Abstract
The misuse of prescription opiates is on the rise with combination therapies (e.g. acetaminophen or NSAIDs) resulting in severe liver and kidney damage. In recent years, cannabinoid receptors have been identified as potential modulators of pain and rewarding behaviors associated with cocaine, nicotine and ethanol in preclinical models. Yet, few studies have identified whether mu opioid agonists and CB2 agonists act synergistically to inhibit chronic pain while reducing unwanted side effects including reward liability. We determined if analgesic synergy exists between the mu-opioid agonist morphine and the selective CB2 agonist, JWH015, in rodent models of acute and chronic inflammatory, post-operative, and neuropathic pain using isobolographic analysis. We also investigated if the MOR-CB2 agonist combination decreased morphine-induced conditioned place preference (CPP) and slowing of gastrointestinal transit. Co-administration of morphine with JWH015 synergistically inhibited preclinical inflammatory, post-operative and neuropathic-pain in a dose- and time-dependent manner; no synergy was observed for nociceptive pain. Opioid-induced side effects of impaired gastrointestinal transit and CPP were significantly reduced in the presence of JWH015. Here we show that MOR + CB2 agonism results in a significant synergistic inhibition of preclinical pain while significantly reducing opioid-induced unwanted side effects. The opioid sparing effect of CB2 receptor agonism strongly supports the advancement of a MOR-CB2 agonist combinatorial pain therapy for clinical trials.
Keywords: Antinociception, CB2 cannabinoid receptor agonist, Inflammation, Morphine, Reward, Synergy
1. Introduction
Prescribed opioids, specifically those that bind mu-opioid receptors (MOR), have become some of the most highly abused drugs with deaths from drug overdose rising steadily over the past two decades (CDC, 2015). In 2007, the cost for prescription opioid abuse in the US was estimated at approximately $55.7 billion (Birnbaum et al. 2011); costs in Europe were nearly €4.2 billion (EMCDDA 2010). Of the 1,244,872 emergency department visits in 2011, almost half involved opioid analgesics (Crane, 2015); 80% of new heroin drug users had previously abused prescription opioids (NPR, 2013). These statistics have led to increases in physician and patient concern over prescribing and using opioids for chronic pain, respectively (Balko, 2012).
Opioids such as morphine derivatives or oxycodone along with a mixture of NSAIDs are commonly prescribed for the treatment of acute and chronic pain (CDC, 2015). Monotherapy opioids are associated with undesirable side effects that include increased somnolence, constipation, cognitive impairment, hyperesthesia, respiratory depression with propensity towards addiction at doses that achieve analgesic efficacy (Chan et al., 1999; Heyman et al., 1988; Koch and Höllt, 2008; Ling et al., 1984). Opioid combination therapies such as Vicodin® (hydrocodone/paracetamol) and Percocet® (oxycodone/paracetamol), although synergistic as analgesics, have been limited due to the significant increase in liver and kidney damage (Mitka, 2014; Watkins et al., 2006). To overcome these opioid dosing obstacles of abuse and toxicity, alternatives to the current opioid or opioid-NSAID combination pain therapies are urgently needed.
Cannabinoid 2 receptors (CB2) are G protein-coupled receptors primarily localized on cells within the immune system (Pertwee, 1997). Activation of CB2 by either endogenous or exogenous agonists attenuates both acute and chronic pain by inhibiting inflammation (Kinsey et al., 2011; Wilkerson and Milligan, 2011). Moreover, CB2 agonists have been shown to significantly inhibit thermal/mechanical hypersensitivity and spontaneous pain in preclinical models of neuropathic and bone cancer pain (Ibrahim et al., 2003; Ibrahim et al., 2005; Lozano-Ondoua, 2013; Lozano-Ondoua et al., 2010). Treatment-resistant neuropathic pain disorders including HIV, multiple sclerosis, and chemotherapy-induced neuropathy have responded to cannabinoid intervention (Anand et al., 2009; Fine and Rosenfeld, 2014; Rahn and Hohmann, 2009). Moreover, CB2 agonists do not elicit many adverse effects including rewarding behavior alone and can reduce cocaine (Xi et al., 2011) nicotine (Navarrete et al., 2013) and ethanol-induced (Ortega-Álvaro et al., 2014) rewarding effects. To date, selective CB2 agonists (i.e., GW842166) have been well-tolerated in human clinical trials for the treatment of inflammatory pain (NIH, 2012). These collective data suggest that the co-administration of a cannabinoid and prescribed opioids would inhibit pain without increasing the misuse of opioids (Perron et al., 2015).
To address the increasing need to advance innovative strategies to treat chronic pain, we investigated whether dual-targeting of the mu opioid (MOR) and CB2 receptors would produce synergistic antinociception in preclinical models of acute, inflammatory, post-operative and neuropathic pain. We also tested whether the combination of a CB2 agonist and morphine would reduce morphine-induced constipation, conditioned place preference and dopamine release; critical limitations that must be addressed for successful clinical translation. Studies here strongly suggest that the combination MOR/CB2 agonists result in a synergistic analgesic effect in chronic models of pain while significantly reducing unwanted opioid side effects.
2. Materials and Methods
2.1. Animals
Male Sprague-Dawley rats weighing 250–300g and male ICR mice weighing 15–25g were obtained from Envigo (Indianapolis, IN). All procedures were approved by the University of Arizona Animal Care and Use Committee, and conform to the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health. Procedures were also in compliance to the guidelines of the International Association for the Study of Pain (Zimmermann, 1983) and are in accordance to ARRIVE guidelines for reporting experiments involving animals or animal tissue (Curtis et al., 2015; McGrath and Lilley, 2015). Animals were maintained on a 12-hour light/dark cycle in a climate-controlled room and were provided with food and water ad libitum. To determine statistical significance, a power analysis was performed using GPower3.1 software to verify the number of animals needed for each experiment (Faul et al., 2009). A total of 273 animals were used herein.
2.2. Drugs
JWH015, a cannabinoid 2 receptor (CB2) agonist (CB2 Ki = 13.8 nM, 30 to 80 fold selectivity versus CB1) was obtained from Cayman Chemical (Ann Arbor, MI). JWH015 was dissolved in a vehicle solution of 10% dimethyl sulfoxide, 10% Tween-80, and 80% saline (Sigma, St. Louis, MO). The mu-opioid agonist (MOR) morphine sulfate was purchased from the NIDA Drug Supply program (Rockville, MD) and was dissolved in saline. Formalin solution was obtained by diluting 1.5% of formaldehyde in saline. Ketamine:xylazine (80 mg/kg: 12 mg/kg; Phoenix Pharmaceutical, St. Joseph, MO) was used to anesthetize animals in order to insert microdialysis probes into the nucleus accumbens. The antibiotic gentamicin (Phoenix Pharmaceutical, St. Joseph, MO) was provided as a single subcutaneous dose (8mg/kg). Drugs were weighed out and dissolved in vehicle daily, prior to use. Cocaine hydrochloride was purchased from the NIDA Drug Supply program (Rockville, MD) and used as a positive control in microdialysis and HPLC studies. Finally, 5% and 2.5% isofluorane (Sigma, St. Louis, MO) mixed in 2.5 L/min of oxygen was delivered through a nose cone and used to induce and maintain anesthesia, respectively, for paw incision and SNI surgeries.
2.3. Antinociceptive responses
2.3.1. Measurement of thermal tail withdrawal latency (tail flick assay)
Male ICR mice were used in all tail flick studies. The distal two-thirds of the tail were immersed in a circulating water bath maintained at 52°C, and latency to withdraw the tail was recorded (tail withdrawal latencies). Following baseline testing, mice were given a systemic injection of vehicle, morphine (1, 3, 10 mg/kg, i.p.), JWH015 (1, 10, 100 mg/kg, i.p.), or a fixed dose combination of morphine and JWH015. As a control, the vehicle for both morphine and JWH015 were injected and tail withdrawal latencies recorded. Mice were re-tested using the tail flick water bath every fifteen minutes over a 1-hour time course. The maximal effect (cut-off latency) was defined at 10 seconds, in order to prevent possible tissue damage. Any animal that reached the cut-off latency was returned to their cage and received the maximum latency score.
2.3.2. Measurement of inflammatory pain (formalin flinch test)
The formalin flinch test is a well-recognized, acute inflammatory pain assay characterized by a biphasic response (Tjølsen et al., 1992). Male ICR mice received an intraplantar injection of 1.5% formalin into their left hind paw 15 minutes before testing. Animals were placed in a Plexiglas chamber and the number of flinches observed was recorded for one hour in 5 min bins. Flinching behavior was characterized as a rapid upward movement of the injected hind paw. As a control, vehicle only (saline) was injected into paws, and flinches recorded. Mice were evaluated for antinociception after systemic (intraperitoneal, i.p.) application of drug treatment as compared to vehicle control. Systemic injections of vehicle, morphine (0.1, 0.3, 0.6, 1 mg/kg, i.p.), JWH015 (0.1, 1, 3, 10, 30, 100 mg/kg, i.p.) or a fixed dose combination of morphine and JWH015 were given to separate mice. The number of flinches in all groups were reassessed for 1 hour. Flinches recorded from 0 to 10 min after formalin is noted as the first phase while the second phase of flinching was recorded from 11 to 60 min.
