Abstract
Background and Objective
Colorectal cancer remains the second deadliest cancer in the United States. Several screening methods exist, however detection of small polyps remains a challenge. Optical coherence tomography has been demonstrated to be capable of detecting lesions as small as 1 mm in the mouse colon, but detection is based on measuring a doubling of the mucosa thickness. The colon microvasculature may be an attractive biomarker of early tumor development because tumor vessels are characterized by irregular structure and dysfunction. Our goal was to develop an endoscopic method of detecting and segmenting colon vessels using Doppler optical coherence tomography to enable future studies for improving early detection and development of novel chemopreventive agents.
Method
We conducted in vivo colon imaging in an azoxymethane (AOM)-treated mouse model of colorectal cancer using a miniature endoscope and a swept-source OCT system at 1040 nm with a 16 kHz sweep rate. We applied the Kasai autocorrelation algorithm to laterally oversampled OCT B-scans to resolve vascular flow in the mucosa and submucosa. Vessels were segmented by applying a series of image processing steps: (1) intensity thresholding, (2) two-dimensional matched filtering, and (3) histogram segmentation.
Results
We observed differences in the vessels sizes and spatial distribution in a mature adenoma compared to surrounding undiseased tissue and compared the results with histology. We also imaged flow in four young mice (2 AOM-treated and 2 control) showing no significant differences, which is expected so early after carcinogen exposure. We also present flow images of adenoma in a living mouse and a euthanized mouse to demonstrate that no flow is detected after euthanasia.
Conclusion
We present, to the best of our knowledge, the first Doppler OCT images of in vivo mouse colon collected with a fiber-based endoscope. We also describe a fast and robust image processing method for segmenting vessels in the colon. These results suggest that Doppler OCT is a promising imaging modality for vascular imaging in the colon that requires no exogenous contrast agents.
Keywords: colorectal cancer, adenoma, image processing, vascular imaging
1. Introduction
Colorectal cancer (CRC) is the second deadliest cancer in the United States, with nearly 50,000 estimated deaths in the United States in 2015.(1) Despite the high prevalence of CRC, the mortality rate has been steadily declining over the last four decades owing to the efficacy of many screening methods. When detected at the local stage, the 5-year survival rate of CRC is 90%. However, this rate drops to just 13% at the distant stage.(1) This exemplifies the importance of early detection. With an overall five-year survival rate of 65%, there is still much need for improved screening methods.
The recommended CRC screening methods can be broadly divided into two types of tests: stool tests and imaging procedures. The stool tests require little preparation by the patient, are non-invasive, and sometimes do not require an office visit. The fecal occult blood test and fecal immunochemical test have high sensitivity and low specificity or vice versa.(2) Stool DNA testing can achieve an area under the receiver operating characteristic (ROC) curve of over 90% given the right combination of tests.(3) Stool DNA testing is particularly convenient because the patient can send samples by mail. However, despite the accuracy and convenience of these tests, they can only detect cancer, not localize the lesions. A positive result still requires the patient to undergo an imaging procedure.
Imaging screening methods, such as flexible sigmoidoscopy, colonoscopy, and virtual (CT) colonoscopy are capable of detecting CRC and localizing lesions, but these methods are more invasive than fecal tests. Virtual colonoscopy is the least invasive imaging modality with similar sensitivity as colonoscopy, but polyps cannot be removed without a more invasive procedure and the patient is exposed to ionizing radiation.(4) Virtual colonoscopy is especially valuable in rural or isolated regions without easy access to gastroenterologists. Optical imaging is more invasive, but polyps can be removed during the procedure for pathological analysis as the gold standard for diagnosing CRC. Colonoscopy has high sensitivity to mature adenoma, but the miss rate for small polyps (<5 mm diameter) is estimated to be over 15%(5) and as high as 26%.(6,7) Flat polyps are particularly easy to miss with white-light imaging.
Optical coherence tomography (OCT) is an attractive modality for CRC imaging because it provides additional information in the form of depth-resolved data. We have demonstrated that it is possible to automatically detect small adenoma in mouse models based on thickening of the mucosa and increased signal attenuation along the depth direction.(8) The additional depth information from OCT can make detecting small, flat polyps easier than in traditional optical modalities like colonoscopy. However, this adenoma detection method using OCT still requires at least a doubling of the thickness of the mucosa, based on the results of a study using blinded experts reading OCT images of the mouse colon (9). Recent advancements in microvascular OCT imaging may enable even earlier detection of adenoma.
