Abstract
Optineurin (Optn) is an adaptor protein with homology to NF-κB essential-modulator (NEMO), the regulatory subunit of the IκB kinase (IKK) complex. Dysregulation of Optn has been linked to neurodegenerative, autoimmune and bone diseases. Optn shares a high degree of homology with NEMO, but is not part of the same high-molecular weight complex containing IKKα and IKKβ. Despite its homology with NEMO and the fact that it has been the subject of extensive study in several cell types, there are no published studies addressing the role of Optn during T cell activation. Here we demonstrate that ectopic expression of Optn down-regulates TCR- induced NF-κB activation and TNF-α production, in a manner dependent on ubiquitin-binding. Conversely, knock-down of Optn enhances NF-κB activation and the production of TNF-α. Consistent with a negative regulatory role for this protein, we observed transient loss of Optn after TCR stimulation in both cell lines and in primary murine T cells. The acute loss of Optn appears to be due to both protein degradation and exocytosis, the latter via activation-induced exosomes. This study therefore provides novel information regarding the role of Optn during TCR activation, suggesting the possible importance of Optn during inflammation and/or autoimmune diseases.
Keywords: Optineurin, T cells, T-cell receptor for antigen (TCR), NF-kappa B (NF-kB), extracellular vesicles
1. Introduction
TCR induced NF-κB nuclear translocation requires activation of the IKK complex, through a combination of NF-κB Essential Modulator (NEMO) K63-polyubiquitination and IKKβ phosphorylation (Tang et al., 2003; Zhou et al., 2004). Although the IKK complex is commonly thought of as a downstream player in TCR signaling and therefore envisioned within a cytosolic compartment, it has been demonstrated that, in response to CD3 and CD28 costimulation, NEMO is recruited to the TCR, which may be important for NF-κB activation. It was also reported that NEMO accumulates at the IS, which may contribute to the regulation of TCR-induced NF-κB activation (Weil et al., 2003).
Optineurin (Optn) is a widely expressed polyubiquitin-binding protein and shares high homology with NEMO, including 53% identity within the ubiquitin binding domain (UBD) (Li et al., 1999). Despite its high level of homology with NEMO, Optn is not part of the same IKK complex with IKKα/β (Schwamborn et al., 2000). The Israël group first showed the functional relevance of the Optn UBD, demonstrating how a chimeric protein, in which the two UBDs of NEMO were substituted with the Optn UBD, retained its ability to associate with K63 ubiquitinated proteins (Laplantine et al., 2009). It has been speculated that upon TNF-α stimulation, Optn may compete with NEMO for a common polyubiquitinated substrate, therefore down-regulating NF-κB activity. In a different model, Optn has been proposed to interact with TAX1BP1 to regulate NF-κB signaling (Journo et al., 2009; Zhu et al., 2007).
Optn has emerged as a regulator of multiple physiological processes, including host defense, cell division, protein secretion and vesicular trafficking (Kachaner et al., 2012a; Kachaner et al., 2012b). Mutations in Optn have been implicated in development of human diseases such as glaucoma (Aung et al., 2003; Ueno et al., 2011), amyotrophic lateral sclerosis and Paget’s disease of the bone (Albagha et al., 2010). At this point, the role of Optn in immune cells is less well understood. It has been reported that in a specific cohort of Crohn’s disease patients a low level of Optn protein results in diminished TNF-α, IFN-γ and IL-6 cytokine secretion after bacterial stimulation (Smith et al., 2014). Several studies have implicated Optn in the negative regulation of NF-κB signaling, although its function in this regard remains controversial (Gleason et al., 2011). Another study (Munitic et al., 2013) showed that endogenous Optn is dispensable for NF-κB activation but necessary for IRF3 activation in bone marrow-derived macrophages stimulated with cytokines or LPS. However, a more recent report did reveal enhanced NF-κB activation in osteoclasts from mice expressing a Ub-binding mutant of Optn (Obaid et al., 2015). Thus, regulation of signaling pathways by Optn may be cell-type specific and may also depend on its precise subcellular localization and interactions with other proteins.
Here we have investigated the role played by Optn during TCR-induced T cell activation, using T cell lines and primary murine and human T cells. We show that TCR/CD28 stimulation regulates post-translational, but not transcriptional, levels of Optn. We further demonstrate that Optn overexpression leads to suppression of NF-κB, but not NFAT or AP-1 activity, and a significant decrease in the production of TNF-α, but not IL-6 or IL-4. We also show that following TCR stimulation T cells release into the extracellular milieu Optn-enriched exosomes that also contain TCR molecules, consistent with previous studies showing the shedding of extracellular vesicular-TCR as a mechanism for dampening TCR-induced T cell activation (Choudhuri et al., 2014).
2. Materials and Methods
2.1 Antibodies and Reagents
Polyclonal anti-TCR ζ chain, mAb to Optn, polyclonal anti-Zap70 and mAb to anti-CD63 were obtained from Santa Cruz Biotechnology. Antibodies to IKKβ, K48-Ubiquitin and pS6 were from Cell Signaling Technology. Anti-human and -mouse CD28, and goat-anti-rabbit Alexa 488-streptavidin were from Invitrogen. Antibody against eGFP was from Rockland Immunochemicals. Anti-mouse CD3 (clone 2C11) and anti-human CD3 (OKT3) were from Bioxcel. Anti-β actin was from Sigma. Biotin-hamster anti-mouse CD3 and biotin anti-mouse CD28 were from BD Biosciences. Clonotypic antibody to the Jurkat TCR (C305) was obtained from A. Weiss (U.C.S.F.). Goat anti mouse-Cy3 was from Jackson Immunoresearch. DAPI dye was from Sigma. Mouse TNF-α and IL-6 ELISA kits were from Biolegend. MG132 was from Calbiochem; bafilomycin and DMSO were from Sigma.
2.2 Cell lines
Jurkat T cells were maintained in RPMI (Cellgro) supplemented with 5% Bovine Growth Serum (Hyclone-Thermo Scientific), 100 units/mL penicillin (Lonza), and 100 ug/mL streptomycin (Lonza) at 37° C in 5% CO2. 3T8 is a CD3+ Jurkat T-cell line that has been sequentially transfected with two reporter constructs containing the human CD14 and the rat Thy1 gene under the control of eight copies NF-κB enhancers. Mutant 8321 is a CD3+ derivative of 3T8 generated by CR191 mutagenesis and subsequent enrichment for cells that do not respond to phorbol myristate acetate (PMA). Cells were maintained in RPMI supplemented with 10% BGS 100U/mL penicillin, and 100ug/mL streptomycin at 37° C in 5% CO2. D10 T cells (a mouse T cell clone) were maintained in RPMI supplemented with 5% Bovine Growth Serum, 100 units/mL penicillin, and 100 μg/mL streptomycin, sodium pyruvate (1%w/v), β-ME (1% w/v), non-essential AA (1% w/v) at 37° C in 5% CO2.
