Abstract
Effects of the constituents from Ginkgo biloba extract (GBE) on the action potentials and the ionic currents in guinea pig ventricular cardiomyocytes were investigated using whole-cell and current-clamp techniques. The constituents, ginkgolides A, B, C and quercetin, had depressant effects at 0.1–3μM on the action potential configuration. Ginkgolide A (1–3 μM) prolonged the action potential (action potential duration: APD) at 75% and 90% repolarizations (APD75 and APD90). However, ginkgolides B and C at low concentrations prolonged APD, but at higher concentrations (>1 μM) shortened APD. Quercetin at 3 μM prolonged the APD, but not at the lower concentrations. These constituents also inhibited the Vmax. The resting potential was unaffected. In voltage-clamp experiments, ginkgolides A and B (0.1–3 μM) markedly and concentration-dependently increased the Ca2+ current (ICa) and the delayed rectifier K+ current (IK), and decreased the inwardly rectifying K+ current (IK1). On the other hand, ginkgolide C failed to affect the ICa but increased the IK by 14.0 ± 2.3% (n = 6, P < 0.05) at 1 μM. Quercetin inhibited ICa, and enhanced IK but decreased IK1. These responses to the constituents were almost reversible (80–90% of control) after a 10- to 20-min washout. These results indicate that even at acute administrations, these constituents produce the effective actions on the APD and the underlying ionic currents in cardiomyocytes. Each constituent does not exhibit a uniform response, although GBE acts as a net.
Keywords: action potential, ionic currents, ginkgolide A, ginkgolide B, ginkgolide C, Ginkgo biloba extract, guinea pig ventricular cardiomyocytes, quercetin
Introduction
The extract from the leaves of Ginkgo biloba (GBE) plays a role in cellular physiological and pharmacological functions. GBE is used for treating impaired brain functions in elderly and peripheral arterial occlusive diseases (1,2). Pharmacological experiments have recently demonstrated that GBE (i) increases cerebral blood flow, decreases viscosity and antagonizes platelet activating factor receptors (3,4); (ii) increases tolerance for anoxia by preventing the decrease in ATP level (5); (iii) improves neurotransmitter disturbances (6,7); and (iv) prevents cell damage induced by free radicals (8–10).
GBE contains many chemical constituents. The major constituents are terpenes such as bilobalide, ginkgolide A, ginkgolide B and ginkgolide C, and flavonoids such as quercetin, kaempferol and isorhamnetin (6,11). In the cardiovascular system, GBE does not affect heart rate and contractility, but produces a concentration-dependent increase in coronary flow (12,13). In our previous experiments, however, we have found that GBE prolongs the action potential duration (APD), whereas bilobalide, a main constituent, shortens the APD in cardiomyocytes (14). The alterations of the action potential configurations clinically would lead to the pathophysiological changes in the electrocardiogram (ECG). In addition, Tamago et al. (15) have shown that ginkgolide B is a PAF-antagonist and acts on the APD, consistent with our results.
The mechanisms of the therapeutic effectiveness of GBE are expected to be extremely complex, because of the large number of its constituent substances (6,8). Unknown mechanisms of these constituents for the ionic channel currents still remain. The aim of the present experiments was to examine, by use of a patch-clamp technique, how these constituents of GBE, other than bilobalide, affect the action potentials and the underlying ionic currents. In addition, the cardiac electropharmacological actions of each constituent were compared.
Materials and Methods
All experiments were carried out according to the guidelines laid down by the Nara Medical University Animal Welfare committee, and also under the terms of the Declaration of Helsinki.
Cell Preparation
Cells were prepared from tissue taken from the ventricle muscle of guinea pig hearts, using methods similar to those described previously (14,16). Under sodium pentobarbital (30 mg/kg, i.p.) anesthesia, the chest was opened and the aorta was cannulated in situ. The heart was dissected out and perfused with normal Tyrode solution on the Langendorff apparatus. After a washout of blood, the heart was perfused with Ca2+-free Tyrode solution, and spontaneous beating ceased. Then, the perfusate was switched to low-Ca2+ (30–60 μM) Tyrode solution containing 0.4 mg/ml collagenase (Type I, Sigma Chemical, St Louis, MO) for about 20 min. The heart was washed out by high-K+ and low-Cl− solution (KB solution), and was dissected with scissors. The temperature of all solutions was maintained at 36°C.
