;86:1–9. doi: 10.1016/j.neuroimage.2013.05.068. http://dx.doi.org/10.1016/j.neuroimage.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts HM, Verté S, Oosterlaan J, Roeyers H, Sergeant Ja. How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism? J Child Psychol Psychiatry. 2004;45:836–854. doi: 10.1111/j.1469-7610.2004.00276.x. http://dx.doi.org/10.1111/j.1469-7610.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- Harada M, Taki MM, Nose A, Kubo H, Mori K, Nishitani H, Matsuda T. Non-invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA-editing proton MR spectroscopy using a clinical 3 tesla instrument. J Autism Dev Disord. 2011;41:447–454. doi: 10.1007/s10803-010-1065-0. http://dx.doi.org/10.1007/s10803-010-1065-0. [DOI] [PubMed] [Google Scholar]
- Hauck M, Fein D, Waterhouse L, Feinstein C. Social initiations by autistic children to adults and other children. J Autism Dev Disord. 1995;25:579–595. doi: 10.1007/BF02178189. http://dx.doi.org/10.1007/BF02178189. [DOI] [PubMed] [Google Scholar]
- Joshi G, Biederman J, Wozniak J, Goldin RL, Crowley D, Furtak S, Lukas SE, Gönenç A. Magnetic resonance spectroscopy study of the glutamatergic system in adolescent males with high-functioning autistic disorder: a pilot study at 4T. Eur Arch Psychiatry Clin Neurosci. 2013;263:379–384. doi: 10.1007/s00406-012-0369-9. http://dx.doi.org/10.1007/s00406-012-0369-9. [DOI] [PubMed] [Google Scholar]
- Kubas B, Kułak W, Sobaniec W, Tarasow E, Łebkowska U, Walecki J. Metabolite alterations in autistic children: a 1H MR spectroscopy study. Adv Med Sci. 2012;57:152–156. doi: 10.2478/v10039-012-0014-x. http://dx.doi.org/10.2478/v10039-012-0014-x. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Cooperrider J, Taylor JS. Spectroscopic brain imaging in autism. In: Casanova MF, El-Baz AS, Suri JS, editors. Imaging the Brain in Autism. Springer; New York, New York, NY: 2013. pp. 231–288. http://dx.doi.org/10.1007/978-1-4614-6843-1. [Google Scholar]
- Leitner Y. The co-occurrence of autism and attention deficit hyperactivity disorder in children - what do we know? Front Hum Neurosci. 2014;8:268. doi: 10.3389/fnhum.2014.00268. http://dx.doi.org/10.3389/fnhum.2014.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Western Psychological Services; Los Angeles: 2001. [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. http://dx.doi.org/10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Near J, Ho YCL, Sandberg K, Kumaragamage C, Blicher JU. Long-term reproducibility of GABA magnetic resonance spectroscopy. Neuroimage. 2014;99:191–196. doi: 10.1016/j.neuroimage.2014.05.059. http://dx.doi.org/10.1016/j.neuroimage.2014.05.059. [DOI] [PubMed] [Google Scholar]
- Nicpon MF, Doobay AF, Assouline SG. Parent, teacher, and self perceptions of psychosocial functioning in intellectually gifted children and adolescents with autism spectrum disorder. J Autism Dev Disord. 2010;40:1028–1038. doi: 10.1007/s10803-010-0952-8. http://dx.doi.org/10.1007/s10803-010-0952-8. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova Ta, Prokofyev AO, Nygren G, Gillberg C, Elam M. Sensory gating in young children with autism: relation to age, IQ, and EEG gamma oscillations. Neurosci Lett. 2008;434:218–223. doi: 10.1016/j.neulet.2008.01.066. http://dx.doi.org/10.1016/j.neulet.2008.01.066. [DOI] [PubMed] [Google Scholar]
- Pellicano E. Individual differences in executive function and central coherence predict developmental changes in theory of mind in autism. Dev Psychol. 2010;46:530–544. doi: 10.1037/a0018287. http://dx.doi.org/10.1037/a0018287. [DOI] [PubMed] [Google Scholar]