2.3 Mouse and human T cell purification and stimulation
Primary murine T cells were purified from spleens and lymph nodes of male C57BL/6 mice (6–12 weeks old), which were obtained from the Jackson Laboratory. Murine T cells isolated from lymph node and spleen were CD4+ enriched at >90% purity with a MACS beads negative selection Kit (Milteny Biotech) according to the manufacturer’s protocol. All experimental protocols for mice were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Healthy adult donor human CD3+ T cells were purified from de-identified blood bank samples. Briefly, PBMC (frozen samples were obtained from Dr. Diana Metes, Univ. of Pittsburgh) were isolated by density gradient centrifugation and CD3+ T cells were sorted by negative selection using a pan T-cell isolation kit (Miltenyi-Biotech). The three experiments represented in Figure 4E–F were conducted on PBMC from three different healthy adult donors.
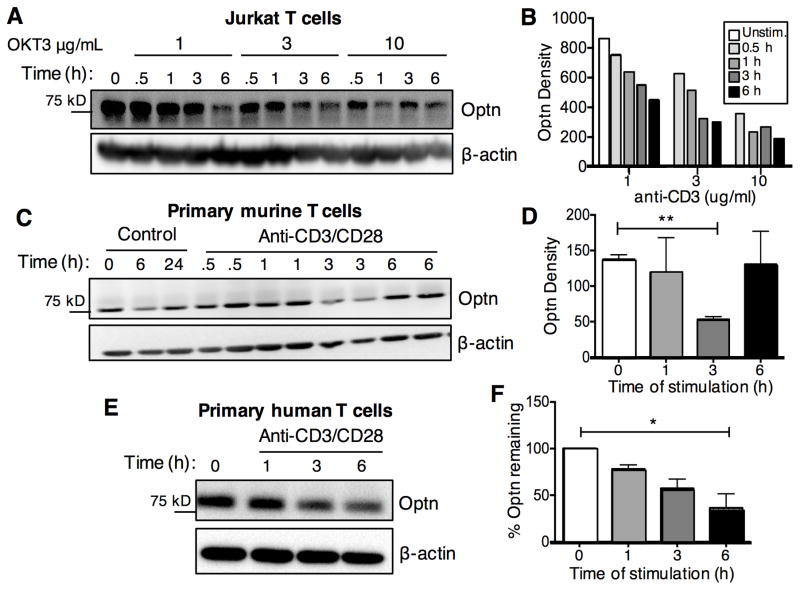
Fig. 4.
Rapid turnover of Optn protein after TCR-induced T cell activation.
Jurkat T cells (A), CD4+ T cells from C57BL/6 mice (C) or primary human CD3+ T cells (E) were stimulated with anti-CD3/CD28 Abs. After the indicated time points cells were harvested and cell lysates were obtained, followed by western blot analysis for Optn and β-actin protein. Panels B, D and F depict quantitative analysis of Optn protein levels following stimulation, from one experiment (A–B) or at least three experiments (C–F). Human T cells were purified from the PBMC of three individual healthy adult donors, with data summarized in panel F, a representative example of which is shown in panel E.
For anti-CD3 stimulations, T cells (2x105) in 0.5 ml medium (RPMI plus 10% FCS, 50 μM b-mercaptoethanol, 2 mM L-glutamine and 50 U/ml penicillin/streptomycin) were added in triplicate to 24-well plates pre-coated with anti-CD3ε antibody (1–3 μg/ml). Where indicated, soluble anti-CD28 (5 μg/ml) was also added at the indicated time points. As a positive control, cells were stimulated with PMA (10 ng/ml) plus ionomycin (125 ng/ml).
2.4 Cell transfection and luciferase assays
Jurkat T cells and D10, 832 and 3T8 cells were transfected by electroporation. Cells (0.4 mL) were added to a 0.4 cm gap cuvette (BioRad) containing plasmid DNA as indicated. Electroporation was carried out at 260V, 950μF, ∞Ω. For electroporation of D10 cells we used different conditions (290V, 950μF). Cells were then transferred to a 6 cm tissue culture plate with 10 ml RPMI containing 5% FCS and cultured for 16–18 h at 37°C, 5% CO2.
For luciferase assays, Jurkat T cells and D10 cells were transiently transfected by electroporation. The total amount for DNA content in each transfection was adjusted to 20 μg. Sixteen hours after transfection, cells were washed with PBS and resuspended at 2 x106 per ml in RPMI containing 5% BGS. Cells were plated in to round-bottom 96-well plates (100μl/well) with RPMI+BGS containing 2x concentration of the indicated stimuli (100 μl/well). After 6h at 37°C, 5% CO2, cells were permeablized for 5 min at room temperature with harvest buffer (10% Triton X-100, 1 mM DTT, 0.2 M KPO4 pH 7.8). Samples were then transferred to an opaque 96-well plate, and 100 μl of luciferase assay buffer was added (10 mM ATP, 20 mM MgCl2, 0.2 M KPO4 pH 7.8). Luciferase activity was determined with a luminometer, with a 10 sec reading, after injection of 50μl of 1.3 mM luciferin (Fisher).
2.5 Plasmids and siRNA
eGFP-Optn (human) plasmid (#27052) was from Addgene. Mutant eGFP-Optn (D474N) was obtain by performing point mutation PCR (Quick Site Direct Mutation Kit, Agilent) using the following primers: Forward: 5’ctgctctttcagcatgaaaattagaacagtaaacttccatctg3’; Reverse: 5’cagatggaagtttactgttctaattttcatgctgaaagagcag3’ following the manufacturer’s instructions. ON-TARGETplus SMARTpool siRNA to murine OPTN was obtained from Dharmacon and was resuspended in sterile, RNAse-free, water. OPTN siRNA (or a non-targeting control pool) was transfected by electroporation, as described above, using 100 nM siRNA per cuvette.
2.6 Western blotting
Whole-cell lysates were prepared by lysing 5x106 cells in lysis buffer (1% NP-40, 150 mM NaCl, 20 mM Tris, pH 7.5 + protease/phosphatase inhibitors). After incubation on ice for 10 min, lysates were spun for 10 min at maximum speed in a microcentrifuge. Post-nuclear supernatants were mixed with 6x reducing sample buffer and boiled for 5 min. Immunoprecipitations were performed with 20 μl of a 50% slurry of protein G–agarose beads (Millipore). Samples were separated on 10% polyacrylamide gels, and then transferred to PVDF membrane (Millipore) using a semi-dry blotting apparatus. Blots were blocked with 5% BSA (Sigma) in wash buffer (250 mM NaCl, 20 mM Tris pH 7.5, 0.05% Tween-20) for 1h at room temperature. Primary antibody was incubated overnight at 4ºC in wash buffer, followed by three 10 min. washes. Horseradish peroxidase-conjugated secondary antibody was diluted to 1:10,000 in wash buffer and incubated with blots two hours at room temperature, followed by three ten minute washes. Immunoblots were developed by enhanced chemiluminescence (Pierce) using a Protein Simple FluoChem M. Densitometry analysis was performed using Alpha View Software.
2.7 Real-time PCR
RNAs extracted with TRIzol reagent (Invitrogen) were reverse-transcribed to generate complementary DNA (cDNA) with and random primers. Quantitative real-time polymerase chain reaction (RT-PCR) assays were performed with Mastercycler Realplex and SYBR Green Master Mix (Eppendorf). The abundance of Optn mRNA was normalized to that of ACTB mRNA (encoding β-actin) or GAPDH, as calculated with the 2−ΔΔ CT method. Pre-designed primers used to perform the reactions were purchased by Qiagen.