2.3.3. Measurement of post-operative pain
For paw incision, male Sprague-Dawley rats were induced at 5% and maintained on 2.5% isoflurane mixed in 2.5 L/min of air delivered through a nose cone. A 1-cm longitudinal incision was made through the skin and fascia on the plantar surface of the left hind paw. The plantaris muscle was elevated and incised longitudinally. Following incision, the muscle remained intact and the skin was affixed with two 3–0 silk sutures. Rats were allowed to recover in their home cages for a 24-hour period before they were behaviorally tested (Brennan et al., 1996). Animals were administered morphine (1, 3, 10 mg/kg, i.p.), JWH015 (1, 3, 10 mg/kg, i.p.) or a fixed dose combination of morphine and JWH015 for behavioral assays and compared to vehicle-treated animals. Behavioral assays included testing for thermal and mechanical hypersensitivity in elevated Plexiglas chambers from a thermal radiant heat source (Hargreaves et al., 1988) and calibrated von Frey filaments (Chaplan et al., 1994) in 30-minute intervals over a 2-hour time course, respectively.
2.3.4. Model of neuropathic pain
Neuropathic pain is characterized as a complex pain disorder that has proven to be very difficult to manage. Spared nerve injury (SNI) is a rodent model of persistent peripheral neuropathic pain (Decosterd and Woolf, 2000). Prior to surgery, baseline behaviors for thermal and mechanical sensitivity were performed. Under isoflurane anesthesia, the common peroneal and tibial terminal distal branches of the sciatic nerve were ligated and axotomized, while leaving the sural nerve intact. As a control, Sham animals underwent surgery and exposure of the sciatic nerve but no ligations were made. One week following surgery, animals (SNI and Sham) were systemically administered vehicle, morphine (1, 3, 10 mg/kg, i.p.), JWH015 (1, 3, 10 mg/kg, i.p.) or a fixed dose combination. Following drug treatment, rats were tested for mechanical and thermal sensitivity over a 2-hour time course and compared to vehicle-treated animals.
2.3.5. Measurement of thermal and mechanical hypersensitivity
Thermal hypersensitivity was determined using the Hargreaves method of assaying thermal nociception as previously described (Hargreaves et al., 1988). Rats were individually placed in clear plastic chambers with a glass floor, and allowed to acclimate to the environment for 30-minutes prior to testing. A radiant heat source was placed directly beneath the hindpaw ipsilateral to injury, and the latency to withdrawal the paw was recorded in seconds. In order to prevent tissue damage, the cut-off time was set at 33 seconds. Thermal antinociception was measured in the ipsilateral hindpaw prior to (baseline) and following drug treatments over a 2-hour time course.
Calibrated von Frey filaments applied to the plantar surface were used to assess paw withdrawal thresholds of the hind paw, ipsilateral to injury. Mechanical hypersensitivity was determined using the Chaplan up-down method (Chaplan et al., 1994). The nonparametric method of Dixon was used to determine the 50% paw withdrawal thresholds (Dixon, 1980).
2.4. Measurement of opioid-induced side effects
2.4.1. Conditioned Place Preference (CPP)
In order to investigate whether JWH015 impacts the rewarding effect of morphine, conditioned place preference (CPP) was conducted (Largent-Milnes et al., 2013). Rats were preconditioned to a 3-chambered testing apparatus (San Diego Instruments) on the first day. They were allowed to freely explore each of the chambers. One end chamber had striped walls and a smooth floor, another end chamber had gray walls with a textured floor, and the middle transition chamber had parallel bars on the floor and a light above. The total time spent in each chamber over 15 minutes was recorded, and only animals that showed no preference (< 80% total time) to any chamber were randomly assigned and counter-balanced to a drug conditioning end-chamber over the next 3 days. The middle chamber acted as a transition chamber and was not assigned for drug pairing. Rats received a systemic (i.p.) injection of vehicle in the opposite end (non-drug paired end) of the box as a control. On test day, after three previous days of drug-chamber pairing (24 hours post-vehicle exposure), animals were allowed to explore all chambers of the box for 15 minutes while the total time spent in each of the ends were recorded to determine chamber preference as compared to vehicle-treated animals.
2.4.2. Brain Microdialysis and High Performance Liquid Chromatography (HPLC)
Extracellular dopamine concentrations in the striatum were collected from freely moving animals via microdialysis (Chefer et al., 2009). Male Sprague-Dawley rats weighing 250–300g were anesthetized with ketamine/xylazine and secured in a stereotaxic apparatus. Guide cannulas (Eicom, San Diego, California) were implanted into the nucleus accumbens (NAc) according to Paxinos and Watson coordinates: AP +1.7mm, ML +1.0mm, and DV −6.0 from bregma. The rats were injected with the antibiotic gentamicin (8 mg/kg, s.c.) and allowed a week for recovery before microdialysis was performed. Artificial cerebral spinal fluid (aCSF) was used to equilibrate within the striatal tissue. Following a 60-minute baseline period, rats were injected with either saline, morphine (10 mg/kg, i.p.), JWH015 (1mg/kg, i.p.), or a combination fixed dose ratio of morphine and JWH015. Dialysate was allowed to flow through the probe at a rate of 0.8 µL/min collected in 30-minute intervals for 3 hours. Cocaine hydrochloride was administered via the dialysate probe at the end of the experiment as a positive microdialysis placement control by measuring increased dopamine levels in the NAc. Collected dialysate samples were chilled in the presence of an antioxidant and injected into the HPLC for neurochemical analysis (Meske et al., 2013). Immediately following microdialysis, rats were sacrificed and their brains were harvested. Microdialysis guide cannula placement was verified post-mortem. Coronal slices (40 µm) of the striatum were cut on a cryostat and placements confirmed after staining with cresyl violet (Hascup et al., 2009). Only rats verified with correct cannula placement were used in the final analysis.
2.4.3. Gastrointestinal Transit
Opioid-induced constipation is a major complaint for pain patients. Our gastrointestinal transit study was used to determine the effect of our drug combination on geometric center and gastric emptying as a representation of GI constipation. Rats were fasted for 16 hours prior to drug treatment in order to empty the contents of their gastrointestinal tract. Animals were treated with vehicle, low (1 mg/kg, i.p.) or high (10 mg/kg, i.p.) doses of morphine, JWH015 (1 mg/kg, i.p.) or a combination treatment of morphine with JWH015. Fifteen minutes following drug administration the animals were fed a nutritive meal of 1.5 ml of 2% milk marked with Cr-51 (p.o.). Cr-51 is used since it does not escape the gut and can easily track GI transit. Fifteen minutes after Cr-51 milk gavage, rats were sacrificed using an overdose of the anesthetic isoflurane, and the GI tract from the stomach to the cecum removed and cut into 10 equal segments. The amount of radioactivity in each segment was determined by a gamma counter and compared to a standard curve for analysis. Plotting drug treatment against the % of total Cr-51 in the small intestine is a measure of gastric emptying. Gastric emptying represents the proportion of the Cr-51 marker in the small intestine segments. Geometric center was used as a measure of gastrointestinal propulsion of the radioactive marker along the small intestine. A low score indicated total inhibition while a high score represented complete transit through the intestine (scored 1–10).
2.5. Statistical analysis
The data and statistical analyses described here adhere to the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Animals were randomly assigned to experimental groups in all studies, and the experimenter was kept blinded to treatment. Power analyses were performed on cumulated data by using GPower3.1 software to estimate the optimal numbers required. We found the adequate statistical separation required for each group in order to detect 0.80 differences between the drugs and control groups at p < 0.05.
GraphPad Prism 5.0 software (Graph Pad Inc., San Diego, CA) was used to analyze and plot data. Responses to drug treatments in tail flick, formalin flinch, gastrointestinal transit, paw incision, and SNI assays were compared using ANOVA, post hoc testing using Student’s t-test with Bonferroni’s multiple comparison test. CPP was analyzed using Student’s t-test. Data were expressed as means ± SEM and statistical significance was defined as p<0.05. Percent of maximal possible effect (%MPE) was calculated based on the following equation:
Isobolographic analyses of tail flick, formalin flinch, post-operative, and neuropathic data were conducted based on principles previously described (Tallarida, 2006). Dose-response relationships were used to provide the magnitude of effect of the drug combination. Following the creation of dose-response curves for morphine and JWH015, linear regression analyses were utilized in order to determine the effective dose at 50% (ED50) for each drug. The effect of least three doses were used for each drug when tested alone or in combination. The ED50 of each individual drug was used to determine the dose ratios for tail flick, formalin flinch, paw incision, and SNI. This allowed us to use the drug potencies (A50) to determine the relative potency when the drugs were combined. If the two drugs produced a similar effect at different doses, their combination likely contributed to the overall effect based on their individual potencies suggesting an additive relationship. A drug interaction occurs when additivity is no longer present, which can be useful in elucidating mechanisms. An isobologram was generated in order to determine whether the drug combination was additive, super-additive (synergistic), or sub-additive (antagonist).