The microvasculature developed to support tumor growth is markedly different from existing, healthy vessels. In particular, the new vessels have variable diameters, dead ends, and form tortuous paths. Furthermore, the abnormal arterioles lack smooth muscle, which prevents them from dilating and constricting to control the blood flow rate in response to a variety of physiological and environmental stimuli.(10) Both the vascular structure and function are potential biomarkers for adenoma detection. Moreover, because the growth of new vessels is one of the earliest morphological changes in the development of adenoma(11), microvascular imaging may improve early polyp detection.
To enable the investigation of the colon microvasculature using OCT, we developed a method of processing the raw data generated from a swept-source OCT system using a lab-built miniature endoscope. The method uses the 2-dimensional Kasai autocorrelation method for measuring bi-directional blood flow.(12–14) The flow images are processed using intensity thresholding, 2D matched filtering, and histogram segmentation to extract vessels for further analysis. We tested the method on the azoxymethane (AOM) mouse model of colorectal cancer. We present, to the best of our knowledge, the first endoscopic Doppler OCT images of in vivo mouse colon.
2. Materials and Methods
2.1. Imaging System
We previously integrated a lab-built miniature endoscope for mouse colon imaging with a commercial swept-source OCT system (OCS1050SS, Thorlabs, Newton, NJ, USA).(15) The system layout and endoscope schematic are shown in Figure 1. Briefly, the source has a central wavelength of 1040 nm and a bandwidth of 80 nm, which produces an axial resolution of 12 μm in air. The A-scan rate is 16 kHz. The endoscope produces a numerical aperture of 0.14 with a glass spacer, gradient-index lens (GRINTECH, Jena, Germany) and 41° prism. The optics are enclosed by a glass envelope with an outer diameter of 2 mm and a wall thickness of 0.1 mm. These dimensions are robust to breakage during mouse colon imaging. However, a future probe for human imaging would require a safer material, such as a biocompatible, optically transparent plastic. The focus is located about 200 μm outside the envelope and has a spot size of 6.1 μm at focus. The measured power exiting the endoscope is 350 μW. The endoscope is connected to a fiber-optic rotary joint that is interfaced with a rotational motor for selecting the rotational angle for longitudinal imaging. The rotary joint and rotational motor are mounted on a linear translation stage that is moved by a linear actuator to control the motion of the endoscope.
Figure 1.
OCT system diagram and endoscope schematic. (a) The x-galvanometer control signal from the OCS1050SS is used to synchronize movement of the endoscope. The red line denotes the beam path in fiber. For the application described in this paper, the linear actuator moves the endoscope along the distal 30 mm of the colon. The rotational motor is used to select the rotational location of the longitudinal B-scan. OCT: OCS1050SS system, SE: synchronization electronics, LA: linear actuator, RM: rotational motor, TS: translation stage, RJ: fiber-optic rotary joint. (b) Schematic of the distal endoscope optics. S: spacer, GL: gradient-index lens, P: 41° rod prism. (c) Photograph of the distal portion of the endoscope.
2.2. Animal Model
A mouse model of colorectal cancer was used for in vivo imaging. A/J mice (The Jackson Laboratory, Bar Harbor, ME, USA) were treated with the colon-specific carcinogen AOM to develop adenoma.(16) Beginning at six weeks of age, the A/J mice were administered 10 mg/kg of AOM for the cancer group and 0.2 mL of saline for the control group via subcutaneous injection weekly for five weeks. These procedures were in accord with a University of Arizona Institutional Animal Care and Use Committee approved protocol.
2.3. Imaging Protocol
Solid food was replaced with a pediatric electrolyte solution 24 hours prior to imaging. Mice were anesthetized for imaging by intraperitoneal injection of 100 mL/kg ketamine and 10 mL/kg} xylazine. The colon was flushed with warm saline immediately prior to imaging. Mice were placed on a heating pad during imaging to maintain constant body temperature. Mice were 53 or 13 weeks of age during imaging.