2.8 Immunofluorescence and Total Internal Reflection Fluorescence Microscopy
Jurkat T cells were seeded on glass bottom micro-well dishes (MatTek) pre-coated with anti-CD3 Ab or left untreated. After the indicated time points cells were washed with PBS and fixed in 2% paraformaldehyde for 15 min at room temperature, rinsed and permeablized with 0.1% Triton X-100 in phosphate BSA 0.5% buffer (PBB) for 15 min at room temperature. After two washes in PBS, non-specific binding was blocked with 5% normal serum from the same species as the secondary antibody (45 min at RT). After five washes, cells were incubated with primary antibody (overnight at 4ºC). The following day cells were washed five times with PBS and incubated with secondary Ab for 45 min at room temperature. Following five washes with PBS, cells were stained with Hoechst stain for 30 sec to stain the nuclei. Then, cells were washed three times with PBS before imaging on an Olympus Fluoview 1000 (Center for Biologic Imaging, University of Pittsburgh).
For total internal reflection fluorescence (TIRF) imaging, Jurkat T cells were transiently transfected with eGFP-Optn and RFP-NEMO as indicated. Cells were seeded on glass bottom micro-well dishes (MatTek) pre-coated with anti-CD3 Ab or left untreated. TIRF images were acquired every five sec; every 30 min an epifluorescence image was taken at a depth of 0.8 μm into the cells. Confocal single plane images were acquired every 5 sec. TIRF images were acquired on a Nikon 2000TE microscope (Melville, NY) with an argon laser (laser bench provided by Prairie Technologies, Madison, WI) and a 60x, 1.45 NA oil immersion objective capable of both epifluorescence and TIRF illumination, using Metamorph 6.1 software (Molecular Devices, Downingtown, PA) and a Retiga-SRV camera (Qimaging) or a Hamamatsu EM CCD C9100 camera. Confocal images were acquired with a spinning-disc confocal microscope (Solamere Technology Group) with a Yokogawa scanhead on a Zeiss Axiovert 200M using QED InVivo software and a QICAM fast 1394 camera (QImaging). Adobe Photoshop was used for image analysis.
2.9 Data and statistical analysis
Data and statistical analyses were carried out using GraphPad Prism, as follows. Luciferase reporter assays: For two-condition experiments, triplicate samples from a representative experiment were analyzed with the Mann-Whitney U test; for three or more conditions, the Kruskal-Wallis test was used. Cytokine measurements: replicates were analyzed with the Mann-Whitney U test, Wilcoxon Signed Rank Test or paired t-test, as described in the Figure Legends. For time course experiments measuring Optn protein or message, the Kruskal-Wallis test was used.
3. Results
3.1 Optn suppresses TCR-induced NF-κB activity
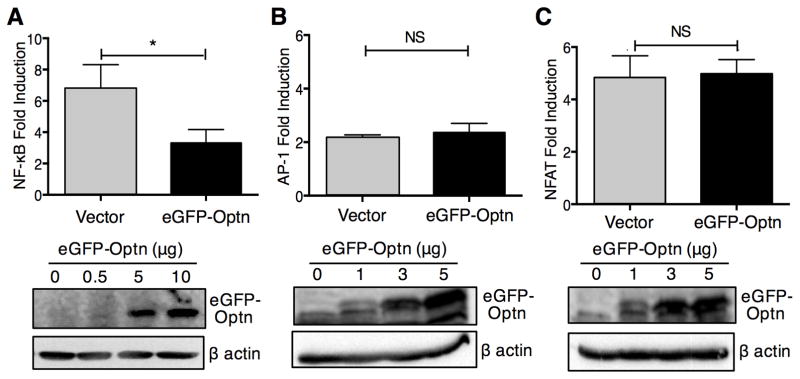
We sought to investigate a possible role for Optn during T cell activation by examining its effects on NF-κB, NFAT and AP-1 transcription, key pathways for T cell activation. We ectopically expressed eGFP-tagged Optn and evaluated TCR-induced NF-κB activity by luciferase reporter assay. As shown in Fig. 1A, ectopic expression of Optn resulted in significant inhibition of NF-κB activity. To assess if Optn may also play a role during induction of NFAT and AP-1 following TCR signaling, we co-transfected D10 cells with eGFP-Optn and AP-1 or NFAT luciferase reporter (Fig. 1B-C). Luciferase assays revealed that ectopic expression of Optn perturbed neither NFAT nor AP-1 activity. We also evaluated the effects of Optn overexpression on a composite NFAT/AP-1 luciferase reporter. Similar to our results with isolated NFAT and AP-1 reporters, ectopic expression of Optn did not alter transcription from a composite NFAT/AP-1 reporter (data not shown).
Fig. 1.
Ectopic expression of Optn specifically suppresses TCR-induced NF-κB activity. D10 T cells were co-transfected with the indicated concentration of eGFP-Optn plasmid, plus (A) NF-κB luciferase reporter plasmid, (B) AP-1 luciferase reporter plasmid or (C) NFAT reporter plasmid. Sixteen hours after transfection, cells were either left untreated or stimulated with anti-CD3 and anti-CD28 Abs for six hours. For luciferase assays, cells transfected with 5 ug of eGFP-Optn plasmid were assessed. (Lower panels) Immunoblotting to assess relative levels of expression of eGFP-Optn in the transfected D10 T cells. Each panel reports the data on triplicate samples from a single experiment, representative of three different experiments. Statistical analysis was carried out using the Mann-Whitney U test. *p<0.05.
