Abstract
Objective
Low birth weight (LBW; below 2500 grams) is a general risk factor for a variety of neurodevelopmental difficulties. However, these children may remain more vulnerable to neurologic and environmental insults occurring years later. This prospective case series reports on children who sustained a mild, moderate, or severe traumatic brain injury (TBI) in middle childhood but who had also been born with birth weights below 2500 grams.
Participants
Participants were 14 children with mild, moderate, or severe traumatic brain injury (TBI), 5 of whom had birth weights under 2500g (LBW) and nine children with normal birth weight (NBW). All participants were drawn from a larger study on the long-term cognitive and behavioral impact of pediatric TBI and were matched on age, estimated socioeconomic status (SES), and severity of TBI (with NBW children actually having a slightly worse overall injury severity).
Results
At baseline, both groups exhibited similar scores on WJ-R Letter Word Identification and Calculations, Tower of London number solved, and CVLT-C total correct. Baseline group differences were observed on the CELF-III Formulated Sentences (NBW>LBW) and on the VABS Adaptive Behavior Composite and Socialization subdomain (LBW>NBW). Over two-years, relative to the NBW group, the LBW group evidenced declines on both WJ-R subtests, CVLT-C total correct, CELF-III Formulated Sentences and VABS Adaptive Behavior Composite and Socialization.
Conclusions
Although preliminary in nature due to small sample size, findings suggest a history of LBW influences the recovery trajectory following childhood TBI. Academic and adaptive functioning and verbal memory appeared particularly affected.
Introduction
More than 20 million children are born low birth weight (LBW) (i.e., having a birth weight below 2500 grams) each year around the world; accounting for about 15.5% of all births (Wardlaw et al, 2004). According to the World Health Organization, children born weighing less than 2500 grams have a significantly higher mortality rate (approximately 20 times that of normal birth weight children), and throughout their lives, LBW children remain at greater risk for a number of deleterious health conditions including increased rates of behavioral disorders (especially attention problems, depression, and anxiety), altered immune function, hypertension, diabetes mellitus, renal failure, and hormonal anomalies (Burnett et al, 2011; Chilcoat & Breslau, 2002; Costello et al, 2007; Lackland et al, 2000; Wüst et al, 2005). In addition, children born with a birth weight below 2500 grams are at higher risk for cognitive, academic, and behavioral difficulties, and these problems can persist long after the early childhood period (Aylward, 2005; Bohnert & Breslau, 2008; Breslau, Chilcoat, DelDotto, Andreski, & Brown, 1996; Breslau, Paneth, & Lucia, 2004; Litt, Taylor, Klein, & Hack, 2005; Taylor, Klein, Minich, & Hack, 2000a; Taylor Klein, Minich, & Hack, 2000b).
In one study, Breslau and colleagues (1996) found a variety of neuropsychological differences in a group of six-year-old LBW children when compared to age-matched children of normal birth weight (i.e., the groups differed on measures of language, visuospatial, fine-motor, and attention skills). Further, these investigators found evidence of gradient effects in this cohort with greater reductions in performance being obtained at progressively lower birth weights. Taylor and collaborators also found evidence of cognitive deficits, academic dysfunction, and greater behavioral problems in a group of middle school aged children born very low birth weight (i.e., <1500 grams). These researchers indicated that, within the extremely low birth weight group, disparities grew more pronounced on measures of global cognitive abilities, basic reading skills, academic performance, and parent-reported measures of behavior and attention as children increased in age. Finally, in a follow-up study to their 1996 paper, Breslau et al. (2004) found that previously LBW children continued exhibiting decreases in academic skills at the age of 17.
In addition, other perinatal neurologic injuries and environmental factors such as low socioeconomic status (SES) and maternal infections may exacerbate these problems (Aylward, 2005; Böhm, Katz-Salamon, Smedler, Lagercrantz, & Forssberg, 2002; Munck et al., 2010; Perlman, 2002). For example, Whitaker et al. (1997) demonstrated that LBW children sustaining an interventricular hemorrhage (IVH) were more likely to exhibit a psychiatric diagnosis (especially attention deficit hyperactivity disorder) at six years of age compared to LBW children who had not sustained a concurrent IVH. Bohnert and Breslau (2008) showed that LBW children growing up under disadvantaged circumstances had a higher rate of attention problems when compared to LBW children growing up in circumstances with more resources.
Taken together, the above studies indicate LBW children are at a significant risk for long-term cognitive and behavioral difficulties and suggest that at least some of these deficits may be influenced by environmental factors and/or exacerbated by other perinatal insults such as an IVH. However, other investigations have suggested that children born LBW demonstrate considerable plasticity and recovery of cognitive skills following birth (Ment et al., 2003), and some studies have suggested that children born late preterm (e.g., between 34 and 36 weeks gestation) who are otherwise healthy do not exhibit any significant decrements in cognitive skills when evaluated years later (Gurka, LoCasale-Crouch, & Blackman, 2010; Romeo et al. 2012).
Few, if any, studies have examined how LBW children respond to future neurologic injuries that occur years after birth. Breslau (1990) addressed a similar question by examining the influence of environmental stress on the rate of psychiatric diagnosis in a group of children with various handicaps. Interestingly, she found no greater adverse effect of environmental stress within the handicapped group suggesting that children with neurological dysfunction did not have greater vulnerability to environmental stress. However, this study was concerned only with increases in the rate of psychiatric diagnosis and was primarily intended to examine relatively low-level, but chronic environmental stress as opposed to a secondary injury. Other investigations have examined the relationship between LBW and complex injuries occurring secondary to premature birth. These investigations indicate that LBW children who also demonstrate brain anomalies are at a significantly higher risk for developmental or motor delays, but most of these investigations are concerned with injuries occurring soon after birth (Pinto-Martin, Whitaker, Feldman, Van Rossem, & Paneth, 1999; Whitaker et al., 1990). An important question is whether LBW status contributes to long-term alterations in brain development that lead to different (i.e., more attenuated) patterns of recovery following a future neurologic insult.