The distal 30 mm of the colon was imaged to produce a single B-scan per mouse. The B-scan consisted of 512,000 A-scans (each with 512 pixels), corresponding to an A-scan spacing of about 58 nm. With a focused spot diameter of 6.1 μm, the number of samples per spot diameter (sampling density) is 104. This oversampling allows the window of measurable flow velocities to be shifted by applying the Kasai autocorrelation to every A-scan, every 2nd A-scan, 3rd A-scan, etc. without undersampling the data in the lateral dimension. Moreover, the high sampling density allows for averaging to reduce artifacts in the flow images due to the swept-source laser phase noise, bulk tissue motion, and vibration in the endoscope. The total imaging time was about 40 seconds.
The 53-week-old mice were euthanized after imaging. The distal 30 mm of the colon was explanted and cut open longitudinally. The tissue was fixed with 10% buffered formalin. Cross sections with 6-μm thickness were cut every 50 μm through the tissue. The sections were stained with hematoxylin and eosin and imaged with a standard laboratory transillumination microscope for histological analysis.
2.4. Image Processing
The raw fringes were recorded linearly in wavenumber. The raw data required numerical dispersion compensation due to an imbalance of dispersive media in the endoscope and the reference arm. We utilized a numerical method that determines the non-linear dispersion by subtracting a linear fit to the phase data as a function of wavenumber.(15) After numerical dispersion correction, the data were processed as described in the following subsections. Dispersion compensation is necessary to improve the axial point-spread function so that it closely matches the expected PSF based on the source spectrum. The numerical dispersion compensation does not affect the results of the Doppler flow map beyond improving the axial resolution. The purpose of the image processing is to extract vessels from the surrounding static tissue.
2.4.1. Doppler OCT
The two-dimensional Kasai autocorrelation method(12–14) was used to generate flow images:
| (1) |
| (2) |
where ΔT is the A-scan period, J and N are number of A-scans and depth pixels summed to reduce degradation due to noise, A is the complex A-scan data resulting from the inverse Fourier transform of the fringes after numerical dispersion correction, λ0 is the central wavelength of the source, fD is the Doppler frequency, v is the flow velocity, n is the refractive index of the sample, and θ is the Doppler angle (angle between the direction of flow and the OCT beam). We used J = 4 and N = 4 for the window size and used every sixth A-scan, corresponding to an A-scan period of 375 μs. By using every sixth A-scan, the product of the A-scans in Eq. (1) is ; however, Eq. (1) is written in the general form. The choice of skipping A-scans for computing the phase change depends on the system (e.g., desired velocity range based on A-scan rate, asymmetric sweep spectra limiting computation of the phase shift on only even or only odd A-scans). The window size was chosen to balance post-processing computation time and noise suppression. The depth window size of 2 pixels reduced the standard deviation of the phase change of stationary 1% Intralipid by about 20%. Further increasing the depth window size showed minimal additional noise suppression and caused a proportional increase in computation time. The larger lateral window size was chosen because phase noise due to synchronization errors between the swept source and the data acquisition manifests across adjacent A-scans. For physiologically relevant flow velocities (< 3 mm/s in mouse colon), applying the Kasai autocorrelation in either axial or transverse directions will accurately measure the flow (14). Due to the asymmetric sweep spectra of the source, the Kasai autocorrelation can only be applied to even or odd A-scans.
With a high sampling density, we can effectively control the range of flow velocities that can be detected. Increasing the A-scan period allows detection of low flow velocities and decreasing the period shifts the flow sensitivity range to higher flow velocities. Because the sampling density is very high, many A-scans can be skipped while still effectively sampling at the same spatial location on the sample. The minimum detectable flow velocity is limited in practice by the phase stability of the imaging system. While a mirror is often chosen as the sample for measuring phase noise, using tissue samples provides a more realistic measure of the system noise due to the decreased SNR of tissue compared to a highly reflective mirror.(17) We plotted histograms of regions of interest containing at least 1000 pixels in several vessel-free regions in the muscularis propria. For each region, we measured the full width at half maximum (FWHM). In static tissue, variance in the measured phase (arg(·) in Eq.(1)) results from system phase noise and bulk motion. The average of the FWHM values was 0.111 rad. If we define the minimum detectable phase change due to flow as 0.111 rad, then this corresponds to a velocity of 17.5 μm/s for θ = 0, ΔT = 375 μs, λ0 = 1040 nm, and n = 1.4. This value is an order of magnitude lower than typical capillary flow velocity. However, most vessels are not parallel to the beam and so the minimum detectable velocity increases as the Doppler angle approaches 90°. The maximum flow velocity at θ = 0 before phase wrapping is 0.5 mm/s.
