Abstract
Agaricus blazei Murill is an edible fungus used in traditional medicine, which has various well-documented medicinal properties. In the present study, we investigated the effects of hemicellulase-derived mycelia extract (Agaricus blazei fraction H: ABH) on the immune system. First, we examined the cytokine-inducing activity of ABH on human peripheral mononuclear cells (PBMC). The results indicated that ABH induced expression of IL-12, a cytokine known to be a critical regulator of cellular immune responses. Flow cytometric analysis demonstrated the induction of IL-12 production by the CD14-positive cell population, consisting of monocytes/macrophages (Mo/Mφ). Furthermore, the elimination of Mo/Mφ attenuated IL-12 production in PBMC. ABH-induced IL-12 production was inhibited by anti-CD14 and anti-TLR4 antibodies but not by anti-TLR2 antibody. The activity of ABH was not inhibited by polymyxin B, while the activity of lipopolysaccharide used as a reference was inhibited. Oral administration of ABH enhanced natural killer (NK) activity in the spleen. These findings suggest that ABH activated Mo/Mφ in a manner dependent on CD14/TLR4 and NK activity.
Keywords: Agaricus blazei Murill; IL-12, Toll-like Receptor; Monocyte/Macrophage
Introduction
Agaricus blazei Murill, an edible mushroom, shows immunomodulatory and antitumor activities (1–5). In Brazil, this fungus is used as a traditional medicine for the prevention of cancer, diabetes, hyperlipidemia, arteriosclerosis and chronic hepatitis. The polysaccharides, proteoglycans, polysaccharide–protein complexes, glycoproteins and steroids from Agaricus blazei Murill mycelia and fruiting body extracts possess antitumor cytotoxic activities (3–10). However, the mechanisms of the antitumor functions of these mushroom components have yet to be determined. The bacterial lipopolysaccharide, mycoplasma lipopeptide (MALP-2), interacts with toll-like receptors (TLRs) and induces cytokine production. Similarly, Dectin-1, a fungal β-glucan receptor subunit, has been shown to interact with TLR2 and mediate cell activation (11,12). Agaricus blazei extract was shown to induce cytokine (IL-12) gene expression. Based on these observations, we hypothesized that Agaricus blazei Murill fraction H (ABH) may interact with TLRs and induce signals for IL-12 production. The specific objectives of this investigation were: (i) to identify the characteristics of the cell population in peripheral blood mononuclear cells (PBMC) stimulated by ABH; (ii) to determine the optimal concentration of ABH required to induce cytokine production in vitro; (iii) to identify the nature of TLR interacting with ABH on the cell surface; and (iv) to evaluate the influence of ABH on natural killer (NK) cell activity.
Subjects and Methods
Preparation of A.blazei Extracts
After 2 weeks in culture, A.blazei mycelia were digested with hemicellulase for 1 h at 45°C. Then, the enzymes were inactivated at 70°C and freeze-dried. This compound is similar to Agaricus Blazei Practical Compound (ABPC; Japan Applied Microbiology Research Institute. Ltd, Yamanashi, Japan) and all ABPC products were made from this compound. Samples of 1.5 g of A.blazei compound were ground and mixed with distilled water to a final concentration of 0.1 g/ml (w/v). After centrifugation, the supernatant was collected and passed through a 0.45 μm filter (Millipore Co., Bedford, MA) for use in the experiments.
Preparation of PBMCs
Heparinized human peripheral blood was obtained from healthy donors. PBMCs were isolated using the Ficoll-Hypaque density-gradient method, as described previously (13). Peripheral blood was centrifuged at 2000 r.p.m. for 10 min to remove plasma. Blood cells were diluted with phosphate buffered saline (PBS), then overlaid onto Ficoll-Hypaque solution (Amersham Pharmacia Biotech UK Ltd., Buckinghamshire, UK) and centrifuged at 2000 r.p.m. for 30 min. The PBMC layers were collected and washed twice with PBS. The cells were resuspended to a concentration of 1 × 106 cells/ml in RPMI-1640 medium supplemented with 10% foetal calf serum, 100 U/ml penicillin and 100 μg/ml streptomycin.
Reagents and Monoclonal Antibodies (mAb)
Anti-human (h) TLR4 mAb HTA125 (mouse IgG2a) and FITC-labelled HTA125 were purchased from BD Biosciences Clontech (San Jose, CA) and eBioscience (San Diego, CA). Anti-TLR2 mAb TL2.1 (mouse IgG2a) and FITC-labelled TL2.1 were purchased from eBioscience and Cascade Bioscience (Winchester, MA).
RNA Preparation Quantification and RNase Protection Assay
After stimulation of human PBMC, total RNA was extracted from the cells using TRIzol (Invitrogen Corp., Carlsbad, CA). RNase protection assay was performed using the hCK-2RiboQuant Multiprobe RNase Protection Assay system (BD Pharmingen, San Jose, CA) according to the manufacturer's instructions. Aliquots of 10 μg of RNA were used for each assay. Gels from three distinct experiments were analyzed using a BAS3000 and Image Gage (Fuji Film, Tokyo, Japan).
