Abstract
Human epidermal growth factor receptor 2 (HER2) overexpression and amplification have been reported as predictive markers for HER2-targeted therapy in breast and gastric cancer, whereas human epidermal growth factor receptor 3 (HER3) is emerging as a potential resistance factor. The aim of this study was to perform a systematic review and meta-analysis of the HER2 and HER3 overexpression and amplification in biliary tract cancers (BTCs). An electronic search of MEDLINE, American Society of Clinical Oncology (ASCO), European Society of Medical Oncology Congress (ESMO), and American Association for Cancer Research (AACR) was performed to identify studies reporting HER2 and/or HER3 membrane protein expression by immunohistochemistry (IHC) and/or gene amplification by in situ hybridization (ISH) in BTCs. Studies were classified as “high quality” (HQ) if IHC overexpression was defined as presence of moderate/strong staining or “low quality” (LQ) where “any” expression was considered positive. Of 440 studies screened, 40 met the inclusion criteria. Globally, HER2 expression rate was 26.5 % (95 % CI 18.9–34.1 %). When HQ studies were analyzed (n = 27 studies), extrahepatic BTCs showed a higher HER2 overexpression rate compared to intrahepatic cholangiocarcinoma: 19.9 % (95 % CI 12.8–27.1 %) vs. 4.8 % (95 % CI 0–14.5 %), respectively, p value 0.0049. HER2 amplification rate was higher in patients selected by HER2 overexpression compared to “unselected” patients: 57.6 % (95 % CI 16.2–99 %) vs. 17.9 % (95 % CI 0.1–35.4 %), respectively, p value 0.0072. HER3 overexpression (4/4 HQ studies) and amplification rates were 27.9 % (95 % CI 9.7–46.1 %) and 26.5 % (one study), respectively. Up to 20 % of extrahepatic BTCs appear to be HER2 overexpressed; of these, close to 60 % appear to be HER2 amplified, while HER3 is overexpressed or amplified in about 25 % of patients. Clinical relevance for targeted therapy should be tested in prospective clinical trials.
Keywords: Biliary tract cancer, Cholangiocarcinoma, HER2 pathway, HER3 pathway, Systematic review, Meta-analysis
Introduction
Background
The prognosis for patients with advanced biliary tract cancers (BTCs) is very poor with a median overall survival of less than 12 months following treatment with systemic chemotherapy [1]. The term BTCs refers to a heterogeneous group of diseases encompassing cholangiocarcinoma (CC) [intrahepatic cholangiocarcinoma (IHCC), extrahepatic cholangiocarcinoma (EHCC)], gallbladder carcinoma (GBC), and ampulla of Vater carcinoma (AC). It is postulated that specific genetic and molecular aberrations vary between these subtypes and thus may provide predictive biomarkers of response to targeted therapy. Unfortunately, unlike other solid tumors, targetable biomarkers are lacking in BTCs and the cisplatin and gemcitabine combination remains gold standard first-line treatment worldwide in patients with advanced disease [2], with no proven benefit from targeted therapies as yet identified [3, 4]. Thus, biomarkers of response are urgently required in this challenging disease. The human epidermal growth factor receptor 2 (HER2), which belongs to the ErbB/(HER) family of receptor tyrosine kinases (TK), is a well-described predictive biomarker for anti-HER2 therapy in breast and gastric cancer [5, 6]. To date, previous clinical reports have suggested some activity of trastuzumab (an anti-HER2 monoclonal antibody) in association with chemotherapy in HER2 upregulated BTCs [7–10]. In contrast, trials exploring the role of anti-EGFR monoclonal antibodies and EGFR tyrosine kinase inhibitors (TKIs) have resulted in disappointing and/or conflicting findings [11–14]. This systematic review aims to quantify the reported HER2 and HER3 expression rates in BTC in order to provide useful data for the development of potential novel systemic-targeted strategies for use in future clinical trials.
HER2/HER3 pathway
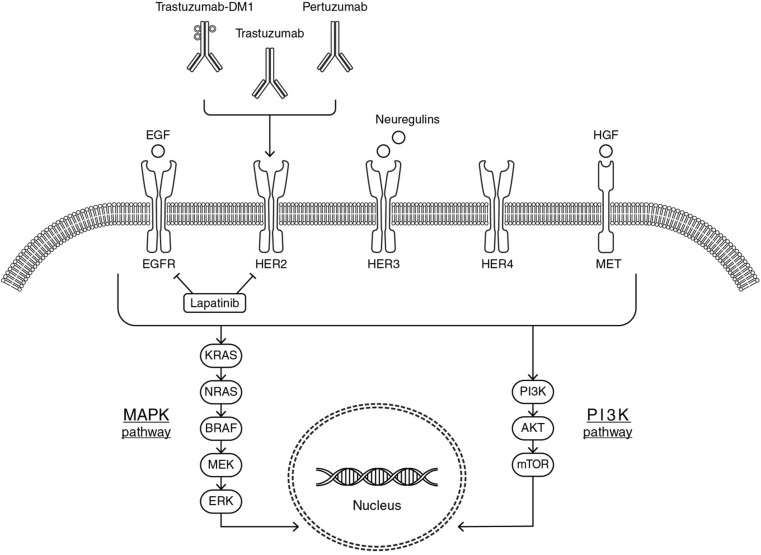
The HER family consists of four receptors [HER1 (EGFR), HER2, HER3, and HER4] with similar structure, consisting of four main parts: an extracellular ErbB ligand-binding domain, a single transmembrane lipophilic segment, an intracellular tyrosine kinase domain, and an intracellular C-terminal tail [15]. The extracellular ligand-binding region contains four domains (I–IV): domains I and III recognize and bind their corresponding ligands and domain II mediates receptor dimerization, whereas domain IV, interacting with domain II, leads to a negative feedback on the dimerization process [16]. Ligand binding to the extracellular domain results in receptor homo- or heterodimerization, a critical step in HER family-mediated signaling. Dimerization induces the activation of the intrinsic tyrosine kinase domain, by phosphorylation of specific tyrosine residues, leading to the activation of different downstream signaling cascades, including the mitogen-activated protein kinase (MAPK) proliferation pathway and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB or Akt) pro-survival pathway [17, 18] (Fig. 1).
