Abstract
Recreational use of substituted cathinones continues to be an emerging public health problem in the United States; cathinone derivatives α-pyrrolidinopentiophenone (α-PVP) and 3,4-methylenedioxypyrovalerone (MDPV), which have been linked to human fatalities and show high potential for abuse liability in animal models, are of particular concern. The objective of this study was to develop an immunotherapeutic strategy for attenuating the effects of α-PVP and MDPV in rats, using drug-conjugate vaccines created to generate antibodies with neutralizing capacity. Immunoconjugates (α-PVP-KLH and MDPV-KLH) or the control carrier protein, keyhole limpet hemocyanin (KLH), were administered to groups (N=12) of male Sprague-Dawley rats on Weeks 0, 2 and 4. Groups were administered α-PVP or MDPV (0.0, 0.25, 0.5, 1.0, 5.0 mg/kg, i.p.) in acute drug challenges and tested for changes in wheel activity. Increased wheel activity produced by α-PVP or MDPV in the controls was attenuated in the α-PVP-KLH and MDPV-KLH vaccinated groups, respectively. Rectal temperature decreases produced by MDPV in the controls were reduced in duration in the MDPV-KLH vaccine group. A separate group (N=19) was trained to intravenously self-administer α-PVP (0.05, 0.1 mg/kg/inf) and vaccinated with KLH or α-PVP-KLH, post-acquisition. Self-administration in α-PVP-KLH rats was initially higher than in the KLH rats but then significantly decreased following a final vaccine booster, unlike the stable intake of KLH rats. The data demonstrate that active vaccination provides functional protection against the effects of α-PVP and MDPV, in vivo, and recommend additional development of vaccines as potential therapeutics for mitigating the effects of designer cathinone derivatives.
Keywords: immunopharmacotherapy, cathinone, wheel running, self-administration, vaccine
1. Introduction
The recreational use of various substituted cathinone stimulants has increased significantly over the past several years. The US Drug Enforcement Administration (DEA) classified 3,4-methylenedioxypyrovalerone (MDPV; aka “bathsalts”) and the closely-related drug α-pyrrolidinopentiophenone (α-PVP; aka “gravel” or “flakka”) as scheduled I compounds (Drug Enforcement Administration, 2011, 2014), in response to an increase in medical emergencies and drug-associated human fatalities (Benzie et al, 2011). Of all the substituted cathinones that have emerged on the market, MDPV and α-PVP show the clearest potential for compulsive, repetitive use in rat self-administration models (Aarde et al, 2015a; Aarde et al, 2013; Aarde et al, 2015b; Schindler et al, 2016; Watterson et al, 2014). These entities are pharmacologically distinct from most of the other cathinone derivatives because they are restricted to monoamine transporter inhibition and do not serve as transporter substrates or monoamine releasers (Baumann et al, 2013; Marusich et al, 2014; Simmler et al, 2013).
Researchers have investigated immunopharmacotherapy for the treatment of drugs of abuse for decades, as reviewed (Moreno and Janda, 2011). The key concept behind this approach involves the creation of drug-protein immunoconjugates, which generate high affinity antibodies in vivo against the target drug, resulting in sequestration of the drug in the peripheral circulation. Vaccines selective for cocaine (Carrera et al, 1995; Kosten et al, 2014), nicotine (Carrera et al, 2004; Roiko et al, 2008) and methamphetamine (MA) (Gentry et al, 2009; Miller et al, 2015; Miller et al, 2013) have all demonstrated efficacy for attenuating the drug-induced changes in locomotor activity and/or self-administration in rodents.
There is currently a lack of targeted therapeutic interventions for substituted cathinones (Glennon, 2014), and the development of novel active vaccines provides a systematic method for targeting various drug effects. The objective of this study was to develop therapeutic vaccines with efficacy against substituted cathinone stimulants, α-PVP and MDPV. The active vaccines were initially evaluated in vivo using an activity wheel model of locomotor activity because locomotor assays have proven to be effective first line tests in identifying the in vivo efficacy of vaccines for cocaine (Carrera et al, 1995), nicotine (Pentel et al, 2000) and methamphetamine (Miller et al, 2013; Shen et al, 2013). Furthermore, both MDPV and α-PVP have been shown to function as locomotor stimulants in rodents (Aarde et al, 2015a; Aarde et al, 2013; Fantegrossi et al, 2013; Gatch et al, 2015; Gatch et al, 2013; Huang et al, 2012; Marusich et al, 2014).
Several studies have also shown efficacy of anti-drug vaccines for modifying drug-taking behavior in the rat model of self-administration. This includes evidence for methamphetamine (Duryee et al, 2009; Miller et al, 2015), cocaine (Kantak et al, 2000), oxycodone (Pravetoni et al, 2014), heroin (Schlosburg et al, 2013), and nicotine (LeSage et al, 2006; Moreno et al, 2010). The present study therefore went on to determine if the α-PVP-KLH conjugate vaccine was capable of altering the intravenous self-administration of α-PVP. This was chosen over MDPV since α-PVP appears to have substantially replaced MDPV amongst US user populations and the vaccine exhibited efficacy at lower doses in the initial activity wheel study.
2. Materials and Methods
2.1 Subjects
Adult male Sprague-Dawley (N=36 wheel; N=19 self-administration) (Charles River, New York) rats were housed in humidity and temperature-controlled vivaria (23±1°C) on 12:12 hour light:dark cycles. Animals entered the laboratory at ~9 weeks of age and were 10–11 weeks old (mean weight 359 g; SEM 4.9) at the start of the study. Animals had ad libitum access to food and water in their home cages. All procedures were conducted under protocols approved by the Institutional Care and Use Committees of The Scripps Research Institute and in a manner consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Immunoconjugate Preparation
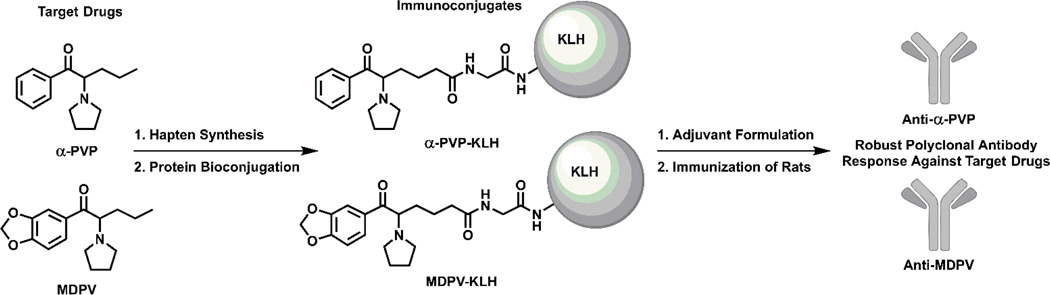
α-PVP or MDPV carboxyl haptens were designed to possess the greatest degree of structural homology to the target cathinone drugs as possible (see Supplemental Materials Fig. S1–2 for synthetic methods). Bioconjugation of the haptens proceeded via an activated N-hydroxysuccinimide (NHS) ester for amide coupling to surface lysines on either bovine serum albumin (BSA) or keyhole limpet hemocyanin (KLH) proteins (Fig. 1). Cathinone haptens (1:1 w/w ratio of hapten to protein, 30 mM in 10% H2O/DMF) were activated using 6 mol equivalents of NHS and 6 mol equivalents of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) over 2 h. Completion of activation was confirmed by liquid chromatopgraphy/mass spectrometry (LC/MS) at which point the hapten solution was added directly to a 1.5 mg/mL solution of protein in pH 7.4 PBS. The solution was gently mixed over 4 h at room temperature and 18 h at 4 °C. The resulting hapten-protein conjugate was dialyzed with 3 buffer changes of pH 7.4 PBS and the purified conjugates were diluted 3:1 with glycerol and stored at 4 °C until injected.
