Abstract
Pavlovian conditioned stimuli can acquire incentive motivational properties, and this phenomenon can be measured in animals using Pavlovian conditioned approach behavior. Drugs of abuse can influence the expression of this behavior, and nicotine in particular exhibits incentive amplifying effects. Both conditioned approach behavior and drug abuse rely on overlapping corticolimbic circuitry. We hypothesize that the orbitofrontal cortex (OFC) regulates conditioned approach, and that one site of nicotine action is in the OFC where it reduces cortical output. To test this, we repeatedly exposed rats to 0.4 mg/kg nicotine (s.c.) during training and then pharmacologically inactivated the lateral OFC or performed in vivo electrophysiological recordings of lateral OFC neurons in the presence or absence of nicotine. In Experiment 1, animals were trained in a Pavlovian conditioning paradigm and behavior was evaluated after inactivation of the OFC by microinfusion of the GABA agonists baclofen and muscimol. In Experiment 2, we monitored phasic firing of OFC neurons during Pavlovian conditioning sessions. Nicotine reliably enhanced conditioned responding to the conditioned cue, and inactivation of the OFC reduced conditioned responding, especially the sign-tracking response. OFC neurons exhibited phasic excitations to cue presentation and during goal tracking, and nicotine acutely blunted this phasic neuronal firing. When nicotine was withheld, both conditioned responding and phasic firing in the OFC returned to the level of controls. These results suggest that the OFC is recruited for the expression of conditioned responses, and that nicotine acutely influences this behavior by reducing phasic firing in the OFC.
1. Introduction
Environmental stimuli associated with nicotine or other drugs of abuse can acquire incentive motivational properties, becoming salient, attractive, and able to motivate behavior (Robinson and Berridge, 1993). In humans attempting to abstain from drug use, encountering these ‘incentives’ - stimuli that acquire motivational properties based on associations with drug rewards (Logan, 1960) - can lead to craving and promote relapse (O’Brien et al., 1992). Bio-behavioral models of substance dependence implicate long-term changes in the brain circuitry that mediates responses to incentives as central to substance use disorders (Di Chiara et al., 1992; Robinson and Berridge, 1993). Preclinical studies have confirmed that frontolimbic circuitry plays a critical role in the motivational effects of many drugs of abuse (Kalivas and Volkow, 2005). This circuit includes ascending dopaminergic projections from the midbrain, including the ventral tegmental area, and descending glutamatergic projections from the frontal cortex, including the anterior cingulate gyrus and prefrontal cortex (PFC). These projections converge on subcortical circuits that include the ventral striatum, ventral pallidum, and subthalamic nucleus.
The PFC has been implicated in substance dependence because of its role in top-down control of behavior, attention, decision making, and other functions that, when compromised, contribute to addiction vulnerability (Perry et al., 2010). Chronic drug use increases the influence of ascending midbrain systems while reducing cognitive control, resulting in an enhanced drive to seek the drug and a decrease in the ability to inhibit drug-seeking (Olausson et al., 2007). The orbitofrontal cortex (OFC), in particular, has been linked to incentive motivation and representations of outcome value or salience in both humans and animals (Gottfried et al., 2003; Ogawa et al., 2013), as well as the expression of behavioral responses and reward-seeking behaviors (Burton et al., 2014; Moorman and Aston-Jones, 2014). While the exact function of the OFC has yet to be precisely defined (see Stalnaker et al., 2015 for review) the OFC has consistently been characterized as involved in behaviors such as impulsivity (Mar et al., 2011; Zeeb et al., 2010) and Pavlovian conditioned approach (Chudasama and Robbins, 2003; Gallagher et al., 1999; Ostlund and Balleine, 2007).
Incentive stimuli that predict both drug and non-drug rewards evoke ‘Pavlovian conditioned approach’ behavior, which can take one of two forms. Approach behaviors oriented toward the location of reward delivery are traditionally referred to as ‘goal tracking,’ whereas behaviors oriented toward the location of the incentive, if it is spatially separated from the reward, are referred to as ‘sign tracking’ (Brown and Jenkins, 1968). Sign tracking has recently come under increasing scrutiny in substance dependence research because of its association with drug abuse vulnerability (Saunders and Robinson, 2013; Tomie et al., 2008). Although both sign and goal tracking rely on the same mesotelencephalic systems implicated in substance dependence (Flagel et al., 2011b; Saunders and Robinson, 2012), individual subjects who display a greater propensity to sign track show increased drug self-administration (Saunders and Robinson, 2011; Versaggi et al., 2016). These individual differences are also linked to variation in stress responses, neurotransmitter release, and neuronal activation in areas including the PFC and the nucleus accumbens (Saunders and Robinson, 2013; Tomie et al., 2008). For example, one study found that c-fos mRNA induction in the OFC was increased only in animals that displayed the sign-tracking response (Flagel et al., 2011a). While it appears that the OFC is involved in Pavlovian conditioned behaviors, there is still much to be learned, including the differential involvement of this region based on specific conditioned responses.
Recent studies from multiple laboratories suggest a special relationship between the effects of nicotine and approach to incentives (Palmatier et al., 2014; Versaggi et al., 2016; Yager and Robinson, 2015). The interaction between nicotine and incentives is especially relevant to tobacco use and dependence because preclinical studies have repeatedly demonstrated that nicotine is a weak primary reinforcer (Foll and Goldberg, 2009; Palmatier et al., 2006). Caggiula, Donny, Chaudhri and others (Caggiula et al. 2001; Donny et al. 2003; Chaudhri et al. 2006) have argued that nicotine self-administration follows from three effects of nicotine on behavior. First, nicotine is a primary reinforcer, albeit a weak one, meaning that nicotine delivery alone supports self-administration. Second, nicotine is a reinforcement enhancer; i.e., nicotine delivery increases responding for non-drug reinforcers (Chaudhri et al. 2007; Donny et al. 2003; Palmatier et al. 2006). Third, serving as a primary reinforcer, nicotine can establish associated non-drug stimuli as ‘conditioned reinforcers’ (i.e., incentives; Palmatier et al. 2008). More recently, Palmatier and colleagues (Palmatier et al., 2014, 2013a, 2012) have argued that the second effect of nicotine, enhanced responding for non-drug reinforcers, reflects an effect of nicotine on underlying neurobiological substrates that mediate responses to incentives, including conditioned stimuli. Accordingly, they have found that nicotine promotes Pavlovian conditioned approach, including sign-tracking (Palmatier et al., 2013b), and that the increase in approach is abolished by dopaminergic antagonists (Palmatier et al., 2014).
The present study sought to more thoroughly explore the neurobiological underpinnings of the incentive-promoting effects of nicotine by evaluating the role of the OFC in sign-and goal-tracking. We hypothesized that the OFC would be directly involved in both sign- and goal-tracking conditioned responses, and that nicotine exposure would reduce the ability of the OFC to exert top down control over this behavior. We tested this hypothesis with pharmacological inactivation of the OFC and by examining OFC firing patterns in vivo during Pavlovian conditioning sessions.
2. Materials and methods
2.1. Animals
Adult, male, Sprague Dawley rats (225–250g on arrival) were purchased from Harlan/Envigo (Indianapolis, IN), pair housed during initial training, and then individually housed after surgery. Experiment 1 used 16 animals and Experiment 2 used 25 animals. Animals were provided with food and water ad libitum during the entire experiment. Rats were housed in a vivarium on a 12:12 hour light:dark cycle, and all experiments were conducted during the light cycle. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
2.2. Behavioral training and nicotine regimen
Before training, animals were allowed 1-hour access to the 20% sucrose (w/v) solution that would be used as the unconditioned stimulus. Animals were then assigned to either a nicotine exposure group (NIC) or a saline control group (SAL). Nicotine hydrogen tartrate salt (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile saline and the pH was adjusted to 7.0 ± 0.2. Animals in the NIC group received one injection of 0.4mg/kg nicotine (s.c., calculated using the freebase form) and animals in the SAL group received an equivalent volume of saline for two days prior to conditioning to habituate them to the injection procedure. This dose was chosen because it is commonly used for repeated subcutaneous injections of nicotine, and we and others have previously shown that this dose influences conditioned responding (e.g., Guy and Fletcher, 2014; Palmatier et al., 2013b). Training sessions were conducted in standard behavioral chambers (MedAssociates, St Albans, VT) assembled with Plexiglas walls. A recessed reward receptacle, stimulus light, and retractable lever directly below the light were located on one wall of the chamber, and a house light was positioned on the opposite wall. A photobeam detector across the reward cup detected head entries into the receptacle. Animals were habituated to the testing chambers during one day of receptacle training, in which they were injected with the assigned drug or control solution, returned to their home cage for 10 min, and then placed in the testing chamber for 5 min before session initiation. During this session, 20% sucrose was dispensed into the receptacle on a variable interval (VI) 120 s schedule. Animals rarely failed to consume the reward, and NIC and SAL groups did not differ in the amount of fluid left in the reward cups at the end of the session (data not shown). Next, 20 (Experiment 1) or 25 (Experiment 2) Pavlovian conditioning sessions were conducted, Monday-Friday, in which the animals were injected with nicotine or saline 15 min before session initiation as described above. The house light was illuminated throughout the session and stimulus-reward pairings occurred on a VI 120 s reinforcement schedule. The conditioned stimulus (cue) consisted of illumination of a cue light and extension of the lever located directly below the light. Cue presentations lasted 30s, and were immediately followed by 0.1ml of 20% sucrose dispensed into the reward receptacle. Each session consisted of 15 cue-reward trials. After these training sessions, all animals were habituated to custom-built Plexiglas chambers inside sound attenuated boxes which had similar components but were optimized for electrophysiology recordings (Fanelli et al., 2013), for an additional 5 days before surgery.
2.3. Surgery
For Experiment 1, rats were anesthetized with isoflurane and implanted bilaterally with 26-gauge stainless steel cannulae (Plastics One, Roanoke, VA) aimed at the lateral OFC (3.7mm anterior, 2.7mm lateral, 4.0mm ventral from bregma). For Experiment 2, rats were implanted with two microwire electrode arrays (NB Labs, Denison, TX), each consisting of 8 stainless-steel, Teflon-coated wires that were 50-μm in diameter and spaced 0.5mm apart in a 2×4 configuration. Fixed-placement arrays were used as we planned to record behavior over several days and conditions, and we aimed to sample the same population of neurons (if not the same individual neurons) across each recording day. Arrays were placed bilaterally into the OFC, (centered at 3.7mm anterior, 2.7mm lateral from bregma, 5.0mm ventral from the adjacent skull surface). For both experiments, animals were allowed to recover from surgery for at least 7 days before resuming Pavlovian conditioning sessions.