HPLC chromatograms were analyzed using Aligent Chemstation data acquisition software (Agilent Technologies, Palo Alto, CA, USA). HPLC data were analyzed using ANOVA followed by the post hoc Dunnett test. Striatal dopamine levels are expressed as the percent of the mean dopamine concentration in the nucleus accumbens obtained from vehicle treated animals, which represented 100%.
3. Results
3.1. Co-administration of morphine and JWH015 produces thermal antinociception
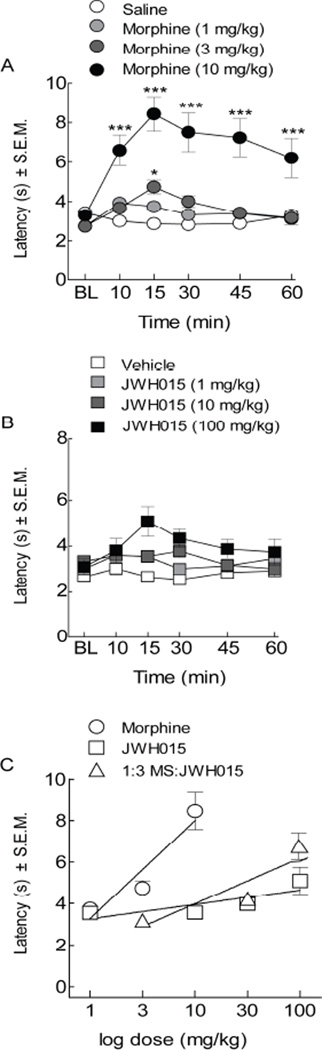
To determine the nature of the interaction between a CB2 and MOR agonist, compounds were administered separately as well as co-administered and tested using a tail-flick assay in mice. Systemic morphine (1, 3, 10 mg/kg, i.p.) dose-dependently increased tail-flick latencies 15 minutes after administration (Figure 1A); morphine achieved full antinociceptive efficacy at 10 mg/kg, i.p. as compared to vehicle-treated animals (A50=4.47, 95% CI ± 2.66; F (3, 20)=16.22). This effect lasted at least 45 minutes. To assess whether our CB2 agonist exerted similar effects on antinociception, tail withdrawal latency was measured in animals treated with JWH015 (1, 10, and 100 mg/kg, i.p.) or its combination with morphine (1:3, morphine:JWH015) using dose ratios.
Figure 1.
The co-administration morphine and JWH015 attenuated nociceptive warm water (52°C) tail-flick assay in an additive manner. Tail-flick latency was assessed after (A) morphine treatment at 1, 3, and 10 mg/kg, i.p. (B) or JWH015 treatment at 1, 10, 100 mg/kg, i.p. resulting in a dose-dependent attenuation of thermal pain signals following treatment with morphine. (C) Dose-response curves revealed a significant left-ward shift with morphine, but not its combination with JWH015 (1:3 morphine:JWH015). Data are expressed as means ± SEM. One-way ANOVA, with Bonferroni’s multiple comparison post hoc test (*p<0.05, n =10).
Data were converted into percent maximum possible effect (%MPE) to generate dose-response curves at the 15 minute time point (Figure 1C). Doing this allowed us to determine the efficacy of the agonists and provided an analgesic index. Even though JWH015 did not result in significant antinociception, we went forward with the combined dose ratio of 1:3 of morphine:JWH015. The combination of JWH015 with morphine did not significantly alter tail withdrawal latencies from predicted single drug administered animals (p=0.12, n=10, Figure 1B, C). Vehicle-treatment did not significantly change baseline tail flick latencies at any time tested.
3.2. Co-administering morphine and JWH015 synergistically attenuates acute inflammatory nociception
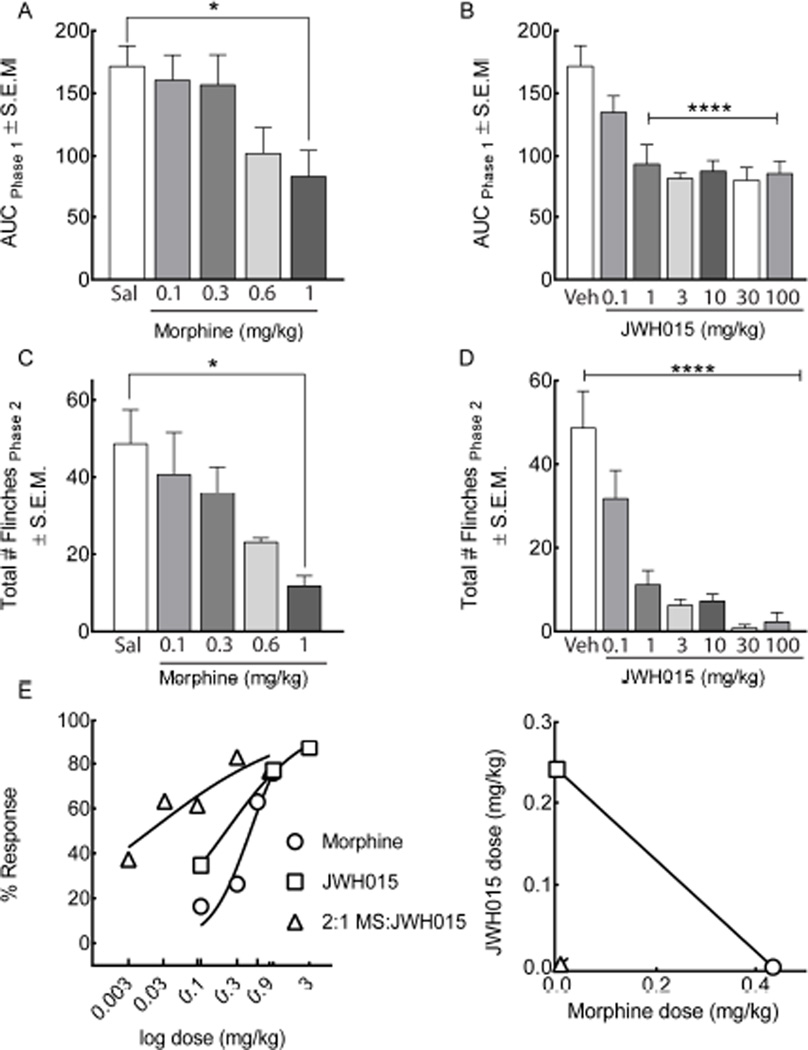
We next investigated the synergistic effect of co-administered morphine and JWH015 to attenuate acute inflammatory pain using the formalin flinch test in male ICR mice. Following a 30-minute habituation period in raised Plexiglas chambers, vehicle or drug was systemically administered to mice. Fifteen minutes later, an intraplantar injection of 1.5% formalin was administered and the number of flinches was recorded for one hour. Intraplantar formalin injection produced a biphasic effect (Figure 2 A–D, n=7–15). The first phase occurred within the first 10 minutes and is characterized by an acute, phasic peak in neuronal firing. The second phase is more prolonged and tonic, and occurs within 11–60 minutes. The second phase is associated with an inflammatory response.
Figure 2.
Combined morphine and JWH015 result in a synergistic inhibition of formalin-induced hind paw flinching. (A–D) Intraplantar injection of 1.5% formalin produced a biphasic effect in the ipsilateral (left) hind paw. Systemic administration of (A,C) morphine at 0.1, 0.3, 0.6, and 1 mg/kg, i.p. or (B,D) JWH015 at 0.1, 1, 3, 10, 30, and 100 mg/kg, i.p. (C,D) dose-dependently attenuated their combination in the second phase. (E) Dose-response analyses illustrate a left-ward shift at a 2:1 morphine:JWH015 dose ratio. Isobologram for the A50 of Morphine plotted against JWH015 (F) indicated a synergistic interaction. The linear line represents the line of additivity. The isobol point was determined from the 2:1 (Morphine:JWH015) dose ratio. Confidence intervals for the theoretical additive and isobol point are shown and can be found in Table 1. Data are expressed as means ± SEM. One-way ANOVA, with Bonferroni’s multiple comparison post hoc test (*p<0.05, n = 7–15).