A flow phantom was used to measure the experimental minimum detectable flow velocity. The phantom was a glass capillary tube with an inner diameter of 0.45 mm. A 27-gauge needle was inserted into one end of the tube. A syringe pump controlled the flow of 5% Intralipid through the capillary tube. A-scans were collected through the center of the capillary tube at a measured A-scan rate of 15.4 kHz without moving the endoscope. Every 6th A-scan was used for processing. The Doppler angle was 80°. After applying numerical dispersion compensation and computing the Kasai autocorrelation with a window size of 2 pixels in depth and 4 pixels in time, the resulting flow profiles were averaged together. Parabolic flow across the entire inner diameter of the capillary tube was seen at a flow velocity of 0.3 mm/s (52.1 μm/s along the beam axis). The maximum flow velocity before phase wrapping was 1.4 mm/s (0.243 mm/s along the beam axis).
After computing the Kasai autocorrelation, the image was resized to produce square pixels using bicubic interpolation (downsampled from 512 × 512000 to 512 × 7680). Due to the high degree of oversampling in the lateral direction, the downsampling removes most of the motion artifacts due to vibration of the endoscope and mouse respiration. This is demonstrated in Figure 2.
Figure 2.
Comparison of flow map before and after downsampling using bicubic interpolation. (a) A flow map after interpolation, (b) the flow map of the region denoted by the box in (a) prior to downsampling. Note that the repetitive red and blue vertical streaks due to the stepper motor motion have been averaged out in (a). Scale bar is 1 mm. Color bar is – π to π radians.
2.4.2. Intensity Thresholding
Signal-void regions produce noisy flow maps when using many Doppler OCT algorithms, including the Kasai method used here. Therefore, we used the structural image intensity to mask the flow image such that signal-void regions are removed. The threshold was 10 dB above the noise floor. Next, a median filter using a 5×5 kernel was applied to the image to reduce speckle noise. The noise floor was computed by averaging the bottom 100 rows in the log-intensity image. At this depth (1.61 mm to 2.00 mm), no signal from the tissue could be seen in the structural image. In addition to removing regions where the signal has been greatly attenuated, this masking step also removes flow data from small, non-scattering cavities in the tissue that might otherwise be confused for vessels. Signal from non-colon tissue (e.g., connective tissue, fluid in the colon, endoscope window) was manually removed as needed.
2.4.3. Two-dimensional Matched Filtering
We used a 2D matched filter to reduce the variability of static, non-circular (i.e., vessel cross sectional) features in the flow map. We manually inspected prominent vessels detected from the flow images and found most to have a diameter of about 7 pixels (27μm). We defined a circular template to extract circular features with sizes around 7 pixels. This template was cross-correlated with the flow image. This step may introduce flow artifacts near the boundaries of the tissue introduced by the intensity threshold. Excessive artifacts should be manually removed prior to histogram segmentation to avoid skewing the histogram.
2.4.4. Histogram Vessel Segmentation
A histogram-based segmentation method was then used to extract vessels using velocity thresholds determined by the descriptive statistics of the pixel values with thresholds set at the mean ± 2 standard deviations. This threshold appears to segment vessels in the flow image while also removing or reducing most of any remaining artifacts caused by bulk tissue or endoscope motion. The result of the vessel segmentation is a “quaternary” image consisting of pixels identified as: (1) flow in the positive direction (red), (2) flow in the negative direction (blue), (3) no flow (white), and (4) pixels removed by intensity thresholding (black). The blue and red colors in the quaternary flow maps indicate flow direction only and do not correspond to specific velocity amplitudes.
3. Results
The matched filter improves performance of the histogram segmentation of the vessels. A profile through two vessels before and after applying the matched filter is shown in Figure 3. The signal-to-noise ratio (SNR), defined as the ratio of the signal mean to the background standard deviation, was computed for the profiles through two vessels in Figure 3 before and after the matched filter operation. The matched filter primarily smoothens the background and reduces the background standard deviation. The SNR of the flow map before applying the matched filter for the positive and negative flow are 13.90 and 18.61, respectively. After the matched filter, the positive and negative flow SNR values are 17.93 and 21.84. This is largely due to the 25% reduction in the background standard deviation. Smoothing the background improves the performance of the histogram segmentation step because local fluctuations due to phase noise are reduced. The cross-correlation operation broadens the vessel size by the diameter of the matched filter. However, the amplitude of this broadened region is significantly lower than the actual vessel region and therefore has a small effect on the sizes of the vessel after histogram segmentation.