2.4. Experiment 1: Intracranial microinfusions
After recovery, rats underwent 3–5 days of Pavlovian conditioning sessions to ensure that behavior remained constant. Two days before the intracranial microinfusions, animals were habituated to the procedure immediately before a standard Pavlovian conditioning session. Rats were held by the experimenter and 33-gauge stainless steel injection cannulae (Plastics One) that protruded 1mm beyond the guide cannulae were inserted and left in place for 4 min, mimicking the subsequent infusion procedure. On the test day, animals were infused with a cocktail of the GABAA receptor agonist muscimol and the GABAB receptor agonist baclofen (Sigma-Aldrich, 0.125μg of each drug/0.5μl) or saline vehicle. These doses were chosen because they have previously been shown to affect OFC-dependent behavior (Zeeb et al., 2010) without influencing locomotor activity (St. Onge and Floresco, 2010). Injection cannulae were left in place for 1 min before and 1 min after the infusion to ensure accurate diffusion of the drug. Each infusion occurred over 2 min, during which 0.5μl of drug cocktail or vehicle was infused into each hemisphere at a rate of 0.25μl/min. Next, animals were immediately injected with the previously assigned solution of either nicotine or saline and Pavlovian conditioning sessions occurred as described above.
2.5. Experiment 2: In vivo electrophysiology
Rats underwent 2–3 Pavlovian conditioning sessions after surgery to confirm that behavior remained consistent before being habituated to a flexible tether connected to the headstage assembly and electrode arrays. After habituation, electrophysiological recordings were conducted during Pavlovian conditioning sessions and test sessions. Electrophysiological recordings were conducted as previously described (Fanelli et al., 2013; Robinson and Carelli, 2008) using a multichannel acquisition processor (MAP system using SortClient software; Plexon Inc, Dallas TX) to record neuronal activity. Briefly, animals were tethered and placed in the recording chamber for 15 min before the start of the session. During this time, a differential reference was manually selected for each electrode array and thresholds were set for all microwires. Pavlovian conditioning sessions were conducted as described above and timestamps from MedAssociates software registering within-session events (cue onset, lever press, receptacle entry, reward delivery) were aligned with neuronal activity recorded with the MAP system. After each session, cell sorting was conducted using Plexon Offline Sorter software. Timestamp data were imported and further analyzed using Neuroexplorer (NEX Technologies, Madison AL) and custom-written programs in MATLAB (Mathworks, Natick, MA).
2.6. Histology
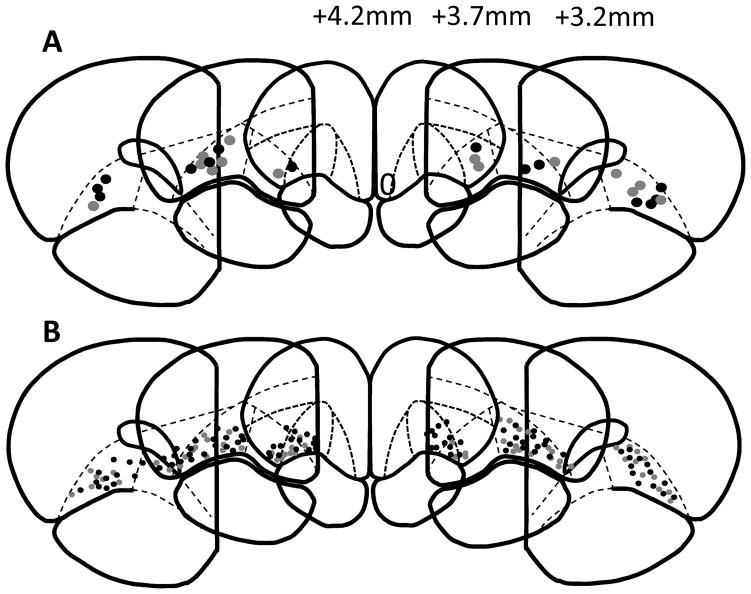
Animals were injected with 1.5 g/kg urethane solution (Sigma-Aldrich, 50% w/w in saline, i.p.). Once anesthetized, a 10μA current was passed through each stainless-steel microwire in rats from Experiment 2 to leave an iron deposit at the location of the electrode tip. All rats were perfused with 10% formaldehyde solution. The brain was removed and fixed in 30% sucrose cryoprotectant before being frozen, 40-μm sections were then taken on a cryostat. Sections were stained with potassium ferracyanide and thionin to visualize individual electrode or cannula placements. Cannula (Figure 1A) and electrode (Figure 1B) placements were marked based on sections from the Paxinos and Watson rat brain atlas (1998).
Figure 1.
Representative schematics of cannula (A) and individual electrode wire (B) placements in rats from Experiments 1 and 2, respectively. AP distances from bregma are indicated in mm. Grey circles represent placements from SAL animals, black circles represent placements from NIC animals. Atlas images are adapted from Paxinos and Watson (1998).
2.7. Behavioral data analysis and statistics
Data from Pavlovian conditioning sessions were collected with MedAssociates software and compiled using custom-written programs in R (R Core Team, version 3.1.2). Lever deflections and latencies to enter the receptacle or press the lever were averaged across trials for each animal. As the reward receptacle was present throughout the entire session, receptacle elevation scores (Palmatier et al., 2013b) were used to assess enhanced receptacle entries that occurred as a result of cue presentation. Elevation scores were calculated by subtracting the number of receptacle entries that occurred 30 s immediately before each cue presentation (pre-cue period) from the number of receptacle entries that occurred during each 30 s cue presentation (cue period). Thus, a positive elevation score indicates that an animal increased this response specifically during cue presentation. Lever and receptacle probability scores were calculated by taking the number of trials during which an animal pressed the lever or entered the receptacle at least once, and dividing by 15 total trials. Comparisons of behavioral responses during training and on test days were conducted using 2-way repeated-measures ANOVA in Sigma Plot (Systat Software Inc, San Jose CA) followed by Tukey’s HSD post-hoc comparisons when appropriate. Probability scores were analyzed with the genmod procedure in SAS (SAS Institute Inc, Cary, NC) using a Wald’s chi-square within the context of a logistic regression model with effects for each drug treatment by week or test day combination and standard error adjusted for multiple observations within rats. Behavioral responses were also compared between NIC and SAL groups on the final day of training (Experiment 1: Day 20, Experiment 2: Day 25) by independent samples t-test or Mann-Whitney U-test, depending on the distribution of the data.
2.8. Cell firing data analysis and statistics
Baseline firing rates of cells for each group were calculated by averaging the spike rates during the 60s immediately before house light illumination that signaled session initiation. Firing rates of individual neurons surrounding within-session events were normalized by dividing the firing rate at the event of interest by the mean firing rate over the whole session. For population analysis, the normalized activity of all cells for each treatment or behavior group was aligned to the event of interest and smoothed with a moving average of 250 ms in 50 ms steps. Statistical analysis of population activity was completed using a Mann-Whitney rank-sum test or Kruskal-Wallis one way ANOVA on ranks in Sigma Plot software.
To identify changes in firing patterns that are not apparent with population analysis, individual neurons that demonstrated phasic activity by significantly changing their firing rate around within-session events were classified using z-scores. For cue onset, firing rates during a 2 s pre-target window (baseline) were compared to firing during a 500ms target window immediately after the cue was presented. For all other events, a change in firing during a target window 500ms before and after the event occurred was compared to a 2 s baseline period. Z-scores were calculated based on the magnitude of the change in firing rate, and cells with a z-score between −2 and 2 were classified as non-phasic. A z-score of >2 was classified as excitatory activity, and a z-score less than −2 was classified as inhibitory activity.
3. Results
Animals were excluded from Experiment 1 if one or both cannula were not placed in the OFC. A total of 16 rats underwent surgery and 2 animals were excluded from the study based on incorrect cannula placements. Placement of cannula tips for remaining animals are depicted in Figure 1A, both the NIC and SAL groups included 7 animals. Individual units recorded in Experiment 2 were excluded if the corresponding wire was not placed in the OFC. Animals were excluded if no cells remained on wires that were correctly placed in the OFC. A total of 25 animals underwent surgery and 1 animal was removed based on incorrect electrode placements. Placements of remaining electrodes are depicted in Figure 1B. One animal was removed from analysis for failing to complete the saline test day. A total of 12 animals in the SAL group and 11 animals in the NIC group were included in the study.