Both morphine (0.1, 0.3, 0.6, 1 mg/kg, i.p., Figure 2A, p=0.02) and JWH015 (0.1, 1, 3, 10, 30, 100 mg/kg, i.p., Figure 2B, p=0.001) treatment resulted in a dose-dependent decrease in area under the curve (AUC) values in the first phase. A significant reduction in the number of flinches was also observed in the inflammatory second phase in response to systemic morphine (Figure 2C, F(4,44)=3.372, p=0.01) and JWH015 (Figure 2D, F (6,96)=16.16, p=0.0001) as compared to vehicle-treated animals. Furthermore, dose-response analyses resulted in a leftward shift in the curve when the two drugs were co-administered (A50=0.01, 95% CI ± 0.024), suggesting a synergistic interaction (Figure 2E). This synergism was confirmed by isobolographic analyses (Figure 2F and Table 1).
Table 1.
Relative potencies of systemic combinations of morphine and JWH015 observed in thermal, inflammatory, postoperative incision, and neuropathic pain models. A p value of <0.05 is indicative of a synergistic relationship. Statistical analyses for all drug interactions were performed using JFlashCalc software (M. Ossipov, University of Arizona)
| Pain Model | Behavioral Assay | Morphine | JWH015 | Morphine:JWH015 | Theoretical Additive |
Drug Interaction |
|---|---|---|---|---|---|---|
| Thermal | Tail flick | 4.47 ± 2.66 | 2.44E4 ± 4.38E5 | 1:3 39.2 ± 43.8 |
28.5 ± 71.7 | Not synergistic |
| Inflammatory | 1.5% Formalin flinch | 0.38 ± 0.33 | 0.10 ± 0.11 | 2:1 0.01 ± 0.02* R2 = 0.9 |
0.34 ± 0.17 | Synergistic |
| Post-operative incision |
Radiant Heat | 3.56 ± 8.61 | 3.73 ± 2.31 | 1:1 0.14 ± 0.36* R2 = 0.9 |
3.65 ± 11.89 | Synergistic |
| von Frey | 5.02 ± 26.86 | 4.52 ± 13.87 | 1:1 0.11 ± 0.41* R2 = 0.7 |
4.75 ± 14.29 | Synergistic | |
| Spared Nerve Injury (SNI) |
Radiant Heat | 3.94 ± 2.99 | 3.73 ± 23.07 | 1:1 0.14 ± 3.36* R2 = 0.9 |
3.83 ± 12.26 | Synergistic |
| von Frey | 2.56 ± 4.68 | 2.92 ± 3.42 | 1:1 0.11 ± 0.41* R2 = 0.7 |
2.72 ± 3.05 | Synergistic |
Relative A50 ± 95% CI
significant difference from the theoretical A50, indicating a synergistic relationship
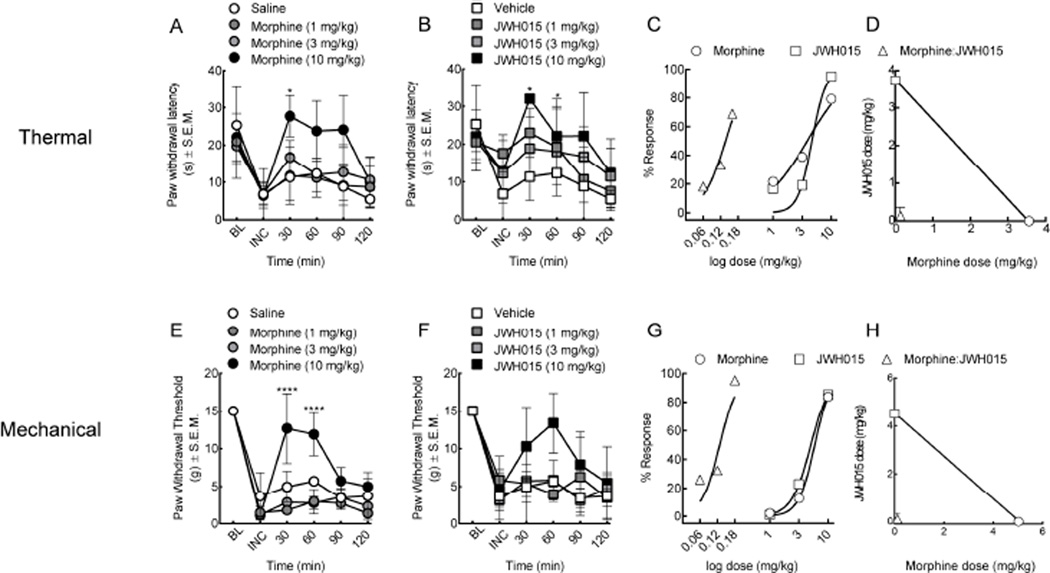
3.3. Synergistic interaction between morphine and JWH015 in a rodent model of postoperative pain
Acute pain from post-operative procedures is a widespread phenomenon. To determine the effect of morphine and JWH015 on post-operative pain, the left hind paw of rats were incised (Brennan, 2010). Twenty-four hours later, rats demonstrated both thermal and mechanical hypersensitivity (Figure 3, incision (INC) baseline, n=7). Systemic morphine (1, 3, 10 mg/kg, i.p.) produced a dose-dependent increase in thermal paw withdrawal latency (A50=3.56 CI ± 8.61, F (5, 89)=12.6, p=0.0001, Figure 3A) and paw withdrawal threshold (A50=5.02 CI ± 26.86, F (3, 95)=56.29, p=0.0001, Figure 3E). Rats treated with JWH015 (1, 3, 10 mg/kg, i.p.) showed a dose-dependent increase in paw withdrawal latency (A50=3.73 CI ± 2.31, F (3, 83)=4.18, p=0.008, Figure 3B) in the thermal assay and increased paw withdrawal thresholds (A50=4.52 CI ± 13.87, F (3, 83)=7.236, p=0.002, Figure 3F) in mechanical testing when compared to vehicle-treated animals. Dose-response and isobolographic analyses revealed a significant left-ward shift in the dose-response curve using a 1:1 (morphine:JWH015) dose ratio at the peak antinociceptive effect for both thermal hypersensitivity at 30 minutes (A50=0.14 CI ± 0.36, Figure 3C,D, Table 1) and allodynia at 60 minutes (A50=0.11 CI ± 0.41, Figure 3G,H, Table 1) as compared to vehicle-controls.
Figure 3.
Synergistic interaction between morphine and JWH015 in post-surgical pain of the plantar surface of the hind paw. (A–D) Thermal hypersensitivity (E–H) and mechanical withdrawal thresholds of the ipsilateral paw 24 hours post-incision. (A, E) Systemic morphine (1, 3, 10 mg/kg, i.p.) and (B, F) JWH015 (1, 3, 10 mg/kg, i.p.) resulted in an anti-thermal and anti-mechanical hypersensitivity. Combination therapy using dose ratios resulted in a significant leftward shift in the dose-response curve for both (C, D) thermal and (G, H) mechanical allodynia, indicative of synergy. BL = baseline; INC = incision. Data are expressed as means ± SEM. One-way ANOVA, with Bonferroni’s multiple comparison post hoc test (*p<0.05, n = 7).