Figure 3.
Comparison of vessel flow profiles before and after applying 2D matched filter. (a) Flow image prior to matched filter; (b) flow image after applying matched filter; (c) profile along black line in (a); profile along black line in (b); (e) histogram segmentation of (a) with upper and lower thresholds set to mean ± 2 standard deviations, excluding black pixels, (f) histogram segmentation of (b) with upper and lower thresholds set to mean ± 2 standard deviations, excluding black pixels. Scale bar is 1 mm. Color bar is – π to π radians (black pixels denote signal-void regions removed during intensity thresholding) and applies to (a) and (b). The blue and red in (e) and (f) indicate positive and negative flow, but not specific flow velocities.
Example structural and segmented flow images from 13-week-old mice are shown in Figure 4 (saline group) and Figure 5 (AOM group). Vessels are present in the mucosa and submucosa in all four mice. Most vessels are circular, suggesting that the OCT image plane was close to perpendicular to most vessels, though a few larger vessels that are more parallel to the images can be seen. The large blue area on the right side of Figure 5b is an artifact from motion that was partially suppressed by the histogram segmentation.
Figure 4.
Structural and segmented flow images from two saline-treated mice. (a,d) Structural OCT images, (b,e) result from matched filter operation after computing Kasai autocorrelation, (c,f) result of histogram segmentation on (b,e). Arrows denote portions of vessels parallel to the image plane and arteriole-venule pairs. Scale bars are 1 mm. Images have been stretched vertically by 25% to improve readability. The blue and red in (c) and (f) indicate positive and negative flow, but not specific flow velocities.
Figure 5.
Structural and segmented flow images from two AOM-treated mice. (a,d) Structural OCT images, (b,e) result from matched filter operation after computing Kasai autocorrelation, (c,f) result of histogram segmentation on (b,e). Arrow denotes a partially suppressed motion artifact. Scale bars are 1 mm. Images have been stretched vertically by 25% to improve readability. The blue and red in (c) and (f) indicate positive and negative flow, but not specific flow velocities.
A comparison of adenoma tissue from a living and a euthanized mouse are shown in Figure 6. Figures 6a and 6b show intensity images of the adenoma, clearly distinguishable as such due to the absence of a visible mucosa-submucosa boundary. The in vivo adenoma has a high density of vessels and several arteriole-venule pairs. The motion artifacts on the left and center of Figure 6c have been suppressed in Figure 6e by the histogram segmentation. As expected, the flow images of Figures 6d and 6f show no vessels in the euthanized tissue. Some vertical streaking artifacts from the endoscope motion can be seen in Figure 6d. These artifacts are nearly fully suppressed by histogram segmentation; Figure 6f shows only a small number of (artifactual) flow pixels.
Figure 6.
Comparison of adenoma tissue from living and euthanized mice. (a) and (b) are OCT images from the living and euthanized mouse, respectively. (c) and (d) are the corresponding flow images after intensity thresholding and matched filtering, (e) and (f) are the corresponding flow images after histogram segmentation. Scale bar is 1 mm. Color bar shows the phase shift in radians and applies to (c) and (d). The blue and red in (e) and (f) indicate positive and negative flow, but not specific flow velocities.