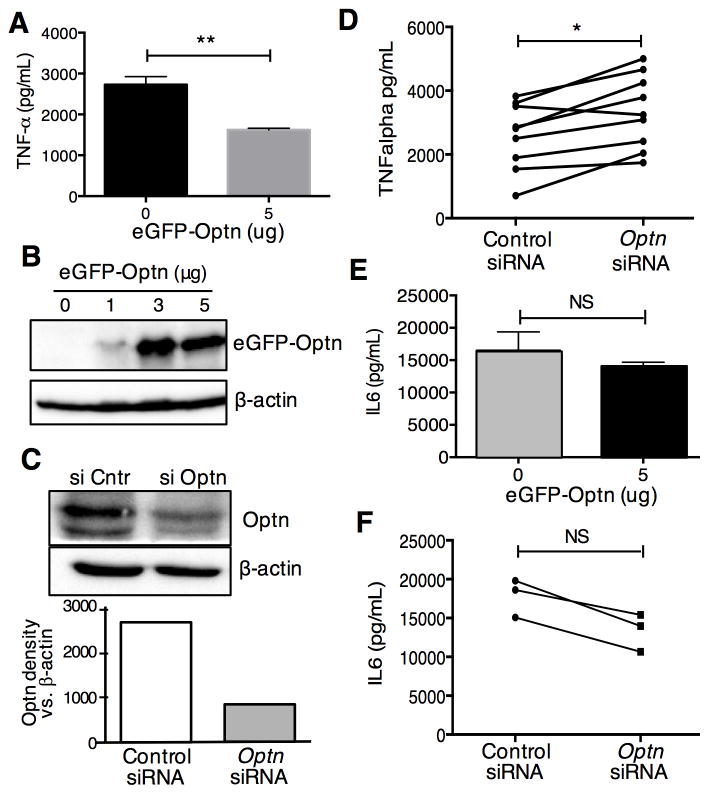
Next we examined the role of Optn in the more complex response of cytokine production. We transfected D10 T cells with eGFP-Optn, followed by stimulation with anti-CD3/CD28 Abs. Supernatants were collected after 24 hours of stimulation and cytokine release was quantified by ELISA. Thus, we observed (Fig. 2A-B) that ectopic expression of Optn resulted in a significant decrease in secretion of TNF-α, a canonical NF-κB regulated cytokine. To further validate the role played by Optn during TCR-induced T cell activation, we investigated the effects of Optn knock-down on cytokine production. Again, we used the murine D10 T cell clone, into which we transfected siRNA targeting Optn mRNA or a control siRNA. Forty-eight hours after siRNA transfection, cells were evaluated for levels of Optn protein, and stimulated with anti-CD3/CD28 Abs for determination of TNF-α release. Thus, we were able to achieve about 70% knock-down of Optn after siRNA treatment (Fig. 2C). After 24 hours of stimulation, supernatants were collected and assessed for cytokine release by ELISA. As might be expected, based on the results of ectopic expression of Optn, we observed a statistically significant increase in the amount of TNF-α produced by T cells with Optn knock-down (Fig. 2D), when controlling for experimental variability in the overall response of the cells to stimulation. The effects of Optn overexpression were apparently not due to general effects on cell health or viability, as production of IL-6 (Fig. 2E) or IL-4 (not shown) was not affected by ectopic expression of Optn. Although IL-4 production was also not affected by siRNA knock-down of Optn (not shown), IL-6 production tended to decrease after Optn knock-down (Fig. 2F), although this did not reach the level of statistical significance. Our findings are consistent with Obaid et al., who noted that TNF-α production was increased in osteoclasts expressing an Optn mutant defective in Ub binding, while IL-6 production was decreased in the same cells (Obaid et al., 2015).
Fig. 2.
Optn suppresses the production of TNF-α in T cells.
(A-B) D10 T cells were transfected with 5μg of eGFP-Optn or empty vector. Sixteen hours after transfection, cells were stimulated with plate-bound anti-CD3 Ab plus soluble anti-CD28 Ab for 24h. Supernatants were collected for ELISA quantitation of TNF-α (A). Expression of eGFP-Optn was assessed by western blotting with an anti-GFP antibody (B). (C–D) D10 T cells were transfected with Optn-specific siRNA or control siRNA. Forty-eight hours after transfection cells were stimulated with plate-bound anti-CD3 Ab plus soluble anti-CD28 Ab for 24h. Endogenous Optn expression was assessed by western blotting (C, upper panel) and quantified by densitometry after normalization to β actin (C, lower panel). Cell-free were collected and ELISA was performed to evaluate the concentration of TNF-α (D). (E–F) D10 T cells were transfected with eGFP-Optn (D) or siRNA (E) and analyzed for IL-6 secretion by ELISA. Each panel is representative of three different experiments, except for panel D, which is representative of eight different experiments. Statistical analysis was carried out on triplicate samples from a single experiment, using the Mann Whitney U test (panels A and E); paired t test on individual samples from nine independent experiments (panel D); or the Wilcoxon matched-pairs signed rank test, on samples from three independent experiments (panel F). *p<0.05; **p<0.01.
3.2 Relationship of Optn to the IKK complex
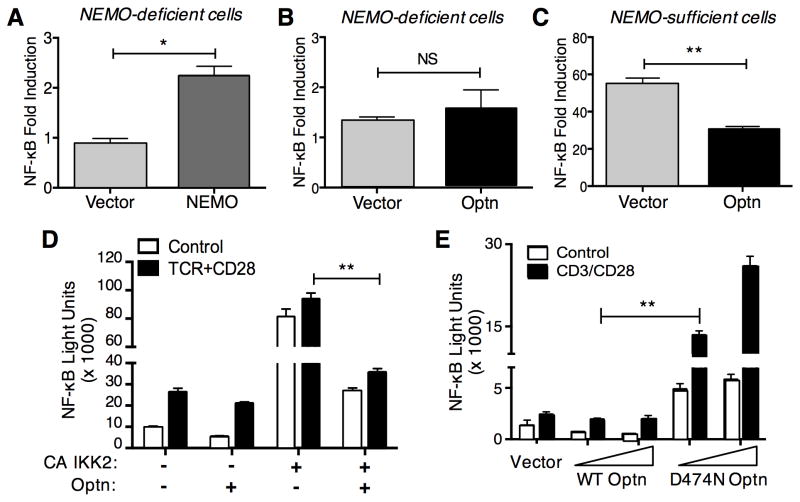
Given the high degree of homology between Optn and the IKKγ/NEMO protein of the IKK complex, we wanted to determine whether Optn could complement a NEMO-deficient T cell line for NF-κB induction. Attempts to study TCR signaling in NEMO-deficient T cell lines have been hindered by the fact that the most commonly studied NEMO deficient cell line available (SVT2C) lacks any detectable cell-surface TCR expression (Weil et al., 2003). Thus, here we employed a different NEMO-deficient T cell line - 8321 and the matched NEMO-expressing 3T8 cell line, which express moderate, but similar, levels of surface TCR (He and Ting, 2002). A previous study showed that in order to achieve significant activation, both the 8321 and 3T8 cell lines need to be stimulated with anti-TCR mAb together with a suboptimal dose of PMA, due to low levels of the co-stimulatory molecule CD28 (He and Ting, 2003).
First we confirmed that we could restore TCR-induced NF-κB activity in 8321 NEMO-deficient cells. Transient transfection of a vector expressing NEMO in 8321 cells resulted in significant increases in NF-κB activity following TCR/PMA stimulation (Fig. 3A), compared with empty vector-transfected 8321 cells. By contrast, transfection of eGFP-Optn did not result in NF-κB activation under the same conditions, suggesting that Optn could not replace NEMO for NF-κB signaling (Fig. 3B). Consistent with the findings described above, transfection of the parental NEMO-expressing 3T8 cells with eGFP-Optn impaired the induction of NF-κB activity after TCR stimulation (Fig. 3C). Taken together, these data demonstrate that Optn cannot replace the critical role played by NEMO during TCR-induced NF-κB activation.
Fig. 3.
Optn regulates NF-κB downstream of the IKK complex and does not substitute for NEMO in TCR-induced NF-κB.
The NEMO-deficient T cell line 8321 was transfected with 1μg of NEMO plasmid (A) or with 5μg of eGFP-Optn plasmid (B), together with NF-κB luciferase reporter plasmid. Sixteen hours after transfection, cells were either left untreated or stimulated with anti-TCR plus PMA for six hours. (C) Control 3T8 cells (8321 cells expressing NEMO) were co-transfected with 5μg of eGFP-Optn together with NF-κB luciferase reporter plasmid or vehicle control. Sixteen hours after transfection cells were left untreated or stimulated with anti-TCR plus PMA for hours. (D) Jurkat cells were co-transfected with 5μg of eGFP-Optn (or empty vector), NF-κB luciferase reporter plasmid and/or IKK2-CA plasmid. Sixteen hours after transfection, cells were either left untreated or stimulated with anti-TCR/CD28 antibodies for six hours. (E) Jurkat T cells were co-transfected with the indicated amount of WT eGFP-Optn or eGFP-D474N Optn, together with NF-κB luciferase reporter plasmid. Sixteen hours after transfection, cells were either left untreated or stimulated with anti-CD3/CD28 Abs for six hours. Each panel is representative of three different experiments. Statistical analysis was carried out on triplicate samples from a representative experiment, using the Mann-Whitney U test (panels A-C); or on multiple samples form a representative experiments using the Kruskal-Wallis test (panels D–E). *p<0.05; **p<0.01.