Quantification of IL-12p40 and p70 in Culture Supernatants
Isolated human PBMC were cultured in media containing ABH, 1 ml of 10% FCS/RPMI1640 with various concentrations of ABH, for 0–16 h. The levels of IL-12p40 in the supernatants were measured using an enzyme-linked immunosorbent assay (ELISA) kit, optEIA™ ELISA kit (BD Pharmingen) according to the manufacturer's protocol. The concentration of IL-12p40 and p70 was determined using the data analysis program Softmax (Molecular Devices, Menlo Park, CA).
NK Activity Assay
NK activities of human PBMC and murine spleen cells were determined by lactate dehydrogenase (LDH) assay. The NK-sensitive mouse cell line YAC-1 was used as target cells. Target and effector cells were mixed at the indicated effector/target (E/T) ratios at 0.2 ml/well using 96-well round-bottomed multi-well plates (BD Labware, Lincoln Park, NJ). After incubation for 4 h, cells were centrifuged at 250 g for 4 min, and then the cell-free supernatant was collected for LDH assay using CytoTox96 (Promega, Madison, WI). The percentage of specific LDH release was calculated by the following formula: % cytotoxicity = [(experimental LDH release) − (spontaneous LDH release by effector and target)/(maximum LDH release) × (spontaneous LDH release)] × 100. For the control experiments, the target cells were incubated either in culture medium alone to determine spontaneous release or in a mixture of 2% Triton X-100 to define the maximum LDH release. The spontaneous release was always <10% of the maximum release. All assays were performed in triplicate.
Results
Treatment of PBMC with ABH induced IL-12 Production
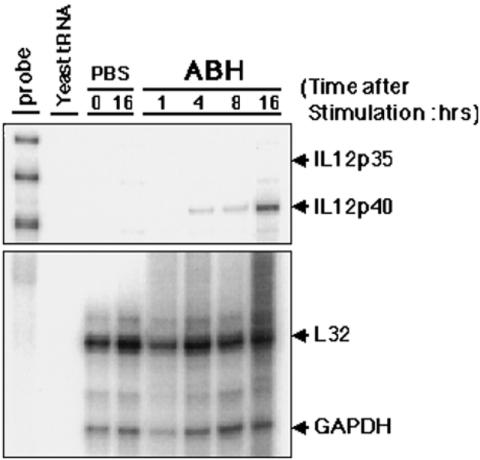
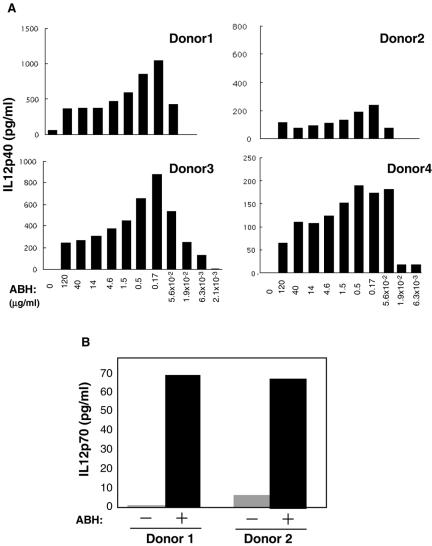
Expression of IL-12 messenger RNA (both p35 and p40 subunits) was determined in PBMC with or without ABH stimulation by RNase protection assay. As shown in Figure 1, IL-12 messenger RNA expression (p40 subunit) was induced within 4 h after ABH stimulation. In unstimulated controls, IL-12 messenger RNA was not induced until 16 h. We quantified the release of IL-12 p40 protein in human PBMC culture supernatants in relation to ABH dose. As shown in Figure 2A, IL-12 p40 was induced significantly in all donors. The levels of IL-12 production were reduced at concentrations of ABH in excess of 170 ng/ml. Figure 2B shows induction of IL-12 p70 (heterodimer of p40 and p35) by ABH. These observations suggest that ABH induces IL-12 production by PMBC.
Figure 1.
ABH induces IL-12 gene expression. Ficoll-isolated human PBMCs were stimulated with 40 ng/ml ABH for the indicated times. RNase protection assay was performed with total RNA from stimulated PBMC. Aliquots of 10 μg of RNA were used for each determination. Lane 1, free probe; lane 2, yeast tRNA; lanes 3 and 4, unstimulated cells (–); lanes 5–8, cells stimulated with Agaricus blazei extract for the times indicated at the top of the panel. The upper panel shows IL-12p35 and p40 mRNA, and the lower panel shows L32 and GAPDH as a control. Data shown are representative of three independent experiments.
Figure 2.