Current- and Voltage-clamp Experiments
Current-clamp and whole-cell voltage-clamp recordings were performed using an Axopatch patch-clamp amplifier (Axon Instruments, Burlingame, CA) and standard techniques. Patch pipettes from borosilicate glass capillaries were fabricated using a two-stage puller, and had a resistance of 5–7 MΩ. The series resistance error was <3–7 mV, and no compensation was used. The liquid junction potential between the pipette solution and the external solution (<10 mV) was corrected for all membrane potential recordings. Experiments were carried out at a temperature of 36.5°C. The data were stored and analyzed on an IBM-AT microcomputer, using the PCLAMP analysis program (Axon Instruments). Current traces were filtered using a cut-off frequency of 1 kHz for plotting. The ICa was measured as the difference between the peak current and the zero current, and the IK1 was the difference between the current at the end of a 1-s test pulse and the zero current. The IK was measured at the peak of the outward tail current. All values are given as mean ± S.E.M. The differences of the mean values were analyzed by Student's t-test and ANOVA for paired data, and a P-value of <0.05 was considered significant.
Experimental Solutions
The composition of the modified Tyrode solution was: 137 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1.0 mM MgCl2, 0.3 mM NaH2PO4, 5.0 mM glucose and 5.0 mM HEPES. The pH was adjusted to 7.4 with NaOH. The constituents of Ginkgo biloba extract, ginkgolides A, B and C, and quercetin (Tokiwa Phytochemical Co., Tokyo, Japan) were dissolved with DMSO. Bath solutions with the desired concentrations were made and superfused. The pipette solution (intracellular) contained: 110 mM K-aspartate, 20 mM KCl, 1 mM MgCl2, 10 mM EGTA, 5 mM Mg-ATP, 5 mM creatine phosphate and 5 mM HEPES (pH 7.2). The pipette solution for the measurement of ICa alone contained: 110 mM CsOH, 20 mM CsCl, 2 mM MgCl2, 10 mM EGTA, 5 mM MgATP, 5 mM creatine phosphate, 100 mM aspartic acid and 5 mM HEPES (pH 7.2).
Results
Gingko biloba Effects on the Action Potentials
To examine the effects of each constituent on the action potential configuration, a current-clamp experiment was carried out. The isolated single cell was stimulated at 1 Hz. As shown in Fig. 1A, ginkgolide A (at >1 μM) markedly prolonged the APD; at 3 μM, 75% and 90% repolarizations of APD (APD75 and APD90) were by +43.8 ± 3.6% (n = 8, P < 0.001) and by +40.9 ± 4.2% (n = 8, P < 0.001), respectively (Fig. 2A). Ginkgolide B at low concentrations (0.1–0.3 μM) markedly prolonged APD75 and APD90, but at high concentrations (1–3 μM) it decreased both of the APDs (although the APDs were still enhanced in comparison with the control) (Fig. 1B). The APD75 and APD90 at 0.3 μM were +63.6 ± 4.8% (n = 9, P < 0.001) and +58.3 ± 3.6% (n = 6, P < 0.001), respectively, and at 3 μM were +16.2 ± 3.9% (n = 9, P < 0.05) and +15.0 ± 3.7% (n = 9, P < 0.05), respectively (Fig. 2B).
Figure 1.
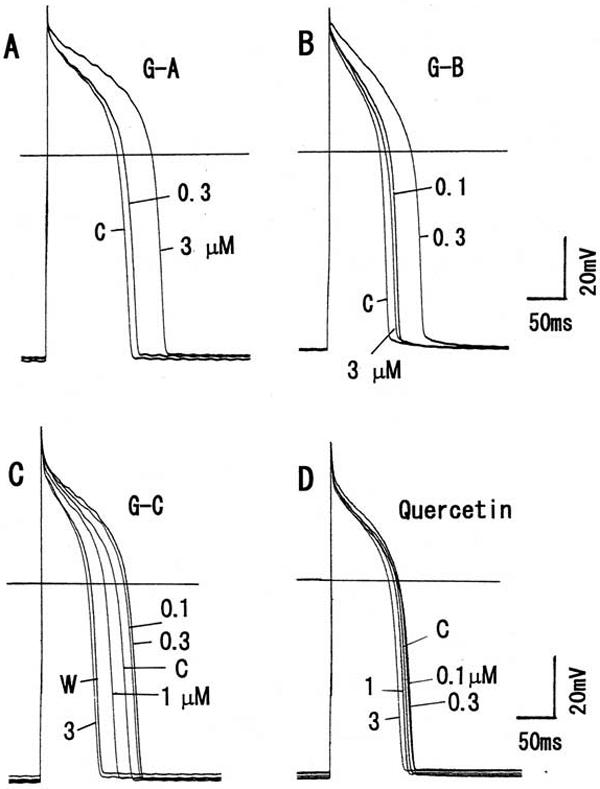
Modulation of the action potentials by the constituents of Ginkgo biloba extract in guinea pig ventricular cardiomyocytes. (A) Effects of ginkgolide A on the action potentials. (B) Action potentials in ginkgolide B. (C) Action potentials in ginkgolide C. (D) Quercetin on action potentials. Each constituent from 0.1–3μM was cumulatively added to bath solution. Horizontal lines indicate 0 mV. Symbols used are control (C) and washout (W).