Since our data indicated that Optn negatively regulates NF-κB induction in T cells, we sought to more precisely determine at what point in the NF-κB signaling cascade Optn is playing a modulatory role. For this purpose, we co-transfected Jurkat T cells with a constitutively active (CA) form of IKKβ (IKK2), together with eGFP-Optn and an NF-κB luciferase reporter, then measured luciferase activity after TCR stimulation. As shown in Fig. 3D, Optn overexpression reduced NF-κB reporter activity about six-fold, compared to IKKCA transfection alone. Thus, Optn appears to act either at the level of the IKK complex itself or at more downstream point, since the effects of a constitutively active IKK are still inhibited.
It has been proposed that Optn may function as an adaptor protein to link deubiquitinases to polyubiquitinated substrates and so inhibit NF-κB activity (Journo et al., 2009). Therefore, we investigated the importance of the Optn Ub-binding domain during TCR signaling. To do so, we generated an eGFP-Optn plasmid with a point mutation in the UBD (D474N), a mutation that has been shown to disrupt binding of the Optn UBD to ubiquitin (Zhu et al., 2007). Jurkat T cells were transfected with increasing amounts of WT or D474N eGFP-Optn and subsequently stimulated with anti-TCR and anti-CD28 Abs. In contrast to the inhibition of NF-κB that we observed with WT Optn, the UBD mutant of Optn actually enhanced activation of NF-κB in conjunction with TCR/CD28 stimulation, suggesting that this mutant was acting in a “dominant negative” fashion (Fig. 3E).
3.3 Regulation of Optn during TCR-induced T cell activation
Since our data suggested a negative regulatory role for Optn, we explored possible changes in Optn expression during T cell activation. We stimulated Jurkat T cells with varying amounts of plate-bound anti-CD3 Ab, with or without anti-CD28 Ab (Fig. 4A). We noted a dose-dependent decrease in Optn protein with anti-CD3 stimulation. In Jurkat T cells, this occurred within 30 minutes following stimulation (Fig. 4B). To further validate this finding, we repeated this experiment in primary murine and human T cells (Fig. 4C,E). Thus, we observed that Optn was degraded after CD3/CD28 stimulation of primary T cells, although the kinetics of degradation were different than what was observed in Jurkat T cells. As shown by quantitative analysis (Fig. 4D,F) significant degradation of Optn occurred by three hours following T cell stimulation of both mouse and human primary T cells.
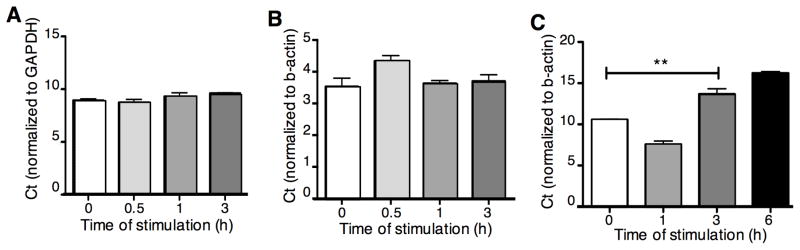
Next, we measured the levels of OPTN mRNA in Jurkat T cells, a murine T cell clone (D10) and primary murine CD4+ T cells. β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as internal controls. Quantitative RT-PCR revealed that TCR stimulation does not significantly modulate OPTN mRNA levels in Jurkat or D10 T cells at the time points investigated (Fig. 5A-B). However, we did observe a slightly decreased level of OPTN mRNA in primary murine CD4+ T cells three hours after anti-CD3 stimulation, followed by a rebound effect three-to-six hours after stimulation (Fig. 5C). Taken together these data show that stimulation of T cells through TCR and CD28 leads to a rapid reduction in Optn protein, with less dramatic effects on OPTN message.
Fig. 5.
Relative levels of Optn mRNA expression after anti-TCR(CD3)/CD28 stimulation of Jurkat T cells (A), D10 T cells (B) and CD3+ primary murine T cells (C), was assessed by real-time PCR. Results are shown as Optn Ct values normalized to the indicated endogenous control. Data are representative of three independent experiments. Statistical analysis was carried out on triplicate samples from a representative experiment, using the Kruskal-Wallis test. **p<0.01.
3.4 Protease-dependent turnover of Optn protein after TCR stimulation
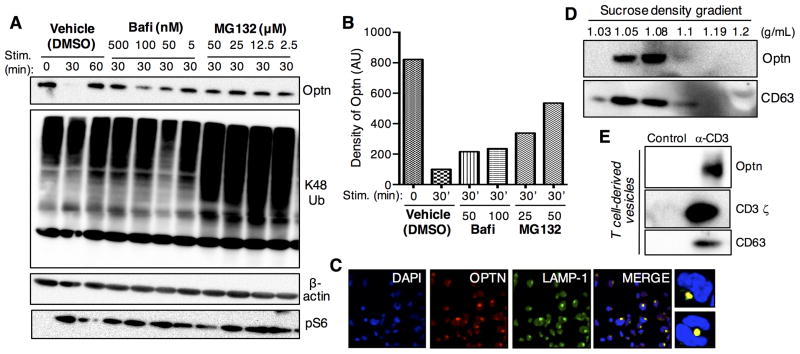
To evaluate whether the reduced levels of endogenous Optn observed in T cells were the result of protein degradation, we tested the roles of proteasomal and lysosomal degradation pathways in Optn turnover (Fig. 6). Jurkat T cells were pretreated with different concentrations of MG132 (proteasome inhibitor) or bafilomycin (a lysosomal protease inhibitor) prior to stimulation with anti-CD3/CD28 Abs. Cell lysates were subjected to western blot analysis for Optn protein. While the vehicle control showed Optn degradation thirty minutes after TCR-induced T cell stimulation, higher levels of Optn were observed in cells treated with either proteasome or lysosome inhibitors (Fig. 6A). To confirm that treatment with MG132 led to proteasome inhibition, cell lysates were subjected to immunoblotting for K48-linked ubiquitin. As shown (Fig. 6A, central panel) more significant ubiquitin accumulation was observed in samples pretreated with MG132 prior to stimulation. Quantitative analysis revealed that while treatment with increasing concentrations of MG132 resulted in a relatively efficient inhibition of Optn degradation (Fig. 6B), treatment with bafilomycin had a less pronounced effect. To rule out that the lack of TCR-induced protein degradation led to general inhibition of T cell activation, we performed immunoblotting for pS6, a downstream target of Akt/mTOR signaling. Thus, we confirmed that pS6 induction was not impaired by either bafilomycin or MG132 (Fig. 6A, bottom panel).
Fig. 6.