Research with other populations has examined the influence of cumulative risk on long-term cognitive outcomes. Research with children who have sustained traumatic brain injuries (TBIs) suggests that these children remain at risk for significantly worse outcomes following a second injury even if one or both of the traumas was relatively minor (Stern et al., 2011). Further, evidence suggests that repetitive, even mild, head traumas in young adulthood may result in very long-term increases in mild cognitive impairment and earlier onset of Alzheimer’s type dementia (Guskiewicz et al., 2005). Thus, it seems plausible that even in the context of apparently intact functioning, LBW children may be at increased risk for an attenuated recovery following future neurologic insults. This small study reports a prospective case-series of children who were part of a larger investigation on the long-term cognitive impact of traumatic brain injury (TBI) but who were also born with birth weights below 2500 grams. We hypothesized that LBW children may exhibit a different pattern of recovery following a mild, moderate, or severe TBI when compared to a group of normal birth weight children who had sustained a TBI of similar severity.
Methods
Participants
All participants were part of a larger project examining cognitive and neuropsychological sequelae following mild, moderate, or severe TBI in children between five and 17 years of age. For a complete description of the cohort and study design, see Hanten et al. (2009). Briefly, children between the ages of five-15 years, who had sustained a closed head injury, were recruited during their hospitalization after they had become medically stable. Recruitment occurred at five major medical centers (three in Texas, the University of California San Diego, and the Hospital for Sick Children in Toronto).
Participants had injuries ranging in severity from mild to severe based on the lowest post-resuscitation Glasgow Coma Scale (GCS) score. The original study cohort was composed of children who experienced mild to severe closed head injuries and who had a recorded GCS (Teasdale & Jennett, 1974) score reported in hospital records. Mild TBI was defined by the lowest GCS score ranging between 13–15. Moderate TBI was defined by lowest GCS scores of 9 to 12 or by GCS scores of 13 to 15 with brain lesions (contusions, hematomas) indicated by computed tomography (CT) scans. GCS scores of 3 to 8 defined severe TBI. Children with a history of neurological involvement (e.g., previous head injuries of any severity, injuries resulting from child abuse, cerebral palsy, epilepsy, brain tumors/stroke, hypoxia, etc.); those with significant sensory impairments (e.g., hearing loss requiring assistive devices or vision loss that was not normalized by corrective lenses); and significant developmental or psychiatric disorders (e.g., mental retardation, autism spectrum disorders, bipolar disorder, or schizophrenia) were excluded from participation in the study. An index score of socioeconomic status (SES) based on four factors (education, income, occupation, and occupational prestige) was measured by the Hollingshead Index (Hollingshead, 1975).
The entire cohort of the original study contained 173 children who had sustained mild (n=47), moderate (n=60), or a severe TBI (n=66). As is typical for childhood TBI studies, the population contained more males (n = 120) than females (n = 53) although there were no gender differences by injury severity. All participants in the current paper were drawn from the TBI group of the initial cohort. There were a total of five LBW participants with birth weights ranging between 1200g and 2500g (approximately 2.5 and 5.5 lbs) in this prospective, longitudinal study. These participants were matched to nine normal birth weight (NBW) participants. Participants were matched on age, estimated SES, and severity of TBI similar or worse than the LBW group. This procedure yielded a total of 14 children with mild to severe TBI. Of note, we were particularly interested in the impact of low birth weight on the trajectory of recovery from a later injury rather than on the impact of TBI on overall cognitive and academic development. Therefore, we chose to compare the LBW group to NBW participants who had also sustained a TBI; thereby partly controlling for injury related factors.
Procedures
All study procedures were approved by and in accordance with the internal review board and human subjects guidelines of the participating institutions. After parental informed consent was obtained and documented, participating children were administered a battery of neuropsychological tests. Assessments occurred on five occasions, at: baseline (within one month following the injury), 3 months, 6 months, 12 months, and 24 months. Most participants from both groups completed all assessments. Of note, despite some individuals not participating at all follow-up intervals, our rate of follow-up for the entire study was not significantly different from other studies of pediatric TBI.
Although other measures were given in the original study, four broad measures of executive and cognitive function were used in the current study. As these measures provide a general overview of a range of cognitive abilities, and were the measures available at each of the time points for this relatively small sample. Specifically, we used standardized measures of: academic functioning (i.e., Woodcock-Johnson Test of Academic Achievement –Revised (WJ-R) Letter-Word Identification and Calculations subtests (Woodcock & Mather, 1989); adaptive functioning (i.e., Vineland Adaptive Behavior Scale (VABS) (Sparrow, Balla, & Cicchetti, 1984); language abilities (i.e., Clinical Evaluation of Language Fundamentals –3rd Edition (CELF-III) (Semel, Wiig, & Secord, 1995); verbal memory (i.e., California Verbal Learning Test–Children’s Version (CVLT-C) (Delis, Kramer, Kaplan, & Ober, 1986); and planning skills (i.e., Tower of London Test (TOL) (Shallice, 1982).
Results
Because of the descriptive/preliminary nature of this investigation and small sample size, no inferential statistics were performed as any analyses would be hampered by a lack of power. The LBW and NBW groups were similar in terms of age and estimated SES. Groups were less well-matched in terms of injury severity with the NBW group having more severe injuries as a whole than the LBW group (see Table 1). At baseline, both groups exhibited similar scores on all measures except the CELF-III Formulated Sentences and VABS (see table 1). Specifically, at baseline, the NBW group scored higher on the CELF-III Formulated Sentences test than the LBW group. The VABS Adaptive Behavior Composite also revealed baseline differences between the groups, with the LBW group scoring higher on the measure than the NBW group. Group comparisons were also performed for the three subtests of the Vineland (see Table 1 for summary statistics). At baseline group differences emerged on the socialization subdomain of the Vineland with LBW scoring higher than NBW, but baseline group differences on the other subdomains of communication and daily living skills were not observed.