Figure 1.
Chemical structures of target synthetic cathinones and corresponding immunoconjugates.
2.3 Immunizations
Immunoconjugates α-PVP-KLH and MDPV-KLH (150 µg per rat per injection) were mixed with CpG oligodeoxynucleotide (CpG ODN) (Eurofins, 100 µg per rat per injection) and Alhydrogel alum (Invivogen, 1 mg per rat per injection) for 30 min. For locomotor activity testing, rats were administered the conjugate vaccines (N=12 per conjugate) or protein-only control (KLH; N=12) on Weeks 0, 2 and 4 with blood samples obtained on Weeks 0–6 and 10. For the intravenous self-administration experiment, rats were vaccinated four days after the last acquisition session (Week 0) and then on Weeks 2 and 4 (as with the locomotor groups) with an additional vaccination boost during Week 8. Vaccines were administered via i.p. injection with an injection volume of 200 uL. Blood samples were obtained on Weeks 1, 3, 4.5 (3 days post-inoculation to minimize impact on IVSA which started 6 days after the Week 4 inoculation), 8 (one day prior to inoculation) and 12. The immunization protocol was adapted from a heroin vaccination protocol previously reported (Bremer et al, 2014).
2.4 Immunologic Assays
Rats were anesthetized with an isoflurane/oxygen vapor mixture (isoflurane 5% induction, 1–3% maintenance), and blood was collected from the jugular vein during Weeks 1–6, and 10. Antibody titer was defined by the dilution required to achieve a 50% signal using enzyme-linked immunosorbent assay (ELISA) with a Biomek 4000 liquid handling robot. 96-well assay plates were coated with 25 µg/well cathinone-BSA conjugate and blocked with skim milk. Twelve 1:1 rat plasma dilutions were added to the plate starting at 1:200 and allowed to incubate for 2 h. Following a wash step, goat anti-rat HRP IgG (SouthernBiotech) at 1:10,000 dilution was incubated in the plates for 18 h at 4 °C. After a second wash step the plates were developed using a 3,3’–5,5’-tetramethylbenzidine (TMB) substrate kit (Thermo Pierce) and 2 M H2SO4 as a stopping solution. The well absorbance values were read at 450 nm and normalized to the highest value for each sample in GraphPad Prism version 6, followed by curve fitting with log(inhibitor) vs. normalized response – variable slope to find the midpoint titer. Competitive ELISA was also performed in a similar manner but with an added step: plasma at the IC80 dilution was incubated with free drug (α-PVP, MDPV or methamphetamine) dilutions of 1 mM to 0.1 nM (eleven 5-fold dilutions) in cathinone-BSA coated plates for 2 h.
2.5 Drugs
Racemic α-pyrrolidinopentiophenone HCl (α-PVP) was obtained from Cayman Chemical Company (Ann Arbor, MI, USA). Racemic 3,4-methylenedioxypyrovalerone HCl (MDPV) was obtained from Fox Chase Chemical Diversity Center (Doylestown, PA, USA). All drugs were expressed as the salt and dissolved in physiological saline (0.9%). For locomotor and temperature studies, rats were administered 0.9% saline vehicle, α-PVP (0.25, 0.5, 1.0 and 5.0 mg/kg) or MDPV (0.25, 0.5, 1.0 and 5.0 mg/kg) via intraperitoneal (i.p.) injection with an injection volume of 1 ml/kg body weight. Dosing order was counterbalanced within each group with 3–6-day intervals between sessions necessitating a 4 week interval for each drug series.
2.6 Activity and Temperature Response Evaluation
One week prior to the start of immunization, rats were given 1 h access to a standard activity wheel attached to clear shoebox cages (Model ENV-046; 35.6 cm diameter; Med Associates, St. Albans, VT) for 2 days to establish baseline activity differences to balance the vaccine groups prior to immunization. A full wheel revolution was equivalent to 1.12 m and quarter-rotations (wheel activity QRs) were recorded. Rats were separated into three treatment conditions (KLH, α-PVP-KLH vaccine, and MDPV-KLH vaccine) and then probed again for baseline activity on two 1 h sessions in Week 7 to verify no major differences in spontaneous wheel activity were caused by the immunizations. Drug challenges were started on Week 9. Following drug injections, rats had 4 h access to the wheel and activity rates were recorded every 5 minutes. Half of the KLH rats (N=6) completed a full dose response series with α-PVP followed by a dose response with MDPV and half completed the MDPV series first and the α-PVP series second. An initial statistical analysis failed to confirm any differences in the effect of either drug across series order thus all KLH animals (N=12) were included in the analysis. Primary analysis focused on the average wheel activity in 1 h intervals as derived from the primary 5 min sampling bins. Rats were also assessed in the same sessions for possible drug-induced thermoregulatory changes using hourly rectal temperature measurement with a lubricated thermistor (VWR Traceable™ Digital Thermometer) as previously described (Gilpin et al, 2011). The procedure room was 21 °C (±2°C) for these studies.
2.7 Intravenous Self-Administration
2.7.1 Intravenous Catheterization
Rats were anesthetized with an isoflurane/oxygen vapor mixture (isoflurane 5 percent induction, 1–3 percent maintenance) and prepared with chronic intravenous catheters as describe previously (Aarde et al, 2015a; Aarde et al, 2013; Miller et al, 2013; Nguyen et al, 2016). Briefly, the catheters consisted of a 14-cm length polyurethane based tubing (MicroRenathane®, Braintree Scientific, Inc, Braintree MA, USA) fitted to a guide cannula (Plastics one, Roanoke, VA) curved at an angle and encased in dental cement anchored to an ~3-cm circle of durable mesh. Catheter tubing was passed subcutaneously from the animal’s back to the right jugular vein. Catheter tubing was inserted into the vein and secured gently with suture thread. A liquid tissue adhesive was used to close the incisions (3M™ Vetbond™ Tissue Adhesive; 1469S B). A minimum of 4 days was allowed for surgical recovery prior to starting an experiment. For the first three days of the recovery period, an antibiotic (cephazolin) and an analgesic (flunixin) were administered daily. During testing and training, intra venous catheters were flushed with ~0.2–0.3 ml heparinized (32.3 USP/ml) saline before sessions and ~0.2–0.3 ml heparinized saline containing cefazolin (100 mg/ml) after sessions. Catheter patency was assessed nearly once a week after the last session of the week via administration through the catheter of ~0.2 ml (10 mg/ml) of the ultra-short-acting barbiturate anesthetic, Brevital sodium (1 percent methohexital sodium; Eli Lilly, Indianapolis, IN). Animals with patent catheters exhibit prominent signs of anesthesia (pronounced loss of muscle tone) within 3 s after infusion. Animals that failed to display these signs were considered to have faulty catheters and were discontinued from the study. Data that was taken prior to failing this test and after the previous passing of this test were excluded from analysis.