ABH induces IL-12p40 and p70. (A) Ficoll-isolated human PBMC (1 × 106/sample) were stimulated with ABH at various concentrations as indicated at the bottom of the panels. After incubation for 16 h, supernatants were collected and the amounts of IL-12 p40 were determined. (B) PBMCs were stimulated with 0.17 ng/ml ABH for 16 h. The supernatant was collected and the amounts of IL-12 p70 were determined. (A and B) IL-12 p40 and p70 in culture supernatants were measured by ELISA. Data of IL-12 p40 and p70 shown are from four and two different donors, respectively.
CD14-positive Cells Produced IL-12 on Stimulation with ABH
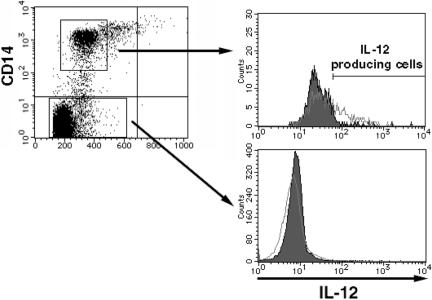
To determine the target population of ABH, we analyzed IL-12-production levels of each population by intracellular cytokine staining method and flow cytometry. After 16-hour culture, only CD14 positive population was induced IL-12p40 (Figure 3). Neither normal media (control) nor peripheral dendritic cells (lin-/CD11c and HLA-DR+) induced production of IL-12 p40 protein (data not shown).
Figure 3.
CD14-positive cells showed induction of IL-12. Human PBMC was stimulated with 0.17 ng/ml ABH for 16 h. IL-12 production was quantified by flow cytometry (right panels). The flow cytometric data in the right panels represent three gated populations of CD14-positive and negative cells. Results of ABH stimulated and unstimulated samples shown as open and closed histograms respectively. The region indicated by the bar represents IL-12-producing cells (shown in left panels). Data shown are representative of three independent experiments.
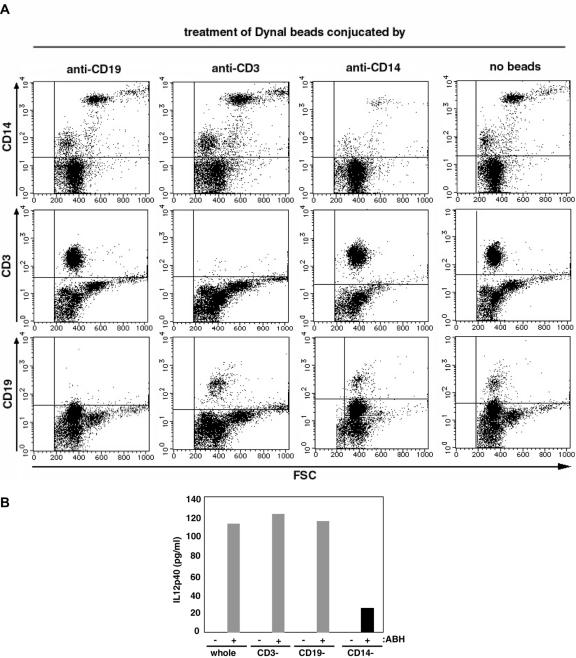
We then added anti-CD3/CD19 and CD14 monoclonal antibody-conjugated Dynal beads to Ficoll PBMC fractions to deplete each fractions. IL-12 production was observed in PBMC from which CD3 and CD19 were depleted.
However, depletion of CD14 caused a 20% reduction in the level of IL-12p40 production (Figure 4).
Figure 4.
Depletion of CD14-positive cells reduced IL-12 production. (A) CD14-, CD3- or CD19-positive cells were depleted using beads conjugated with monoclonal antibodies specific for each surface marker, as indicated at the top of the panels. After depletion, CD14-, CD3- and CD19-positive cells were quantified by flow cytometry. (B) After treatment, PBMCs were stimulated with 0.17 ng/ml ABH for 16 h. Supernatants were examined by ELISA. Data shown are representative of three independent experiments.
Involvement of CD14 and TLR4 in IL-12 Induction by ABH
Human TLRs have been shown to transduce intracellular signals by some mycobacterial ligands. These reports suggest that TLRs initiate human innate immune responses and pattern recognition by TLRs regulates the nature of not only innate but also adaptive immune responses (14–16).