Figure 2.

Changes in the action potential durations (75% and 90% repolarizations) in the presence of the constituents (0.1–3μM). Maximum responses at different concentrations of each constituent are plotted. Values are represented as mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001, with respect to control value.
On the other hand, ginkgolide C at low concentrations (0.3μM) prolonged APD, whereas at high concentrations (1–3 μM) it markedly shortened APD (Fig. 1C). The APD75 and APD90 at 0.3 μM were altered by +15.5 ± 3.7% (n = 8, P < 0.05) and +9.9 ± 2.6% (n = 8, P < 0.05), respectively, and at 3 μM they were altered by −38.5 ± 2.8% (n = 8, P < 0.001) and −37.2 ± 3.5% (n = 8, P < 0.01), respectively (Fig. 2C). Quercetin at 0.1–1 μM tended to prolong the APD, and at 3 μM prolonged the APD75 by +11.9 ± 3.4% (n = 8, P < 0.05) and APD90 by +17.9 ± 3.0% (n = 8, P < 0.05) (Figs 1D and 2D).
The maximum rate of deporization (Vmax) significantly decreased by ∼18–20% (n = 8–9) at higher concentrations (1–3 μM) of these constituents. Other action potential parameters were also unaffected in the presence of all the constituents. The modulation of the action potential configurations by the constituents is summarized in Fig. 3. A washout of the drugs for 15–20 min recovered to ∼70–80% of the control value.
Figure 3.
Summary of the effects on action potential parameters. (A) Ginkgolide A, (B) ginkgolide B, (C) ginkgolide C, (D) quercetin. APA, action potential amplitude; MDP, maximum diastolic potential; APD75, 75% repolarization of the action potential duration; APD90, 90% repolarization of the action potential duration; Vmax, maximum rate of depolarization. Values are represented as mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001, with respect to control value.
Constituents of Gingko biloba and Quercetin Exert Contrasting Effects on Ionic Currents
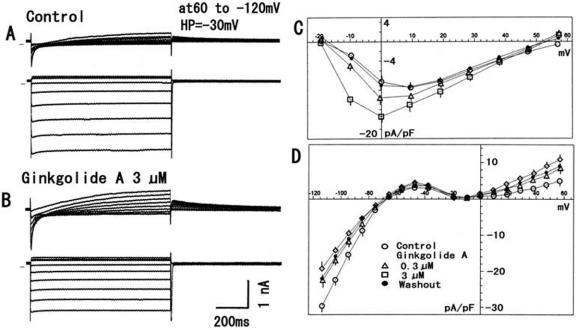
Whole-cell patch voltage-clamp experiments were performed to examine the effects of the constituents on the underlying ionic currents. Test pulses (1 s duration) were applied to −20 to 60 mV and −40 to −120 mV from a holding potential of −30 mV. The average capacitance was 86.1 ± 2.0 pF (n = 44). As shown in Fig. 4, ginkgolide A (1 μM) enhanced both the Ca2+ current (ICa), by 68.2 ± 2.8% (n = 7, P < 0.001), and the delayed rectifier K+ current (IK) by 66.3 ± 3.8% (n = 7, P < 0.001). Each constituent (0.1–3 μM) was cumulatively administrated to the bath solution. Ginkgolide B at 0.3 μM also increased the ICa at 10 mV by 58.1 ± 2.8% (n = 6, P < 0.001) (Fig. 5). Increasing the concentrations (1–3 μM) still enhanced the ICa, but the maximal response at 0.3 μM decreased. The IK at 60 mV increased by 89.3 ± 3.8% (n = 6, P < 0.001) at 3 μM, but the inwardly rectifying K+ current (IK1) was not affected significantly. Ginkgolide C had a smaller or no effect on the ICa, but markedly increased the IK by 59.2 ± 2.8% (n = 7, P < 0.001) at 3 μM (Fig. 6).