2.7.2 Self-Administration Procedure
Drug self-administration was conducted in operant boxes (Med Associates) located inside sound-attenuating chambers located in an experimental room (ambient temperature 22±1°C; illuminated by red light) outside of the housing vivarium. To begin a session, the catheter fittings on the animals’ backs were connected to polyethylene tubing contained inside a protective spring suspended into the operant chamber from a liquid swivel attached to a balance arm. Each operant session started with the extension of two retractable levers into the chamber. Following each completion of the response requirement (response ratio), a white stimulus light (located above the reinforced lever) signaled delivery of the reinforcer and remained on during a 20 s post-infusion timeout, during which responses were recorded but had no scheduled consequences. Drug infusions were delivered via syringe pump. The training dose (0.05 mg/kg/infusion; ~0.1ml/infusion) and session duration of 1 h were selected from a prior self-administration study (Aarde et al, 2015a). The per-infusion dose was increased to 0.1 mg/kg/infusion after 15 sessions because of low and inconsistent self-administration compared with the prior study and a potential role of rat strain (Wistar rats were used in the prior study). Following acquisition training (30 sessions), rats were separated into two treatment conditions (KLH and α-PVP-KLH vaccine) based on balancing their final five sessions of acquisition. Following the vaccination regimen, both groups were first permitted to self-administer saline on two sequential sessions to ensure that all animals would still respond on the drug-associated lever. The rats were then allowed access to α-PVP (0.025 mg/kg/inf) in daily (M-F) 1 h sessions. Rats were evaluated for wheel running responses to non-contingent injection of α-PVP (0.0, 0.25, 0.5 mg/kg, i.p.) after the first 9 self-administration sessions and then again after the Week 8 immunization (0.0, 0.5, 1.0, 5.0 mg/kg, i.p.), prior to the initiation of the final self-administration sessions.
2.8 Data Analysis
Antibody titer data in the wheel activity experiment were analyzed within group with two-way repeated-measure Analysis of Variance (rmANOVA) with Vaccine and Time (week) as within-subjects factors. The wheel activity data were first analyzed within group with repeated-measure rmANOVA with Drug Treatment Condition (dose) and Time (post-injection) as within-subjects factors. Significant rmANOVA effects were followed with post hoc multiple comparisons analysis using the Dunnett procedure within-group and Tukey procedure between-groups to correct for multiple comparisons. The body temperature data were analyzed with rmANOVA with Time (post-injection) as the within-subjects factor and a between-groups factor for both group and dose. Antibody titer data in the intravenous self-administration experiment were analyzed using one-way rmANOVA with pre-planned comparisons of changes from beginning to end of the original inoculation regimen (Week 1 vs Week 4.5) and after the final booster inoculation (Week 8 vs Week 12). The intravenous self-administration acquisition data were analyzed with rmANOVA with Time (sessions) as a within-subjects factor. The post-vaccination self-administration data were analyzed with rmANOVA with Vaccine Treatment as a between-subjects factor and Time (bins of sessions) as a within-subjects factor. The criterion for significant results was set at P < 0.05 and all analyses were conducted using Prism 6 or 7 for Windows (v. 6.02, 7.00; GraphPad Software, Inc, San Diego CA).
3. Results
3.1 Antibody Titer
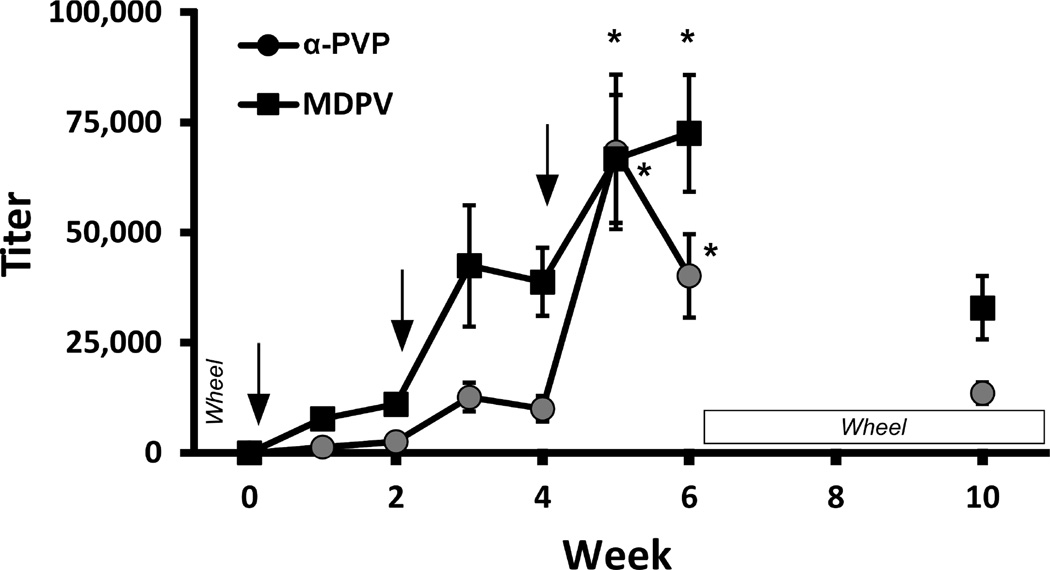
The mean plasma antibody titer increased across the initial 6 weeks in a step-wise manner after each injection with the α-PVP-KLH and MDPV-KLH vaccines (Fig. 2). The ANOVA confirmed a significant effect of Time [F (6, 132) = 15.64; P < 0.0001] and of Vaccine [F (1, 22) = 8.685; P < 0.01], and the post-hoc analysis confirmed that titers significantly increased during week 5 and 6 compared to week 1. The titers were lower in Week 10 but were still approximately commensurate with the levels obtained after the second injection.
Figure 2.
Vaccination schedule and plasma titer (dilution producing 50% signal in ELISA analysis). Rats were inoculated with the α-PVP-KLH vaccine or the MDPV-KLH vaccine on Weeks 0, 2 and 4. Plasma samples (n=12 ±SEM) were collected during Weeks 1–6 and 10. Arrows indicate timing of vaccine injections, and labels indicate the timing of the activity wheel behavioral experiments. Significant difference from week 1 are indicated by *.
The relative affinities for α-PVP, MDPV and methamphetamine are reported in Table 1 for each group and these data show that the α-PVP-KLH and MDPV-KLH vaccines were selective for their respective target drugs compared with the other cathinone and methamphetamine. We note that the relative affinities for each drug-hapten-vaccine candidate as reported in Table 1 were modest (micomolar). However, we attribute this to the competition ELISA format, which greatly underestimates relative affinity for antibody-small molecule interactions (Collins and Janda, 2014).
Table 1.
Relative affinities of vaccinated rat plasma for various drugs as determined by competitive ELISA.
| Drug Affinity (µM) | |||
|---|---|---|---|
| Vaccine | α-PVP | MDPV | Methamphetamine |
| α-PVP-KLH | 86.6 ± 28.9* |
3220 | >1,000,000 |
| MDPV-KLH | 6520 | 28.9 ± 6.9* |
>1,000,000 |
Values were obtained using individual plasma samples from N=12 rats per treatment group. Other values were obtained using pooled plasma samples for each treatment group.