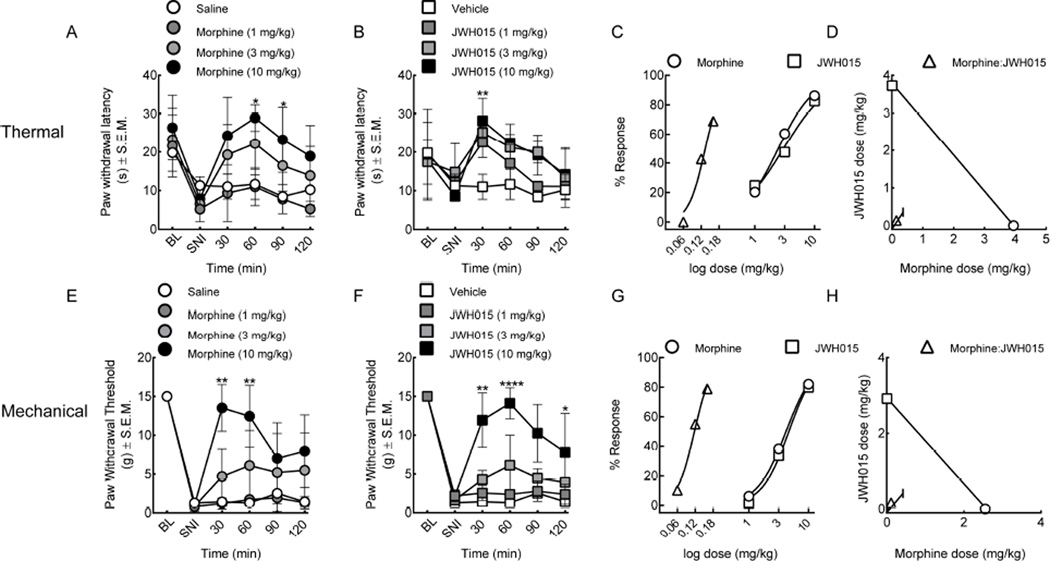
3.4. Synergistic drug interaction in a model of peripheral neuropathic pain
Neuropathic pain was induced using the spared nerve injury (SNI) of the sciatic nerve. On day 7, following SNI, baseline measurements of thermal and mechanical withdrawal latencies were assessed. The rats exhibited a significant decrease in both paw withdrawal latencies and mechanical thresholds as compared to their non-injured baselines (Figure 4, n=6). Rats given an intraperitoneal injection of morphine (1, 3, 10 mg/kg, i.p., Figure 4A, E) or JWH015 (1, 3, 10 mg/kg, i.p., Figure 4B,F) resulted in a dose-dependent increase in thermal paw withdrawal latencies and mechanical thresholds as compared to sham-operated and vehicle-treated controls. Dose-dependent increases in paw withdrawal thresholds (morphine, A50=3.94 CI ± 2.99, F (3, 88)=18.52, p<0.0001 and JWH015, A50=3.73 CI ± 27.07, F (3, 66)=5.001, p=0.003, Figure 4 A, B) and mechanical hypersensitivity (morphine, A50=2.56 CI ± 4.68, F (3, 84)=23.87, p<0.0001 and JWH015, A50=2.92 CI ± 3.42, F (3, 60)=33.35, p<0.0001, Figure 4E, F) were observed in the nerve-injured animals. Dose-response curves were constructed and used to determine A50 values and for isobolographic analysis from data collected at the 30 minute time point. The co-treatment of morphine and JWH015 synergistically inhibited thermal (A50=0.14 CI ± 3.36, Figure 4C,D; Table 1) and mechanical (A50=0.11 CI ± 0.41, Figure 4G, H, Table 1) hypersensitivities.
Figure 4.
Co-treatment with morphine and JWH015 alleviates neuropathic pain. Spared nerve injury (SNI) resulted in robust hind paw mechanical and thermal hypersensitivity in rats on day 7. (A–D) Thermal latencies and (E–H) withdrawal thresholds significantly decreased after SNI. (A, E) Morphine (1, 3, 10 mg/kg, i.p.) and (B, F) JWH015 (1, 3, 10 mg/kg, i.p.) when given systemically resulted in a significant reversal of the SNI-induced thermal and mechanical hypersensitivities. Combined therapies using fixed dose ratios resulted in a leftward shift of the dose response curves and isobolographic analyses indicated synergism in response to a (C–D) thermal stimulus and (G–H) mechanical allodynia. BL = baseline, SNI = spared nerve injury. Data are expressed as means ± SEM. One-way ANOVA, with Bonferroni’s multiple comparison post hoc test (*p<0.05, n = 6).
3.5. JWH015 co-administration attenuates the rewarding effects of morphine
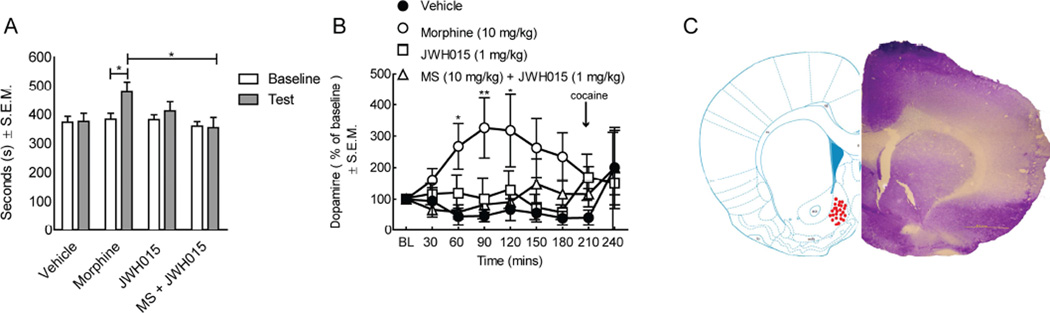
Opioids like morphine carry reward liability and abuse potential. We next asked whether JWH015 would attenuate the rewarding effect of morphine as measured by a conditioned place preference (CPP) paradigm (Figure 5, n=24). Rats spent a relatively equal amount of time in the chambers at baseline (mean 374.5 seconds/group). Significantly more time (p = 0.03) was spent in the chamber paired with morphine (10 mg/kg, i.p.) when compared to vehicle-treated animals. Conversely, the time spent in the chamber paired with the CB2 agonist, JWH015, was not significantly increased when compared to pre-conditioning baseline in this group of rats. Rats co-administered morphine and JWH015 spent similar time in the drug-paired chamber as compared to vehicle-treated animals; this was statistically less time in the putative chamber compared to morphine treated rats (Figure 5A; p = 0.02). These findings suggest a role for CB2 agonism in preventing the induction of place preference to morphine.
Figure 5.
JWH015 attenuates morphine-induced condition place preference and morphine-induced dopamine release. Rats were conditioned for 3 days in a drug-paired chamber using morphine (10 mg/kg, i.p.), JWH015 (1 mg/kg, i.p.), vehicle or a combination of morphine and JWH015. (A) Vehicle did not result in animals remaining in one chamber longer than another, yet morphine resulted in animals spending a significant more time in the chamber paired with morphine. JWH015 alone did not result in a positive CPP and when co-administered with morphine resulted in a significant attenuation of a positive CPP. (B) In vivo microdialysis of the nucleus accumbens (bilaterally) to measure dopamine levels was performed in male SD rats. Systemic administration of morphine alone (10 mg/kg, i.p.) significantly increased dopamine release in the nucleus accumbens that was significantly attenuated by the co-administration of JWH015 (1 mg/kg, i.p.) JWH015 alone had no significant change on dopamine levels. ( ) Represents time of cocaine (20 mg/kg, i.p.) administration. (C) Brain slices corresponding to dopamine release in microdialysis studies. Coronal sections of the rat ventral striatum were sliced at 40 µm and stained with cresyl violet. (•) Represents individual animal placement in nucleus accumbens shell. Data are expressed as means ± SEM. One-way ANOVA, with Bonferroni’s multiple comparison post hoc test (*p<0.05, n = 24).
We next asked if MOR/CB2 agonist co-administration prevention of CPP induction was reflected in reduced dopamine release in the nucleus accumbens (NAc). Mcrodialysis of the NAc and HPLC measurements for dopamine were performed. Analyses of dialysate collected from the NAc revealed a significant reduction of dopamine release from the striatum in rats co-treated with morphine-JWH015 (10:1) when compared to morphine-treated animals (Figure 5B,C). Cocaine hydrochloride (20 mg/kg, i.p.) when given via the microdialysate into the NAc induced dopamine release in all rats and served as a positive control. Probe location was verified with placement detailed in Figure 5C. This result suggests, on a neurochemical level, that the co-administration of morphine with JWH015 is likely to have a lower abuse liability if administered to a clinical population.