Figure 4.
Modulation by ginkgolide A of the ionic currents. (A) Current traces in control. (B) Current traces in ginkgolide A (3μM). Test pulses for 1 s were applied to −20 to 60 mV and −40 to −120 mV from a holding potential of −30 mV. Horizontal line before the current traces indicates zero current level. (C) Current–voltage relationship for Ca2+ current. (D) Current–voltage relationship for the delayed rectifier K+ current and the inwardly rectifying K+ current. Symbols used are control (open circles), 0.3 μM (triangles) and 1 μM (squares) of ginkgolide A, and washout (filled circles).
Figure 5.
Modulation by ginkgolide B of the ionic currents. (A) Current traces in control. Test pulses for 1 s were applied to −20 to 60 mV and −40 to −120 mV from a holding potential of −30 mV. (B) Current traces in ginkgolide B (0.3 μM). Test pulses for 1 s were applied to −20 to 60 mV. Holding potential was −30 mV. (C) Current traces at 3 μM. Test pulses for 1 s were applied to −20 to 60 mV and −40 to −120 mV from a holding potential of −30 mV. Horizontal line before the current traces indicates zero current level. (D) Current–voltage relationship for Ca2+ current. (E) Current–voltage relationship for the delayed rectifier K+ current and the inwardly rectifying K+ current. Symbols used are control (open circles), 0.1 μM (triangles), 0.3 μM (squares) and 1 μM (filled circles) of ginkgolide B.
Figure 6.
Modulation by ginkgolide C of the ionic currents in guinea pig ventricular cardiomyocytes. (A) Current traces in control. (B) Current traces in ginkgolide C (3μM). Test pulses for 1 s were applied to −20 to 60 mV and −40 to −120 mV from a holding potential of −30 mV. Horizontal line before the current traces indicates zero current level. (C) Current–voltage relationship for the Ca2+ current. (D) Current–voltage relationship for the delayed rectifier K+ current and the inwardly rectifying K+ current. Symbols used are control (open circles), 0.3 μM (triangles) and 3 μM (squares) of ginkgolide C.
On the other hand, the application of quercetin (0.1–3 μM) inhibited the ICa (Fig. 7A and B). The ICa at 10 mV decreased by 34.9 ± 3.2% (n = 8, P < 0.05) at 0.3 μM and by 56.8 ± 3.3% (n = 8, P < 0.05) at 3 μM. The responses were produced in a concentration-dependent manner. Simultaneously, the IK at 60 mV increased by 60.4 ± 2.7% (n = 8, P < 0.001) at 0.3 μM and by 89.7 ± 3.3% (n = 8, P < 0.001) at 3 μM. Quercetin simultaneously did not affect the IK1 to a significant extent (by ∼10–15%) at low concentrations, but at 3 μM inhibited the IK1 by 12.4 ± 2.1% (n = 8, P < 0.05). The responses to the constituents for the ICa and IK currents are summarized in Fig. 8. The responses to all the constituents were recovered to ∼80–90% of the control value after a 20-min washout.
Figure 7.

Modulation by quercetin of the ionic currents in guinea pig ventricular cardiomyocytes. (A) Current traces in control. (B) Current traces in quercetin (3μM). Test pulses for 1 s were applied to −20 to 60 mV and −40 to −120 mV. Holding potential was −30 mV. Horizontal line before the current traces indicates zero current level. (C) Current–voltage relationship for Ca2+ current. (D) Current–voltage relationship for the delayed rectifier K+ current and the inwardly rectifying K+ current. Symbols used are control (open circles), 0.3 μM (triangles) and 3 μM (squares) of quercetin.
Figure 8.