3.2 Wheel Activity
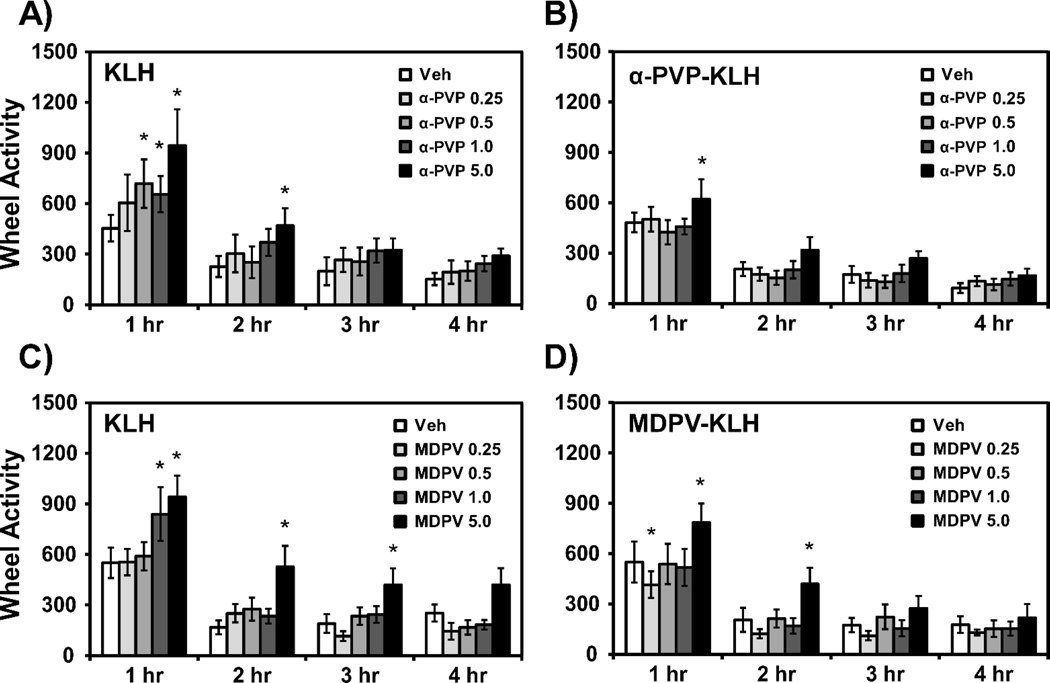
The acute administration of α-PVP (0.5–5.0 mg/kg, i.p.) significantly increased wheel activity in KLH rats (Fig. 3A). The ANOVA confirmed a significant effect of Time [F (3, 33) = 21.97; P < 0.0001] and of Drug Condition [F (4, 44) = 4.90; P < 0.01]. The post hoc analysis confirmed that wheel rotations were increased over the vehicle condition for the 0.5 mg/kg (1 h post-injection), 1.0 mg/kg (1 h post-injection) and 5.0 mg/kg (1–2 h post-injection) α-PVP doses. In contrast the α-PVP-KLH vaccine rats exhibited significantly increased wheel activity only following administration of 5.0 mg/kg α-PVP (Fig. 3B). The ANOVA confirmed a significant effect of Time [F (3, 33) = 55.93; P < 0.0001] and of Drug [F (4, 44) = 3.95; P < 0.01] within this group and the post hoc analysis confirmed that wheel rotations were increased over the vehicle condition for the 5.0 mg/kg (1–2 h post-injection) α-PVP dose.
Figure 3.
Mean (n=12 ±SEM) wheel quarter rotations following acute administration of α-PVP (0.25–5.0 mg/kg, i.p.) in A) KLH, and B) α-PVP-KLH vaccine rats, or administration of MDPV (0.25–5.0 mg/kg, i.p.) in C) KLH, D) MDPV-KLH vaccine rats. Significant differences from vehicle at corresponding time point are indicated by *.
As shown in Fig. 3C, the acute administration of MDPV significantly increased wheel activity in KLH rats in a dose dependent manner. The ANOVA confirmed a significant effect of Time [F (3, 33) = 45.27; P < 0.0001] and Drug Condition [F (4, 44) = 7.0; P < 0.001] and of the Time × Drug Condition interaction [F (12, 132) = 1.91; P < 0.05] on wheel activity. The post hoc analysis confirmed that wheel rotations were increased over the vehicle condition for the 1.0 mg/kg (1 h post-injection) and 5.0 mg/kg (1–3 h post-injection) MDPV doses. In contrast the MDPV-KLH vaccine rats exhibited increases in wheel activity only following administration of 5.0 mg/kg MDPV (Fig. 3D) as confirmed by the post hoc test following a significant effect of Time [F (3, 33) = 19.23; P < 0.0001] and Drug Condition [F (4, 44) = 5.19; P < 0.01] and of the Time × Drug interaction [F (12, 132) = 1.96; P < 0.05] on the ANOVA. The post hoc analysis also confirmed that wheel activity was decreased for the 0.25 mg/kg (1 h post-injection) MDPV dose and was increased over the vehicle condition for the 5.0 mg/kg (1–2 h post-injection) MDPV doses within the MDPV-KLH group.
3.3 Body Temperature
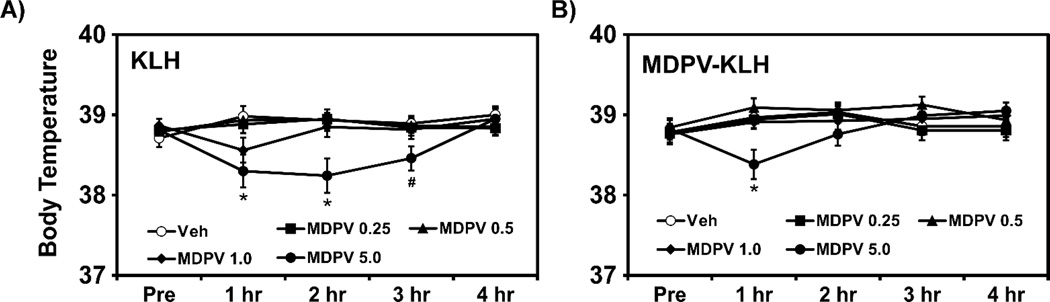
Acute administration of MDPV (5.0 mg/kg, i.p.) decreased body temperature in KLH and MDPV-KLH rats, as shown in Fig. 4. The ANOVA confirmed significant between group main effect of Time [F (4, 440) = 3.899; P < 0.01] and of Time × Group interaction [F (36, 440) = 3.291; P < 0.0001]. The post hoc analysis confirmed lower body temperature than the vehicle condition for the 5.0 mg/kg MDPV dose in KLH rats (1 and 2 h post-injection) but for only 1 h post-injection in MDPV-KLH vaccine rats. Analysis also confirmed significant difference between KLH and MDPV-KLH vaccine rats following the 5.0 mg/kg MDPV dose at the 3 h post-injection. There was no significant effect of α-PVP on body temperature in KLH or α-PVP-KLH vaccine rats (not shown).
Figure 4.
Mean (n=12 ±SEM) rectal temperature following acute administration of MDPV (0.25–5.0 mg/kg, i.p.) 4 h post injection in A) KLH and B) MDPV-KLH vaccine rats. Significant differences from vehicle at corresponding time point are indicated by *. Significant difference between groups within drug identity and dose are indicated by #.
