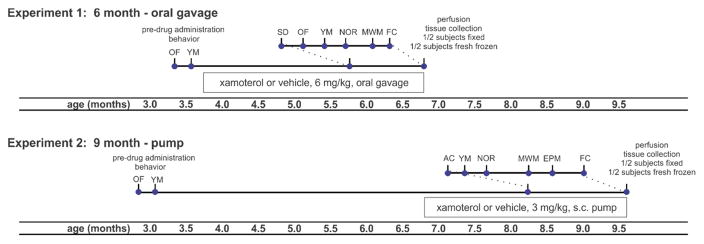
Fig. 1.
Timeline of in vivo studies indicates age, drug administration and behavioral testing schedules for Experiment 1 and Experiment 2. Two independent cohorts of 5XFAD and wildtype mice were chronically administered a selective partial ADRB1 agonist, xamoterol, or vehicle, 6 mg/kg oral gavage from 4.0 to 6.5 months of age or 3 mg/kg/day subcutaneous pump from 7.0 to 9.5 months of age. Experiment 1 is referred to as “6 month - oral gavage” as behavior and tissue collection occurs during the 6th month. Experiment 2 is likewise referred to as “9 month - pump”. Behavioral testing was performed in the indicated order during the final 4 to 5 weeks of administration. At the end of testing, mice were perfused and brains collected for neurobiochemical endpoints. Sample size = 10 to 11 per group for behavior and 4–6 per group for neurobiological endpoints. Experiments were analyzed independently. Abbreviations: OF, open field, AC, activity chamber; YM, Y-maze; SD, social discrimination; NOR, novel object recognition; MWM, Morris Water Maze; EPM, elevated plus maze; FC, fear conditioning; s.c., subcutaneous.