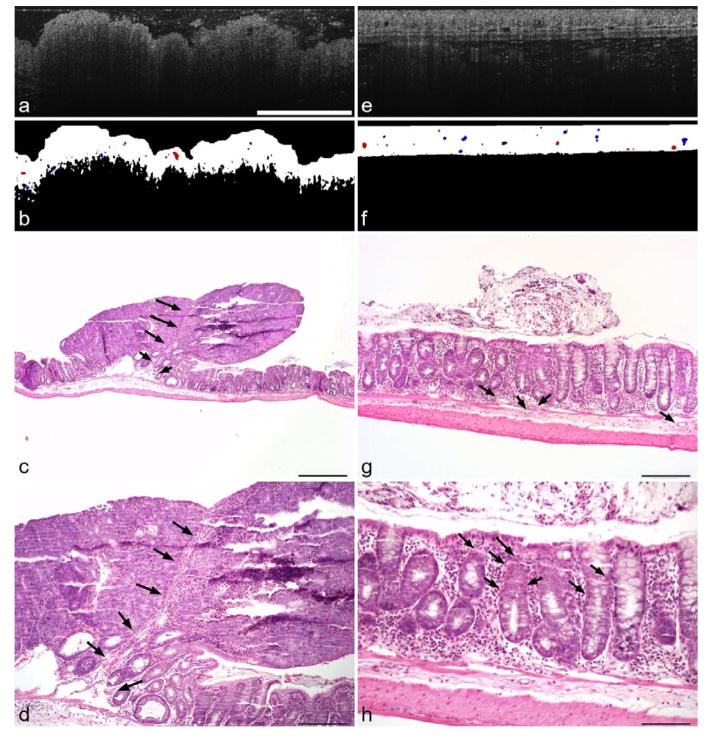
A comparison of an adenoma and surrounding undiseased tissue from a single 53-week-old mouse is shown in Figure 7. The segmented vessels in the undiseased tissue vary in size with a qualitatively uniform distribution of small, medium, and large vessels. However, the vessels in the adenoma are mostly either small or large. The adenoma also has fewer vessels total and the vessels are mostly located in the center and left of center. The large vessel in Figure 7b can be seen in the 4× (Figure 7c) and 10× (Figure 7d) histology images. Very few other vessels were found in the adenoma histology. In contrast, the vessels are homogeneously distributed in the undiseased flow image shown in Figure 7(f) and are located in both the mucosa and submucosa. Some arterioles and venules near the submucosa are denoted with arrows in the corresponding 10× histology image (Figure 7g). Small vessels throughout the depth of the mucosa are shown with arrows in the 20× histology image (Figure 7h).
Figure 7.
Comparison of adenoma and undiseased tissue. (a) Structural OCT image of adenoma, (b) segmented flow image of adenoma, (c,d) histology cross-section of adenoma at 4× and 10× magnification, (e) structural OCT image of undiseased colon tissue, (f) segmented flow image of undiseased tissue, (g,h) histology cross-section of undiseased tissue at 10× and 20× magnification. Arrows denote blood vessels. Scale bars are: 1 mm (a,b,e,f), 0.5 mm (c), 0.2 mm (d,g), and 0.1 mm (h). The blue and red in (b) and (f) indicate positive and negative flow, but not specific flow velocities.
4. Discussion
The method described here can enable rapid measurements such as total vessel count, vessel density, and extracting flow velocities from individual vessels. Using a standard PC running Matlab (2014b, Mathworks, Natick, MA, USA), all processing steps after the Kasai autocorrelation were completed in about 1 second. Real-time Doppler processing using Kasai autocorrelation in software has been previously demonstrated.(18) By implementing the methods described in this paper in a precompiled programming language and using real-time Doppler processing, many vascular measurements could be made in real time.
The matched filter operation smoothens the static tissue regions to improve the performance of the histogram segmentation. This is particularly useful for swept-source lasers because they typically have more phase noise than other OCT sources such as superluminescent diodes. A series of filter templates may enable more accurate vessel detection and orientation (e.g., determining the Doppler angle) at the expense of greater computation time.
The image processing steps described here reduce, but do not completely remove, artifacts due to motion. By oversampling the data in the lateral direction and averaging many A-scans, artifacts from repetitive motions like breathing and endoscope vibration can be reduced or removed. In cases where motion artifacts are not sufficiently suppressed with histogram segmentation, other bulk-motion correction methods (e.g., histogram mean/median or referencing all phase measurements to the tissue surface) may be implemented. Oversampling can be implemented in 3D imaging with a high-speed OCT system,(19) and the additional information from surrounding regions can improve the accuracy of vessel detection. Circularly shifting flow values to center the mode of the flow histogram can further reduce bulk motion with a modest increase in computation time.(20)
The differences in vascular size and spatial distribution in adenoma and undiseased colon tissue are similar to those reported in the literature. The spatial distribution of vessels is more homogeneous in the undiseased tissue than in the adenoma. Also, the adenoma has a large vessel in the center and a few other small vessels while the undiseased tissue vessels range from small to large. Konerding et al. have shown similar results using corrosion casts of normal and adenoma tissue in the human colon.(10) We do not see significant differences in vessel sizes or spatial distribution in the 13-week-old AOM- and saline-treated mice. Based on our previous experience, we would not expect adenomas to develop so shortly after administering AOM. Additionally, it is difficult to ensure that the same location is imaged over multiple imaging sessions without volumetric imaging.