3.6. Co-treatment with low dose morphine and JWH015 improves gastrointestinal transit
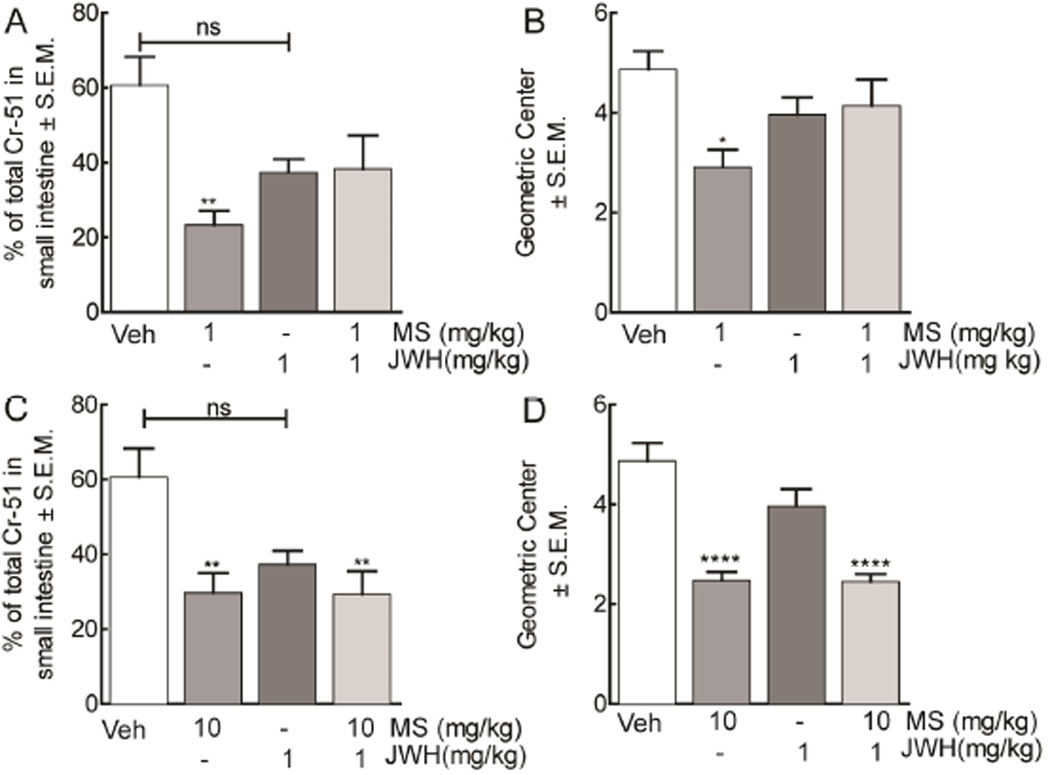
Opioids, like morphine, are known to significantly inhibit gastrointestinal transit, resulting in constipation that is both painful and uncomfortable for patients. Here we investigated whether the co-administration of morphine and JWH015 would significantly alter GI transit and geometric center. Animals were treated with a low (1 mg/kg, i.p.) and high (10 mg/kg, i.p.) dose of morphine to investigate whether it would produce constipation in the presence of a CB2 agonist (Figure 6, n=6). Low dose morphine significantly (F (3, 21)=6.232, p=0.001) decreased GI transit, as indicated by a lower % of the total radioactivity found in the small intestine, (23.4% CI ± 3.8) when compared to JWH015 (37.4% CI ± 3.6) and control (60.7% CI ± 7.6); co-treatment attenuated morphine-induced slowing of gastric propulsion in these animals (38.3% CI ± 8.9, Figure 6A). Next we investigated the effect of high morphine treatment (10 mg/kg, i.p.) on gastric transit in the presence or absence of JWH015 (Figure 6C). High dose morphine (29.7 CI ± 5.3) decreased gastric emptying when compared to control animals (60.7 CI ± 7.6). This was not significantly increased by treatment with JWH015 (37.6 CI ± 3.6) or restored by combination treatment in these animals (29.3 CI ± 6.2). Morphine significantly reduced geometric center (2.5 CI ± 0.2, F (3, 21)=4.373, p=0.001) when compared to controls (4.9 CI ± 0.4) that was ameliorated with JWH015 (3.9 CI ± 0.3, Figure 6B,D). A combination dose with JWH015 and low dose morphine mobilized total Cr-51 through the small intestine when compared to control (Figure 6B). This effect was not observed with high morphine treatment (2.4 CI ± 0.2, Figure 6D).
Figure 6.
Gastrointestinal transit time in animals treated with morphine and JWH015. (A, C) Gastrointestinal transit was evaluated in rats treated with either vehicle, low (1 mg/kg, i.p.) or high (10 mg/kg, i.p.) dose of morphine, JWH015 (1 mg kg, i.p.), or their combination by measuring gastric emptying of a milk meal 51 Cr (radioactivity/vol, p.o.) into the intestine and calculating the (B, D) geometric center to determine distance of propulsion in cm. Morphine treatment decreased transit time and geometric center of the gastrointestinal tract. JWH015 had no effect alone and reinstated gastric transit and geometric center to vehicle-treated levels with low, but not high, doses of morphine. Data are expressed as means ± SEM. One-way ANOVA, with Bonferroni’s multiple comparison post hoc test (*p<0.05, n = 6).
4. Discussion
Our present study shows for the first time that the MOR agonist, morphine, and the CB2 agonist, JWH015 interact synergistically to reverse inflammatory, post-operative, and neuropathic pain while preventing induction of opioid-induced rewarding behaviors and reducing constipation. Our data support the use of opioid-CB2 multimodal therapy in treating chronic pain while limiting abuse liability.
Given that humans are living longer, millions more of individuals are suffering from chronic pain and therefore being treated with analgesics over longer periods of time (CDC, 2015). Opioids comprise the mainstay of analgesic therapy, and many clinically available formulations take advantage of two mechanisms (opioids and NSAIDs) to inhibit pain including the popular medications such as Percocet® (oxycodone and acetaminophen) and Vicodin® (hydrocodone and paracetamol); these were the most commonly prescribed drugs in the U.S. with 136 million prescriptions (NIDA, 2014). While combinations of opioids and NSAIDs are effective analgesics for the treatment of moderate to severe pain, they are implicated in the spreading epidemic of prescribed narcotic drug abuse with over 5% of the adult population using them non-medically (Informatics; Young et al., 2012). This trend of narcotic prescription abuse has increased with the number of prescriptions for all narcotics reaching over 250 million in 2012 (CDC, 2015). There is growing evidence suggesting that prescription narcotics are leading to heroin abuse in the United States (SAMHSA, 2013). The addiction propensity of narcotics has led to enhanced concerns in both patients (36%) and physicians (68%) (SAMHSA, 2013).
Novel strategies to combat chronic pain, while limiting misuse, abuse, unwanted side effects, and toxicities often associated with opioid intervention are in high demand. We determined if multimodal targeting of the CB2 and the MOR during pain was beneficial compared to engaging the MOR alone. CB2 receptors are primarily on immune cells in both the periphery and the central nervous system with some evidence of up-regulation on neurons after injury (Galiègue et al., 1995; Wotherspoon et al., 2004). Previous work using selective CB2 agonists as well as enzyme inhibitors to increase the endogenous CB2 agonist, 2-archidonylglyceryol (Ignatowska-Jankowska et al., 2015), have resulted in significant analgesia in several preclinical models of pain (Ibrahim et al., 2006; Ibrahim et al., 2005; Malan et al., 2001). In the present study, the combination of MOR agonist + CB2 agonist was more effective in alleviating pain in preclinical models with inflammatory components (e.g. inflammation, post-operative) compared to acute nociception.
Pain due to nerve injury is often treatment resistant (Dworkin et al., 2008). In the current study, we found that notable synergy occurred in the SNI neuropathic pain model. Traumatic nerve injury-induced increases in pro-inflammatory mediators and glial activation is likely due to spontaneous activity of sensory neurons in the CNS, an effect that can be alleviated by targeting peripheral endocannabinoids (Fox et al., 2001; Mitrirattanakul et al., 2006; Seltzman et al., 2016) and/or CB2 activation (Watkins et al., 2007). Repeated treatment with the selective CB2 agonists JWH133 (Elmes et al., 2004) or NESS400 (Luongo et al., 2010) reduced hypertrophic microglia in a significant manner, while importantly, sparing microglial cell number in rodent models of SNL/SNI. CB2 activation is also correlated with increasing anti-inflammatory gene expression in the dorsal horn and significant reductions in mechanical and thermal hypersensitivity (Luongo et al., 2010). Thus, the synergy observed between JWH015 and morphine is likely due to the activation of CB2 receptors on immune cells and subsequent inhibition of the inflammatory process (Hsieh et al., 2010; Kinsey et al., 2011; Yao et al., 2007) coupled with morphine’s well-characterized ability to inhibit nociceptive signaling (Fields, 2004; McCabe et al., 2014). These data strongly suggest that the combination of a MOR agonist along with a CB2 agonist may inhibit neuropathic pain at much lower doses than a MOR agonist alone, offering an effective therapeutic option for a difficult to manage pain state. In addition to pain, previous studies have demonstrated that CB2 may play a role in the rewarding pathways of the CNS (Ignatowska-Jankowska et al., 2013; Zhang et al., 2014). The work from Gardner’s group demonstrated that the CB2 agonist JWH133 significantly inhibited cocaine-induced self-administration and elevated levels of dopamine in the NAc (Zhang et al., 2014) suggesting that CB2 agonists may attenuate the rewarding effects of drugs of abuse. Presently, we showed that the CB2 agonist alone did not significantly change conditioned place preference or conditioned place aversion suggesting a lack of rewarding behavior and dysphoria, respectively. In line with our CPP data, the systemic administration of JWH015 significantly reduced morphine-induced dopamine release in the nucleus accumbens. Although the mechanism of action of how CB2 receptor activation may attenuate morphine rewarding effects and elevated levels of dopamine in rewarding centers has yet to be elucidated, several hypotheses have been suggested. Research has shown that morphine-induced activation of MOR promotes the release of pro-inflammatory cytokines like IL-1b and TNFa in activated microglial cells, the activity of which is attenuated by CB2 activation (Merighi et al., 2012). Recent studies have demonstrated CB2 expression on neurons within in various brain regions including the prefrontal cortex, striatum, midbrain (Zhang et al., 2014) and hippocampus (Li and Kim, 2015). Moreover, CB2 protein is expressed in the VTA (Zhang et al., 2014), and it appears that activation of microglia in the VTA may alter the release of dopamine to drugs of abuse like opioids and cocaine (Taylor et al., 2015). CB2 may inhibit reward through actions in the VTA, indicated by significant reductions of tyrosine hydroxylase, the rate-limiting step in the synthesis of dopamine, in animals that lack CB2 receptors (García et al., 2015; Navarrete et al., 2013). These actions of CB2 may also have important implications for CNS-related disorders, such as Parkinson’s disease, where lesioned areas of the substantia nigra are significantly reduced by the activation of CB2 on microglia (Gómez-Gálvez et al., 2016), or in Alzheimer’s disease, where upregulated CB2 in microglial cells target neuro-inflammation (Fagan and Campbell, 2014).