Table 1.
Demographics and Neuropsychological Test Performance of LBW and NBW children from Baseline to 24-months Post-Injury
| Table 1 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Task | Occasion | S1 | S2 | S3 | S4 | S5 | LBW (N=5) | NBW (N=9) | Effect Size Cohen’s D | 95% CI for Cohen’s D | |||||
| Mean | Median | SD | Mean | Median | SD | ||||||||||
|
WoodCock
Johnson Letter-Word ID Standard Score |
BL | 80 | 85 | 113 | 128 | 123 | 105.8 | 113.0 | 22.0 | 109.5 | 106.5 | 23.2 | 0.16 | [.04, 1.32] | |
| 12 M | 89 | 71 | 103 | 117 | 126 | 101.2 | 103.0 | 22.0 | 110.8 | 105.0 | 18.9 | 0.48 | [.06, 1.69] | ||
| 24 M | 87 | 72 | 121 | --- | 121 | 100.3 | 104.0 | 24.7 | 116.0 | 110.0 | 15.6 | 0.82 | [.12, 3.08] | ||
|
WoodCock
Johnson Calculation Standard Score |
BL | --- | 89 | 124 | 118 | 115 | 111.5 | 116.5 | 15.5 | 107.1 | 108.0 | 18.7 | 0.25 | [.03, 1.68] | |
| 12 M | 105 | 80 | 102 | 121 | 111 | 103.8 | 105.0 | 15.2 | 98.5 | 95.5 | 17.8 | 0.31 | [.05, 2.03] | ||
| 24 M | 106 | 71 | 112 | --- | 107 | 99.0 | 106.5 | 18.9 | 107.3 | 107.0 | 12.5 | 0.55 | [.10, 1.72] | ||
|
Tower of
London Number Solved |
BL | 10 | 17 | 15 | 16 | 14 | 14.4 | 15.0 | 2.7 | 15.3 | 17.0 | 5.6 | 0.18 | [.04, 1.86] | |
| 12 M | 18 | 14 | 20 | 20 | 18 | 18.0 | 18.0 | 2.5 | 18.8 | 18.5 | 1.2 | 0.43 | [.06, 1.56] | ||
| 24 M | 20 | 20 | 20 | --- | 16 | 19.0 | 20.0 | 2.0 | 19.1 | 20.0 | 1.2 | 0.09 | [.05, 1.47] | ||
|
CELF3 Formulated Sentences Scale Score |
BL | --- | 9 | 9 | 7 | --- | 8.3 | 9.0 | 1.2 | 9.4 | 10.0 | 2.3 | 0.53 | [.06, 3.16] | |
| 12 M | 7 | 9 | 10 | 8 | 8 | 8.4 | 8.0 | 1.1 | 9.7 | 9.0 | 2.1 | 0.73 | [.10, 1.78] | ||
| 24 M | 5 | 6 | 12 | --- | 8 | 7.8 | 7.0 | 3.1 | 10.7 | 11.0 | 2.4 | 1.11 | [.20, 3.05] | ||
|
CVLT-C Monday List T-Score |
BL | 42 | 40 | 33 | 47 | 60 | 44.4 | 42.0 | 10.1 | 48.9 | 49.5 | 8.3 | 0.50 | [.08, 2.05] | |
| 12 M | 45 | 45 | 26 | 72 | 62 | 50.0 | 45.0 | 17.7 | 59.0 | 58.5 | 7.3 | 0.74 | [.06, 2.68] | ||
| 24 M | 48 | 33 | 25 | --- | 48 | 38.5 | 40.5 | 11.4 | 58.4 | 62.0 | 11.8 | 1.71 | [.76, 4.16] | ||
| BABS | Composite Standard Score |
BL | 100 | 96 | 115 | 91 | 121 | 104.6 | 100.0 | 12.8 | 97.3 | 96.0 | 13.9 | 0.54 | [.05, 1.78] |
| 12 M | 100 | 86 | 94 | 104 | 122 | 101.2 | 100.0 | 13.5 | 96.5 | 93.0 | 20.4 | 0.26 | [.04, 1.42] | ||
| 24 M | 95 | 85 | 91 | --- | 101 | 93.0 | 93.0 | 6.7 | 102.7 | 96.0 | 17.2 | 0.67 | [.10, 1.63] | ||
| Communication Standard Score |
BL | 89 | 95 | 123 | 97 | 121 | 105.0 | 97.0 | 15.8 | 101.9 | 100.0 | 17.2 | 0.19 | [.03, 1.45] | |
| 12 M | 87 | 81 | 114 | 100 | 125 | 101.4 | 100.0 | 18.3 | 99.6 | 95.0 | 20.8 | 0.09 | [.04, 1.34] | ||
| 24 M | 85 | 84 | 115 | --- | 110 | 98.5 | 97.5 | 16.3 | 105.7 | 101.0 | 15.9 | 0.45 | [.06, 1.77] | ||
| Daily Living Standard score |
BL | 109 | 99 | 107 | 86 | 123 | 104.8 | 107.0 | 13.6 | 99.3 | 103.0 | 12.4 | 0.43 | [.04, 1.51] | |
| 12 M | 108 | 91 | 85 | 106 | 125 | 103.0 | 106.0 | 15.7 | 96.9 | 94.0 | 18.8 | 0.35 | [.03, 1.49] | ||
| 24 M | 102 | 90 | 76 | --- | 105 | 93.3 | 96.0 | 13.2 | 98.3 | 96.0 | 10.1 | 0.45 | [.07, 1.97] | ||
| Socialization Standard score |
BL | 104 | 98 | 104 | 98 | 102 | 101.2 | 102.0 | 3.0 | 93.6 | 90.0 | 8.8 | 1.03 | [.39, 2.32] | |
| 12 M | 106 | 96 | 89 | 104 | 99 | 98.8 | 99.0 | 6.8 | 95.6 | 99.0 | 10.5 | 0.34 | [.04, 1.18] | ||
| 24 M | 104 | 92 | 91 | --- | 88 | 93.8 | 91.5 | 7.0 | 102.6 | 98.0 | 13.6 | 0.75 | [.13, 1.99] | ||
| Demographics | |||||||||||||||
| Age at Injury (years) | 5.1 | 6.9 | 12 | 7.1 | 5.2 | 7.3 | 6.9 | 2.7 | 7.78 | 7.32 | 2.66 | .09 | |||
| GCS | 15 | 8 | 14 | 15 | 14 | 13.2 | 14.0 | 3.0 | 11.56 | 15.0 | 5.10 | −.36 | |||
| Birth Weight (lb) | 3.9 | 4.5 | 5.4 | 5.4 | 5.4 | 4.9 | 5.38 | 0.7 | 7.42 | 7.47 | .57 | 4.55 | |||
LBW = Low Birth Weight group; NBW = Normal Birth Weight Group; SD = Standard Deviation; CVLT-C = California Verbal Learning Test –Children’s version; CELF-III = Clinical Evaluation of Language Fundamentals –3rd Edition; VABS = Vineland Adaptive Behavior System.