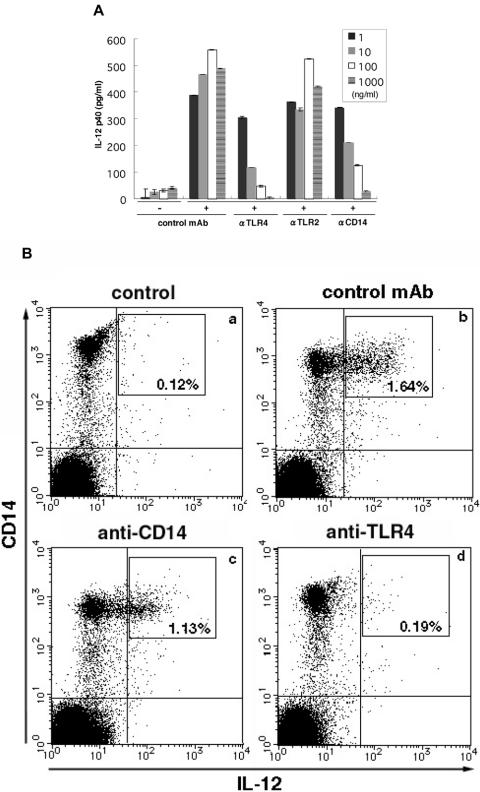
Using neutralising antibodies (anti-hTLR4:HTA125, anti-hTLR2:TL2.1 and anti-CD14), we examined whether TLR2, TLR4 and CD14 are involved in the induction of IL-12 expression by ABPC. Preincubation of PBMC with anti-hTLR4 mAb (HTA125) or anti-CD14 mAb (61D3) clearly inhibited IL-12 production in a dose-dependent manner. PBMC from three different donors were used, and the same results were observed in all cases. In contrast, pre-treatment with anti-hTLR2 (TL2.1) showed no effect (Fig. 5A).
Figure 5.
Anti-CD14 and anti-TLR4 monoclonal antibodies neutralized the IL-12-inducing effect of ABH. (A) PBMCs were pre-incubated with anti-CD14, anti-TLR4, anti-TLR2 or control monoclonal antibody at four different doses (1000, 100, 10, 1 ng). Agaricus blazei extract was added after 4 h and incubation was continued for 16 h. IL-12 p40 in supernatants was quantified by ELISA. Data from three independent donors are shown. (B) Cells pre-treated with 1 μg of control monoclonal antibody (b), 1 μg of anti-CD14 antibody (c) or 1 μg of anti-TLR4 antibody (d) were stimulated with ABH for 16 h (b–d). Then, IL-12-producing cells were quantified by flow cytometry. Unstimulated PBMCs (a) were included as controls. Data shown are representative of three independent experiments.
Furthermore, flow cytometry was performed to quantify the IL-12-producing cells. In PBMC pre-treated with anti-hTLR4 and anti-hCD14 monoclonal antibodies, the levels of IL-12 production by CD14-positive cells were reduced to 1.33% and to 0.19% in samples treated with anti-hTLR4 and anti-hCD14 mAb, respectively. No effect was observed in samples treated with control antibody. There were no differences in the ratio of CD14-positive cells in PBMC before and after antibody treatment (Fig. 5B). These results suggested that anti-hTLR4 and anti-hCD14 mAbs blocked signal transduction via TLR4 and CD14.
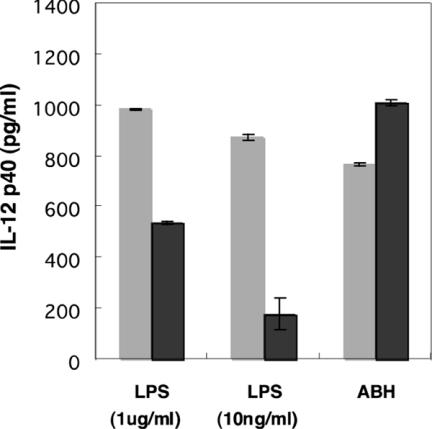
Induction of IL-12 by ABH was Not Inhibited by Polymyxin B
CD14 and TLR4 have been reported as subunits of the lipopolysaccharide (LPS) receptor complex. The CD14-TLR4-MD2 heterotrimer recognises the LPS/LBP-complex (17–20). Our results indicated that CD14 and TLR4 were essential components for induction of IL-12 by ABH. To exclude the possibility that cellular activation by ABH was a result of endotoxin contamination in the extracts, the abilities of ABH and reference LPS to induce IL-12 production by human PBMC were examined in the presence and absence of polymyxin B. Polymyxin B showed little effect on IL-12 induction by ABH, whereas that of LPS was inhibited completely (Fig. 6). We measured the amount of endotoxin in the ABH extract using an Endospecy kit for endotoxin quantification (Seikagaku Corp., Tokyo, Japan). The ABH extract used in the present study was essentially free from endotoxin contamination (data not shown).
Figure 6.
Influence of polymyxin B on IL-12-inducing activity of ABH. ABH (0.17 ng/ml) and reference LPS (10 ng/ml) were pre-treated with 5 μg of polymyxin B for 30 min, and then used to stimulate PBMC for 16 h. The IL-12 secreted into the supernatant was measured by ELISA.