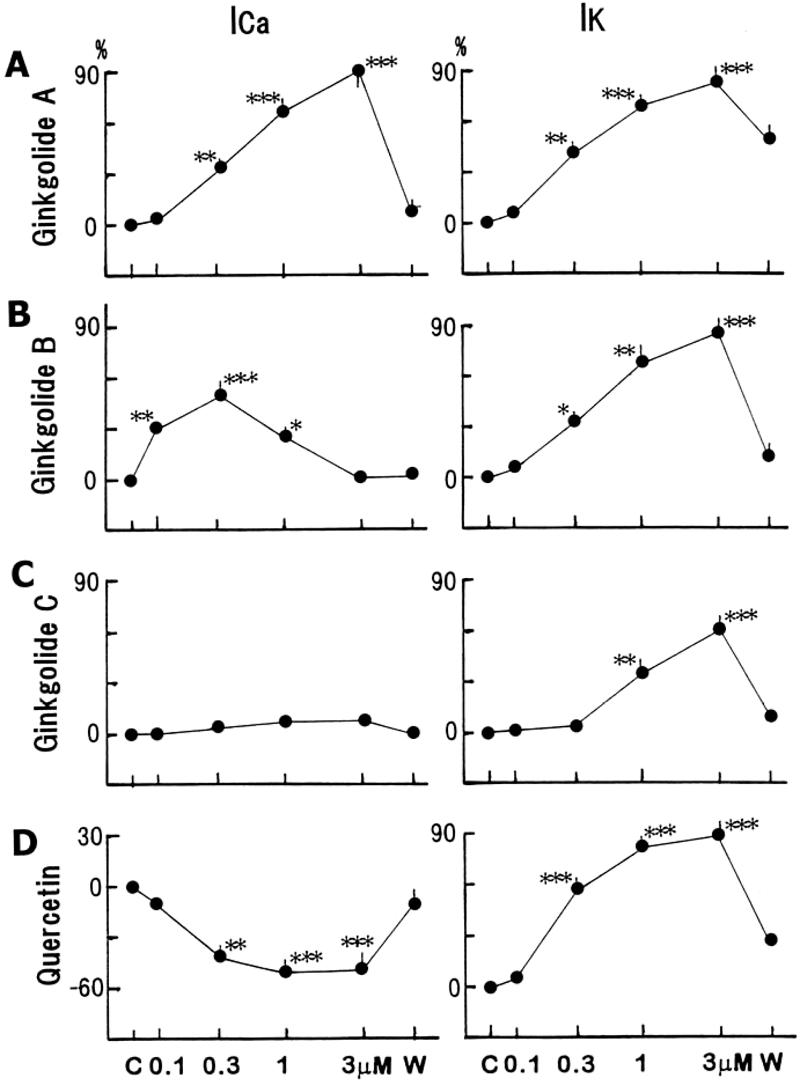
Concentration-dependent responses to the constituents on the Ca2+ and delayed rectifier K+ currents. (A–D) Percentage changes in control and at 0.3 and 3 μM of ginkgolide A, B, C and quercetin. Maximum values for the currents at different concentrations of each constituent are plotted. Values are represented as means ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001, with respect to control value.
Discussion
The present experiments showed that: (i) ginkgolide A prolonged the APD, but ginkgolides B and C shortened APD; (ii) quercetin prolonged the APD; (iii) the constituents also inhibited the Vmax and the RP was unaffected; (iv) ginkgolides A and B increased ICa and IK, and decreased IK1; (v) ginkgolide C failed to affect the ICa but increased the IK; (vi) quercetin inhibited ICa, and enhanced IK but decreased IK1; and (vii) these responses were almost reversible after a washout. These results indicate that acute administrations of these constituents can produce the effective actions on cardiomyocytes. Each constituent never exhibits a uniform response.
In recent clinical and experimental experiments, GBE has been found to be effective against ischemic brain injury (11,17) and cerebral disorders due to aging (18). The mechanisms of the beneficial effects of GBE are considered to be improvements in: (i) hemodynamic disorders (6,7,19); (ii) PAF-associated abnormalities (3,4,20,21); (iii) cell damage induced by free radicals (8–10); and (iv) a decrease in ATP level during anoxia (5). In fact, these effects have recently been recognized clinically by several double-blind studies with GBE versus placebo (22–25).
APD Regulation
In experiments for the cardiovascular system, GBE does not affect heart rate and contractility, but produces a concentration-dependent increase in coronary flow (12,13). In our laboratory, however, GBE caused a negative chronotropic effect in rabbit SA nodal cells (unpublished data). Also, GBE inhibited ICa and IK, but bilobalide (a constituent) increased them in guinea pig ventricular cardiomyocytes (14). Simultaneously, GBE prolonged the APD, whereas bilobalide shortened it. In the present experiments, a major action of the constituents was the alteration of APD involved with the effects on the ionic channels. The APD means a period for the repolarization of the membrane, making T wave on ECG. Thus, the APD of cardiac myocytes is clinically reflected directly to the QT interval. The QT interval is a reflection of the repolarization of action potential, which is mainly responsible for the modulation of IK. The APD prolongation increases the refractory period and simultaneously elevates the cellular Ca2+ concentration (26–28). Therefore, the constituents would play an important role as ionic channel modulators of cadiomyocytes, although the effects of each constituent on the APD and the ionic currents were not exhibited in a uniform direction. Quercetin, a kind of flavonoid, had a smaller or no effect on the action potentials, but caused the APD prolongation only at high concentrations. Also, quercetin inhibited ICa and enhanced IK. Quercetin has been reported to possess many pharmacological effects (29,30); the modification of eicosanoid synthesis, prevention of platelet aggregation, and vasorelaxation due to the inhibition of PK-C.