3.1. Experiment 1: Inactivation of the OFC
3.1.1. Training
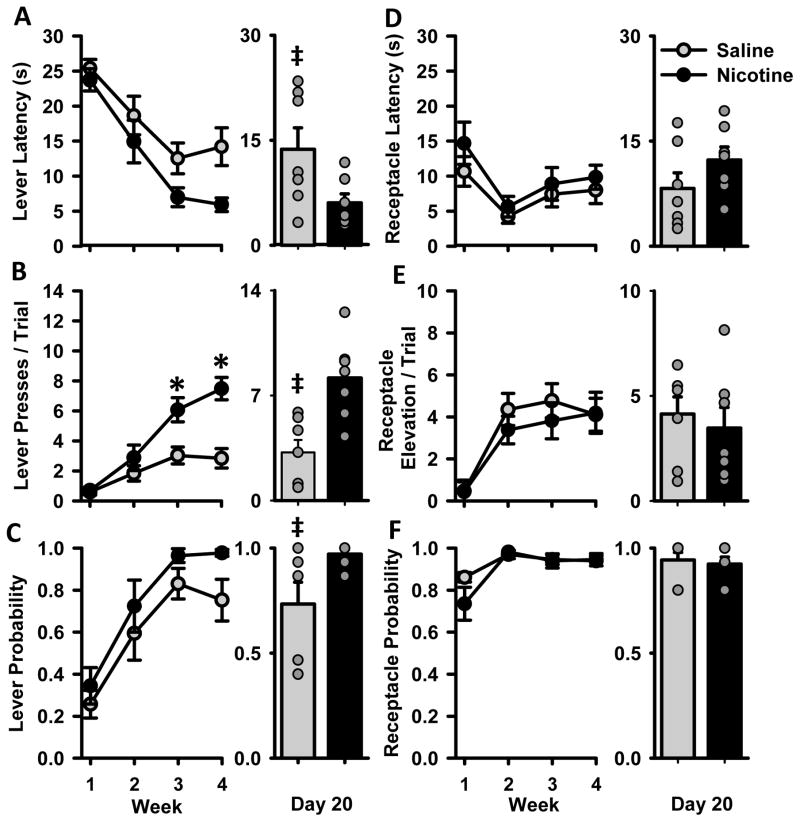
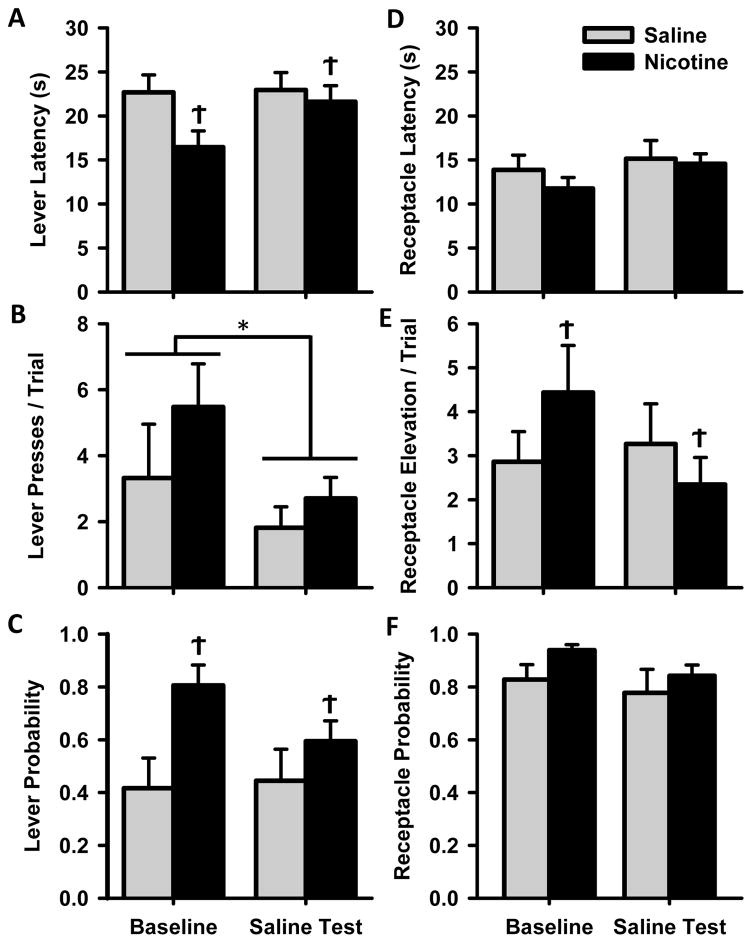
To assess differences in acquisition of Pavlovian conditioned approach behavior, NIC and SAL animals were compared over 20 days of training. Measures of conditioned responding are collapsed across 4 weeks and means ± SEM are presented in Figure 2. Over time, both experimental groups developed conditioned responses to cue presentation, approaching both the lever and reward receptacle. Rats in the NIC group were more likely to exhibit sign tracking behaviors than controls. Both groups decreased the latency to approach the reward receptacle and increased both the number of lever presses per trial and the probability of pressing the lever over 4 weeks of training. NIC rats, relative to SAL rats, showed a non-significant decrease in lever latency over 4 weeks of training [Fig 2A, F(1,12) = 4.3, p=0.061], and a statistically significant decrease on the final day of training [t(12) = 2.3, p<0.05]. NIC rats exhibited more lever pressing than SAL rats over the last two weeks of training [Fig 2B, group × week interaction F(3,36) = 13.1, p<0.001], which was also visible on the final day of training [t(12) = 3.8, p<0.01]. During acquisition, both groups of animals were similarly likely to approach and press the lever at least once per trial [Fig 2C, Χ2(4) = 6.2, p>0.05], but a difference in lever probability emerged by the final day of acquisition [t(12) = 2.3, p<0.05]. There were no differences between groups or any interactions for the goal-tracking measures of receptacle latency, receptacle elevation score, or receptacle probability (Figs 2D–F).
Figure 2.
Cue-evoked behavior during acquisition of Pavlovian conditioned approach behavior for animals in Experiment 1. Rats were trained for 20 days; data are collapsed across 4 weeks and presented as the mean ± SEM for SAL (grey circles) and NIC (black circles) rats. Figures A–F represent separate measures of sign and goal tracking behavior with bar graphs comparing groups only on the last day of training (Day 20): (A) Latencies to approach the lever (B) lever presses per trial (C) probability of pressing the lever, (D) latency to approach the receptacle (E) receptacle elevation scores per trial (F) probability of entering the receptacle. The right side of each panel depicts group behavior (mean ± SEM) on the last day of training (Day 20). Behavior of individual animals in each group on that day are represented by grey circles (note that some circles overlap, especially in the probability graphs). * week × group interaction p<0.05, ‡ difference between NIC and SAL groups on the last day of training, p<0.05.
While we hypothesized that nicotine would increase the likelihood that an animal will show sign-tracking behaviors, we anticipated that significant individual variability would arise within treatment groups. Individual rats can develop sign- and goal-tracking behaviors regardless of drug treatment, and this individual variability emerged during training. To illustrate this, we plotted the behavior of each individual rat in the NIC and SAL groups on the last day of training (Figs 2A–F, right). NIC animals displayed more sign tracking behavior on average, but individuals within both drug exposure groups revealed a diverse behavioral profile, including both sign- and goal-tracking conditioned responses.
3.1.2. Pharmacological inactivation of the OFC
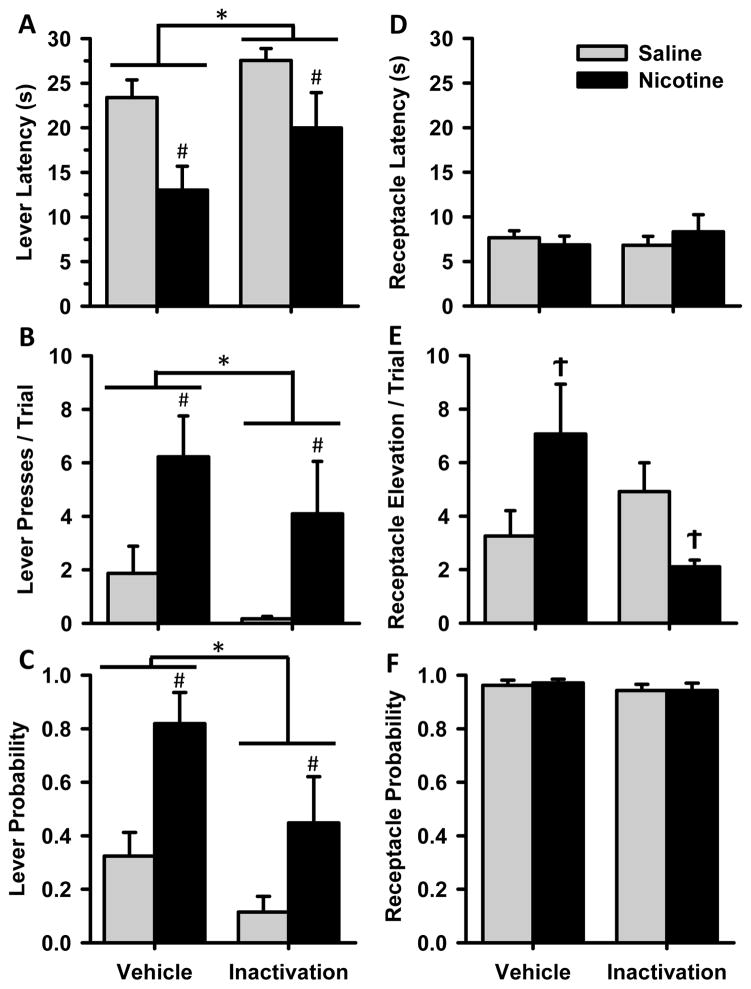
To assess the involvement of the OFC in mediating sign- and goal-tracking conditioned responses and the ability of nicotine exposure to influence OFC control of these behaviors, animals received intra-OFC infusions of the GABA receptor agonists baclofen and muscimol. OFC inactivation reduced conditioned responding in both NIC and SAL animals compared to vehicle infusion, but did not completely abolish behavior (Figure 3). In fact, there were selective changes in some behaviors, and no difference in responding in others. Both NIC and SAL groups showed a reduction in sign-tracking behaviors after OFC inactivation, compared to vehicle infusion. Group differences that were present after vehicle infusion remained after inactivation. There was an increase in lever latency after inactivation (Fig 3A), with a main effect of infusion [F(1,12) = 8.6, p<0.05] and a main effect of group [F(1,12) = 7.3, p<0.05]. Lever presses per trial (Fig 3B) were reduced in both groups after inactivation [F(1,12) = 4.9 p<0.05] while group differences remained [F(1,12) = 5.8, p<0.05]. The same held for lever probability (Fig 3C) with main effects of infusion [Χ2(1) = 7.3, p<0.01] and group [Χ2(2) = 6.6, p<0.05]. The reduction but not complete loss of sign-tracking conditioned responses suggests that the OFC influences the expression of this behavior.
Figure 3.
Cue-evoked behavior after pharmacological inactivation of the OFC. Data are presented as mean ± SEM for NIC (black bars) and SAL (grey) groups after infusion of either vehicle or the GABA receptor agonists baclofen and muscimol into the OFC. Behavioral measures (A–F) are as described in Figure 2. * main effect of infusion p<0.05, # main effect of group p<0.05, Ϯ group × infusion interaction p<0.05.
There were fewer effects of inactivation on goal-tracking behaviors. For receptacle elevation score (Fig 3E) only NIC animals reduced their elevation score after inactivation of the OFC, as demonstrated by a group × infusion interaction [F(1,12) = 6.0 p<0.05]. There was no difference in latency to approach the reward cup (Fig 3D), or probability of performing a receptacle entry after GABA agonist infusion compared to control conditions (Fig 3F). Thus, while both NIC and SAL animals reduced their sign-tracking behaviors after inactivation of the OFC, there were few changes to goal-tracking behaviors, suggesting that the change in behavior was not due to gross deficits in locomotor activity. While measures of receptacle latency and probability of entering the receptacle did not change after OFC inactivation in either group, NIC animals did show a decrease in receptacle elevation score while SAL animals were unchanged.