ABH Extract Enhanced NK Activity
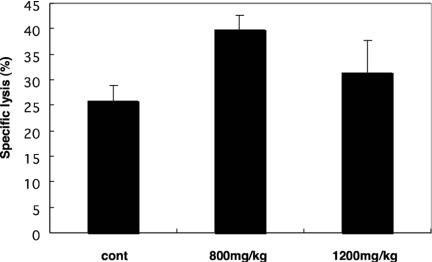
IL-12 is a critical factor in immune responses against pathogens and tumors as it is the most potent promoter of type 1 responses in CD4 T cells (Th1 responses). Th1 responses regulate proliferation, interferon-γ (IFN-γ) production and cytotoxic activities by T cells and natural killer cells (21–23). To evaluate its effects in vivo, we examined the effect of oral administration of ABH on NK cytotoxic activity of murine spleen cells.
ABH-treated mice showed significantly higher levels of NK cytotoxic activity than controls; the ABH-treated group showed 40% cytolysis, while the control group showed 25% cytolysis (Fig. 7). This result suggested that oral administration of ABH can induce NK activity in vivo.
Figure 7.
Oral administration of ABH enhanced NK cytotoxic activity in the spleen. The indicated amounts of ABH were administered for 21 consecutive days. Control mice were administered water. NK activity of spleen cells was quantified using CytoTox96 reagent (Promega). Results are expressed as means ± SEM, n = 6.
Discussion
Hemicellulase-digested A.blazei extract showed a very high degree of water solubility. Almost 95% by dry weight was extracted in water, in contrast to non-digested samples, which showed only about 20% extraction (data not shown). Various methods were employed to extract the components from A.blazei, including treatment with acid and organic solvent. These methods for extraction were not suitable for evaluation and analysis of total activity of all components of A.blazei. The high degree of water solubility obtained by hemicellulase digestion is a major advantage for analysis of the effects of Agaricus. In the present study, we were able to analyze the total effect of Agaricus extract as we could extract almost all of the components.
As the first step to determine the effects of ABH, we attempted to identify inducible or suppressible cytokines. We found the IL-12-inducing activity in the water extract. The optimum concentration of ABH for induction of IL-12 was from 170 to 510 ng/ml, and higher concentrations showed lower levels of induction.
Agaricus blazei extract was reported previously to show a suppressive effect against proliferation and cytokine expression using PBMC (24). The effects of elements with the ability to suppress cell activity may appear at high doses. Suppressive elements did not have sufficient effects to suppress the induction of IL-12 to <170 ng/ml.
The results of intracellular cytokine staining and population-specific elimination showed CD14 positive cells, monocytes/ macrophages (Mo/Mφ) to be the target population of ABH. We detected no IL-12 production by peripheral dendritic cells (DC). Peripheral circulating Mo circulate for 1–3 days before entering tissues, where they differentiate into mature resident Mφ or DCs (25). Our results suggested that ABH possessed the potential to activate immature Mo and promote the Th1 response in tissue. As a result of this activity, ABH maintains the Th1 response level and cellular immunity. ABH-treated mice have been reported to show higher levels of NK activity in the spleen than untreated controls. The ability of DCs to produce cytokines differs among DC subsets depending on the tissue to which they belong (26,27). Peripheral Mo heterogeneity was reported previously (28–30). Thus, we speculate that ABH may have different effects on different subsets of Mo and DC. Therefore, the effects of ABH should be analyzed from various viewpoints.
Various models to explain the mechanism of the immunomodulatory effects of many mycobacterial extracts and components have been reported. These include cell activation by intracellular signals transduced by TLRs. The results of the present study indicate that the activity of ABH to induce IL-12 production is dependent on CD14 and TLR4 (Fig. 5). LPS causes activation of Mo/Mφ via the CD14/TLR4/MD2 receptor complex (31). The Limulus activity of ABH was 80 pg/ml, equivalent to E.coli LPS (data not shown). Therefore, the endotoxin content in the ABH used in the present study was <1 pg/ml, which was completely inactive in our system. On the other hand, polymyxin B-treated ABH also induced IL-12 production at almost the same level as untreated ABH (Fig. 6). This excluded the possibility that the activation of Mo/Mφ was due to response to contamination of the ABH extract by LPS.
TLR2 was also reported to be a signal transducer for components of fungi (11,12,32). The anti-TLR2 mAb TL2.1 did not inhibit induction of IL-12 production by ABH. Agaricus blazei is rich in polysaccharides that can stimulate immune cells. One of these, β-1,6-D-glucan, has been reported to be the main component responsible for this activity. Ohno et al. demonstrated that the functional centre of β-1,6-D-glucan is a region rich in β-1,3 links (33–35). β-Glucans activate cells via TLR2 (32). The mannan fraction from Candida albicans and Saccharomyces cerevisiae induced TNF-α production in a manner dependent on CD14 and TLR4 (36). TLR4 is also involved in pattern recognition of soluble branched β-(1,4)-glucans from Acetobacter AC-1 (37).
We speculate on two possible explanations for this observation: β-Glucan in ABH may be recognised by a different receptor complex, or other components, such as mannan, may be mainly responsible for the IL-12-inducing activity of ABH. As ABH is a mixture of various components, it is necessary to identify and isolate the elements responsible for the action of ABH.