Some challenges exist for translating endoscopic OCT colon imaging to humans. The small diameter of the mouse colon allows for the tissue to remain in contact with the endoscope window. However, the imaging system will need to be modified for imaging in the larger human colon in order to keep the colon within the imaging depth of field. OCT typically uses low numerical aperture probes, so the depth of field is already on the order of the imaging depth imposed by light scattering in the tissue. Two methods could be used to keep the tissue within the depth of field. The first would be to use a balloon catheter inflated to hold the endoscope in the center of the colon, thus maintaining a mostly uniform distance from the probe to the tissue. A second method, termed adaptive ranging (21), would be to design the reference arm to continuously adjust length to keep the zero optical path difference location near the surface of the tissue. Another challenge to clinical translation is optimizing presentation of images and measurements for physicians. Rapid display of volumetric angiograms may enable assessment of tumors based on visual judgment of vessel tortuosity. We have presented, to the best of our knowledge, the first endoscopic in vivo Doppler OCT images of the mouse colon and described a simple, robust, and fast image processing method for extracting vessels. Future studies will use volumetric imaging to co-register vessels across multiple time points using a larger mouse population for a statistically significant study. Angiogenesis of dysfunctional vessels form at the earliest stages of pre-malignant tumorigenesis in the colon and many other organs (11,22). Therefore, Doppler OCT may be capable of discriminating pre-malignant tumors based on changes of blood flow in response to vasodilators or vasoconstrictors. The tumors in this AOM-treated mouse model are adenoma and do not progress to malignant adenocarcinoma. Future work will use a model with malignant potential.
Acknowledgments
We thank Photini Faith Rice and Caitlin Howard for assistance with drug treatment and animal imaging.
Funding Acknowledgements: This research was supported by the National Cancer Institute and the National Heart, Lung, and Blood Institute of the National Institutes of Health under award numbers R01CA109385 and T32HL007955, respectively, the American Society for Laser Medicine and Surgery Student Research Grant, and the Arizona TRIF Imaging Initiative. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Weston A. Welge, College of Optical Sciences, The University of Arizona, Tucson, AZ 85721.
Jennifer K. Barton, College of Optical Sciences, The University of Arizona, Tucson, AZ 85721. Department of Biomedical Engineering, The University of Arizona, Tucson, AZ 85721.
References
- 1.Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015. Internet. Available from: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. [Google Scholar]
- 2.Burch JA, Soares-Weiser K, StJohn DJB, Duffy S, Smith S, Kleijnen J, et al. Diagnostic accuracy of faecal occult blood tests used in screening for colorectal cancer: a systematic review. J Med Screen. 2007 Sep 1;14(3):132–7. doi: 10.1258/096914107782066220. [DOI] [PubMed] [Google Scholar]
- 3.Yang H, Xia B-Q, Jiang B, Wang G, Yang Y-P, Chen H, et al. Diagnostic value of stool DNA testing for multiple markers of colorectal cancer and advanced adenoma: A meta-analysis. Can J Gastroenterol Hepatol. 2013 Aug 1;27(8):467–75. doi: 10.1155/2013/258030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal Cancer: CT Colonography and Colonoscopy for Detection—Systematic Review and Meta-Analysis. Radiology. 2011 May;259(2):393–405. doi: 10.1148/radiol.11101887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn SB, Han DS, Bae JH, Byun TJ, Kim JP, Eun CS. The Miss Rate for Colorectal Adenoma Determined by Quality-Adjusted, Back-to-Back Colonoscopies. Gut Liver. 2012 Jan;6(1):64–70. doi: 10.5009/gnl.2012.6.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006 Feb;101(2):343–50. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 7.Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of Adenomas Missed by Optical Colonoscopy. Ann Intern Med. 2004 Sep 7;141(5):352–9. doi: 10.7326/0003-4819-141-5-200409070-00009. [DOI] [PubMed] [Google Scholar]