3.2 Experiment 2 – Single-unit recording from the OFC during Pavlovian conditioning
3.2.1. Training
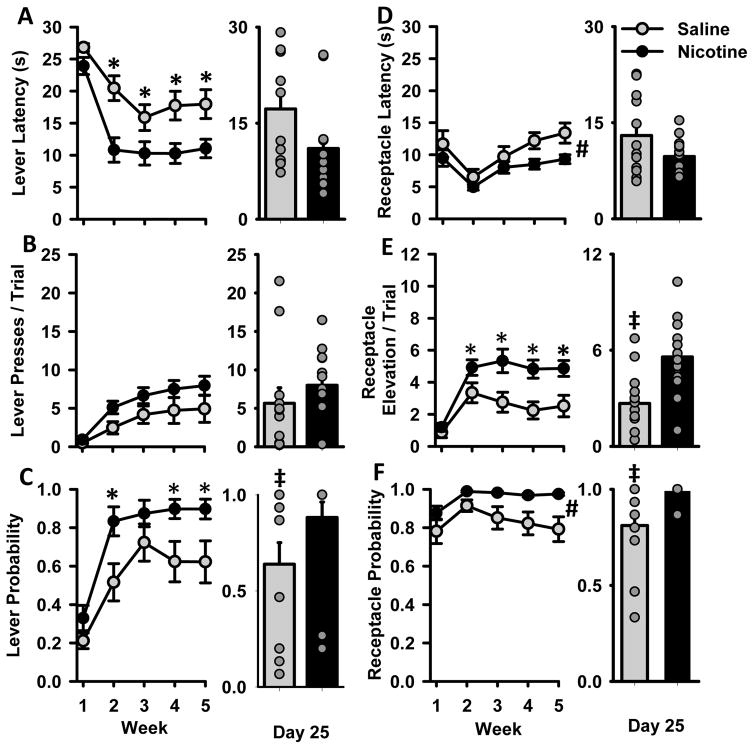
A second cohort of rats was trained with the purpose of conducting in vivo electrophysiology during Pavlovian conditioning sessions. This group of animals trained as described for Experiment 1, but training occurred over 25 days and behavioral data were collapsed across 5 weeks (Fig 4). Additionally, we include comparisons between treatment groups on the last day of training and plot individual behavioral responses on that day (Fig 4A–F, right). In this cohort, NIC animals displayed increased sign tracking and goal tracking behaviors. On measures of sign tracking, NIC animals decreased their lever latency during acquisition [Fig 4A, group × week interaction F(4,92) = 38.6, p<0.05 and showed an increase in lever probability [Fig 4C, group × week interaction X2(4) = 13.3 p<0.05] Post-hoc tests indicate that NIC animals were significantly faster to approach and more likely to press the lever during the last 4 weeks of training. On the last day of training (Day 25), group differences between NIC and SAL animals did not reach significance for lever latency [MWU=42.0, p=0.053], but NIC rats demonstrated a higher probability of lever pressing on this day [MWU=28, p<0.01]. There was no difference in lever presses per trial between NIC and SAL animals, although the mean lever presses for NIC animals was consistently higher than SAL animals each week. On the last day of training, the difference between NIC and SAL groups in number of lever presses did not reach statistical significance [Fig 4B, MWU=44.5, p=0.072]. The lack of a difference on this measure can be attributed to the high variability in SAL animals, and the existence of high-pressing SAL animals. NIC animals also showed increases in goal-tracking behaviors. NIC animals were generally faster to and more likely to approach the receptacle, as demonstrated by main effects of group for receptacle latency [Fig 4D, F(1,23) = 4.4, p<0.05] and receptacle probability [Fig 4F, Χ2(5) = 11.9, p<0.05]. For receptacle elevation score, NIC animals showed a higher elevation score over the last 4 weeks of training [Fig 4E, F(4,92) = 3.5, p<0.05]. On the last day of training, NIC and SAL groups did not differ in terms of latency to press the lever [Fig 4D, MWU=59.0, p>0.05]. However, animals in the NIC group showed a higher receptacle elevation score [Fig 4E, t(23)=3.3, p<0.01] and a greater probability of pressing the lever [Fig 4F, MWU=24.5, p<0.01].
Figure 4.
Cue-evoked behavior during Pavlovian conditioned approach training for animals in Experiment 2. Rats were trained for 25 days and data are collapsed across 5 weeks and presented as mean ± SEM. Panels A–F depict behavior of NIC (black) and SAL (grey) groups, as described in Figure 2. The right side of each panel depicts group behavior (mean ± SEM) on the last day of training (Day 25). Behavior of individual animals in each group on that day are represented by grey circles (note that some circles overlap, especially in the probability graphs). * group × week interaction p<0.05, # main effect of group p<0.05, ‡ difference between groups on Day 25 of training.
3.2.2. Single-unit recording from the OFC
We next performed in vivo single unit recordings of OFC neurons during a standard Pavlovian conditioning session. We analyzed 153 neurons from 12 SAL animals and 120 neurons from 11 NIC animals. 2 animals from the NIC group were removed from analysis for not completing the saline test day, or due to incorrect electrode placements. Basal firing rates in NIC and SAL rats were analyzed immediately before session initiation. No significant differences in basal firing rate arose between groups (mean SAL firing rate: 4.7 ± 0.25 mean NIC firing rate: 5.2 ± 0.3 Hz, MWU statistic = 7972.5, p = 0.231). There was also no difference in average firing rate across the whole session (mean SAL firing rate: 4.6 ± 0.23 Hz, mean NIC firing rate: 5.2 ± 0.29 Hz, MWU statistic =7744.0, p=0.118).
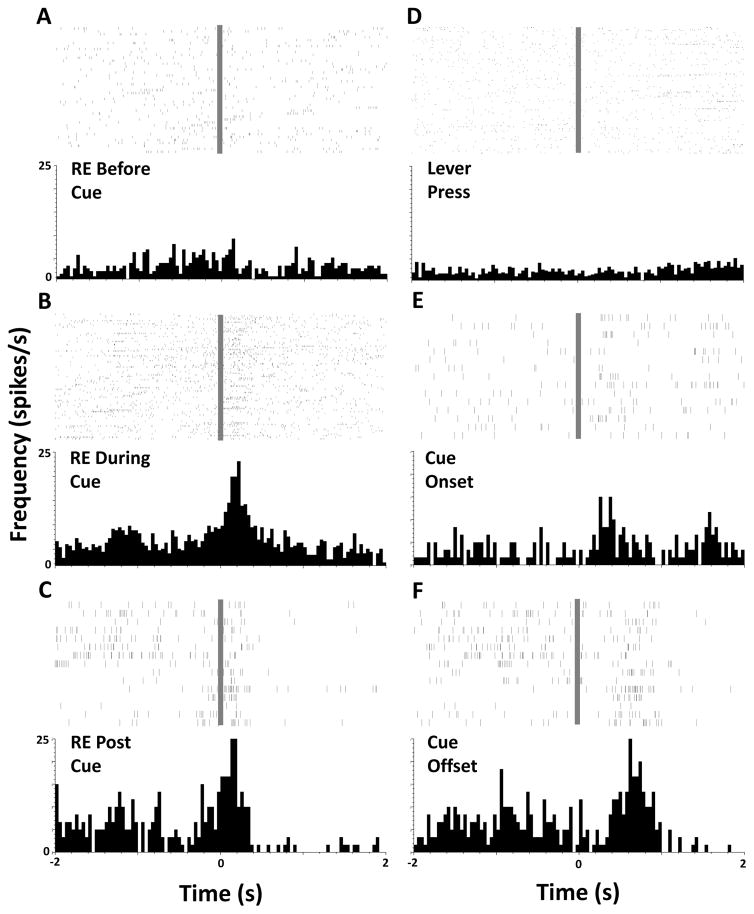
Example raster plots and peristimulus time histograms for a single cell from one NIC animal are presented in Figure 5, depicting firing patterns centered on behavioral responses and within-session events. This cell did not exhibit a change in firing rate during a receptacle entry prior to cue presentation (Fig 5A) but showed an increase in firing rate when the animal performed a goal tracking conditioned response (Fig 5B) and during reward retrieval (Fig 5C). There was no change in firing rate when the animal pressed the lever (Fig 5D), but the cell exhibited changes in firing rate at cue presentation (Fig 5E), and during the first second after cue offset (Fig 5F). Similar firing patterns were visible when population activity was analyzed.
Figure 5.
Rasters and perievent histograms depicting phasic activity of one individual example neuron. Panels A–F represent individual spikes and averaged firing rate during a 4-second period surrounding an event of interest at time=0 s (grey bar). Events of interest are noted on each panel, RE = receptacle entry.