3.4 Self-Administration
3.4.1-Acquisition
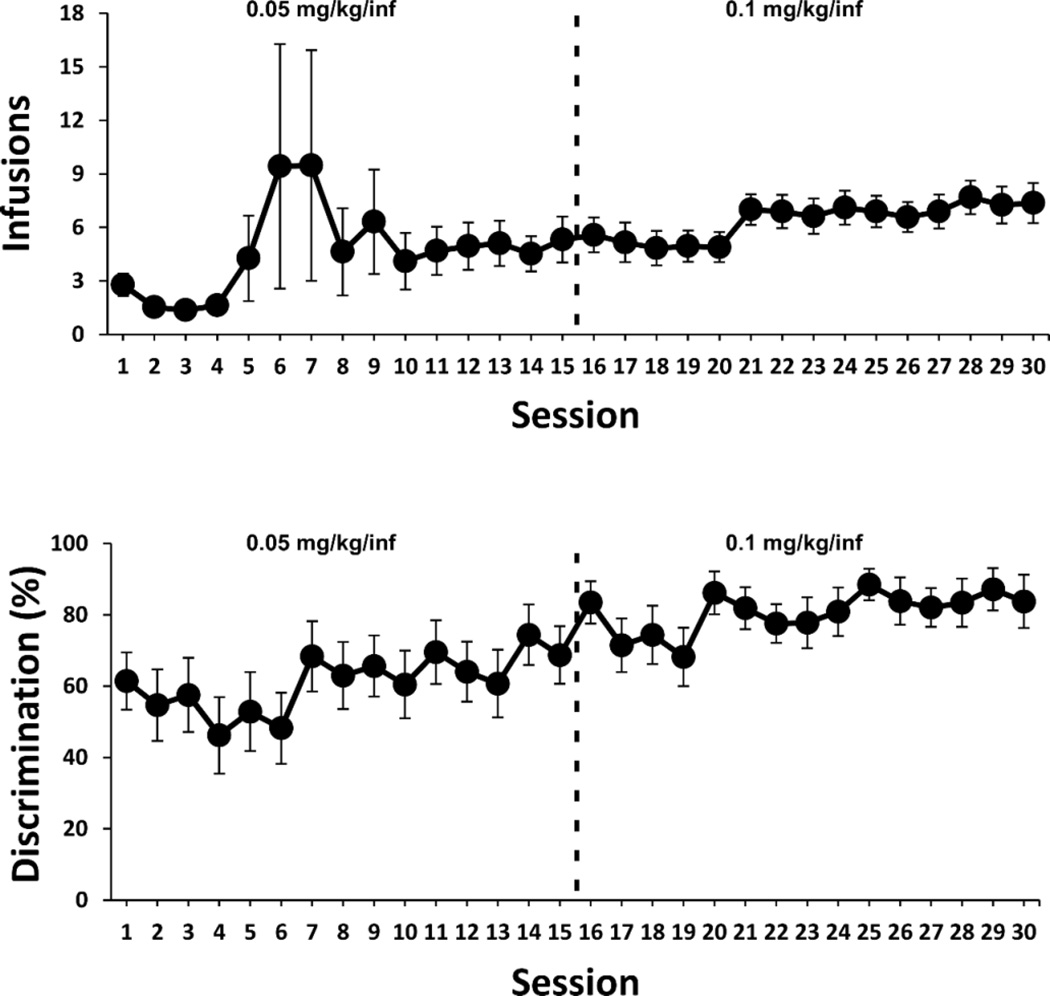
The mean number of infusions and the discrimination ratios (drug-paired lever responses / all lever responses) during acquisition are shown in Fig. 5. One rat responded for an unusually elevated number of infusions on three sessions (see large deviations in mean and variance on sessions 5–7), which may represent isolated voluntary binge behavior as has been observed for α-PVP and MDPV in prior studies (Aarde et al, 2015a; Aarde et al, 2015b); however, it cannot be excluded that this may have been involuntary repetitive responding (stereotypy). The per-infusion dose was increased from 0.05 mg/kg/inf to 0.1 mg/kg/inf after sessions 15 due to an apparent discrepancy with prior results (Aarde et al, 2015a) in terms of session intake and consistency of acquisition across the group. This was judged potentially due to the strain difference (Wistar rats were used in the prior study) in drug sensitivity. Drug-lever discrimination significantly increased across the 30-session acquisition interval, as confirmed by a significant main effect of Session [F (8.445, 152.0) = 2.836; P = 0.005], and was >80% for the final 7 sessions.
Figure 5.
Acquisition of α-PVP (0.05–0.1 mg/kg/inf) self-administration. Mean (n=19 ±SEM) drug infusions obtained per session (upper panel) and drug-paired lever discrimination ratios (lower panel) across sessions under a fixed-ratio 1 (FR1) response contingency.
3.4.2-Titer
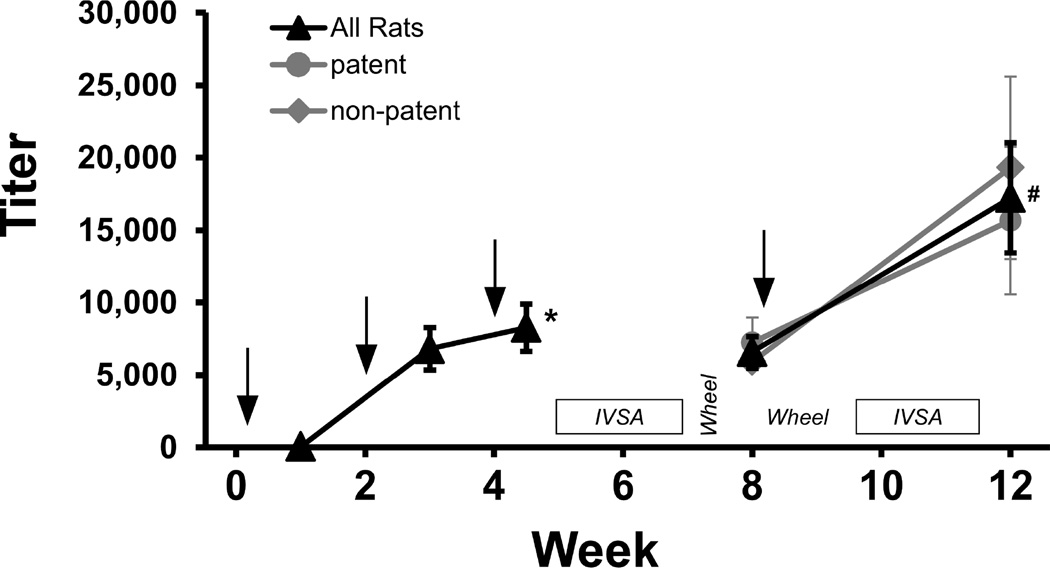
The mean antibody titers for the IVSA group (N=10) are depicted in Figure 6. The ANOVA confirmed a main effect of the Week of blood draw [F (1.538, 13.84) = 11.47; P<0.005] and the pre-planned comparison confirmed that titer increased significantly from Week 1 to 4.5 and from Week 8 to 12. The titer means for rats with patent (N=6) catheters included in the final post-boost IVSA data (see below) and those with catheter failures (N=4) are also depicted separately for the Week 8 and 12 analyses; no significant differences were observed.
Figure 6.