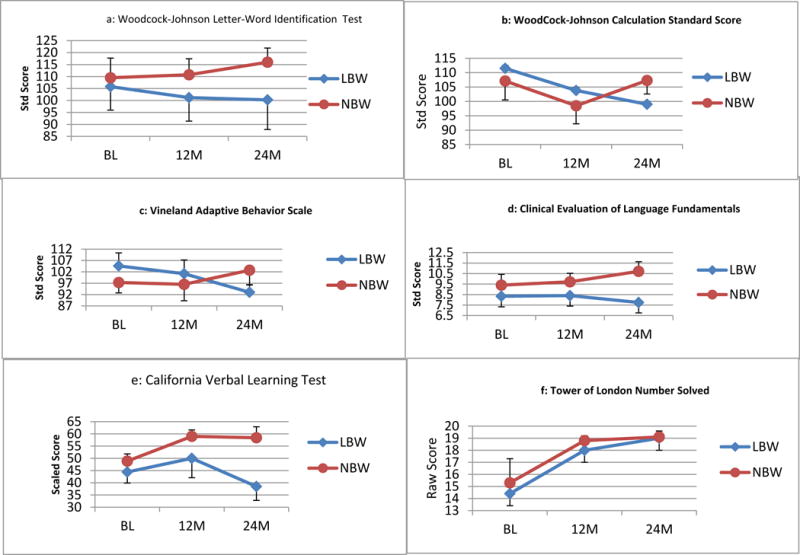
Over the two-year follow-up period, relative to the NBW group, the LBW group evidenced relative declines (i.e., declines in their standard scores) on both WJ-R subtests, as well as CVLT-C total correct, CELF-III Formulated Sentences, and VABS ABC and Socialization. Essentially, the difference between NBW and LBW groups was increasing with time post injury especially on the WJ-R Calculations and VABS socialization. LBW participants also exhibited notable decreases on the WJ-R Letter-word Identification and CVLT-C total correct between baseline and 24 months post-injury compared to modest improvements in these measures within the NBW group.(see Figure 1) No apparent differences on the TOL problem solving emerged. Please see table 1 for a list of specific scores for LBW participants for each measure and occasion, for mean and median scores for both groups, and for Cohen’s d with confidence intervals obtained via a bootstrap analysis that resampled the population 500 times with replacement.
Figure 1.
Discussion
The current preliminary study presents a small case series of 14 children who sustained a mild, moderate, or severe TBI and five of whom were also born with a birth weight below 2500 grams. When compared to a group of age and SES matched normal birth weight children each of whom had similar or worse injuries, the LBW children seemed to exhibit attenuated recovery despite appearing relatively well matched at baseline. That is, there were negligible differences in the groups at baseline in terms of their academic skills, verbal memory abilities, and problem solving skills. NBW children performed better at baseline on a measure of language skills, but LBW children had better parent-reported adaptive skills in the baseline assessment. Nevertheless, throughout the two-year follow-up period, the LBW group demonstrated a decline, relative to their age-matched peers, in their academic skills, verbal memory abilities, and, in particular, their adaptive (especially socialization) skills. This latter finding is particularly salient because at baseline, the LBW group was actually judged to have better developed adaptive/social abilities when compared to the NBW children. NBW superiority in terms of language skills continued to be evidenced throughout the follow-up period, and no differences on measures of problem solving emerged. Because of its limited number of participants and the convenience nature of the sample, this investigation can only be considered a preliminary study, but it provides interesting data regarding the potential for differences in how LBW children respond to later neurologic insult.
Despite many baseline similarities, the pattern that emerged during the follow-up period suggests that children who were born LBW, but appeared to be functioning generally within the average range years later, exhibit an attenuated pattern of recovery following a much later brain insult. Of note, the present results do not suggest that children within the LBW group actually suffered a loss of cognitive skills throughout the follow-up interval, but rather, that these individuals may have failed to make age-expected gains, even more so than other children who had also sustained a TBI. Failure to make age expected gains in various cognitive domains is a common pattern emerging in long-term studies of TBI survivors (Anderson et al., 2006; Hanten et al., 2009; Schmidt et al., 2012). The differences that emerged between the groups in the current small case series appears to suggest that this trend was accentuated in LBW children although it may also be the case that children within the LBW group exhibit an extended window of recovery that goes beyond the two-year follow-up period of the current investigation. Regardless, these conjectures can only be disentangled by additional larger-scale investigations.