TCR-induced Optn turnover is due to both Optn degradation and release via exosomes. Jurkat T cells were pretreated with the lysosomal inhibitor bafilomycin, the proteosomal inhibitor MG-132 or DMSO as a control. (A) Protein expression was analyzed by immunoblotting. Jurkat T cells were pre-treated for 30 min at 37°C with increasing concentration of bafilomycin (5–500 nM) or MG-132 (2.5–50 μM) or DMSO. After inhibitor pretreatment, Jurkat T cells were washed with PBS and stimulated for 30 min on anti-CD3 (OKT3)-coated plates plus soluble anti-CD28. To validate the efficiency of proteasomal inhibition, we performed immunoblotting for K48-ubiquitin. T cell activation was assessed by immunoblotting for pS6. (B) Protein expression densitometry of Optn expression after 30min of TCR stimulation was normalized to β-actin expression, and then normalized to non-stimulated cells. (C) Optn localizes within a late endosomal compartment. Jurkat T cells were settled onto glass slides and stained for the indicated markers (larger panels). Smaller right panels show two magnified regions. (D) Exosomes were isolated from anti-CD3-stimulated Jurkat T cells (6 hr stim). After centrifugation, supernatant was seeded on a 30% sucrose gradient. Pellets were collected and western blotting was performed as indicated for Optn and CD63 (exosome control). (E) Exosomes were isolated as above and the peak exosome fraction was analyzed by western blotting for Optn, CD63 and TCR zeta, as a surrogate for TCR/CD3. Each panel is representative of two independent experiments.
To confirm whether the lower levels of Optn protein in bafilomycin-treated samples were not the result of cell death, we pretreated Jurkat T cells with a lower concentration of bafilomycin and followed the extent of Optn degradation after TCR-induced T cell stimulation for a longer time; we observed no difference between bafilomycin-treated samples and vehicle controls (DMSO) (data not shown). Therefore, we conclude that TCR stimulation triggers the degradation of endogenous Optn through both proteasomal and lysosomal pathways. Nonetheless, we were not able to completely rescue Optn levels by protease inhibition, suggesting the existence of other mechanisms of Optn turnover. It has been reported that when the amount of protein targeted for proteasomal degradation saturates the proteasomal machinery, subsequent accumulation of proteins may activate autophagic-lysosomal degradation (Yao, 2010).
3.5 Subcellular localization and release of Optn from T cells
Optn has been reported to bind to Rab8, huntington and myosin VI, important regulators of vesicle transport and secretory vesicle fusion at the plasma membrane (Peranen et al., 1996; Vaibhava et al., 2012). It has been shown that Optn can be associated with the Golgi apparatus (Sahlender et al., 2005). We performed confocal immunofluorescence imaging, with Abs to Optn and markers of the selected endosome compartments, to investigate the steady-state subcellular localization of Optn. We speculated that since Optn is enriched in an exosome-like population of vesicles, we would be able to detect Optn and LAMP-1 co-localization. Our results indicate that during steady-state conditions Optn localizes, at least in part, in late endosomal compartments (Fig. 6C).
Vesicular late endosomal compartments, also called multivesicular bodies (MVB), fuse with the plasma membrane to release their content (exosomes) in the extracellular milieu (Colombo et al., 2014; Denzer et al., 2000; Raposo and Stoorvogel, 2013). Exosomes are enriched in MHC II together with co-stimulatory molecule CD86 and in several tetraspan proteins, including CD37, CD53, CD53, CD81 and CD82. These are localized on the internal membrane on the MVB, whereas other molecules such as Lamp-1 and Lamp-2 are mainly localized to the limiting membrane. Thus, we investigated if TCR-induced T cell activation results in release of Optn in exocytic vesicles. We stimulated Jurkat T cells with anti-CD3 Ab for six hours, and collected cell supernatants for vesicle preparation. To characterize the vesicle population associated with Optn exocytosis, we performed sucrose gradient ultracentrifugation (Montecalvo et al., 2012). We found that the Optn-enriched vesicle population isolated from stimulated Jurkat T cells corresponds to the sucrose gradient fractions associated with exosomes (Fig. 6D), which have previously been shown to include TCR complexes (Blanchard et al., 2002). As shown in Figure 6E, immunoblot analysis revealed the presence of Optn-associated vesicles in the supernatant of stimulated T cells, whereas we did not detect CD63- or Optn containing vesicles in the supernatant of resting T cells. Finally, as also shown in Figure 6E, activated T cell-derived Optn-containing vesicles also contain the ζ protein, consistent with these vesicles being exosomes.
The findings described above suggest that vesicles enriched with Optn and TCR molecules are indeed associated with exocytosis following TCR stimulation. Previous results indicated that NEMO and Optn do not directly interact (Schwamborn et al., 2000), so Optn and NEMO may be enriched in different vesicular populations. Therefore, we co-transfected Jurkat T cells with eGFP-Optn and RFP-NEMO plasmids and performed live cell imaging on transfected T cells activated on anti-TCR-coated dishes, using TIRF microscopy. As shown in the Video, vesicular Optn appears to be distinct from the vesicular pool of NEMO protein, consistent with the notion that Optn and NEMO are part of different T cell activation complexes. Interestingly, vesicular trafficking of Optn exhibits a characteristic exocytosis pattern. NEMO-containing vesicles appear bigger in size than the Optn cargo vesicles and also show slower and more confined movements. Upon closer analysis, we observed that Optn-containing vesicles appeared to move along microtubules from the cytosolic compartment to the plasma membrane, where they disappear. Thus, our data suggest that Optn and NEMO traffic through different vesicular compartments, and that Optn undergoes exocytosis following TCR activation.
4. Discussion
This study provides new insights into the role played by Optn during TCR-induced T cell activation. Interestingly, we observed that TCR triggering induces rapid turnover of Optn protein, which may ultimately serve as a mechanism to modulate T cell activation, and is consistent with a negative regulatory role for Optn. We investigated the nature of the TCR-induced Optn turnover and demonstrate that Optn degradation in T cells occurs through both lysosomal and proteasomal degradation. We speculate that upon TCR triggering Optn may be targeted to the proteasomal machinery for degradation, but since Optn can be organized into large protein clusters, this may saturate the proteasomal machinery and then be shunted to the lysosomal compartment. Upon TCR stimulation, T cells can also release vesicles into the extracellular milieu (Blanchard et al., 2002). Thus, we show for the first time that T cell-derived exosomes are enriched with Optn, which may constitute a mechanism by which TCR signaling is dampened. In the same vein, it has been shown that Optn binds to other proteins involved in vesicular trafficking to the plasma membrane, such as myosin VI and Rab8 (Chibalina et al., 2010; Chibalina et al., 2008). Optn has also been reported to localize to the Golgi compartment (Sahlender et al., 2005; Sippl et al., 2014).
Our results show that Optn down-regulates NF-κB induced TCR signaling at the level of the IKK complex or downstream, and that the Ub-binding domain seems to play an important role in this process. Moreover, we show that negative regulation of NF-κB by Optn is reflected in corresponding changes in the levels of TNF-α secretion upon T cell stimulation. Although there is some overlap in their regulation, IL-6 and IL-4 are under the regulation of different combinations of transcription factors. Indeed, we report here that following TCR activation Optn does not regulate NFAT and AP-1 signaling. Of note, we observed increased levels of TNF-α secretion after Optn knock-down, which leads us to conclude that the inhibitory effects of ectopic expression of Optn indeed reflect an inhibitory role for Optn in this pathway.