Vaccination schedule and plasma titer (dilution producing 50% signal in ELISA analysis). Rats were inoculated with the α-PVP-KLH vaccine on Weeks 0, 2, 4 and 8. Plasma samples (n=10 ±SEM) were collected on Weeks 1, 3, 4.5, 8 and 12. Arrows indicate timing of vaccine injections, and labels indicate the timing of post-inoculation intravenous self-administration (IVSA) and activity wheel behavioral experiments. A significant difference from Week 1 is indicated by * and a significant difference from Week 8 by #.
3.4.3-Post-Vaccination
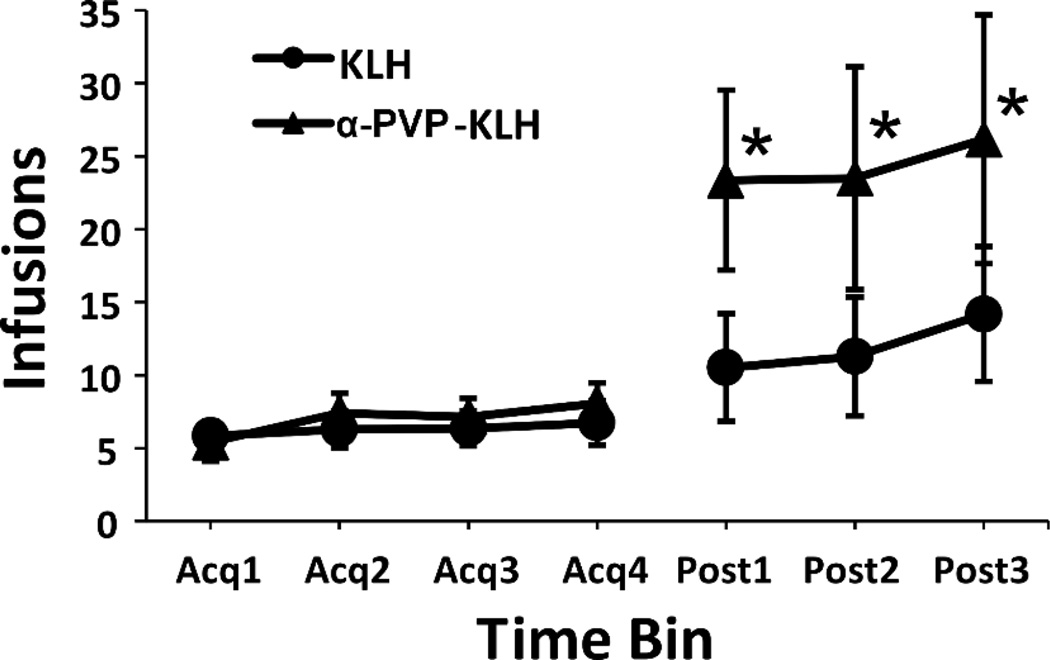
As shown in Fig. 7, there was an initial increase in drug infusions obtained (data are shown as sequential 3 session averages) following the 4 week break for the vaccination regimen. This was consistent with the reduction in the per-infusion dose to 0.025 mg/kg/inf, but interestingly was more pronounced in the α-PVP-KLH group. The ANOVA confirmed a significant effect of Time [F (6, 102) = 8.91; P < 0.0001] and of the interaction of Time and Vaccine group [F (6, 102) = 2.23; P < 0.05]. The post hoc analysis confirmed that infusions were significantly greater in α-PVP-KLH rats following active vaccination (Post 1–3) compared to all four within-group pre-vaccination time bins.
Figure 7.
Increase in self-administration at Weeks 5–6 following active vaccination at Weeks 0, 2 and 4. Mean (n=9–10) infusions of α-PVP (0.025 mg/kg/inf) obtained per 3 sessions under a fixed-ratio 1 (FR1) response contingency. Significant differences within group from all acquisition conditions are indicated by *.
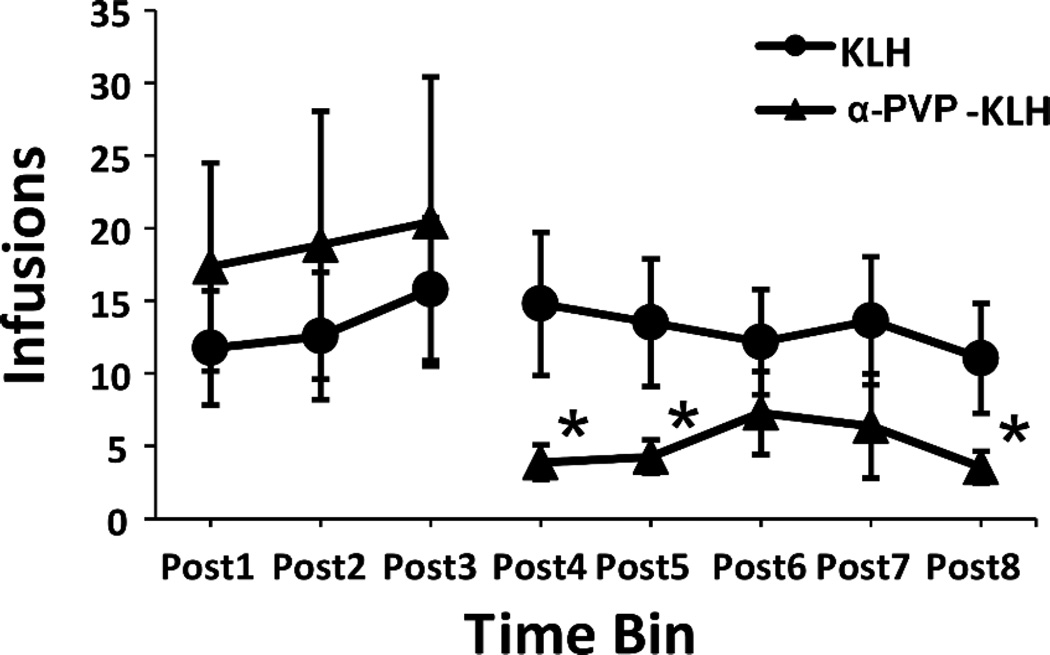
Following the final inoculation during Week 8, there was a significant attenuation of α-PVP self-administration (Fig. 8). Four rats (1 KLH, 3 α-PVP-KLH) that completed the acquisition interval did not complete the post-vaccination self-administration phase due to catheter patency failure, and one α-PVP-KLH rat was lost from the study due to illness. The ANOVA confirmed a significant effect of Time [F (7, 84) = 3.91; P < 0.005] and the interaction of Time and Vaccine group [F (1, 12) = 3.36; P < 0.005]. The post hoc analysis confirmed that significantly fewer infusions were obtained by α-PVP-KLH rats in the 15 sessions that followed the Week 8 vaccination (Post 4, 5 and 8), compared to the nine pre-vaccination sessions for this group. Self-administration did not change in the KLH control group.
Figure 8.
Attenuation of α-PVP self-administration at Weeks 9–11 following Week 8 booster vaccination. Mean (n=6–8 ±SEM) infusions of α-PVP (0.025 mg/kg/inf) obtained per 3 sessions under a fixed-ratio 1 (FR1) response contingency. Significant differences within group from all pre-vaccination conditions are indicated by *.