Modulation of the Ionic Currents
Furthermore, these constituents also acted on the Vmax, although they did not affect other action potential parameters. In general, the Vmax is used as an activator of the Na+ channel current (INa). Thus, the constituents have an inhibitory action on the INa, resulting in an inhibition of the conduction velocity and a suppression of excitability. In addition, the constituents simultaneously modulated the ionic channel currents such as ICa, IK, IK1 and INa. These effects might cause antiarrhythmic actions. In all the constituents, IK enhancement was finally produced, indicative of a cell protection due to an APD shortening and a decline of cellular Ca2+ concentration.
The channel activity of single myocytes might be caused a run-down, especially Ca2+ channel and rapidly activating K+ channel. The cells not causing run-down were chosen and used for the experiments. However, there may be a limitation to patch-clamp experiments. The responses to the constituents were considered to be due to the effects of constituents, because of the reversible response. Therefore, these constituents would exert many helpful and protective actions upon cardiac cells.
Clinical Uses and Summary
GBE can be administered to patients with mild to moderate symptoms of cerebral insufficiency (1,2). The half-life of GBE is 2–3 h (11). In pharmacokinetic analysis, the bioavailabilities of ginkgolides A and B are practically high, but that of ginkgolide C is very low (31). Of the GBE-containing flavonoids, quercetin has the highest percentage (8.91%). With regard to the distribution of radioactivity in the cardiovascular system, the tissues in areas such as vein, heart and aorta are relatively higher 3–7 h after oral administration (32). Quercetin possesses a vasodilating action (33,34). Also, the histamine-induced contraction of isolated guinea pig intestine was inhibited by a mixture of flavonoids (35). In our laboratory, GBE as a mixture exhibited a potent vasodilatory action (34). Therefore, these responses are most likely produced by the modulation of ionic channel currents that have been demonstrated in this study.
It has been reported that a single dose of GBE does not produce potent pharmacological activities, and repeated doses are needed to produce beneficial effects over a long period of administration (36–38). In the present in vitro experiments, however, acutely single administrations produced marked actions on the ionic channel currents and the action potentials. Since GBE is a mixture of its constituents, the total response to GBE would be a result of complex interactions with the constituents. Therefore, the constituents from GBE would play an important role in modulating the strong APD prolongation by interacting with each other. Although relatively higher concentrations were used in this study, because the pharmacological effects on the ionic channels were so remarkable, GBE and its constituents would be helpful in the treatment of cerebral disorders due to their effects on central nervous system (CNS) neurons. Further experiments are needed to elucidate the detailed mechanisms of the cardiac actions of Ginkgo biloba.
Acknowledgments
The author wishes to thank Tokiwa Phytochemical Co. Ltd. for providing the constituents of Ginkgo biloba extract (ginkgolides A, B and C, quercetin).