Although we only examined a limited subset of cognitive domains, when compared to the NBW TBI group, some of the most vulnerable areas within the LBW group appeared to be in terms of adaptive skills (especially socialization skills), academic skills, and verbal memory. Previous studies have indicated that all of these areas are commonly affected in pediatric TBI (Catroppa & Anderson, 2007; Catroppa, Anderson, Morse, Haritou, & Rosenfeld, 2008; Ewing-Cobbs et al., 2004; Max et al., 1998; Mottram & Donders, 2006; Roman et al., 1998; Rivara et al., 2011), and children with severe injuries appear most vulnerable for persistent deficits (Ewing-Cobbs et al., 2004; Fay et al., 2009). The decrease in terms of socialization skills following a TBI for the LBW participants is particularly concerning as social skill deficits are some of the most intractable difficulties arising following a closed head injury. These deficits can be associated with significant problems with peer and family relationships and overall rehabilitation success (Yeats et al., 2007).
Despite the range of injury severities (including several mild TBI patients) represented in the LBW group, the pattern observed suggests a recovery trajectory similar to that seen following severe TBI. That is, the data presented here suggests that the recovery trajectory of children born with LBW may be worse than would be expected given their injury severity. Interestingly, recent research suggests that pre-injury functioning may be just as important as injury severity in influencing outcomes (Anderson et al., 2012). The current findings complement these observations by suggesting that other pre-injury variables may also affect long-term outcomes even when pre-injury functioning appears relatively similar.
Language skills did not exhibit much relative change over the follow-up period. However, this is not to say that these skills were preserved, but rather that the groups performance on these measures did not change relative to each other over the follow-up interval. In fact, LBW participants experienced a decrease in their performance on this measure although their baseline performance was already below that of the NBW group complicating interpretation of this result. It is striking that differences in TOL performance were not observed between the groups, as executive skills are extremely vulnerable to closed head injuries (Levin & Hanten, 2005). However, it may be that the impact of the injury was so significant as to swamp any differences between the NBW or LBW groups on this measure. It is also possible that the TOL task is more complex and thus less sensitive to executive dysfunction in this age range.
Other investigations have examined outcomes in LBW children who also incur brain injuries such as white matter injury (Pinto-Martin et al., 1999). This investigation reported that children born below 2000 grams and who demonstrated evidence of white matter injury on a neonatal cranial ultrasound exhibited lower motor skills as late as nine years of age despite apparently normal cognitive development. Studies using animal models have demonstrated the concept of continued vulnerability of brain structures that may already be in somewhat of a weakened state. Rao and colleagues (1999, 2007) demonstrated that animals exposed to fetal/neonatal iron deficiency were more vulnerable to subsequent hypoxic/ischemic injuries sustained in the early postnatal period.
As described above, many of these investigations typically involve injuries that occur soon after birth or assess functioning within the first several years of life. A few investigations that have examined long-term cognitive, learning, and psychiatric outcomes in LBW children have suggested interactions between LBW status and environmental factors. For example, Casey and collaborators (2006) found that children born small for gestational age (SGA) were essentially undistinguishable from typically developing children in terms of growth, cognitive development, and academic achievement unless these children also experienced postnatal growth restriction (i.e., were described as failure to thrive). These investigators showed that children who were both SGA and failed to thrive performed significantly worse than all other groups on measures of cognitive development and academic achievement. (Casey, Whiteside-Mansell, Barrett, Bradley, & Gargus, 2006).
Roberts and colleagues (2007) described a cumulative risk model whereby children born LBW had an increased probability of being diagnosed with a learning disability if they also exhibited certain other risk factors. In another study, Bohnert and Breslau (2008), found that children born LBW and who grew up in an urban community had a higher rate of externalizing disorders. The authors suggested that these findings provided evidence of an interaction between the child’s LBW status at birth and environmental/genetic factors in development. Conversely, in one of the few studies of its kind, a study by Donders and Strom (1997) found that children with a history of learning disability who had sustained a subsequent TBI exhibited a significant decrease in their performance on a measure of intellectual functioning when compared to age and injury matched controls without a history of learning disability. This study suggests that children with a history of learning differences may be at an increased risk of long-term cognitive changes following a closed head injury (Donders and Strom, 1997), and is the closest parallel to the current case series we noted in our review of the literature.
Each of the aforementioned investigations have suggested that children born LBW may remain vulnerable to future environmental influences that affect neurological development; however, in all of the studies, conclusions were necessarily cautious because genetic influences could not firmly be ruled out. While we urge similar caution in the interpretation of the current findings, these results provide some tentative support for the hypothesis that LBW children respond differently to subsequent neurologic insults despite apparently normal development.
The current findings are somewhat in contrast to other studies suggesting relatively normal development of larger LBW children. That is, although not entirely consistent, many previous investigations have indicated that larger LBW children (i.e., children with a birth weight between 2000–2500 grams) and/or children born between 35–37 weeks gestation are generally undistinguishable from their NBW or full-term peers in the absence of significant perinatal complications (Cheatham, Bauer, & Georgieff, 2006; Gurka et al., 2010). Similarly, Rose and colleagues suggest that although cognitive differences in processing speed and attention may continue to be present in formerly LBW children at one year of age, many of the risks associated with medical complications appear to have been minimized by this point in development (Rose, Feldman, & Jankowski 2001, 2002). Most of the children involved in the current study were relatively large LBW or late preterm births. Therefore, these findings potentially suggest that children born LBW (even if at the upper end of this range) may display an atypical pattern of recovery following subsequent neurological injuries, at least until middle childhood. If supported by future, larger investigations, these findings may suggest that there are differences in the long-term brain development of these children even out of the neonatal period, and may help to bolster the previous observations of other research that indicate interactions between LBW status and environmental factors (Bohnert and Breslau, 2008; Casey et al., 2006; Roberts et al., 2007).
It remains unclear as to why formerly LBW children who appear to be typically developing may exhibit an atypical pattern of recovery following a subsequent neurologic injury; however, a recent investigation has suggested a possible mechanism that may explain this pattern. Pitcher et al. (2012) used transcranial magnetic stimulation to investigate mechanisms of neuroplasticity in a group of healthy adolescents who were born preterm. These investigators found evidence for reduced long-term depression (a process thought to be critical to learning and memory formation) in the formerly preterm adolescents and conjectured that their findings were suggestive of reduced neuroplasticity in this group. Although obviously speculative, alterations in the mechanisms of neuroplasticity could affect the long-term recovery pattern of children sustaining a TBI and may provide one potential explanation of the current findings.