- Puts NAJ, Edden RAE. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog Nucl Magn Reson Spectrosc. 2012;60:29–41. doi: 10.1016/j.pnmrs.2011.06.001. http://dx.doi.org/10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Singel D, Steinmetz S, Hepburn S, Brown MS. Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. Neuroimage. 2014;86:28–34. doi: 10.1016/j.neuroimage.2013.01.045. http://dx.doi.org/10.1016/j.neuroimage.2013.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. http://dx.doi.org/10.1034/j.1601-183X.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rump KM, Giovannelli JL, Minshew NJ, Strauss MS. The development of emotion recognition in individuals with autism. Child Dev. 2009;80:1434–1447. doi: 10.1111/j.1467-8624.2009.01343.x. http://dx.doi.org/10.1111/j.1467-8624.2009.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F, Mottron L, Soulières I, Zeffiro Ta. Enhanced visual functioning in autism: an ALE meta-analysis. Hum Brain Mapp. 2012;33:1553–1581. doi: 10.1002/hbm.21307. http://dx.doi.org/10.1002/hbm.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer GB, Lutz RE. Diagnostic yield in the clinical genetic evaluation of autism spectrum disorders. Genet Med. 2006;8:549–556. doi: 10.1097/01.gim.0000237789.98842.f1. http://dx.doi.org/10.1097/01.gim.0000237789.98842.f1. [DOI] [PubMed] [Google Scholar]
- Simpkins CA, Simpkins AM. Neurons and neurotransmitters. In: Simpkins CA, Simpkins AM, editors. Neuroscience for Clinicians. Springer; New York, New York, NY: 2013. pp. 77–91. 〈 http://dx.doi.org/:10.1007/978-1-4614-4842-6_7〉. [Google Scholar]
- Snijders TM, Milivojevic B, Kemner C. Atypical excitation–inhibition balance in autism captured by the gamma response to contextual modulation. Neuroimage Clin. 2013;3:65–72. doi: 10.1016/j.nicl.2013.06.015. http://dx.doi.org/10.1016/j.nicl.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares DP, Law M. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clin Radiol. 2009;64:12–21. doi: 10.1016/j.crad.2008.07.002. http://dx.doi.org/10.1016/j.crad.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Soulières I, Dawson M, Samson F, Barbeau EB, Strangman GE, Zeffiro TA, Mottron L. Enhanced visual processing contributes to matrix reasoning in autism. Hum Brain Mapp. 2009;30:4082–4107. doi: 10.1002/hbm.20831. http://dx.doi.org/10.1002/hbm.20831.Enhanced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. http://dx.doi.org/10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Tirouvanziam R, Obukhanych TV, Laval J, Aronov PA, Libove R, Banerjee AG, Parker KJ, O’Hara R, Herzenberg LA, Herzenberg LA, Hardan AY. Distinct plasma profile of polar neutral amino acids, leucine, and glutamate in children with autism spectrum disorders. J Autism Dev Disord. 2012;42:827–836. doi: 10.1007/s10803-011-1314-x. http://dx.doi.org/10.1007/s10803-011-1314-x. [DOI] [PubMed] [Google Scholar]
- Tu WJ, Chen H, He J. Application of LC-MS/MS analysis of plasma amino acids profiles in children with autism. J Clin Biochem Nutr. 2012;51:248–249. doi: 10.3164/jcbn.12-45. http://dx.doi.org/10.3164/jcbn.12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. anual for the Wechsler Intelligence Scale for Children. 3rd. The Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Zikopoulos B, Barbas H. Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front Hum Neurosci. 2013;7:609. doi: 10.3389/fnhum.2013.00609. http://dx.doi.org/10.3389/fnhum.2013.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]