References
- 1.Christen Y, Costentin J, Lacour H. Effects of Ginkgo biloba Extract (Egb 761) on the Central Nervous System. Paris: Elsevier; 1992. [Google Scholar]
- 2.Kleijnen J, Knipschild P. Ginkgo biloba for cerebral insuffiency. Br J Clin Parmacol. 1992;34:352–358. doi: 10.1111/j.1365-2125.1992.tb05642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oberpichler H, Beck T, Abdel-Rahman MM, Bielenberg GW, Krieglstein J. Effects of Ginkgo biloba constituents related to protection against brain damage caused by hypoxia. Pharmacol Res Commun. 1988;20:349–368. doi: 10.1016/s0031-6989(88)80011-0. [DOI] [PubMed] [Google Scholar]
- 4.Braquet P, Shen TY, Vargaftig BB. Perspectives in platelet-activatng factor research. Pharmacol Rev. 1987;39:97–145. [PubMed] [Google Scholar]
- 5.Janssens D, Michiels C, Delative E, Eliaers F, Drieu K, Remacle J. Protection of hypoxia-induced ATP decrease in endothelial cells by Ginkgo biloba extract and bilobalide. Biochem Pharmacol. 1995;50:991–999. doi: 10.1016/0006-2952(95)00227-q. [DOI] [PubMed] [Google Scholar]
- 6.DeFeudis FV. Ginkgo biloba Extract (EGB 761): Pharmacological Activities and Clinical Applications. In: DeFeudis FV, editor. Clinical Studies and Clinical Pharmacology with Egb. 761. Paris: Elsevier; 1991. pp. 97–142. [Google Scholar]
- 7.Klein J, Chatterjee SS, Loffelholz K. Phospholipid breakdown and choline release under hypoxic conditions: inhibition by bilobalide, a consituent of Ginkgo biloba. Brain Res. 1997;755:347–350. doi: 10.1016/s0006-8993(97)00239-4. [DOI] [PubMed] [Google Scholar]
- 8.Drieu K. Preparation et definition de de l'extrait de Ginkgo biloba. Press Med. 1986;15:1455–1457. [PubMed] [Google Scholar]
- 9.Schoilcher H. Ginkgo biloba L. Untersuchung zur Qualitat, Wirkung, Wirksamkeit und Unbedenkichkeit. Zeitschr Phytother. 1988;9:119–127. [Google Scholar]
- 10.Wagner H, Bladt S, Hartmann U, Daily A, Berkulin W. Ginkgo biloba. DC- und HPLC-analyse von Ginkgo-extrakten und Ginkgo-extrakte enthaltenden phytopraparaten. Deutisch Apothek Zeit. 1989;129:2421–2424. [Google Scholar]
- 11.Kleijnen J, Knipschild P. Ginkgo biloba. Lancet. 1992;340:1136–1139. doi: 10.1016/0140-6736(92)93158-j. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee SS, Gabard B. Studies on mechanism of action of an extract of Ginkgo biloba, a drug used for treatment of ischemic vascular diseases. Naunyn-Schmiedeberg's Arch Pharmacol. 1982;321:207. [Google Scholar]
- 13.Chatterjee SS, Noldner M. Behavioural observations demonstrating influences of the extract of Ginkgo biloba (EGB-761) on some specific central cholinergic systems. Naunyn-Schmiedeberg's Arch Pharmacol. 1989;339:425. [Google Scholar]
- 14.Satoh H. Effects of Ginkgo biloba extract and bilobalide, a main constituent, on the ionic currents in Guinea pig ventricular cardiomyocytes. Artzmitt Forsh. 2003;53:407–413. doi: 10.1055/s-0031-1297128. [DOI] [PubMed] [Google Scholar]
- 15.Tamago J, Delgado CM, Diez J, Delpon E. Cardiac electrophysiology of PAF-acether and PAF-acether antagonists. In: Braquet P, Prous JR, editors. Ginkgoliges vol. 1. Barcelona: 1988. pp. 417–431. [Google Scholar]
- 16.Satoh H. Taurine modulates IKr but not IKs in guinea pig ventricular cardiomyocytes. Br J Pharmacol. 1999;126:87–92. doi: 10.1038/sj.bjp.0702308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang WR, Hayashi T, Kitagawa H, et al. Protective effect of Ginkgo extract on rat brain with transient middle cerebral artery occlusion. Neurol Res. 2000;22:517–521. doi: 10.1080/01616412.2000.11740713. [DOI] [PubMed] [Google Scholar]
- 18.Taillandier J, Ammar A, Rabourdin JP, et al. Traitement des traubles du vieillissement cerebral par l'extrait de Ginkgo biloba. Press Med. 1986;15:1583–1587. [PubMed] [Google Scholar]
- 19.Arrigo A, Cattaneo S. Clinical and psychometric evaluation of Ginkgo biloba extract in chronic cerebro-vascular diseases. In: Agnoli A, Rapin JR, Scapagnini V, Weitbrecht WV, editors. Effects of Ginkgo Biloba Extract on Organic Cerebral Impairment. Montrouge: John Libby Urotext; 1985. pp. 85–89. [Google Scholar]