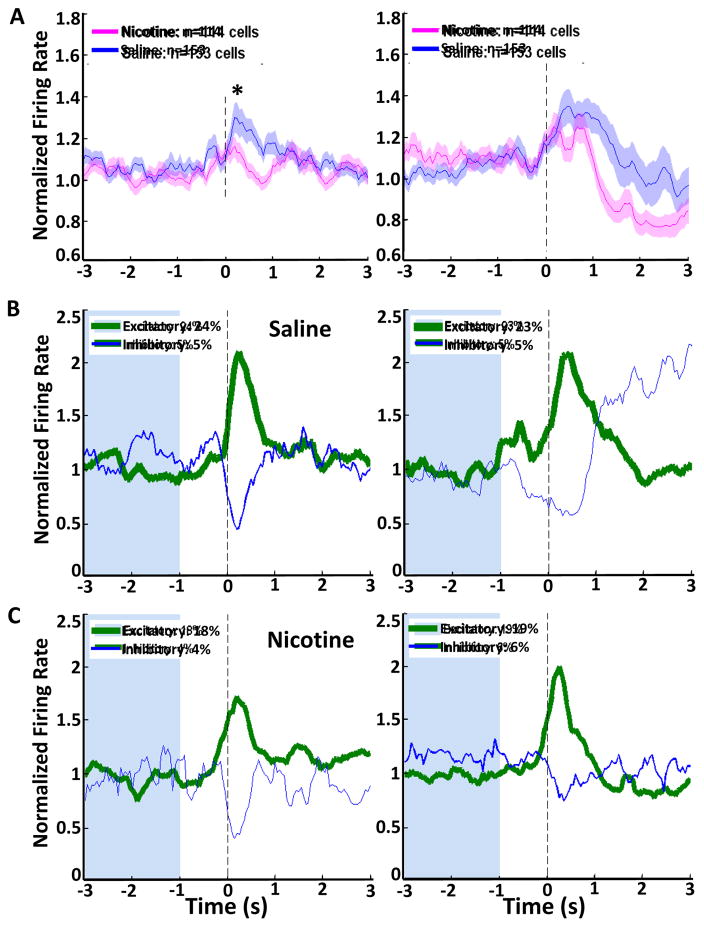
Mean population activity for NIC and SAL groups for those same events are presented in Figures 6(A) and 7(A–D). Figure 6A depicts peristimulus time histograms centered at cue onset and cue offset for NIC and SAL groups. These events are both predictors of reward, as cue onset inspires measurable conditioned responding, and cue offset is a more proximal predictor of reward availability. Firing in both NIC and SAL animals increased in response to cue onset and in response to cue offset. Cells from NIC animals increased their firing rate less than cells from SAL animals did at cue onset. Analysis of the peak firing rate during the 1s after cue onset by Kruskal-Wallis one way ANOVA on ranks indicates a difference between NIC and SAL groups (Fig 6A, left; H(1) = 9.1, p<0.01). There was no difference in firing rate during the 1s after cue offset (Fig 6A, right). It is possible that the observed changes in OFC neuronal firing were due to alterations in the testing environment, and therefore not specific to the presentation of reward-predictive stimuli. To test this possibility, we analyzed population activity of OFC cells to an additional stimulus: the house light illumination that marked the start of the conditioning session (Supplemental figure S1). We found no increase in OFC firing rate upon illumination of the house light.
Figure 6.
Single unit electrophysiological recordings at cue onset or offset during a Pavlovian conditioning session. (A) Neuronal population activity in the OFC is presented as mean firing rate (±SEM, shaded) and normalized to whole session firing rate for nicotine-exposed (pink) and saline (blue) animals at cue onset and cue offset. (B, C) Phasic firing patterns of neurons that significantly changed their firing rate surrounding either cue offset or cue onset, for SAL (B) and NIC (C) groups. Green histograms represent cells that increased their firing rate, and blue histograms represent cells that decreased their firing rate, line thickness represents the proportion of cells displaying each phasic pattern. (*) significant difference in peak firing rate between groups, p<0.05.
Figure 7.
Single unit electrophysiological recordings during behavioral responses in a Pavlovian conditioning session. (A–D, left column) Neuronal population activity in the OFC is presented as mean firing rate (±SEM, shaded) and normalized to whole session firing rate for nicotine-exposed (pink) and saline-control (blue) animals centered on behavioral responses. Phasic firing patterns of neurons that significantly changed their firing rate surrounding behavioral events are depicted for SAL (center column) and NIC (right column) groups. Green histograms represent cells that increased their firing rate, and blue histograms represent cells that decreased their firing rate, line thickness represents the proportion of cells displaying each phasic pattern. (*) significant difference in peak firing rate between groups, p<0.05.
Next we examined individual neuronal firing patterns at cue onset and offset. Figures 6B and 6C depict mean firing rates for phasically active neurons at cue onset and offset for SAL (Fig 6B) and NIC (Fig 6C) groups. Phasically active neurons are classified as those cells that significantly increased or decreased their firing rate compared to a 2s baseline (blue shaded area) prior to the time of the stimulus. Phasic cells from SAL and NIC animals displayed a primarily excitatory response at cue onset and cue offset. For cells from SAL animals, 24% were excited and 5% were inhibited at cue onset, while 23% were excited and 5% were inhibited at cue offset. The same pattern was seen in cells from NIC rats, where 18% of cells were excited and 4% were inhibited at cue onset, and 19% were excited and 6% were inhibited at cue offset.
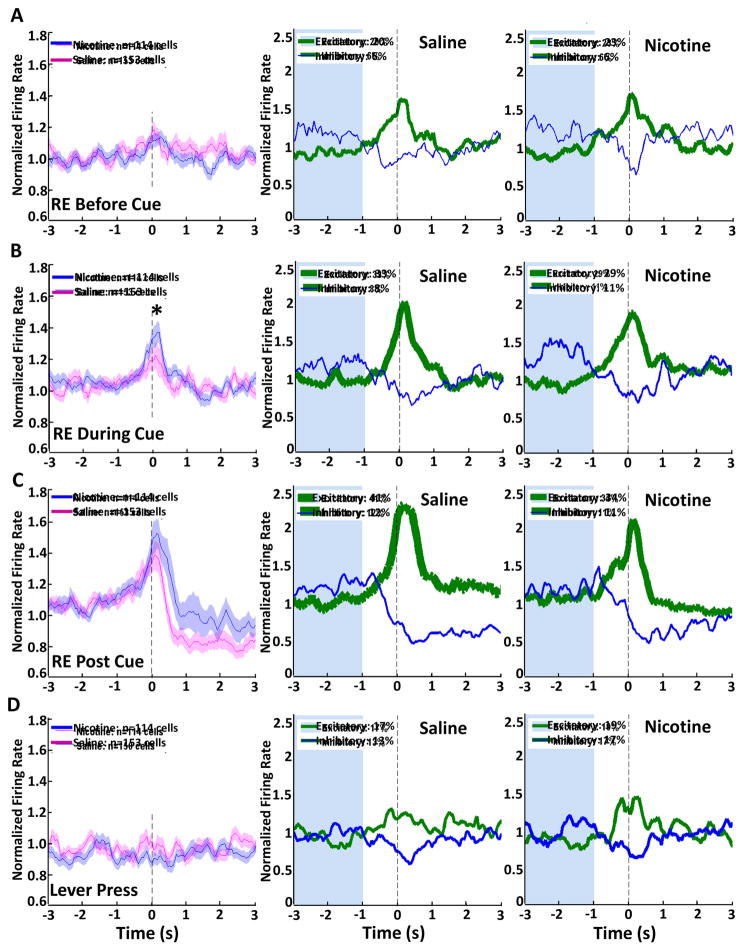
In addition to changes in firing rates due to cue presentation, we analyzed OFC neuronal firing during conditioned responses in the same Pavlovian conditioning session. Events of interest were the first receptacle entries in the 30 s before cue onset, during each trial, or immediately after cue offset, as well as the first lever press of each trial. Population and phasic activity were analyzed and compared for NIC and SAL groups. OFC neurons showed an increase in firing rate particularly during receptacle entries that occur as part of a goal-tracking conditioned response and when retrieving the reward after the cue presentation, but not during receptacle entries that occurred before cue presentation (Fig 7). Population activity centered on receptacle entries that occur in the absence of the cue indicate no change in mean firing rate during this behavior. Analysis of phasic activity in both NIC and SAL animals indicates that a proportion of neurons are excited surrounding this behavior, with 20% and 6% of SAL cells and 23% and 6% of NIC cells being excited or inhibited, respectively (Fig 7A). During the first receptacle entry that occurred during cue presentation, OFC neurons exhibited a pronounced increase in firing rate. The peak firing rate for SAL cells was significantly higher than that of NIC cells (Kruskal-Wallis H(1) = 5.7, p<0.05). In addition, 33% of SAL units and 29% of NIC units were phasically excited, while 8% and 11% were inhibited (Fig 7B). No peak differences arose between NIC and SAL cells during the first receptacle entry immediately after cue offset, when the animal retrieves the reward. The highest proportion of phasically excited cells for each group was present during this period, with 41% of SAL cells and 34% of NIC cells being excited, and 12% of SAL cells and 11% of NIC cells being inhibited (Fig 7C). Finally, when the first lever press of each trial was analyzed, we found that there was no increase in population firing rate surrounding this action. This can be explained by the roughly equal proportion of cells that were excited and inhibited, with 17% and 13% of SAL cells being excited or inhibited, and 19% and 17% of NIC cells showing excitation or inhibition surrounding the event (Fig 7D). Thus, the increase in peak firing and proportion of phasically active cells during a cue-evoked conditioned response compared to a general receptacle entry suggests that the OFC encodes these actions differently. In comparison, there was a much less distinct change in population activity during a lever press, although OFC neurons were both phasically excited and inhibited during this behavior.
3.2.3. Pavlovian conditioned approach behavior in the absence of nicotine
We next injected NIC animals with saline instead of nicotine before testing to investigate whether the observed effects on Pavlovian conditioned responding were due to acute exposure to the drug or to lasting effects of repeated nicotine exposure. SAL animals received a saline injection as always, and served as a control for the reproducibility of this behavior. On the saline test day (Figure 8), NIC animals exhibited less conditioned responding on both sign- and goal-tracking measures while SAL animals did not change their behavior across the two days. There was a main effect of test day for lever presses [Fig 8B, F(1,21) = 8.5, p<0.01], which is explained by high variability in two animals in the SAL group that drastically reduced their lever pressing from the baseline day to the test day, while all other SAL animals remained within ± 2.4 lever presses per trial between the two days. There was a group × test day interaction for lever latency [Fig 8A, F(1,21) = 8.4, p<0.01], lever press probability [Fig 8C, Χ2 (1) = 6.3, p<0.05], and receptacle elevation score [Fig 8E, F(1,21) = 6.7, p<0.05]. Post-hoc comparisons indicate that NIC animals displayed more of these behaviors on the baseline day and reduced their conditioned behaviors on the saline test day while SAL animals showed no change. These results suggest that the acute effect of nicotine is responsible for the enhanced conditioned responding, as NIC animals reduced their behavior to SAL levels in the absence the drug.
Figure 8.
Cue-evoked behavior during the saline test session. Data are presented as mean ± SEM for NIC and SAL groups after injection with saline during the saline test session, or on a baseline day in which animals received the assigned drug or control injection. Behavioral measures (A–F) are as described in Figure 2. * main effect of test day p<0.05, Ϯ group × test day interaction p<0.05.