It is well-established that chronic morphine treatment is associated with adverse effects including emesis/vomiting, somnolence, constipation, respiratory depression, and abuse. Of these, constipation is a major compliant among patients (Harned and Sloan, 2016). Here we demonstrated that a CB2 agonist alone does not slow GI transit and reports in humans did not observe GI disturbances or bleeding (clinical trial.gov), yet morphine alone resulted in a significant inhibition of GI transit. When the CB2 agonist, JWH015, was combined with a low dose of morphine (1 mg/kg, i.p.), the low-dose morphine-induced slowing of gastrointestinal transit and geometric center was significantly blocked. This CB2 mediated blockade of morphine induced GI slowing was overcome by increasing the dose of morphine (10 mg/kg, i.p). Since the combination of a MOR agonist with a CB2 agonist synergistically inhibits inflammatory and chronic pain thus reducing the doses of opioids required, we anticipate that opioid-induced constipation will also be lessened in patients subjected to long-term opioid exposure.
Taken together, our findings indicate that the systemic co-administration of morphine and JWH015 is able to significantly inhibit the transmission of inflammatory, postoperative and neuropathic pain in a synergistic manner. Moreover, this combination of a MOR agonist and a CB2 agonist at optimal ratios results in the significant reduction of: individual compound dosing, potential rewarding behaviors, and opioid-induced GI slowing without causing GI bleeding, in contrast to opioid-NSAID combinations. CB2 agonists are currently in Phase II clinical trials and have, with compounds such as GW842166 and LY2828360 (NIH, 2012) not resulted in any serious reported side effects. Data here suggest the combination of a mu and CB2 agonists are likely to act synergistically to reduce multiple types of pain while attenuating unwanted side effects.
Research Highlights.
Opioid use is associated with undesirable side effects, including addiction.
Dual-targeting MOR and CB2 receptors results in analgesic synergy.
Co-administration of MOR-CB2 reduces opioid-induced increases in reward.
Acknowledgments
This research was supported by the National Institutes of Health (R01CA142115).
Abbreviations
- 2-AG
2-arachidonoylglycerol
- %MPE
percent maximal possible effect
- A50
potency
- a-CSF
artificial spinal fluid
- CB1
cannabinoid 1 receptor
- CB2
cannabinoid 2 receptor
- CPP
conditioned place preference
- ED50
effective dose at 50%
- HPLC
high performance liquid chromatography
- MOR
mu opioid receptor
- SNI
spared nerve injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
SA Grenald, MA Young, Y Wang performed the research
SA Grenald, TM Largent-Milnes and TW Vanderah designed the research study
TW Vanderah and MM Ibrahim contributed essential tools
SA Grenald and MH Ossipov analyzed the data
SA Grenald, TM Largent-Milnes, MM Ibrahim and TW Vanderah wrote the paper
Chemical compounds studied in this article: Cocaine hydrochloride (PubChem CID: 656832); JWH015 (PubChem CID: 4273754); Morphine Hydrochloride (PubChem CID: 5464110)
Conflicts of Interest
The authors state no conflict of interest.
References
- Anand P, Whiteside G, Fowler CJ, Hohmann AG. Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain Res Rev. 2009:255–266. doi: 10.1016/j.brainresrev.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balko R. Painkiller Access Debated as Patients Suffer. Huffington Post. 2012 [Google Scholar]
- Brennan TJ. Pathophysiology of postoperative pain. Pain. 2010:S33–S40. doi: 10.1016/j.pain.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- CDC. Fatal Injury Reports. 2015. [Google Scholar]
- Chan R, Irvine R, White J. Cardiovascular changes during morphine administration and spontaneous withdrawal in the rat. Eur J Pharmacol. 1999:25–33. doi: 10.1016/s0014-2999(98)00984-4. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Thompson AC, Zapata A, Shippenberg TS. Overview of brain microdialysis. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.ns0701s47. pp. Unit7-Unit1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane EH. Emergency department visits involving narcotic pain relievers. 2015 [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA, Gilchrist A, Hoyer D, Insel PA, Izzo AA, Lawrence AJ, MacEwan DJ, Moon LDF, Wonnacott S, Weston AH, McGrath JC. Experimental design and analysis and their reporting: new guidance for publication in BJP. British Journal of Pharmacology. 2015:3461–3471. doi: 10.1111/bph.12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000 doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 1980:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Elmes SJ, Jhaveri MD, Smart D, Kendall DA, Chapman V. Cannabinoid CB2 receptor activation inhibits mechanically evoked responses of wide dynamic range dorsal horn neurons in naive rats and in rat models of inflammatory and neuropathic pain. Eur J Neurosci. 2004;20:2311–2320. doi: 10.1111/j.1460-9568.2004.03690.x. [DOI] [PubMed] [Google Scholar]
- Fagan SG, Campbell VA. The influence of cannabinoids on generic traits of neurodegeneration. British Journal of Pharmacology. 2014:1347–1360. doi: 10.1111/bph.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. Nature Publishing Group. 2004:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Fine PG, Rosenfeld MJ. Cannabinoids for neuropathic pain. Curr Pain Headache Rep. 2014:451–451. doi: 10.1007/s11916-014-0451-2. [DOI] [PubMed] [Google Scholar]
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, James I. The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- García MC, Cinquina V, Palomo-Garo C, Rábano A, Fernández-Ruiz J. Identification of CB2 receptors in human nigral neurons that degenerate in Parkinson's disease. Neurosci Lett. 2015:1–4. doi: 10.1016/j.neulet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Gómez-Gálvez Y, Palomo-Garo C, Fernández-Ruiz J, García C. Potential of the cannabinoid CB(2) receptor as a pharmacological target against inflammation in Parkinson's disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016:200–208. doi: 10.1016/j.pnpbp.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Harned M, Sloan P. Safety concerns with long-term opioid use. Expert Opin Drug Saf. 2016:1–8. doi: 10.1080/14740338.2016.1177509. [DOI] [PubMed] [Google Scholar]
- Hascup ER, Bjerkén Sa, Hascup KN, Pomerleau F, Huettl P, Strömberg I, Gerhardt GA. Histological studies of the effects of chronic implantation of ceramic-based microelectrode arrays and microdialysis probes in rat prefrontal cortex. Brain Res. 2009:12–20. doi: 10.1016/j.brainres.2009.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman JS, Williams CL, Burks TF, Mosberg HI, Porreca F. Dissociation of opioid antinociception and central gastrointestinal propulsion in the mouse: studies with naloxonazine. J. Pharmacol. Exp. Ther. 1988:238–243. [PubMed] [Google Scholar]
- Hsieh GC, Pai M, Chandran P, Hooker BA, Zhu CZ, Salyers AK, Wensink EJ, Zhan C, Carroll WA, Dart MJ, Yao BB, Honore P, Meyer MD. Central and peripheral sites of action for CB2 receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in rats. British Journal of Pharmacology. 2010:428–440. doi: 10.1111/j.1476-5381.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim, Rude, Stagg, Mata, Lai, Vanderah, Porreca, Buckley, Makriyannis, Malan CB2 cannabinoid receptor mediation of antinociception. Acute Pain. 2006:1–1. doi: 10.1016/j.pain.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, Vanderah TW, Lai J, Porreca F, Makriyannis A, Malan TP., Jr Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan TP. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proceedings of the National Academy of Sciences. 2005 doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowska-Jankowska B, Wilkerson JL, Mustafa M, Abdullah R, Niphakis M, Wiley JL, Cravatt BF, lichtman AH. Selective Monoacylglycerol Lipase Inhibitors: Antinociceptive versus Cannabimimetic Effects in Mice. Journal of Pharmacology and Experimental Therapeutics. 2015:424–432. doi: 10.1124/jpet.114.222315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Muldoon PP, lichtman AH, Damaj MI. The cannabinoid CB2 receptor is necessary for nicotine-conditioned place preference, but not other behavioral effects of nicotine in mice. Psychopharmacology (Berl) 2013:591–601. doi: 10.1007/s00213-013-3117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Informatics IIfH. HSRN DATA BRIEF: NATIONAL PRESCRIPTION AUDIT. imshealth.com.