Some studies of the anti-tumor effects of A.blazei have demonstrated direct cytotoxic effects (1). The chloroform/ methanol extracts of A.blazei were shown to have antitumor activity against solid tumors. Ergosterol in this extract inhibits angiogenesis and causes the death of tumor cells by preventing neovascularisation (5). When the particles from fruiting bodies of A.blazei Murill (ABP-F) were reacted with human serum, the formation of complement-opsonised ABP, iC3b-ABP-F complexes, and binding of the complexes to human PBMCs were observed. In addition, PBMCs incubated in the presence of iC3b-ABP-F complexes inhibited the proliferation of a human tumor cell line in vitro (38).
These reports indicated that components of A.blazei have various activities. It was reported that A.blazei extract acts mainly through modulation of the immune system, activating macrophages, neutrophils and lymphocytes (3,4,8). On the other hand, ethanol extracts of A.blazei mycelia were shown to reduce the cytopathic effects of the western equine encephalitis (WEE) virus (39). Several fungi incubated with immune cells showed activation of some cells, especially antigen-presenting cells, and induced cytokine production (40–42,44–48). The ethanol extracts of A.blazei stimulated macrophages and induced expression of the cytokines IL-8 and TNF (49).
This report indicates that the extract of A.blazei mycelia induces IL-12 production. IL-12 exerts multiple biological activities, which include activation of CD8+ CTLs, differentiation of CD4+ T lymphocytes, induction of nitorogen oxide production by macrophages, induction of type 1 responses by helper T lymphocytes and NK cell activation (50,51). IL-12 has also been shown to possess potent antitumor activity in a wide variety of murine tumor models (49,52–55). Recent reports have demonstrated that the in vivo antitumor capacity of IL-12 is mediated via NK and/or NKT cells (56,57). The findings of the present study will help in furthering our understanding of the immunomodulatory and anti-viral effects of A.blazei. IL-12 promotes and sustains the cellular immune system. Oral administration of ABH was shown to enhance the NK activity of mouse spleen cells (Fig. 7). It is notable that ABH modulates immune responses not only in vitro but also in vivo. In the context of A.blazei administration as a functional food, the induction of IL-12 and enhancement of cellular immunity are important for various biological activities.
In conclusion, we have demonstrated that hemicellulase-derived ABH induces IL-12 production by human peritoneal monocytes via TLR4-CD14 and enhances cytotoxic activity against tumor cells in vivo. These responses may be involved in the biological activities of ABH, such as its anti-tumor, anti-infection and anti-allergic effects. ABH is a non-proliferative and non-pathogenic microbial component that can trigger an immune response that is useful in the fight against infectious diseases and cancer. The accumulation of such data concerning the effects of components and clarification of mechanisms of their action are essential to understand features of mushroom such as the biological response modifier (BRM).
References
- 1.Itoh H, Ito H, Amano H, Noda H. Inhibitory action of action of a (1–6)-β-D-glucan-protein complex (F III-2-b) isolated from Agaricus blazei Murill (‘Himematsutake’) on Meth A fibrosarcoma-bearing mice and its antitumor mechanism. Jpn J Pharmacol. 1994;66:265–271. doi: 10.1254/jjp.66.265. [DOI] [PubMed] [Google Scholar]
- 2.Osaki Y, Kato T, Yamamoto K, Okubo J, Miyazaki T. Antimutagenic and bactericidal substances in fruit body of a basidiomycete Agaricus blazei. Yakugaku Zasshi. 1994;114:342–350. doi: 10.1248/yakushi1947.114.5_342. [DOI] [PubMed] [Google Scholar]
- 3.Fujimiya Y, Suzuki Y, Oshiman K, et al. Selective tumoricidal effect of soluble proteoglucan extracted from the basidiomycete, Agaricus blazei Murill, mediated via natural killer cell activation and apoptosis. Cancer Immunol Immunother. 1998;46:147–159. doi: 10.1007/s002620050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujimiya Y, Suzuki Y, Katakura R, Ebina T. Tumor-specific cytocidal and immunopotentiating effects of relatively low molecular weight products derived from the basidiomycete Agaricus blazei Murill. Anticancer Res. 1999;19:119–128. [PubMed] [Google Scholar]
- 5.Takaku T, Kimura Y, Okuda H. Isolation of an antitumor compound from Agaricus blazei Murill and its mechanism of action. J Nutr. 2001;131:1409–1413. doi: 10.1093/jn/131.5.1409. [DOI] [PubMed] [Google Scholar]