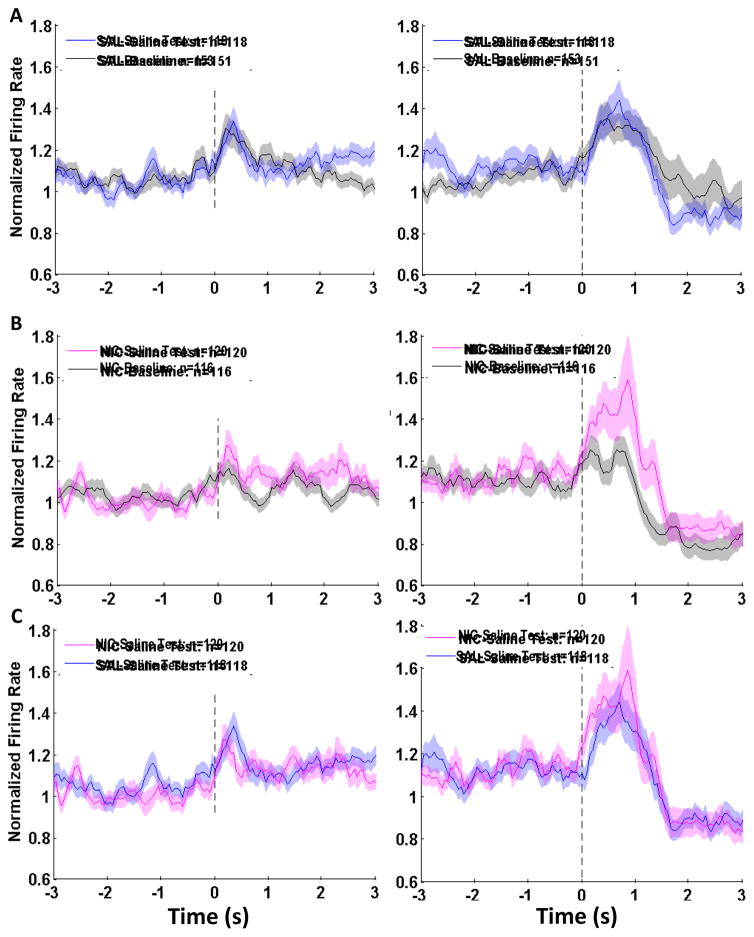
3.2.4. Phasic cell firing in the absence of nicotine
In addition to behavior, we also measured neuronal firing during the saline test day. Analysis of basal firing rates recorded prior to session initiation for SAL animals did not yield any statistically significant differences (mean baseline day: 4.7 ±0.25 Hz, mean saline test day: 4.2 ±0.26 Hz, MWU statistic = 8201.0, p= 0.197) nor were there any differences in whole session firing rate (mean baseline day: 4.6 ±0.23 Hz, mean saline test day: 4.1 ±0.23 Hz, MWU statistic = 8286.0, p=0.247). There was a difference in basal firing rate in NIC animals on the regular Pavlovian session compared to the saline test session (mean baseline day: 5.2 ±0.3 Hz, mean saline test day: 4.0 ±0.27Hz, MWU statistic = 5226.5, p<0.01) and in whole session firing rate (mean baseline day: 5.2 ± 0.29 Hz, mean saline test day: 3.9 ±0.25 Hz, MWU statistic = 5048.0, p<0.01). However, basal firing rates and whole session firing rates did not differ between NIC and SAL animals on either the baseline day or the saline test day.
Peak normalized firing rates to within-session events were compared for each exposure group on the saline test day and baseline day. There were no statistically significant differences for SAL animals across the two test days on any measure (Figure 9A). For cells from NIC animals, there was an increase in peak firing at cue onset and [Kruskal-Wallis H(1) = 7.5, p<0.01] and at cue offset [Kruskal- Wallis H(1) = 4.8, p<0.05] after injection with saline instead of nicotine (Figure 9B). When peak firing rates for NIC and SAL animals were compared on the saline test day, there was no difference between them (Figure 9C). Thus, withholding the nicotine injection resulted in a reduction in conditioned responding and an increase in OFC neuronal firing to within-session events.
Figure 9.
Single unit electrophysiological recordings at cue onset or offset during the saline test session. Neuronal population activity in the OFC is presented as mean firing rate (±SEM, shaded) and normalized to whole session firing rate for nicotine-exposed and saline-control animals at cue onset and cue offset. (A, B) Population firing rates in SAL animals on the saline test day (Panel A, blue histograms) and NIC animals (Panel B, pink histograms) compared to the baseline recording session depicted in Figure 6 (grey histograms). (C) Comparison of population activity from SAL (blue) and NIC (pink) animals on the saline test day. (*) significant difference in peak firing rate between groups, p<0.05.
4. Discussion
In two cohorts of animals, we found that nicotine increased conditioned responding; sign-tracking behaviors were elevated in both Experiments 1 and 2 while goal-tracking behaviors were increased in Experiment 2. Inactivation of the OFC primarily reduced sign tracking and additionally reduced goal tracking in NIC rats. Electrophysiological recordings indicated that the OFC is active in response to the onset of a reward-predictive cue and during the retrieval of the reward. Goal tracking was encoded in about 30% of OFC neurons via phasic excitations that were time-locked to the behavior. In contrast, less than 20% of OFC neurons exhibited phasic excitations to sign-tracking behaviors, which was not sufficient to produce a change in population activity. Chronic treatment with nicotine blunted the increase in OFC population firing rate at cue onset and this reduction in firing was recovered when nicotine treatment was discontinued, suggesting that nicotine acutely reduces phasic firing of OFC neurons. Nicotine-induced enhancement of conditioned approach also declined to control levels when nicotine treatments were discontinued, suggesting that the changes in firing rate observed in the OFC may play a role in nicotine-enhanced conditioned approach. However, this association cannot be explicitly discerned from the inactivation study and should be validated empirically.
Our model produced sign- and goal-tracking behavior similar to previous reports (Flagel et al., 2009; Palmatier et al., 2013b; Versaggi et al., 2016), as control animals exhibited individual preferences for the sign- or goal-tracking behavior. In addition, nicotine exposure increased the likelihood that an animal would display a sign-tracking response in Experiments 1 and 2. This enhancement in approach to the conditioned cue in NIC animals fits with previous accounts of nicotine’s ability to enhance the incentive value of a conditioned cue, even one that is not particularly associated with delivery of nicotine itself (Chaudhri et al., 2006; Palmatier et al., 2013b; Yager and Robinson, 2015). We see that nicotine increased goal tracking in Experiment 2 but not Experiment 1, possibly because of the smaller number of animals in Experiment 1. Many studies that observe populations of animals that sign and goal track include much larger cohorts of animals to achieve the full range of behavior (Meyer et al., 2012). Previous work has also pointed to the effect of colony and vendor differences on the behavioral traits of animals (Fitzpatrick et al. 2013), although all animals from this study were obtained from the same vendor and location. We have previously reported enhanced goal tracking in NIC-exposed animals (Palmatier et al., 2013b), similar to the Experiment 2 results reported here. This interesting result prompts further investigation into the nature of nicotine’s incentive amplifying effects, as other drugs of abuse, such as cocaine and alcohol, have been shown to preferentially enhance sign tracking (Krank et al., 2008; McClory and Spear, 2014; Uslaner et al., 2006). Nicotine is capable of increasing conditioned approach in animals pre-classified as sign and goal trackers, but on tests of conditioned reinforcement, nicotine enhances conditioned reinforcement in sign tracking animals specifically (Yager and Robinson, 2015). This suggests that while nicotine can enhance both conditioned responses, drug exposure may impact animals differently based on individual predispositions.
We aimed to discern the function and involvement of the OFC in both sign- and goal-tracking components of Pavlovian conditioned approach. Previous studies involving permanent lesions of the OFC indicated involvement of this region in approach to the location of reward delivery and noted deficits in behavior when updating stimulus-outcome associations and representations of the value of the cue or reward (e.g., Ostlund & Balleine 2007; Chudasama & Robbins 2003b). Here, we show that inactivation of the OFC by infusion of GABAA and GABAB receptor agonists (Experiment 1) reduced expression of sign tracking regardless of nicotine exposure. Yet, when neuronal firing rates were analyzed surrounding a lever press (Experiment 2), we saw little phasic firing of OFC cells. In one study (Flagel et al., 2011a), OFC activation was measured by c-fos mRNA expression when the cue was presented without reward after 3 days of extinction to the context, and only sign-tracking animals displayed an increase in OFC c-fos expression. Our pharmacological inactivation experiment agrees with this study, in that there was a reduction in sign tracking after OFC inactivation. This result, tempered by the lack of population activity or robust phasic firing during expression of the behavior, suggests that the OFC is involved in promoting the conditioned response, but that the behavior itself is not explicitly encoded in the firing rate of OFC neurons. The OFC is part of a much broader circuit that stimulates the sign-tracking response, particularly in terms of mesocorticolimbic circuitry that includes the nucleus accumbens (Cooch et al., 2015) and is required for the attribution of incentive salience to a cue (Flagel et al., 2011b). The function of the OFC during sign tracking may be to represent the association between the cue and expected outcome and encode the anticipatory state evoked by cue presentation, allowing other components of the circuit to access this representation and invoke the actual behavioral response (Gallagher et al., 1999). Recent descriptions of the function of the OFC, which suggest that it serves to integrate a multitude of cortical, subcortical, and sensory inputs to create a representation of the current task state (Wilson et al., 2014), may provide an explanation for the role of the OFC during sign tracking.
Conversely, inactivation (Experiment 1) produced a limited reduction in goal tracking that only occurred in NIC animals. Physiologically (Experiment 2), there was a time-locked excitation of OFC neurons during cue-evoked receptacle entries as well as during retrieval of the reward, but not during receptacle entries that occurred in the absence of the cue. This suggests that neurons in the OFC are specifically encoding receptacle entries associated with anticipation of the expected outcome, which aligns with previous reports of single-unit activity in the OFC (Schoenbaum et al., 1998; Stalnaker et al., 2014). Therefore, while the OFC may contribute to both types of conditioned response, OFC neurons explicitly encode in their firing patterns the goal-tracking conditioned response that more closely represents the expected outcome.