Obviously, this case series investigation is limited by a small convenience sample, problems with estimation of premorbid abilities (as in all studies of TBI), and by a restricted range of cognitive measures. The inability to access birth records, the absence of measurements of head circumference and/or head/body growth trajectories, characterization of what, if any, were the additional pre and perinatal complications of the LBW group (e.g., maternal substance abuse, maternal diabetes, presence of other complications of prematurity, etc.), and lack of information regarding any pre-existing brain abnormalities are other significant limitations of the present investigation. In addition, the current study would have been strengthened if children who were born LBW but who had not sustained a TBI were also evaluated and compared to the other two groups. Unfortunately, there were insufficient numbers of age-matched LBW children within the typically developing group employed as a control group in the initial investigation to make this approach feasible. However, because of the relatively stringent exclusion criteria and the generally consistent performance of the groups at baseline, it is likely that none of the participants in either group had significant pre-existing neurologic, motor, sensory, intellectual, or psychiatric issues that would have otherwise skewed the current findings. It is also possible that the current findings reflect a developmental decline in cognitive abilities within the LBW group that would have occurred regardless of these children sustaining a TBI although previous longitudinal research with late pre-term populations does not support this contention (see Gurka et al., 2010; Romeo et al., 2012). Finally, it should be emphasized that the injuries sustained by the NBW group were either the same or worse than those of LBW participants; thereby increasing the likelihood of more negative outcomes within the NBW group.
Future studies would benefit from the addition of functional (e.g., functional magnetic resonance imaging or resting state imaging) and structural (diffusion tensor imaging or structural MRI) imaging technology to track the pattern of recovery in children born LBW who sustain a later neurologic insult. These type of studies could help to clarify if the brains of children born LBW respond in a fundamentally different way to neurologic insult (i.e., if they exhibit different patterns of neuroplasticity following a subsequent injury) or if any differences that emerge in recovery are secondary to preexisting structural anomalies present in the formerly LBW group.
Future studies should also examine other high-risk neonatal populations to assess if these children are at increased risk for long-term cognitive difficulties following other injuries such as shaken baby syndrome, environmental toxicity, or nutritionally related deficits. Although not explicitly examined by the current study, the findings suggest that children born late preterm who sustain even a mild future injury may experience cognitive complications despite apparently intact premorbid skills. If confirmed by additional larger investigations, this finding has importance given the substantial numbers of concussions and other mild head injuries sustained by children and adolescents in the United States.
The trend of the data appear to suggest that children born LBW have a different (i.e., shallower) trajectory of recovery following subsequent neurologic damage many years after their birth. This was observed despite indications that these children were well functioning prior to their injury and being well matched to controls in most cognitive domains at baseline. If supported by future research, these findings would suggest that even relatively larger LBW children may have differences in terms of their overall brain development (especially under circumstances of challenge) when compared to their NBW peers. Although obtained with small numbers, non-optimal control groups, and limited background information, the current results appear to highlight the need for clinicians and researchers to not ignore the long-term implications of pre and perinatal history when describing current functioning or characterizing the impact of recent injuries, and suggest that early pre/perinatal complications may continue to have a pervasive if subtle affect on brain development long after birth.
Acknowledgments
This research was supported by NINDS grant # NS-21889 to HSL. We would also like to appreciate the contributions of Ms. Amanda Barnes and Ms. Sophie Lin for assistance in manuscript preparation.
References
- Anderson VA, Catroppa C, Dudgeon P, Morse SA, Haritou F, Rosenfeld JV. Understanding predictors of functional recovery and outcome 30 months following early childhood head injury. Neuropsychology. 2006;20(1):42–57. doi: 10.1037/0894-4105.20.1.42. [DOI] [PubMed] [Google Scholar]
- Anderson V, Le Brocque R, Iselin G, Eren S, Dob R, Davern TJ, McKinlay L, Kenardy J. Adaptive ability, behavior and quality of life pre and posttraumatic brain injury in childhood. Disability and Rehabilitation. 2012 Mar 15; doi: 10.3109/09638288.2012.656789. 2012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Aylward GP. Neurodevelopmental outcomes of infants born prematurely. Journal of Developmental and Behavioral Pediatrics. 2005;26(6):427–440. doi: 10.1097/00004703-200512000-00008. [DOI] [PubMed] [Google Scholar]
- Bohnert KM, Breslau N. Stability of psychiatric outcomes of low birth weight: a longitudinal investigation. Archives of General Psychiatry. 2008;65(9):1080–1086. doi: 10.1001/archpsyc.65.9.1080. [DOI] [PubMed] [Google Scholar]
- Böhm B, Katz-Salamon M, Institute K, Smedler AC, Lagercrantz H, Forssberg H. Developmental risks and protective factors for influencing cognitive outcome at 5½ years of age in very-low-birthweight children. Developmental Medicine and Child Neurology. 2002;44(8):508–516. doi: 10.1017/s001216220100247x. [DOI] [PubMed] [Google Scholar]