- 6.Kawagishi H, Inagaki R, Kanao T, Mizuno T, Shimura K, Itoh H, et al. fractionation an antitumor activity of the water-insoluble residue of Agaricus blazei fruiting bodies. Carbohydrate Res. 1989;186:267–273. doi: 10.1016/0008-6215(89)84040-6. [DOI] [PubMed] [Google Scholar]
- 7.Ito H, Shimura K, Itoh H, Kawade M. Antitumor effects of a new polysaccharide-protein complex (ATOM) prepare from Agaricus blazei (Iwade Strain 101) ‘Himematsutake’ and its mechanisms in tumor-bearing mice. Anticancer Res. 1997;17:277–284. [PubMed] [Google Scholar]
- 8.Mizuno M, Morimoto M, Minato K, Tsuchida H. Polysaccharides from Agaricus blazei stimulate lymphocyte T-cell subsets in mice. Biosci Biotech Biochem. 1998;63:434–437. doi: 10.1271/bbb.62.434. [DOI] [PubMed] [Google Scholar]
- 9.Mizuno T, Hasegawa T, Nakamura T, et al. Antitumor activity and some properties of water-soluble polysaccharides from Himematsutake, the fruiting body of Agaricus blazei Murill. Agric Biol Chem. 1990;54:2889–2896. [Google Scholar]
- 10.Kawagishi H, Katsumi R, Sazawa T, Mizuno T, Hagiwara T, Nakamura T. Cytotoxic steroid from the mashroom Agaricus blazei. Phytochemistry. 1988;27:2777–2779. [Google Scholar]
- 11.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He L, Terunuma H, Hanabusa H, et al. Interleukin 18 and interleukin 1beta production is decreased in HIV type 1-seropositive hemophiliacs but not in HIV type 1-seropositive nonhemophiliacs. AIDS Res Hum Retroviruses. 2000;16:345–353. doi: 10.1089/088922200309223. [DOI] [PubMed] [Google Scholar]
- 14.Miyake K. Innate recognition of lipopolysaccharide by CD14 and toll-like receptor 4-MD-2: unique roles for MD-2. Int Immunopharmacol. 2003;3:119–128. doi: 10.1016/s1567-5769(02)00258-8. [DOI] [PubMed] [Google Scholar]
- 15.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 16.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 17.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 18.Kirschning CJ, Wesche H, Merrill Ayres T, Rothe M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 20.Qureshi ST, Lariviere L, Leveque G, et al. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 22.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 23.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 24.Kuo YC, Huang YL, Chen CC, Lin YS, Chuang KA, Tsai WJ. Cell cycle progression and cytokine gene expression of human peripheral blood mononuclear cells modulated by Agaricus blazei. J Lab Clin Med. 2002;140:176–187. doi: 10.1067/mlc.2002.126717. [DOI] [PubMed] [Google Scholar]
- 25.Volkman A, Gowans JL. The origin of macrophages from bone marrow in the rat. Br J Exp Pathol. 1965;46:62–70. [PMC free article] [PubMed] [Google Scholar]
- 26.Liu YJ, Kanzler H, Soumelis V, Gilliet M. Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol. 2001;2:585–589. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- 27.Weiner HL. The mucosal milieu creates tolerogenic dendritic cells and T(R)1 and T(H)3 regulatory cells. Nat Immunol. 2001;2:671–672. doi: 10.1038/90604. [DOI] [PubMed] [Google Scholar]
- 28.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- 29.Weber C, Belge KU, von Hundelshausen P, et al. Differential chemokine receptor expression and function in human monocyte subpopulations. J Leukoc Biol. 2000;67:699–704. doi: 10.1002/jlb.67.5.699. [DOI] [PubMed] [Google Scholar]
- 30.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 31.Schletter J, Heine H, Ulmer AJ, Rietschel ET. Molecular mechanisms of endotoxin activity. Arch Microbiol. 1995;164:383–389. doi: 10.1007/BF02529735. [DOI] [PubMed] [Google Scholar]
- 32.Ozinsky A, Underhill DM, Fontenot JD, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohno N, Furukawa M, Miura NN, Adachi Y, Motoi M, Yadomae T. Antitumor beta glucan from the cultured fruit body of Agaricus blazei. Biol Pharm Bull. 200;24:820–828. doi: 10.1248/bpb.24.820. [DOI] [PubMed] [Google Scholar]
- 34.Oshiman K, Fujimiya Y, Ebina T, Suzuki I, Noji M. Orally administered beta-1,6-D-polyglucose extracted from Agaricus blazei results in tumor regression in tumor-bearing mice. Planta Med. 2002;68:610–614. doi: 10.1055/s-2002-32904. [DOI] [PubMed] [Google Scholar]
- 35.Dong Q, Yao J, Yang XT, Fang JN. Structural characterization of a water-soluble beta-D-glucan from fruiting bodies of Agaricus blazei Murr. Carbohydr Res. 2002;337:1417–1421. doi: 10.1016/s0008-6215(02)00166-0. [DOI] [PubMed] [Google Scholar]