In addition to measuring OFC firing during conditioned responses, we also measured increases in firing rate immediately following presentation of the reward predictive cue. Multiple studies report OFC excitations to reward predictive cues in both primate and rodent models (Moorman and Aston-Jones, 2014; O’Doherty et al., 2002; Schoenbaum and Roesch, 2005; Tremblay and Schultz, 1999), and OFC cells in the present study displayed excitations to both cue presentation and cue offset. Both aspects of the cue provide valuable information about the timing of reward receipt, with cue onset signaling pending reward delivery, and cue offset being the most proximal signal of immediate reward availability. However, it is possible that OFC cells are firing due to salient changes in the testing chamber, and not specifically to stimuli that predict reward. To address this, we analyzed firing in response to house light illumination, which occurs at the beginning of every conditioning session. The house light represents a salient change in the testing environment, but not one that is paired with immediate reward delivery. We found that OFC neurons did not show a time-locked excitation to the house light, which stands in contrast to their increased firing rate within the first second after cue presentation. A limitation of the present study is the lack of an unpaired stimulus for comparison, but a recent study that included an unpaired stimulus (Moorman and Aston-Jones, 2014) reported that OFC neurons exhibit reduced excitation to unpaired stimuli relative to reward-predictive stimuli. The inclusion of an unpaired stimulus would also allow us to discern the effects of nicotine on neuronal firing and behavior, as nicotine might enhance the reinforcing properties of an unpaired stimulus. However, we have previously demonstrated that nicotine exposure did not enhance the reinforcing properties of a non-reinforcing stimulus (Palmatier et al., 2012, 2007).
In this study, we found that peak firing rates in NIC animals were lower than those in SAL animals at cue onset and during a goal-tracking conditioned response. When nicotine was not injected prior to session initiation, NIC animals exhibited a higher peak firing to both cue presentation and cue offset. Similar to a previous report (Guy and Fletcher, 2014), a reduction in conditioned responding accompanied these physiological effects, suggesting that the behavioral and physiological effects of nicotine resulted from acute actions of the drug.
At the time of testing, rats in this experiment had been receiving single daily injections of nicotine, 5 days on and 2 days off, for at least 8 weeks. Animals received enough nicotine to produce locomotor sensitization (Benwell and Balfour, 1992; Cadoni and Di Chiara, 2000; Miller et al., 2001) but would not have achieved the long-lasting increase in blood concentration of nicotine seen with self-administration. Studies of the effects of chronic exposure to nicotine, either through self-administration of the drug or through passive exposure paradigms, have demonstrated changes on a cellular and behavioral level in humans and animals (Barik and Wonnacott, 2009; Perry et al., 1999). Acute or low dose administration of the drug can also result in changes to gene expression (Mychasiuk et al., 2013) as well as receptor expression and behavior (Barik and Wonnacott, 2009; Vezina et al., 2007). Although we cannot be sure of the extent of neuroadaptations induced by nicotine exposure in our paradigm, it is clear that this exposure resulted in physiological adaptations that were alleviated in the absence of nicotine.
Nicotine acts on nicotinic acetylcholine receptors (nAchRs) that are comprised of combinations of receptor subunits that display variations in receptor level physiology resulting in differences in affinity and rates of receptor desensitization or upregulation (Feduccia et al., 2012; Picciotto et al., 2008). NAChRs are located on multiple cell types in the PFC, including fast spiking and non-fast spiking interneurons (Poorthuis et al., 2013). Activation of nAchRs on interneurons can lead to the increase in GABAergic transmission within the PFC (Couey et al., 2007) and could reduce firing of pyramidal neurons, which would provide an explanation for the reduction in peak firing rate that we observed. We found that inactivation of the OFC by microinfusion of GABAergic agonists resulted in a slight reduction in goal tracking, specifically in NIC animals. This might have resulted from the compounded interaction of nicotine on GABAergic signaling, along with the increase in GABA receptor activation caused by drug infusion. Future studies could utilize techniques that do not target neurotransmitter signaling, such as chemogenetic inactivation of the region immediately before conditioned responding. Overall, we expect that the intricate pattern of nicotinic receptor activation, desensitization, and upregulation led to the nicotine-induced changes in cell firing that we observed,, but further studies are necessary to complete our understanding of the effect of receptor-level plasticity on real-time neuronal firing patterns.
The complex activation profile of nAChRs within the PFC alone could provide an explanation for the physiological results we observed. However, nicotine is capable of acting on nAChRs present throughout the brain, particularly within corticolimbic regions involved in reward processing and motivated behavior (Markou, 2008). Activation of nAChRs within regions that project to the OFC, such as the ventral tegmental area, can influence both behavior and cell firing within the OFC. In the ventral tegmental area, activation of nAChRs on dopaminergic projection neurons can lead to the release of dopamine in the PFC (Livingstone et al., 2009). With this study, we begin to elucidate the effects of nicotine on phasic firing patterns in the OFC, but additional studies will be required to form a complete picture of the circuit-level effects of nicotine on both behavior and the underlying neuronal activity.
This is the first set of experiments to systematically explore the neurophysiology of nicotine-enhanced sign and goal tracking; therefore, several questions about the effects of nicotine and the neurobiological underpinnings of its effects on motivated behavior remain. For example, is it problematic that nicotine appears to enhance approach to both the sign and the goal in this paradigm? Most substance dependence models have emphasized sign tracking and its role in vulnerability, yet we (Palmatier et al. 2013) have shown that nicotine can increase both sign tracking and goal tracking to a sucrose-paired stimulus, and others (Yager and Robinson, 2015) have shown that sign trackers and goal trackers approach a nicotine-paired stimulus equally. In addition, while we have begun to explore the role of the OFC in these behaviors, the recruitment of the broader mesocorticolimbic circuit should also be investigated. Further clarification could be garnered by specifically targeting corticolimbic pathways thought to be associated with this behavior. Lastly, the precise actions of nicotine that lead to the measured behavioral and physiological changes are beyond the scope of this study. Future investigations of the cellular events, triggered by repeated exposure to nicotine, will bolster our understanding of the process by which nicotine modifies cue-evoked behavior.
5. Conclusions
Although we utilized an animal model of conditioned responding to probe the ability of nicotine to influence approach to a reward-predictive cue, these results have translational relevance as the same phenomenon has been observed in studies of similar cue-evoked behavior in humans. Specifically, nicotine use can enhance attentional bias to drug-associated cues in smokers (Chanon et al., 2010; Field et al., 2004) and increased cue-related activity in mesotelencephalic systems can predict relapse to nicotine use (Janes et al., 2010; McClernon et al., 2007; Versace et al., 2014). Moreover, smoking nicotine-containing cigarettes can also enhance ratings of facial attractiveness in smokers, relative to smoking de-nicotinized cigarettes (Attwood et al., 2009). In studies of smokers who were presented with both food and cigarette cues and then asked about craving, a strong cue-induced craving for food was correlated with craving cigarettes (Mahler and de Wit, 2010; Styn et al., 2013). Thus, using animal models to investigate sources of biological variability in nicotine’s enhancement of incentive stimuli can contribute to potential targets for intervention in the treatment of nicotine addiction.
In summary, using an established model of Pavlovian conditioned approach behavior that is enhanced by nicotine, we have shown that the OFC neuronal firing is recruited primarily during the goal-tracking conditioned response and that nicotine exposure acutely blunts firing in the OFC. These findings further our understanding of the ability of drugs of abuse to amplify existing variation in behavioral and physiological responses to conditioned cues.
Supplementary Material
Highlights.
Nicotine enhances Pavlovian conditioned responses.
The orbitofrontal cortex modulates conditioned responding.
Nicotine acutely blunts phasic cell firing to Pavlovian conditioned cues.
Acknowledgments
Funding: This research was funded by the National Institutes of Health [P60 AA011605, Project #3] and the UNC Bowles Center for Alcohol Studies. SJS was supported on 5P60AA011605-17S1.
The authors would like to thank Drs. Aric Madayag and Tatiana Shnitko for critical comments on the manuscript, Dr. Rebecca Fanelli and Kevin Caref for programming assistance, Dr. Margaret Broadwater, Brandi Lawrence, Yue Dong, Jesse Sharp, and Eric Bloomquist for outstanding technical assistance and Chris Wiesen from the UNC Odum Institute for statistical consultation.