- Breslau N. Does brain dysfunction increase children’s vulnerability to environmental stress? Archives of General Psychiatry. 1990;47(1):15–20. doi: 10.1001/archpsyc.1990.01810130017003. [DOI] [PubMed] [Google Scholar]
- Breslau N, Chilcoat H, DelDotto J, Andreski P, Brown G. Low birth weight and neurocognitive status at six years of age. Biological Psychiatry. 1996;40(5):389–397. doi: 10.1016/0006-3223(95)00399-1. [DOI] [PubMed] [Google Scholar]
- Breslau N, Paneth NS, Lucia VC. The lingering academic deficits of low birth weight children. Pediatrics. 2004;114(4):1035–1040. doi: 10.1542/peds.2004-0069. [DOI] [PubMed] [Google Scholar]
- Burnett AC, Anderson PJ, Cheong J, Doyle LW, Davey CG, Wood SJ. Prevalence of psychiatric diagnoses in preterm and full-term children, adolescents and young adults: a meta-analysis. Psychological Medicine. 2011;20:1–12. doi: 10.1017/S003329171100081X. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Casey PH, Whiteside-Mansell L, Barrett K, Bradley RH, Gargus R. Impact of prenatal and/or postnatal growth problems in low birth weight preterm infants on school-age outcomes: an 8-year longitudinal evaluation. Pediatrics. 2006;118(3):1078–1086. doi: 10.1542/peds.2006-0361. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Anderson V. Recovery in memory function, and its relationship to academic success, at 24 months following pediatric TBI. Child Neuropsychology. 2007;13(3):240–261. doi: 10.1080/09297040600837362. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Anderson VA, Morse SA, Haritou F, Rosenfeld JV. Outcome and predictors of functional recovery 5 years following pediatric traumatic brain injury (TBI) Journal of Pediatric Psychology. 2008;33(7):707–718. doi: 10.1093/jpepsy/jsn006. [DOI] [PubMed] [Google Scholar]
- Cheatham CL, Bauer PJ, Georgieff MK. Predicting individual differences in recall by infants born preterm and full term. Infancy. 2006;10(1):17–42. doi: 10.1207/s15327078in1001_2. [DOI] [PubMed] [Google Scholar]
- Cheatham CL, Sesma HW, Bauer PJ, Georgieff MK. The development of declarative memory in infants born preterm. Advances in Child Development and Behavior. 2010;38:111–135. doi: 10.1016/b978-0-12-374471-5.00005-2. [DOI] [PubMed] [Google Scholar]
- Chilcoat HD, Breslau N. Low birth weight as a vulnerability marker for early drug use. Experimental and Clinical Psychopharmacology. 2002;10(2):104–112. doi: 10.1037//1064-1297.10.2.104. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Worthman C, Erkanli A, Angold A. Prediction from low birth weight to female adolescent depression: a test of competing hypotheses. Archive of General Psychiatry. 2007;64(3):338–344. doi: 10.1001/archpsyc.64.3.338. [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test: Children’s Version. San Antonio, TX: The Psychological Corporation; 1986. [Google Scholar]
- Donders J, Strom D. The effect of traumatic brain injury on children with learning disability. Pediatr Rehabil. 1997;1(3):179–184. doi: 10.3109/17518429709167356. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Landry SH, Kramer L, DeLeon R. Executive functions following traumatic brain injury in young children: a preliminary analysis. Developmental Neuropsychology. 2004;26(1):487–512. doi: 10.1207/s15326942dn2601_7. [DOI] [PubMed] [Google Scholar]
- Fay TB, Yeates KO, Wade SL, Drotar D, Stancin T, Taylor HG. Predicting longitudinal patterns of functional deficits in children with traumatic brain injury. Neuropsychology. 2009;23(3):271–282. doi: 10.1037/a0014936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurka MJ, LoCasale-Crouch J, Blackman JA. Long-term cognition, achievement, socioemotional, and behavioral development of healthy late-preterm infants. Archives of pediatrics & adolescent medicine. 2010;164(6):525. doi: 10.1001/archpediatrics.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, Jordan BD. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57(4):719–726. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- Hanten G, Li X, Newsome MR, Swank P, Chapman SB, Dennis M, Barnes M, Ewing-Cobbs L, Levin HS. Oral reading and expressive language after childhood traumatic brain injury: trajectory and correlates of change over time. Topics in Language Disorders. 2009;29(3):236–248. [Google Scholar]
- Hollingshead AA. Four-factor index of social status. Yale University; New Haven, CT: 1975. Unpublished manuscript. [Google Scholar]
- Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJ. Low birth weights contribute to high rates of early-onset chronic renal failure in the Southeaster United States. Archives of Internal Medicine. 2000;160(10):1472–1476. doi: 10.1001/archinte.160.10.1472. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G. Executive functions after traumatic brain injury in children. Pediatric Neurology. 2005;33(2):79–93. doi: 10.1016/j.pediatrneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Litt J, Taylor HG, Klein N, Hack M. Learning disabilities in children with very low birthweight: prevalence, neuropsychological correlates, and educational interventions. Journal of Learning Disabilities. 2005;38(2):130–141. doi: 10.1177/00222194050380020301. [DOI] [PubMed] [Google Scholar]
- Max JE, Koele SL, Lindgren SD, Robin DA, Smith WL, Jr, Sato Y, Arndt S. Adaptive functioning following traumatic brain injury and orthopedic injury: a controlled study. Archives of Physical Medicine and Rehabilitation. 1998;79(8):893–899. doi: 10.1016/s0003-9993(98)90084-3. [DOI] [PubMed] [Google Scholar]
- Ment LR, Vohr B, Allan W, Katz KH, Schneider KC, Westerveld M, Makuch RW. Change in cognitive function over time in very low-birth-weight infants. JAMA: the journal of the American Medical Association. 2003;289(6):705–711. doi: 10.1001/jama.289.6.705. [DOI] [PubMed] [Google Scholar]
- Mottram L, Donders J. Cluster subtypes on the California verbal learning test-children’s version after pediatric traumatic brain injury. Developmental Neuropsychology. 2006;30(3):865–883. doi: 10.1207/s15326942dn3003_6. [DOI] [PubMed] [Google Scholar]