4. Discussion
This investigation is the first to demonstrate the efficacy of active vaccination against the effects of any of the synthetic cathinone derivative psychostimulants, in vivo. The vaccines in this study were directed against α-PVP and MDPV because these two compounds have been shown to have very high reinforcing potential in rat models of self-administration (Aarde et al, 2013; Aarde et al, 2015b; Schindler et al, 2016; Watterson et al, 2014), locomotor stimulant effects in both rats and mice (Aarde et al, 2015a; Baumann et al, 2013; Fantegrossi et al, 2013; Gatch et al, 2015; Gatch et al, 2013; Huang et al, 2012; Marusich et al, 2014) and therefore likely have high abuse liability in humans. The antibody titer levels increased over the course of 4.5–6 weeks following each of three vaccinations. Each vaccine demonstrated good affinity for their respective target drug and importantly very poor affinity for the alternate cathinone and for methamphetamine, thereby demonstrating specificity. These data are a testament to the successful design and synthesis of the two novel cathinone immonoconjugates.
The in vivo efficacy of the vaccines was first evaluated in a wheel activity assay identical to that previously used to show efficacy of active vaccination against MA-induced psychomotor stimulation (Miller et al, 2013). Although the pharmacokinetic parameters of α-PVP in rats are currently unknown, an investigation of the distribution and metabolism of MDPV after subcutaneous administration in rats (Anizan et al, 2014) found a half-life of 77.8 – 83.8 minutes for 1.0 and 2.0 mg/kg doses respectively, with increases in locomotor activity lasting up to 140 min. The locomotor stimulant effects of α-PVP and MDPV in the KLH control group using the wheel-running assay were dose-dependent and were consistent with prior results using an open-field or radio-telemetric assays (Aarde et al, 2015a; Aarde et al, 2013; Baumann et al, 2013; Fantegrossi et al, 2013; Gatch et al, 2015; Gatch et al, 2013; Huang et al, 2012; Marusich et al, 2014). The locomotor stimulant effects of MDPV or α-PVP were lesser in the respectively vaccinated groups of rats since only the highest dose of MDPV or α-PVP significantly increased activity within MDPV-KLH and α-PVP-KLH groups whereas lower doses of MDPV (1.0 mg/kg) and α-PVP (0.5–1.0 mg/kg) were effective in the control group. Thus, the vaccinated animals were protected against effects of the target drug.
The first study also found that reductions of body temperature were produced by the 5.0 mg/kg dose of MDPV, similar to a prior finding using radio-telemetry (Aarde et al 2015), although unlike that prior work no significant thermoregulatory effects of α-PVP were found in this study. The temperature response was attenuated in the MDPV-KLH vaccine group since the rate of temperature recovery was more rapid, thereby demonstrating an additional facet of vaccine efficacy. Importantly, the fact that the effect was observed following a dose of MDPV that did not result in group differences on the locomotor assay confirms that reliance on a single in vivo assays is insufficient to fully describe the potential effects of anti-drug vaccines. That is, different behavioral or physiological effects of the target drug may be ameliorated by vaccination at different doses. For example, a prior report found that temperature and locomotor effects of anti-MA vaccination did not coincide at the same MA dose (Miller et al, 2013).
The rats were readily trained in the intravenous self-administration (IVSA) procedure, with some animals reaching active/inactive lever discrimination criteria at the 0.05 mg/kg/infusion dose and the full group well trained once 0.1 mg/kg was available in each infusion. This is only the second report of α-PVP IVSA in rats (see Aarde et al, 2015a) and thus it is not clear if this apparent difference from the first report (where Wistar rats were trained to over 80% active-lever responses after 5 sessions using 0.05 mg/kg/infusion) is due to rat strain or some other experimental variable. Nevertheless, this is a further confirmation of the efficacy of α-PVP as a reinforcer in the rat IVSA model. These studies also outlined the minimum conditions for maintenance of IVSA of α-PVP. For example, cumulative drug intake in the Acquisition 4 bin of sessions (with the 0.1 mg/kg per-infusion dose) was 0.75 mg/kg/session for both groups. Upon the return to IVSA post-vaccination at the 0.025 mg/kg per-infusion dose), behavioral compensation was only partial with the KLH control animals averaging 0.29–0.40 mg/kg/session (the alpha-PVP-KLH animals averaged 0.43–0.51 mg/kg/session). The KLH animals continued to average 0.28–0.37 mg/kg/session across the 5 post-boost session bins (while the alpha-PVP-KLH averaged 0.09–0.18 mg/kg/session).
The finding that the initial vaccination with α-PVP-KLH resulted in an increase in drug-seeking behavior is consistent with prior results from two groups in which the self-administration of methamphetamine in vaccinated rats was initially higher than controls and then decreased over time (Duryee et al, 2009; Miller et al, 2013). The present results are congruent with α-PVP-specific antibodies sequestering drug into the peripheral circulation, with the increase in drug self-administration interpreted as a compensatory behavior. This initial compensation may be particularly likely when high rates of behavioral responding have been established by prior lever training with food (Duryee et al, 2009; Miller et al, 2013) or drug (present study) as the reinforcer. A significant decrease in drug infusions following the additional Week 8 vaccination was also observed in α-PVP-KLH rats, with no change in the KLH-only control group. This pattern most likely reflects greater antibody-drug sequestering capacity generated by the final booster injection and extinction of the behavior in the absence of a reinforcing effect.
As a caveat, the attenuation of drug-induced hyperlocomotion by the vaccines in the first study was partially (α-PVP) or completely (MDPV) surmountable by the highest dose administered since in each within-group analysis, activity following the 5.0 mg/kg dose was higher than in the vehicle condition. In the case of the α-PVP-KLH vaccine animals, the first hour of wheel activity after the 5.0 mg/kg dose was significantly lower than the wheel activity of the control animals following the same dose of α-PVP so some protection was still observed. In the self-administration animals, the absence of wheel running response to non-contingent injections of α-PVP (0.0–5.0 mg/kg, i.p.) in both groups (data not shown) could suggest the development of tolerance following extensive history of intravenous drug exposure.
In summary, this study showed that active vaccination of rats with conjugate vaccines directed against α-PVP and MDPV was successful in mounting a robust and specific antibody response against both drugs. The circulating antibodies acted effectively as ‘immunoantagonists’, attenuating locomotor stimulation caused by each drug, thermoregulatory disruption caused by MDPV and α-PVP self-administration. Overall, this study demonstrates the efficacy of α-PVP-KLH and MDPV-KLH vaccines and supports further investigation into immunopharmacotherapies for substituted cathinone drugs.
Supplementary Material
Highlights.
The novel drugs alpha-pyrrolidinopentiophenone (α-PVP) and 3,4-methylenedioxypyrovalerone (MDPV) have high abuse potential.
There are no currently available therapies to treat stimulant abuse, including MDPV and α-PVP.
Drug-conjugate vaccines were created to generate antibodies to neutralize MDPV and α-PVP.
Increased wheel activity and temperature decreases were produced by α-PVP or MDPV in the controls but not the vaccinated groups.
Self-administration of α-PVP was disrupted in the vaccinated group.