Footnotes
Notes: The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Attwood AS, Penton-voak IS, Munafò MR. Effects of acute nicotine administration on ratings of attractiveness of facial cues. 2009;11:44–48. doi: 10.1093/ntr/ntn006. [DOI] [PubMed] [Google Scholar]
- Barik J, Wonnacott S. Molecular and cellular mechanisms of action of nicotine in the CNS. Handb Exp Pharmacol. 2009:173–207. doi: 10.1007/978-3-540-69248-5_7. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol. 1992;105:849–56. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Jenkins HM. Auto-shaping of the pigeon’s key-peck. J Exp Anal Behav. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton AC, Kashtelyan V, Bryden DW, Roesch MR. Increased firing to cues that predict low-value reward in the medial orbitofrontal cortex. Cereb Cortex. 2014;24:3310–3321. doi: 10.1093/cercor/bht189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G. Differential changes in accumbens shell and core dopamine in behavioral sensitization to nicotine. Eur J Pharmacol. 2000;387:1999–2001. doi: 10.1016/S0014-2999(99)00843-2. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/S0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Chanon VW, Sours CR, Boettiger CA. Attentional bias toward cigarette cues in active smokers. Psychopharmacology (Berl) 2010;212:309–320. doi: 10.1007/s00213-010-1953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: Impact of nicotine dose and reinforcement schedule. Psychopharmacology (Berl) 2007;190:353–362. doi: 10.1007/s00213-006-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 2006;189:27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable Contributions of the Orbitofrontal and Infralimbic Cortex to Pavlovian Autoshaping and Discrimination Reversal Learning: Further Evidence for the Functional Heterogeneity of the Rodent Frontal Cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooch NK, Stalnaker TA, Wied HM, Bali-Chaudhary S, McDannald MA, Liu TL, Schoenbaum G. Orbitofrontal lesions eliminate signalling of biological significance in cue-responsive ventral striatal neurons. Nat Commun. 2015;6:7195. doi: 10.1038/ncomms8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couey JJ, Meredith RM, Spijker S, Poorthuis RB, Smit AB, Brussaard AB, Mansvelder HD. Distributed Network Actions by Nicotine Increase the Threshold for Spike-Timing-Dependent Plasticity in Prefrontal Cortex. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Acquas E, Carboni E. Drug motivation and abuse: a neurobiological perspective. Ann N Y Acad Sci. 1992;654:207–19. doi: 10.1111/j.1749-6632.1992.tb25969.x. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Fanelli RR, Klein JT, Reese RM, Robinson DL. Dorsomedial and dorsolateral striatum exhibit distinct phasic neuronal activity during alcohol self-administration in rats. Eur J Neurosci. 2013;38:2637–48. doi: 10.1111/ejn.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feduccia AA, Chatterjee S, Bartlett SE. Neuronal nicotinic acetylcholine receptors: neuroplastic changes underlying alcohol and nicotine addictions. Front Mol Neurosci. 2012;5:1–18. doi: 10.3389/fnmol.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Mogg K, Bradley BP. Eye movements to smoking-related cues: effects of nicotine deprivation. Psychopharmacology (Berl) 2004;173:116–23. doi: 10.1007/s00213-003-1689-2. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Gopalakrishnan S, Cogan ES, Yager LM, Meyer PJ, Lovic V, Saunders BT, Parker CC, Gonzales NM, Aryee E, Flagel SB, Palmer AA, Robinson TE, Morrow JD. Variation in the form of pavlovian conditioned approach behavior among outbred male sprague-dawley rats from different vendors and colonies: sign-tracking vs. goal-tracking. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56:139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience. 2011;196:80–96. doi: 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers Ca, Clinton SM, Phillips PEM, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–7. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine Psychopharmacology. Springer Berlin Heidelberg; Berlin, Heidelberg: 2009. Effects of Nicotine in Experimental Animals and Humans: An Update on Addictive Properties; pp. 335–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Mcmahan RW, Schoenbaum G. Orbitofrontal Cortex and Representation of Incentive Value in Associative Learning. J Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–7. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Guy EG, Fletcher PJ. The effects of nicotine exposure during Pavlovian conditioning in rats on several measures of incentive motivation for a conditioned stimulus paired with water. Psychopharmacology (Berl) 2014;231:2261–2271. doi: 10.1007/s00213-013-3375-3. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, de Frederick BB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain Reactivity to Smoking Cues Prior to Smoking Cessation Predicts Ability to Maintain Tobacco Abstinence. Biol Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Krank MD, O’Neill S, Squarey K, Jacob J. Goal- and signal-directed incentive: conditioned approach, seeking, and consumption established with unsweetened alcohol in rats. Psychopharmacology (Berl) 2008;196:397–405. doi: 10.1007/s00213-007-0971-0. [DOI] [PubMed] [Google Scholar]
- Livingstone PD, Srinivasan J, Kew JN, Dawson LA, Gotti C, Moretti M, Shoaib M, Wonnacott S. Alpha7 and Non-Alpha7 Nicotinic Acetylcholine Receptors Modulate Dopamine Release in Vitro and in Vivo in the Rat Prefrontal Cortex. Eur J Neurosci. 2009;29:539–550. doi: 10.1111/j.1460-9568.2009.06613.x. EJN6613 [pii]\r. [DOI] [PubMed] [Google Scholar]
- Logan FA. Incentive: How the Conditions of Reinforcement Affect the Performance of Rats. Yale University Press; 1964. [Google Scholar]
- Mahler SV, de Wit H. Cue-reactors: Individual differences in cue-induced craving after food or smoking abstinence. PLoS One. 2010;5:1–3. doi: 10.1371/journal.pone.0015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar AC, Walker ALJ, Theobald DE, Eagle DM, Robbins TW. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J Neurosci. 2011;31:6398–404. doi: 10.1523/JNEUROSCI.6620-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A. Review. Neurobiology of nicotine dependence. Philos Trans R Soc L B Biol Sci. 2008;363:3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Liu J, Salley AN, Behm FM, Rose JE. Selectively reduced responses to smoking cues in amygdala following extinction-based smoking cessation: Results of a preliminary functional magnetic resonance imaging study. Addict Biol. 2007;12:503–512. doi: 10.1111/j.1369-1600.2007.00075.x. [DOI] [PubMed] [Google Scholar]
- McClory AJ, Spear L. Effects of Ethanol Exposure During Adolescence or in Adulthood on Pavlovian Conditioned Approach in Sprague-Dawley Rats. Alcohol. 2014:1–30. doi: 10.1016/j.alcohol.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One. 2012;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DK, Wilkins LH, Bardo MT, Crooks PA, Dwoskin LP. Once weekly administration of nicotine produces long-lasting locomotor sensitization in rats via a nicotinic receptor-mediated mechanism. Psychopharmacology (Berl) 2001;156:469–476. doi: 10.1007/s002130100747. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orbitofrontal Cortical Neurons Encode Expectation-Driven Initiation of Reward-Seeking. J Neurosci. 2014;34:10234–10246. doi: 10.1523/JNEUROSCI.3216-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychasiuk R, Muhammad A, Ilnytskyy S, Kolb B. Persistent gene expression changes in NAc, mPFC, and OFC associated with previous nicotine or amphetamine exposure. Behav Brain Res. 2013;256:655–661. doi: 10.1016/j.bbr.2013.09.006. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/S0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Mclellan AT, Ehrman R. Classical Conditioning in Drug-Dependent Humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984. [DOI] [PubMed] [Google Scholar]
- Ogawa M, van der Meer MAA, Esber GR, Cerri DH, Stalnaker TA, Schoenbaum G. Risk-Responsive Orbitofrontal Neurons Track Acquired Salience. Neuron. 2013;77:251–258. doi: 10.1016/j.neuron.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Krueger DD, Tronson NC, Nairn AC, Taylor JR. Orbitofrontal cortex and cognitive-motivational impairments in psychostimulant addiction: evidence from experiments in the non-human primate. Ann N Y Acad Sci. 2007;1121:610–38. doi: 10.1196/annals.1401.016. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J Neurosci. 2007;27:4819–25. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Coddington SB, Liu X, Donny EC, Caggiula AR, Sved AF. The motivation to obtain nicotine-conditioned reinforcers depends on nicotine dose. Neuropharmacology. 2008;55:1425–1430. doi: 10.1016/j.neuropharm.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Kellicut MR, Sheppard BA, Brown RW, Robinson DL. The incentive amplifying effects of nicotine are reduced by selective and non-selective dopamine antagonists in rats. Pharmacol Biochem Behav. 2014;126C:50–62. doi: 10.1016/j.pbb.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Lantz JE, O’Brien LC, Metz SP. Effects of nicotine on olfactogustatory incentives: preference, palatability, and operant choice tests. Nicotine Tob Res. 2013a;15:1545–54. doi: 10.1093/ntr/ntt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Marks KR, Jones SA, Freeman KS, Wissman KM, Sheppard BA. The effect of nicotine on sign-tracking and goal-tracking in a Pavlovian conditioned approach paradigm in rats. Psychopharmacology (Berl) 2013b;226:247–59. doi: 10.1007/s00213-012-2892-9. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, Donny EC, Sved AF. The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug Alcohol Depend. 2007;89:52–59. doi: 10.1016/j.drugalcdep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, O’Brien LC, Hall MJ. The role of conditioning history and reinforcer strength in the reinforcement enhancing effects of nicotine in rats. Psychopharmacology (Berl) 2012;219:1119–1131. doi: 10.1007/s00213-011-2439-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. Increased Nicotinic Receptors in Brains from Smokers: Membrane Binding and Autoradiography Studies. J Pharmacol Exp Ther. 1999;289:1545–1552. [PubMed] [Google Scholar]
- Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, Huettl P, Dwoskin LP, Bardo MT. Prefrontal cortex and drug abuse vulnerability : Translation to prevention and treatment interventions. Brain Res Rev. 2010;65:124–149. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorthuis RB, Bloem B, Schak B, Wester J, de Kock CPJ, Mansvelder HD. Layer-specific modulation of the prefrontal cortex by nicotinic acetylcholine receptors. Cereb Cortex. 2013;23:148–61. doi: 10.1093/cercor/bhr390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Carelli RM. Distinct subsets of nucleus accumbens neurons encode operant responding for ethanol versus water. Eur J Neurosci. 2008;28:1887–94. doi: 10.1111/j.1460-9568.2008.06464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in resisting temptation: Implications for addiction. Neurosci Biobehav Rev. 2013;37:1955–1975. doi: 10.1016/j.neubiorev.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012;36:2521–32. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual Variation in the Motivational Properties of Cocaine. Neuropsychopharmacology. 2011;36:1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch M. Orbitofrontal Cortex, Associative Learning, and Expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Prefrontal cortical contribution to risk-based decision making. Cereb Cortex. 2010;20:1816–1828. doi: 10.1093/cercor/bhp250. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Cooch NK, McDannald MA, Liu T-L, Wied H, Schoenbaum G. Orbitofrontal neurons infer the value and identity of predicted outcomes. Nat Commun. 2014:5. doi: 10.1038/ncomms4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Cooch NK, Schoenbaum G. What the orbitofrontal cortex does not do. Nat Neurosci. 2015;18:620–627. doi: 10.1038/nn.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styn MA, Bovbjerg DH, Lipsky S, Erblich J. Cue-induced cigarette and food craving: A common effect? Addict Behav. 2013;38:1840–1843. doi: 10.1016/j.addbeh.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–8. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Acerbo MJ, Jones Sa, Robinson TE. The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behav Brain Res. 2006;169:320–4. doi: 10.1016/j.bbr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Versace F, Engelmann JM, Robinson JD, Jackson EF, Green CE, Lam CY, Minnix JA, Karam-hage MA, Brown VL, Wetter DW, Cinciripini PM. Prequit fMRI responses to pleasant cues and cigarette-related cues predict smoking cessation outcome. Nicotine Tob Res. 2014;16:697–708. doi: 10.1093/ntr/ntt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versaggi CL, King CP, Meyer PJ. The tendency to sign-track predicts cue-induced reinstatement during nicotine self-administration, and is enhanced by nicotine but not ethanol. Psychopharmacology (Berl) 2016 doi: 10.1007/s00213-016-4341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, McGehee DS, Green WN. Exposure to nicotine and sensitization of nicotine-induced behaviors. Prog Neuro-Psychopharmacology Biol Psychiatry. 2007;31:1625–1638. doi: 10.1016/j.pnpbp.2007.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RC, Takahashi YK, Schoenbaum G, Niv Y. Orbitofrontal cortex as a cognitive map of task space. Neuron. 2014;81:267–278. doi: 10.1016/j.neuron.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. Individual variation in the motivational properties of a nicotine cue: sign-trackers vs. goal-trackers. Psychopharmacology (Berl) 2015;232:3149–3160. doi: 10.1007/s00213-015-3962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Floresco SB, Winstanley CA. Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology (Berl) 2010;211:87–98. doi: 10.1007/s00213-010-1871-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.