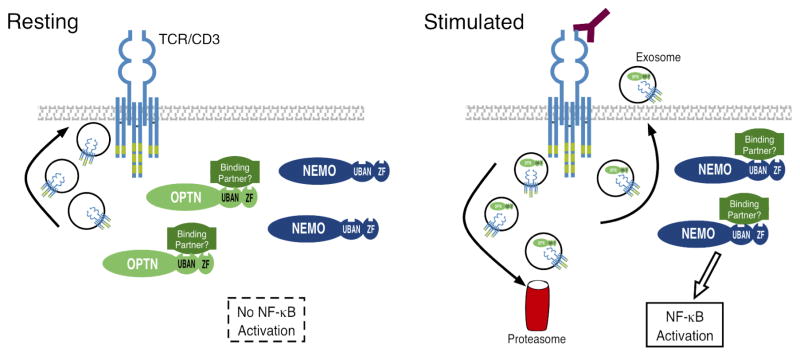
A model illustrating our findings is presented in Figure 7. Thus, during the steady state, TCR molecules transit through a recycling pool, as a result of internalization, endocytosis and cycling from and to the plasma membrane, via vesicular transport (Clark and Griffiths, 2003; Das et al., 2004; Liu et al., 2000; Minami et al., 1987; Myers et al., 2005). It has been speculated that constant TCR recycling during the steady-state, together with other biochemical events, contribute to control T cell homeostasis (Boding et al., 2009). In addition, the rapid cycling of surface receptors allows T cells to respond rapidly to stimuli by controlling responsiveness to peptide/MHC ligands (Harding and Unanue, 1990). We propose that during the steady state Optn might sequester an intermediate signaling mediator of NF-κB shared with NEMO, which could, through the aforementioned mechanisms, regulate T cell responsiveness to TCR signaling. Upon TCR stimulation, Optn undergoes degradation and exocytosis, therefore altering the balance between Optn and its putative targets in NF-κB signaling. Exocytosis of Optn might also contribute to TCR cycling through exocytosis. Our data show the presence of Optn and TCR in exosomes released upon T cell stimulation, although further investigation is needed to establish if Optn regulates the TCR itself, or if Optn- and TCR- containing vesicles are overlapping or distinct and serve different roles.
Fig. 7.
Model for the negative regulation of NF-κB signaling by Optn, and for downregulating levels of Optn protein during T cell activation. UBAN refers to the ubiquitin-binding domain of Optn and NEMO, while ZF refers to the zinc finger domain.
In support of our model, TCR-mediated NF-κB induction was suppressed in cells that overexpress Optn, relative to cells with only endogenous levels of Optn. As stated above, Optn overexpression might sequester an intermediate NF-κB signaling substrate shared with NEMO to inhibit NEMO-dependent NF-κB signaling. When we impaired the ability of Optn to bind to ubiquitinated substrate, with the D674N ubiquitin-binding domain (UBD) mutation, we noted that TCR-induced NF-κB activity was actually increased, even over that of cells transfected with empty vector. Thus, the UBD mutant of Optn appears to act as a “dominant negative,” interfering with the activity of endogenous Optn. This is another piece of evidence that supports a negative regulatory function for Optn during T cell activation.
Conflicting conclusions have been drawn regarding the function of Optn in the immune system (Kachaner et al., 2012a; Munitic et al., 2013; Obaid et al., 2015). Our study in T cells has revealed that Optn activity is down-regulated after TCR stimulation, consistent with the observed ability of Optn to inhibit NF-κB downstream of the TCR and CD28. Future studies will address the specific targets of the ubiquitin-binding domain of Optn and the role of this protein in T cell activation in vivo.
Supplementary Material
Highlights.
Optineurin (Optn) is expressed in murine and human T cells
Ectopic expression of Optn suppresses TCR-mediated induction of NF-κB and production of TNF-α
Knock-down of Optn enhances NF-κB induction and TNF-α production in T cells
T cell activation leads to rapid loss of endogenous Optn protein, through both degradation and exocytosis in microvesicles
Acknowledgments
Supported by NIH grant AI103022 (to L.P.K.). Author contributions: A.M. designed and performed experiments and co-wrote the manuscript; S.C.W. performed TIRF imaging. L.P.K. provided funding, designed experiments and co-wrote the manuscript. Conflicts of interest: The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albagha OM, Visconti MR, Alonso N, Langston AL, Cundy T, Dargie R, Dunlop MG, Fraser WD, Hooper MJ, Isaia G, Nicholson GC, del Pino Montes J, Gonzalez-Sarmiento R, di Stefano M, Tenesa A, Walsh JP, Ralston SH. Genome-wide association study identifies variants at CSF1, OPTN and TNFRSF11A as genetic risk factors for Paget's disease of bone. Nature genetics. 2010;42:520–524. doi: 10.1038/ng.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung T, Ebenezer ND, Brice G, Child AH, Prescott Q, Lehmann OJ, Hitchings RA, Bhattacharya SS. Prevalence of optineurin sequence variants in adult primary open angle glaucoma: implications for diagnostic testing. J Med Genet. 2003;40:e101. doi: 10.1136/jmg.40.8.e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. Journal of immunology. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- Boding L, Bonefeld CM, Nielsen BL, Lauritsen JP, von Essen MR, Hansen AK, Larsen JM, Nielsen MM, Odum N, Geisler C. TCR down-regulation controls T cell homeostasis. Journal of immunology. 2009;183:4994–5005. doi: 10.4049/jimmunol.0901539. [DOI] [PubMed] [Google Scholar]
- Chibalina MV, Poliakov A, Kendrick-Jones J, Buss F. Myosin VI and optineurin are required for polarized EGFR delivery and directed migration. Traffic. 2010;11:1290–1303. doi: 10.1111/j.1600-0854.2010.01101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibalina MV, Roberts RC, Arden SD, Kendrick-Jones J, Buss F. Rab8-optineurin-myosin VI: analysis of interactions and functions in the secretory pathway. Methods Enzymol. 2008;438:11–24. doi: 10.1016/S0076-6879(07)38002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhuri K, Llodra J, Roth EW, Tsai J, Gordo S, Wucherpfennig KW, Kam LC, Stokes DL, Dustin ML. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature. 2014;507:118–123. doi: 10.1038/nature12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R, Griffiths GM. Lytic granules, secretory lysosomes and disease. Current opinion in immunology. 2003;15:516–521. doi: 10.1016/s0952-7915(03)00113-4. [DOI] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Das V, Nal B, Dujeancourt A, Thoulouze MI, Galli T, Roux P, Dautry-Varsat A, Alcover A. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity. 2004;20:577–588. doi: 10.1016/s1074-7613(04)00106-2. [DOI] [PubMed] [Google Scholar]
- Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. Journal of cell science. 2000;113(Pt 19):3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- Gleason CE, Ordureau A, Gourlay R, Arthur JS, Cohen P. Polyubiquitin binding to optineurin is required for optimal activation of TANK-binding kinase 1 and production of interferon beta. The Journal of biological chemistry. 2011;286:35663–35674. doi: 10.1074/jbc.M111.267567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding CV, Unanue ER. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature. 1990;346:574–576. doi: 10.1038/346574a0. [DOI] [PubMed] [Google Scholar]