- 36.Tada H, Nemoto E, Shimauchi H, et al. Saccharomyces cerevisiae- and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiol Immunol. 2002;46:503–512. doi: 10.1111/j.1348-0421.2002.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 37.Saito K, Yajima T, Nishimura H, et al. Soluble branched beta-(1,4)glucans from Acetobacter species show strong activities to induce interleukin-12 in vitro and inhibit T-helper 2 cellular response with immunoglobulin E production in vivo. J Biol Chem. 2003;278:38571–38578. doi: 10.1074/jbc.M304948200. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu S, Kitada H, Yokota H, et al. Activation of the alternative complement pathway by Agaricus blazei Murill. Phytomedicine. 2002;9:536–545. doi: 10.1078/09447110260573047. [DOI] [PubMed] [Google Scholar]
- 39.Sorimachi K, Ikehara Y, Maezato G, et al. Inhibition by Agaricus blazei Murill fractions of cytopathic effect induced by western equine encephalitis (WEE) virus on VERO cells in vitro. Biosci Biotechnol Biochem. 2001;65:1645–1647. doi: 10.1271/bbb.65.1645. [DOI] [PubMed] [Google Scholar]
- 40.Johansson C, Eshaghi H, Linder MT, Jakobson E, Scheynius A. Positive atopy patch test reaction to Malassezia furfur in atopic dermatitis correlates with a T helper 2-like peripheral blood mononuclear cells response. J Invest Dermatol. 2002;118:1044–1051. doi: 10.1046/j.1523-1747.2002.01758.x. [DOI] [PubMed] [Google Scholar]
- 41.Buentke E, Heffler LC, Wallin RP, Lofman C, Ljunggren HG, Scheynius A. The allergenic yeast Malassezia furfur induces maturation of human dendritic cells. Clin Exp Allergy. 2001;31:1583–1593. doi: 10.1046/j.1365-2222.2001.01199.x. [DOI] [PubMed] [Google Scholar]
- 42.d'Ostiani CF, Del Sero G, Bacci A, et al. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J Exp Med. 2000;191:1661–1674. doi: 10.1084/jem.191.10.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reis e Sousa C, Stahl PD, Austyn JM. Phagocytosis of antigens by Langerhans cells in vitro. J Exp Med. 1993;178:509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stubbs AC, Martin KS, Coeshott C, et al. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat Med. 2001;7:625–629. doi: 10.1038/87974. [DOI] [PubMed] [Google Scholar]
- 45.Boyer A, Andreu G, Romet-Lemonne JL, Fridman WH, Teillaud JL. Generation of phagocytic MAK and MAC-DC for therapeutic use: characterization and in vitro functional properties. Exp Hematol. 1999;27:751–761. doi: 10.1016/s0301-472x(98)00070-8. [DOI] [PubMed] [Google Scholar]
- 46.Bozza S, Gaziano R, Spreca A, et al. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J Immunol. 2002;168:1362–1371. doi: 10.4049/jimmunol.168.3.1362. [DOI] [PubMed] [Google Scholar]
- 47.Grazziutti M, Przepiorka D, Rex JH, Braunschweig I, Vadhan-Raj S, Savary CA. Dendritic cell-mediated stimulation of the in vitro lymphocyte response to Aspergillus. Bone Marrow Transplant. 2001;27:647–652. doi: 10.1038/sj.bmt.1702832. [DOI] [PubMed] [Google Scholar]
- 48.Sorimachi K, Akimoto K, Ikehara Y, Inafuku K, Okubo A, Yamazaki S. Secretion of TNF-alpha, IL-8 and nitric oxide by macrophages activated with Agaricus blazei Murill fractions in vitro. Cell Struct Funct. 2001;26:103–108. doi: 10.1247/csf.26.103. [DOI] [PubMed] [Google Scholar]
- 49.Brunda MJ, Luistor L, Warrier RR, et al. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178:1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adoptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 51.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-15, IL-12, IFN-{gamma}) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 52.Brunda MJ. Interleukin-12. J Leukocyte Biol. 1994;55:280–288. doi: 10.1002/jlb.55.2.280. [DOI] [PubMed] [Google Scholar]
- 53.Zou JP, Yamamoto N, Fuki T, et al. Systemic administration of rIL-12 induces complete tumor regression and protective immunity: response is correlated with a striking reversal of suppressed IFN-{gamma} production by antitumor T cells. Int Immunol. 1995;7:1135–1145. doi: 10.1093/intimm/7.7.1135. [DOI] [PubMed] [Google Scholar]
- 54.Robertson MJ, Ritz J. Interleukin 12: basic biology and potential applications in cancer treatment. Oncologist. 1996;1:88–97. [PubMed] [Google Scholar]
- 55.Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-{gamma} production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 56.Smyth MJ, Thia KY, Street SE, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smyth MJ, Taniguchi M, Street SE. The antitumor activity of IL-12: mechanisms of innate immunity that are dose and model dependent. J Immunol. 2000;165:2665–2670. doi: 10.4049/jimmunol.165.5.2665. [DOI] [PubMed] [Google Scholar]