- Munck P, Haataja L, Maunu R, Parkkola R, Rikalainen H, Lapinleimu H, Lehtonen L, PIPARI Study Group Cognitive outcome at 2 years of age in Finnish infants with very low birth weight born between 2001 and 2006. Acta paediatrica. 2010;99(3):359–366. doi: 10.1111/j.1651-2227.2009.01589.x. [DOI] [PubMed] [Google Scholar]
- Perlman JM. Cognitive and behavioral deficits in premature graduates of intensive care. Clinics in Perinatology. 2002;29(4):779–797. doi: 10.1016/s0095-5108(02)00051-9. [DOI] [PubMed] [Google Scholar]
- Pinto-Martin JA, Whitaker AH, Feldman JF, Van Rossem R, Paneth N. Relation of cranial ultrasound abnormalities in low-birthweight infants to motor or cognitive performance at age 2, 6, and 9 years. Developmental Medicine and Child Neurology. 1999;41(12):826–833. doi: 10.1017/s0012162299001644. [DOI] [PubMed] [Google Scholar]
- Pitcher JB, Riley AM, Doeltgen SH, Kurylowicz L, Rothwell JC, McAllister SM, Ridding MC. Physiological evidence consistent with reduced neuroplasticity in human adolescents born preterm. The Journal of Neuroscience. 2012;32(46):16410–16416. doi: 10.1523/JNEUROSCI.3079-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R, de Ungria M, Sullivan D, Wu P, Wobken JD, Nelson CA, Georgieff MK. Perinatal brain iron deficiency increases the vulnerability of rat hippocampus to hypoxic ischemic insult. The Journal of Nutrition. 1999;129(1):199–206. doi: 10.1093/jn/129.1.199. [DOI] [PubMed] [Google Scholar]
- Rao R, Tkac I, Townsend EL, Ennis K, Gruetter R, Georgieff MK. Perinatal iron deficiency predisposes the developing rat hippocampus to greater injury from mild to moderate hypoxia-ischemia. Journal of Cerebral Blood Flow and Metabolism. 2007;27(4):729–240. doi: 10.1038/sj.jcbfm.9600376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivara FP, Koepsell TD, Wang J, Temkin N, Dorsch A, Vavilala MS, Durbin D, Jaffe KM. Disability at 3, 12 and 24 months after traumatic brain injury among children and adolescents. Pediatrics. 2011;128(5):e1129–1138. doi: 10.1542/peds.2011-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G, Bellinger D, McCormick MC. A cumulative risk factor model for early identification of academic difficulties in premature and low birth weight infants. Maternal and Child Health Journal. 2007;11(2):161–172. doi: 10.1007/s10995-006-0158-z. [DOI] [PubMed] [Google Scholar]
- Roman MJ, Delis DC, Willerman L, Magulac M, Demadura TL, de la Peña JL, Loftis C, Walsh J, Kracun M. Impact of pediatric traumatic brain injury on components of verbal memory. Journal of Clinical and Experimental Neuropsychology. 1998;20(2):245–258. doi: 10.1076/jcen.20.2.245.1168. [DOI] [PubMed] [Google Scholar]
- Romeo DM, Guzzardi S, Ricci D, Cilauro S, Brogna C, Cowan F, Mercuri E. Longitudinal cognitive assessment in healthy late preterm infants. European Journal of Pediatric Neurology. 2012;16(3):243–247. doi: 10.1016/j.ejpn.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Visual short-term memory in the first year of life: capacity and recency effects. Developmental Psychology. 2001;37(4):539–549. doi: 10.1037//0012-1649.37.4.539. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Processing speed in the 1st year of life: a longitudinal study of preterm and full-term infants. Developmental Psychology. 2002;38(6):895–902. doi: 10.1037//0012-1649.38.6.895. [DOI] [PubMed] [Google Scholar]
- Schmidt AT, Hanten GR, Li X, Vasquez AC, Wilde EA, Chapman SB, Levin HS. Decision making after pediatric traumatic brain injury: trajectory of recover and relationship to age and gender. International Journal of Developmental Neuroscience. 2012;30(3):225–230. doi: 10.1016/j.ijdevneu.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. The Clinical Evaluation of Language Fundamentals, Third Edition. San Antonio, TX: The Psychological Corporation; 1995. [Google Scholar]
- Shallice T. Specific impairments of planning. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Sparrow S, Balla D, Cicchetti D. The Vineland Adaptive Behavior Scales: Interview Edition, Survey Form Manual. Circle Pines, MN: American Guidance Services; 1984. [Google Scholar]
- Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, McKee AC. Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM&R: Journal of Injury, Function, and Rehabilitation. 2011;3(10 Suppl 2):S460–S467. doi: 10.1016/j.pmrj.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Klein N, Minich NM, Hack M. Middle-school-age outcomes in children with very low birthweight. Child Development. 2000a;71(6):1495–1511. doi: 10.1111/1467-8624.00242. [DOI] [PubMed] [Google Scholar]
- Taylor GH, Klein N, Minich NM, Hack M. Verbal memory deficits in children with less than 750 g birth weight. Child Neuropsychology. 2000b;6(1):49–63. doi: 10.1076/0929-7049(200003)6:1;1-B;FT049. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Wardlaw TM, editor. Low Birth Weight: Country, Regional and Global Estimates. New York, New York: UNICEF; 2004. [Google Scholar]
- Whitaker A, Johnson J, Sebris S, Pinto J, Wasserman G, Kairam R, Shaffer D, Paneth N. Neonatal cranial ultrasound abnormalities: association with developmental delay at age one in low birth weight infants. Journal of Developmental and Behavioral Pediatrics. 1990;11(5):253–260. [PubMed] [Google Scholar]
- Whitaker AH, Van Rossem R, Feldman JF, Schonfeld IS, Pinto-Martin JA, Tore C, Shaffer D, Paneth N. Psychiatric outcomes in low-birth-weight children at age 6 years: relation to neonatal cranial ultrasound abnormalities. Archives of General Psychiatry. 1997;54(9):847–856. doi: 10.1001/archpsyc.1997.01830210091012. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Mather N. Woodcock-Johnson Tests of Achievement Manual. Allen, TX: DLM Teaching Resources; 1989. [Google Scholar]
- Wüst S, Entringer S, Federenko IS, Schlotz W, Hellhammer DH. Birth weight is associated with salivary cortisol responses to psychosocial stress in adult life. Psychoneuroendocrinology. 2005;30(6):591–598. doi: 10.1016/j.psyneuen.2005.01.008. [DOI] [PubMed] [Google Scholar]