- He KL, Ting AT. A20 inhibits tumor necrosis factor (TNF) alpha-induced apoptosis by disrupting recruitment of TRADD and RIP to the TNF receptor 1 complex in Jurkat T cells. Molecular and cellular biology. 2002;22:6034–6045. doi: 10.1128/MCB.22.17.6034-6045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He KL, Ting AT. Essential role for IKKgamma/NEMO in TCR-induced IL-2 expression in Jurkat T cells. European journal of immunology. 2003;33:1917–1924. doi: 10.1002/eji.200323650. [DOI] [PubMed] [Google Scholar]
- Journo C, Filipe J, About F, Chevalier SA, Afonso PV, Brady JN, Flynn D, Tangy F, Israel A, Vidalain PO, Mahieux R, Weil R. NRP/Optineurin Cooperates with TAX1BP1 to potentiate the activation of NF-kappaB by human T-lymphotropic virus type 1 tax protein. PLoS pathogens. 2009;5:e1000521. doi: 10.1371/journal.ppat.1000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachaner D, Genin P, Laplantine E, Weil R. Toward an integrative view of Optineurin functions. Cell Cycle. 2012a;11:2808–2818. doi: 10.4161/cc.20946. [DOI] [PubMed] [Google Scholar]
- Kachaner D, Laplantine E, Genin P, Weil R. Optineurin: a new vision of cell division control. Cell Cycle. 2012b;11:1481–1482. doi: 10.4161/cc.20116. [DOI] [PubMed] [Google Scholar]
- Laplantine E, Fontan E, Chiaravalli J, Lopez T, Lakisic G, Veron M, Agou F, Israel A. NEMO specifically recognizes K63-linked poly-ubiquitin chains through a new bipartite ubiquitin-binding domain. The EMBO journal. 2009;28:2885–2895. doi: 10.1038/emboj.2009.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kang J, Friedman J, Tarassishin L, Ye J, Kovalenko A, Wallach D, Horwitz MS. Identification of a cell protein (FIP-3) as a modulator of NF-kappaB activity and as a target of an adenovirus inhibitor of tumor necrosis factor alpha-induced apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1042–1047. doi: 10.1073/pnas.96.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Rhodes M, Wiest DL, Vignali DA. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity. 2000;13:665–675. doi: 10.1016/s1074-7613(00)00066-2. [DOI] [PubMed] [Google Scholar]
- Minami Y, Samelson LE, Klausner RD. Internalization and cycling of the T cell antigen receptor. Role of protein kinase C. The Journal of biological chemistry. 1987;262:13342–13347. [PubMed] [Google Scholar]
- Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, Watkins SC, Morelli AE. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munitic I, Giardino Torchia ML, Meena NP, Zhu G, Li CC, Ashwell JD. Optineurin insufficiency impairs IRF3 but not NF-kappaB activation in immune cells. Journal of immunology. 2013;191:6231–6240. doi: 10.4049/jimmunol.1301696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MD, Dragone LL, Weiss A. Src-like adaptor protein down-regulates T cell receptor (TCR)-CD3 expression by targeting TCRzeta for degradation. The Journal of cell biology. 2005;170:285–294. doi: 10.1083/jcb.200501164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid R, Wani SE, Azfer A, Hurd T, Jones R, Cohen P, Ralston SH, Albagha OM. Optineurin Negatively Regulates Osteoclast Differentiation by Modulating NF-kappaB and Interferon Signaling: Implications for Paget's Disease. Cell reports. 2015;13:1096–1102. doi: 10.1016/j.celrep.2015.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peranen J, Auvinen P, Virta H, Wepf R, Simons K. Rab8 promotes polarized membrane transport through reorganization of actin and microtubules in fibroblasts. The Journal of cell biology. 1996;135:153–167. doi: 10.1083/jcb.135.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. The Journal of cell biology. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlender DA, Roberts RC, Arden SD, Spudich G, Taylor MJ, Luzio JP, Kendrick-Jones J, Buss F. Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. The Journal of cell biology. 2005;169:285–295. doi: 10.1083/jcb.200501162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwamborn K, Weil R, Courtois G, Whiteside ST, Israel A. Phorbol esters and cytokines regulate the expression of the NEMO-related protein, a molecule involved in a NF-kappa B-independent pathway. The Journal of biological chemistry. 2000;275:22780–22789. doi: 10.1074/jbc.M001500200. [DOI] [PubMed] [Google Scholar]
- Sippl C, Zeilbeck LF, Fuchshofer R, Tamm ER. Optineurin associates with the podocyte Golgi complex to maintain its structure. Cell Tissue Res. 2014 doi: 10.1007/s00441-014-1968-8. [DOI] [PubMed] [Google Scholar]
- Smith A, Sewell G, Levine A, Chew T, Dunne J, O'Shea N, Smith P, Harrison P, Macdonald C, Bloom S, Segal A. Disruption of macrophage pro-inflammatory cytokine release in Crohn's disease is associated with reduced optineurin expression in a subset of patients. Immunology. 2014 doi: 10.1111/imm.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang ED, Wang CY, Xiong Y, Guan KL. A role for NF-kappaB essential modifier/IkappaB kinase-gamma (NEMO/IKKgamma) ubiquitination in the activation of the IkappaB kinase complex by tumor necrosis factor-alpha. The Journal of biological chemistry. 2003;278:37297–37305. doi: 10.1074/jbc.M303389200. [DOI] [PubMed] [Google Scholar]
- Ueno H, Kobatake K, Matsumoto M, Morino H, Maruyama H, Kawakami H. Severe brain atrophy after long-term survival seen in siblings with familial amyotrophic lateral sclerosis and a mutation in the optineurin gene: a case series. J Med Case Rep. 2011;5:573. doi: 10.1186/1752-1947-5-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaibhava V, Nagabhushana A, Chalasani ML, Sudhakar C, Kumari A, Swarup G. Optineurin mediates a negative regulation of Rab8 by the GTPase-activating protein TBC1D17. Journal of cell science. 2012;125:5026–5039. doi: 10.1242/jcs.102327. [DOI] [PubMed] [Google Scholar]
- Weil R, Schwamborn K, Alcover A, Bessia C, Di Bartolo V, Israel A. Induction of the NF-kappaB cascade by recruitment of the scaffold molecule NEMO to the T cell receptor. Immunity. 2003;18:13–26. doi: 10.1016/s1074-7613(02)00506-x. [DOI] [PubMed] [Google Scholar]
- Yao TP. The role of ubiquitin in autophagy-dependent protein aggregate processing. Genes & cancer. 2010;1:779–786. doi: 10.1177/1947601910383277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Wertz I, O'Rourke K, Ultsch M, Seshagiri S, Eby M, Xiao W, Dixit VM. Bcl10 activates the NF-kappaB pathway through ubiquitination of NEMO. Nature. 2004;427:167–171. doi: 10.1038/nature02273. [DOI] [PubMed] [Google Scholar]
- Zhu G, Wu CJ, Zhao Y, Ashwell JD. Optineurin negatively regulates TNFalpha- induced NF-kappaB activation by competing with NEMO for ubiquitinated RIP. Current biology : CB. 2007;17:1438–1443. doi: 10.1016/j.cub.2007.07.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.