- 8.Winkler AM, Rice PFS, Drezek RA, Barton JK. Quantitative tool for rapid disease mapping using optical coherence tomography images of azoxymethane-treated mouse colon. J Biomed Opt. 2010;15(4):041512–041512-10. doi: 10.1117/1.3446674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hariri LP, Qiu Z, Tumlinson AR, Besselsen DG, Gerner EW, Ignatenko N, et al. Serial endoscopy in azoxymethane treated mice using ultra-high resolution optical coherence tomography. Cancer Biol Ther. 2007 Nov 1;6(11):1753–62. doi: 10.4161/cbt.6.11.4852. [DOI] [PubMed] [Google Scholar]
- 10.Konerding MA, Fait E, Gaumann A. 3D microvascular architecture of pre-cancerous lesions and invasive carcinomas of the colon. Br J Cancer. 2001 May;84(10):1354–62. doi: 10.1054/bjoc.2001.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staton CA, Chetwood ASA, Cameron IC, Cross SS, Brown NJ, Reed MWR. The angiogenic switch occurs at the adenoma stage of the adenoma–carcinoma sequence in colorectal cancer. Gut. 2007 Oct 1;56(10):1426–32. doi: 10.1136/gut.2007.125286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasai C, Namekawa K, Koyano A, Omoto R. Real-Time Two-Dimensional Blood Flow Imaging Using an Autocorrelation Technique. IEEE Trans Sonics Ultrason. 1985 May;32(3):458–64. [Google Scholar]
- 13.Zhang J, Chen Z. In vivo blood flow imaging by a swept laser source based Fourier domain optical Doppler tomography. Opt Express. 2005 Sep 19;13(19):7449–57. doi: 10.1364/opex.13.007449. [DOI] [PubMed] [Google Scholar]
- 14.Morofke D, Kolios MC, Vitkin IA, Yang VXD. Wide dynamic range detection of bidirectional flow in Doppler optical coherence tomography using a two-dimensional Kasai estimator. Opt Lett. 2007 Feb 1;32(3):253–5. doi: 10.1364/ol.32.000253. [DOI] [PubMed] [Google Scholar]
- 15.Welge WA, Barton JK. Expanding Functionality of Commercial Optical Coherence Tomography Systems by Integrating a Custom Endoscope. PLOS ONE. 2015 Sep 29;10(9):e0139396. doi: 10.1371/journal.pone.0139396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papanikolaou A, Wang QS, Papanikolaou D, Whiteley HE, Rosenberg DW. Sequential and morphological analyses of aberrant crypt foci formation in mice of differing susceptibility to azoxymethane-induced colon carcinogenesis. Carcinogenesis. 2000 Aug;21(8):1567–72. [PubMed] [Google Scholar]
- 17.Leitgeb RA, Werkmeister RM, Blatter C, Schmetterer L. Doppler Optical Coherence Tomography. Prog Retin Eye Res. 2014 Jul;41:26–43. doi: 10.1016/j.preteyeres.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu G, Qi W, Yu L, Chen Z. Real-time bulk-motion-correction free Doppler variance optical coherence tomography for choroidal capillary vasculature imaging. Opt Express. 2011 Feb 14;19(4):3657–66. doi: 10.1364/OE.19.003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi W, Mohler KJ, Potsaid B, Lu CD, Liu JJ, Jayaraman V, et al. Choriocapillaris and Choroidal Microvasculature Imaging with Ultrahigh Speed OCT Angiography. PLOS ONE. 2013 Dec 11;8(12):e81499. doi: 10.1371/journal.pone.0081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang VXD, Gordon ML, Mok A, Zhao Y, Chen Z, Cobbold RSC, et al. Improved phase-resolved optical Doppler tomography using the Kasai velocity estimator and histogram segmentation. Opt Commun. 2002 Jul 15;208(4–6):209–14. [Google Scholar]
- 21.Iftimia NV, Bouma BE, de Boer JF, Park BH, Cense B, Tearney GJ. Adaptive ranging for optical coherence tomography. Opt Express. 2004;12(17):4025. doi: 10.1364/opex.12.004025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raica M, Cimpean AM, Ribatti D. Angiogenesis in pre-malignant conditions. Eur J Cancer. 2009 Jul;45(11):1924–34. doi: 10.1016/j.ejca.2009.04.007. [DOI] [PubMed] [Google Scholar]