- Kinsey SG, Mahadevan A, Zhao B, Sun H, Naidu PS, Razdan RK, Selley DE, Damaj MI, lichtman AH. The CB2 cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Neuropharmacology. 2011:244–251. doi: 10.1016/j.neuropharm.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch T, Höllt V. Role of receptor internalization in opioid tolerance and dependence. Pharmacol Ther. 2008:199–206. doi: 10.1016/j.pharmthera.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Largent-Milnes TM, Brookshire SW, Skinner DP, Hanlon KE, Giuvelis D, Yamamoto T, Davis P, Campos CR, Nair P, Deekonda S, Bilsky EJ, Porreca F, Hruby VJ, Vanderah TW. Building a Better Analgesic: Multifunctional Compounds that Address Injury-Induced Pathology to Enhance Analgesic Efficacy while Eliminating Unwanted Side Effects. Journal of Pharmacology and Experimental Therapeutics. 2013:7–19. doi: 10.1124/jpet.113.205245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kim J. Neuronal expression of CB2 cannabinoid receptor mRNAs in the mouse hippocampus. Neuroscience. 2015:253–267. doi: 10.1016/j.neuroscience.2015.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling GS, MacLeod JM, Lee S, Lockhart SH, Pasternak GW. Separation of morphine analgesia from physical dependence. Science. 1984:462–464. doi: 10.1126/science.6541807. [DOI] [PubMed] [Google Scholar]
- Lozano-Ondoua A. Disease modification of breast cancer-induced bone remodeling by cannabinoid 2 receptor agonists. J Bone Miner Res. 2013;28:92–107. doi: 10.1002/jbmr.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Ondoua AN, Wright C, Vardanyan A, King T, Largent-Milnes TM, Nelson M, Jimenez-Andrade JM, Mantyh PW, Vanderah TW. A cannabinoid 2 receptor agonist attenuates bone cancer-induced pain and bone loss. Life Sci. 2010:646–653. doi: 10.1016/j.lfs.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luongo L, Palazzo E, Tambaro S, Giordano C, Gatta L, Scafuro MA, Rossi FS, Lazzari P, Pani L, de Novellis V, Malcangio M, Maione S. 1-(2’,4’-dichlorophenyl)-6-methyl-N-cyclohexylamine-1,4-dihydroindeno[1,2-c]pyraz ole-3-carboxamide, a novel CB2 agonist, alleviates neuropathic pain through functional microglial changes in mice. Neurobiol Dis. 2010;37:177–185. doi: 10.1016/j.nbd.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Malan TP, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, Porreca F, Makriyannis A. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Schulenberg JE, O'Malley PM, Patrick ME, Kloska DD. Non-medical use of prescription opioids during the transition to adulthood: a multi-cohort national longitudinal study. Addiction. 2014:102–110. doi: 10.1111/add.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E. Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. British Journal of Pharmacology. 2015:3189–3193. doi: 10.1111/bph.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi S, Gessi S, Varani K, Fazzi D, Mirandola P, Borea PA. Cannabinoid CB(2) receptor attenuates morphine-induced inflammatory responses in activated microglial cells. British Journal of Pharmacology. 2012:2371–2385. doi: 10.1111/j.1476-5381.2012.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meske DS, Xie JY, Oyarzo J, Badghisi H, Ossipov MH, Porreca F. Opioid and noradrenergic contributions of tapentadol in experimental neuropathic pain. Neurosci Lett. 2013 doi: 10.1016/j.neulet.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitka M. FDA Asks Physicians to Stop Prescribing High-Dose Acetaminophen Products. JAMA. American Medical Association. 2014:563–563. doi: 10.1001/jama.2014.716. [DOI] [PubMed] [Google Scholar]
- Mitrirattanakul S, Ramakul N, Guerrero AV, Matsuka Y, Ono T, Iwase H, Mackie K, Faull KF, Spigelman I. Site-specific increases in peripheral cannabinoid receptors and their endogenous ligands in a model of neuropathic pain. Pain. 2006;126:102–114. doi: 10.1016/j.pain.2006.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete F, Rodríguez-Arias M, Martín-García E, Navarro D, García-Gutiérrez MS, Aguilar MA, Aracil-Fernández A, Berbel P, Miñarro J, Maldonado R, Manzanares J. Role of CB2 cannabinoid receptors in the rewarding, reinforcing, and physical effects of nicotine. Neuropsychopharmacology. 2013:2515–2524. doi: 10.1038/npp.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIDA. America’s Addiction to Opioids: Heroin and Prescription Drug Abuse | National Institute on Drug Abuse (NIDA) 2014 ezproxy1.library.arizona.edu.
- NIH. An Imaging Study to Investigate the Distribution of GW842166X in the Brain - Full Text View - ClinicalTrials.gov. ezproxy1.library.arizona.edu. 2012
- NPR. With rise of painkiller abuse, a closer look at heroin. 2013. [Google Scholar]
- Ortega-Álvaro A, Ternianov A, Aracil-Fernández A, Navarrete F, García-Gutiérrez MS, Manzanares J. Role of cannabinoid CB2 receptor in the reinforcing actions of ethanol. Addict Biol. 2014:43–55. doi: 10.1111/adb.12076. [DOI] [PubMed] [Google Scholar]
- Perron BE, Bohnert K, Perone AK, Bonn-Miller MO, Ilgen M. Use of prescription pain medications among medical cannabis patients: comparisons of pain levels, functioning, and patterns of alcohol and other drug use. J Stud Alcohol Drugs. 2015:406–413. doi: 10.15288/jsad.2015.76.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: From the bench to the bedside. Neurotherapeutics. 2009:713–737. doi: 10.1016/j.nurt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA SAMHSA. Associations of Nonmedical Pain Reliever Use and Initiation of Heroin Use in the United States. 2013 [Google Scholar]
- Seltzman HH, Shiner C, Hirt EE, Gilliam AF, Thomas BF, Maitra R, Snyder R, Black SL, Patel PR, Mulpuri Y, Spigelman I. Peripherally Selective Cannabinoid 1 Receptor (CB1R) Agonists for the Treatment of Neuropathic Pain. J Med Chem. 2016 doi: 10.1021/acs.jmedchem.6b00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ. An Overview of Drug Combination Analysis with Isobolograms. Journal of Pharmacology and Experimental Therapeutics. 2006:1–7. doi: 10.1124/jpet.106.104117. [DOI] [PubMed] [Google Scholar]
- Taylor AMW, Castonguay A, Taylor AJ, Murphy NP, Ghogha A, Cook C, Xue L, Olmstead MC, De Koninck Y, Evans CJ, Cahill CM. Microglia disrupt mesolimbic reward circuitry in chronic pain. Journal of Neuroscience. 2015:8442–8450. doi: 10.1523/JNEUROSCI.4036-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Milligan ED, Maier SF. “Listening” and “talking” to neurons: implications of immune activation for pain control and increasing the efficacy of opioids. Brain Res Rev. 2007;56:148–169. doi: 10.1016/j.brainresrev.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins PB, Kaplowitz N, Slattery JT, Colonese CR, Colucci SV, Stewart PW, Harris SC. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA. 2006:87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- Wilkerson JL, Milligan ED. The Central Role of Glia in Pathological Pain and the Potential of Targeting the Cannabinoid 2 Receptor for Pain Relief. ISRN Anesthesiol. 2011 doi: 10.5402/2011/593894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2004:235–245. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Xi Z-X, Peng X-Q, Li X, Song R, Zhang H-Y, Liu Q-R, Yang H-J, Bi G-H, Li J, Gardner EL. Brain cannabinoid CB2 receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao BB, Hsieh GC, Frost JM, Fan Y, Garrison TR, Daza AV, Grayson GK, Zhu CZ, Pai M, Chandran P, Salyers AK, Wensink EJ, Honore P, Sullivan JP, Dart MJ, Meyer MD. In vitro and in vivo characterization of A-796260: a selective cannabinoid CB2 receptor agonist exhibiting analgesic activity in rodent pain models. British Journal of Pharmacology. 2007:390–401. doi: 10.1038/sj.bjp.0707568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A, McCabe SE, Cranford JA, Ross-Durow P, Boyd CJ. Nonmedical use of prescription opioids among adolescents: subtypes based on motivation for use. J Addict Dis. 2012;31:332–341. doi: 10.1080/10550887.2012.735564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H-Y, Gao M, Liu Q-R, Bi G-H, Li X, Yang H-J, Gardner EL, Wu J, Xi Z-X. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proceedings of the National Academy of Sciences. 2014:E5007–E5015. doi: 10.1073/pnas.1413210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. Pain. 1983:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]