Acknowledgments
Funding and Disclosure: This work was funded by support from the United States Public Health Service National institutes of Health (DA024705, DA042211 and DA037709) which had no direct input on the design, conduct, analysis or publication of the findings. The authors declare no competing financial interests for this work. This manuscript is #29279 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA. In vivo potency and efficacy of the novel cathinone alpha-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology (Berl) 2015a;232(16):3045–3055. doi: 10.1007/s00213-015-3944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Dickerson TJ, Taffe MA. Binge-like acquisition of 3,4-methylenedioxypyrovalerone (MDPV) self-administration and wheel activity in rats. Psychopharmacology (Berl) 2015b;232(11):1867–1877. doi: 10.1007/s00213-014-3819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anizan S, Ellefsen K, Concheiro M, Suzuki M, Rice KC, Baumann MH, et al. 3,4-Methylenedioxypyrovalerone (MDPV) and metabolites quantification in human and rat plasma by liquid chromatography-high resolution mass spectrometry. Anal Chim Acta. 2014;827:54–63. doi: 10.1016/j.aca.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. Powerful cocaine-like actions of 3,4-Methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive 'bath salts' products. Neuropsychopharmacology. 2013;38(4):552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie F, Hekman K, Cameron L, Wade DR, Smolinske S. Emergency department visits after use of a drug sold as "bath salts" --- michigan, november 13, 2010--march 31, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(19):624–627. [PubMed] [Google Scholar]
- Bremer PT, Schlosburg JE, Lively JM, Janda KD. Injection route and TLR9 agonist addition significantly impact heroin vaccine efficacy. Mol Pharm. 2014;11(3):1075–1080. doi: 10.1021/mp400631w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Hoffman TZ, Isomura S, Wirsching P, Koob GF, et al. Investigations using immunization to attenuate the psychoactive effects of nicotine. Bioorg Med Chem. 2004;12(3):563–570. doi: 10.1016/j.bmc.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. Suppression of psychoactive effects of cocaine by active immunization. Nature. 1995;378(6558):727–730. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- Collins KC, Janda KD. Investigating hapten clustering as a strategy to enhance vaccines against drugs of abuse. Bioconjug Chem. 2014;25(3):593–600. doi: 10.1021/bc500016k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration DoJ. Schedules of controlled substances: temporary placement of three synthetic cathinones in Schedule I. Final Order. Federal Register. 2011;76(204):65371–65375. [PubMed] [Google Scholar]
- Drug Enforcement Administration DoJ. Schedules of controlled substances: temporary placement of 10 synthetic cathinones into Schedule I. Final order. Federal register. 2014;79(45):12938–12943. [PubMed] [Google Scholar]
- Duryee MJ, Bevins RA, Reichel CM, Murray JE, Dong Y, Thiele GM, et al. Immune responses to methamphetamine by active immunization with peptide-based, molecular adjuvant-containing vaccines. Vaccine. 2009;27(22):2981–2988. doi: 10.1016/j.vaccine.2009.02.105. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused 'bath salt' constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38(4):563–573. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ. Comparative Behavioral Pharmacology of Three Pyrrolidine-Containing Synthetic Cathinone Derivatives. J Pharmacol Exp Ther. 2015;354(2):103–110. doi: 10.1124/jpet.115.223586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of 'bath salt' cathinones. Behav Pharmacol. 2013;24(5–6):437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry WB, Ruedi-Bettschen D, Owens SM. Development of active and passive human vaccines to treat methamphetamine addiction. Hum Vaccin. 2009;5(4):206–213. doi: 10.4161/hv.5.4.7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Wright MJ, Jr, Dickinson G, Vandewater SA, Price JU, Taffe MA. Influences of activity wheel access on the body temperature response to MDMA and methamphetamine. Pharmacol Biochem Behav. 2011;99(3):295–300. doi: 10.1016/j.pbb.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA. Bath salts, mephedrone, and methylenedioxypyrovalerone as emerging illicit drugs that will need targeted therapeutic intervention. Advances in pharmacology. 2014;69:581–620. doi: 10.1016/B978-0-12-420118-7.00015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug Alcohol Depend. 2012;126(1–2):168–175. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology (Berl) 2000;148(3):251–262. doi: 10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Shen XY, Kinsey BM, Kosten TR, Orson FM. Attenuation of cocaine-induced locomotor activity in male and female mice by active immunization. Am J Addict. 2014;23(6):604–607. doi: 10.1111/j.1521-0391.2014.12152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Hieda Y, Collins G, Burroughs D, Le C, et al. Effects of a nicotine conjugate vaccine on the acquisition and maintenance of nicotine self-administration in rats. Psychopharmacology (Berl) 2006;184(3–4):409–416. doi: 10.1007/s00213-005-0027-2. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. Pharmacology of novel synthetic stimulants structurally related to the "bath salts" constituent 3,4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014;87:206–213. doi: 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Aarde SM, Moreno AY, Creehan KM, Janda KD, Taffe MA. Effects of active anti-methamphetamine vaccination on intravenous self-administration in rats. Drug Alcohol Depend. 2015;153:29–36. doi: 10.1016/j.drugalcdep.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Moreno AY, Aarde SM, Creehan KM, Vandewater SA, Vaillancourt BD, et al. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol Psychiatry. 2013;73(8):721–728. doi: 10.1016/j.biopsych.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno AY, Azar MR, Warren NA, Dickerson TJ, Koob GF, Janda KD. A critical evaluation of a nicotine vaccine within a self-administration behavioral model. Mol Pharm. 2010;7(2):431–441. doi: 10.1021/mp900213u. [DOI] [PubMed] [Google Scholar]
- Moreno AY, Janda KD. Current challenges for the creation of effective vaccines against drugs of abuse. Expert review of vaccines. 2011;10(12):1637–1639. doi: 10.1586/erv.11.145. [DOI] [PubMed] [Google Scholar]
- Nguyen JD, Grant Y, Creehan KM, Vandewater SA, Taffe MA. Escalation of intravenous self-administration of methylone and mephedrone under extended access conditions. Addict Biol. 2016 doi: 10.1111/adb.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentel PR, Malin DH, Ennifar S, Hieda Y, Keyler DE, Lake JR, et al. A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacol Biochem Behav. 2000;65(1):191–198. doi: 10.1016/s0091-3057(99)00206-3. [DOI] [PubMed] [Google Scholar]
- Pravetoni M, Pentel PR, Potter DN, Chartoff EH, Tally L, LeSage MG. Effects of an oxycodone conjugate vaccine on oxycodone self-administration and oxycodone-induced brain gene expression in rats. PLoS One. 2014;9(7):e101807. doi: 10.1371/journal.pone.0101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiko SA, Harris AC, Keyler DE, Lesage MG, Zhang Y, Pentel PR. Combined active and passive immunization enhances the efficacy of immunotherapy against nicotine in rats. J Pharmacol Exp Ther. 2008;325(3):985–993. doi: 10.1124/jpet.107.135111. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, et al. Reinforcing and neurochemical effects of the "bath salts" constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology (Berl) 2016;233(10):1981–1990. doi: 10.1007/s00213-015-4057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Vendruscolo LF, Bremer PT, Lockner JW, Wade CL, Nunes AA, et al. Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc Natl Acad Sci U S A. 2013;110(22):9036–9041. doi: 10.1073/pnas.1219159110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XY, Kosten TA, Lopez AY, Kinsey BM, Kosten TR, Orson FM. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend. 2013;129(1–2):41–48. doi: 10.1016/j.drugalcdep.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler L, Buser T, Donzelli M, Schramm Y, Dieu LH, Huwyler J, et al. Pharmacological characterization of designer cathinones in vitro. British Journal of Pharmacology. 2013;168(2):458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, et al. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2014;19(2):165–174. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.