- 20.Braquet P, Esane A, Buisine E, Hosford D, Broquet C, Koltai M. Recent progress in ginkgolide research. Med Res Rev. 1991;11:295–355. doi: 10.1002/med.2610110303. [DOI] [PubMed] [Google Scholar]
- 21.Krieglstein J, Beck T, Seibert A. Influence of an extract of Ginkgo biloba on cerebral blood flow and metabolism. Life Sci. 1986;39:2327–2334. doi: 10.1016/0024-3205(86)90663-6. [DOI] [PubMed] [Google Scholar]
- 22.Bauer U. 6-Month double-blind randomized clinical trial of Ginkgo biloba extract versus placebo in two parallel group in patients suffering from peripheral arterial insufficiency. Arzneimitt Forsch. 1984;34:121–1125. [PubMed] [Google Scholar]
- 23.Hamann KF. Physikalische Therapie des vestibularen Schwindels in Verbindung mit GBE. Therapie. 1985;35:4586–4590. [Google Scholar]
- 24.Weitbrecht WV, Jansen W. Doubleblind and comparative (Ginkgo biloba versus placebo) therapeutic study in geriatric patients with primary degererative dementia—a preliminary evaluation. In: Agnoli A, Rapin JR, Scapagnini V, Weitbrecht WV, editors. Effects of Ginkgo Biloba Extract on Organic Cerebral Impairment. Montrouge: John Libby Eurotext; 1985. pp. 91–99. [Google Scholar]
- 25.Meyer B. Etude multicentrique randomisee a double insu face au placebo du traitement des acouphenes par l'extrait de Ginkgo biloba. Press Med. 1986;15:1562–1564. [PubMed] [Google Scholar]
- 26.Satoh H, Hashimoto K. An electrophysiological study of amiloride on sino-atrial node cells and ventricular muscle of rabbit and dog. Naunyn-Schmiedeberg's Arch Pharmacol. 1986;333:83–90. doi: 10.1007/BF00569665. [DOI] [PubMed] [Google Scholar]
- 27.Satoh H, Ishii M, Hashimoto K. Effect of cibenzoline, a class I antiarrhythmic drug, on action potential in canine ventricular muscle. Jpn J Pharmacol. 1987;44:113–119. doi: 10.1254/jjp.44.113. [DOI] [PubMed] [Google Scholar]
- 28.Satoh H, Tsuchida K, Hashimoto K. Electrophysiological actions of A23187 and X-537A in spontaneously beating and in voltage-clamped rabbit sino-atrial node preparations. Naunyn-Schmiedeberg's Arch Pharmacol. 1989;339:320–326. doi: 10.1007/BF00173586. [DOI] [PubMed] [Google Scholar]
- 29.Middleton E., Jr The flavonoids. Trends Biol Sci. 1984;5:335–338. [Google Scholar]
- 30.Pathak K, Pathak A, Singla A. Flavonoids as medical agents. Fitoterapie. 1991;62:371–389. [Google Scholar]
- 31.Fourtillan JB, Brisson AM, Girault J, Ingrand I, Decourt JP. Pharmacokinetic properties of bilobalide and ginkgolide A and B in healthy subjects after intraveneous and oral administration of Ginkgo biloba extract (Egb 761) Therapie. 1995;50:137–144. [PubMed] [Google Scholar]
- 32.Moreau JP, Eck J, McCabe J, Skinner S. Absorption, distribution et elimination de l'extrait marque de Ginkgo biloba chez le rat. Press Med. 1986;15:1458–1463. [PubMed] [Google Scholar]
- 33.Kubota Y, Tanaka N, Umegaki K, et al. Ginkgo biloba extract-induced relaxation of rat aorta is associated with increase in endothelial intracellular calcium level. Life Sci. 2001;69:2327–2336. doi: 10.1016/s0024-3205(01)01303-0. [DOI] [PubMed] [Google Scholar]
- 34.Nishida S, Satoh H. Mechanisms for the vasodilations induced by Ginkgo biloba extract and its main constituent, bilobalide, in rat aorta. Life Sci. 2003;72:2659–2667. doi: 10.1016/s0024-3205(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 35.Peter H, Fisel ZJ, Weisser W. Zur Pharmakologie der Wirkstoffe aus Ginkgo biloba. Arzneimitt Forsch. 1966;16:719–725. [PubMed] [Google Scholar]
- 36.Bauer U. A two-year study of Ginkgo biloba extract in the treatment of peripheral arterial disease (Fontaine Stage IIb) Proc Int Union Angiol. 1986:531–532. [Google Scholar]
- 37.Halama P, Bartsch G, Meng G. Hirnleistungsstorung vaskularer Genese. Randomisierte Doppelblindstudie zur Wirksamkeit von Ginkgo-biloba-Extrakt. Fortsch Med. 1988;106:408–412. [PubMed] [Google Scholar]
- 38.Hofferberth B. Einfluss von Ginkgo biloba-Extrakt auf neurophysiologische und sychometrische Messergebnisse bei Patienten mit hirnorganischem Psychosyndrom: eine Doppelblindstudie gegen Plazebo. Arzneimitt Forsch. 1989;39:918–922. [PubMed] [Google Scholar]