Fig. 1.
HER2/HER3 pathway and targeted therapy interaction
HER2 and HER3 are known to have characteristics distinct from other HER family receptors. HER2 lacks a specific ligand, so it can form heterodimers only if it is trans-activated from other activated HER receptors (such as EGFR, HER3, and HER4) [19]. In addition to abnormal overexpression, HER2 is also able to spontaneously homodimerize [16]. In contrast to HER2, HER3 can bind multiple ligands (neuregulins) [20] but it lacks a functioning kinase domain [21] and is, therefore, unable to homodimerize and to induce downstream signaling pathway activation on its own. However, in the presence of HER3 ligands, HER3 may promote the kinase activity of EGFR or HER2 and thereby induce phosphorylation of the HER3 C-terminal tail inducing the PI3K/Akt pathway activation by creating heterodimers [20]. Although all four HER family receptors are capable of dimerizing with each other, HER2 is the preferred dimerization partner [19] and the HER2–HER3 dimer seems to be the most potent HER family dimer [22, 23]. Finally, HER3 has the ability to dimerize with both HER family members and non-HER family members such as mesenchymal epithelial transition (MET) receptor [24, 25], contributing to anti-HER2 therapy resistance. Thus, dysregulation of HER-mediated signaling pathways, through this complex mechanism, results in the growth and spread of cancer cells.
HER2 and HER3 determination
The most commonly used methods to determine the HER2 and HER3 status, in formalin-fixed paraffin-embedded tissue, are (a) immunohistochemistry (IHC), which measures the number of HER2 and HER3 receptors on the cell surface and therefore detects receptor overexpression and (b) fluorescence or chromogenic in situ hybridization (FISH and CISH, respectively), which detects gene amplification by measuring the number of copies of the HER2 and HER3 gene in the nuclei of tumor cells.
Currently, standard clinical practice guidelines for HER2 status assessment are available for breast and gastric cancer only. In contrast, because HER3 status is not routinely analyzed, IHC and ISH techniques for assessing HER3 status have not been standardized.
The IHC and FISH scoring criteria are different for breast and gastric cancer [26, 27], reflecting intrinsic biological differences, including higher heterogeneity of HER2 membranous immunoreactivity in gastric cancer. In addition, in gastric cancer, different scales are used depending on the nature of the diagnostic specimen (surgical specimen vs. biopsy sample) [6, 27]; these criteria are summarized in Table 1.
Table 1.
Standardized guidelines for HER2 analysis, adjusted from 2013 ASCO/CAP guidelines for the HercepTest™ scoring system in breast cancer [26] and standardized guidelines (for both surgical and biopsy specimen) for gastric adenocarcinoma [6, 27]
| 0+ (negative) | 1+ (weak; negative) | 2+ (moderate; equivocal) | 3+ (strong; positive) | |
|---|---|---|---|---|
| HER2 expression (IHC) | ||||
| Breast cancer | No staining observed or membrane staining that is incomplete and is faint/barely perceptible and within ≤10 % of the invasive tumor cells | Incomplete membrane staining that is faint/barely perceptible and within >10 % of the invasive tumor cells | Circumferential membrane staining that is incomplete and/or weak/moderate and within >10 % of the invasive tumor cells or complete and circumferential membrane staining that is intense and within ≤10 % of the invasive tumor cells | Circumferential membrane staining that is complete and intense in >10 % of the cancerous cells |
| Gastric cancer; surgical specimens | No reactivity or membranous reactivity in <10 % of cells | Faint⁄barely perceptible membranous reactivity in >10 % of cells; cells are reactive only in part of their membrane | Weak to moderate complete or basolateral membranous reactivity in >10 % of tumor cells | Moderate to strong complete or basolateral membranous reactivity in >10 % of tumor cells |
| Gastric cancer; biopsy specimens | No reactivity or no membranous reactivity in any tumor cell | Faint/barely perceptible membranous reactivity irrespective of percentage of tumor cells | Weak to moderate complete, basolateral or lateral membranous reactivity irrespective of percentage of tumor cells | Strong complete, basolateral or lateral membranous reactivity irrespective of percentage of tumor cells |
| HER2 amplification (ISH) | ||||
| Breast cancer | HER2 FISH testing (gene copy number and HER2-to-CEP17 ratio) positive: HER2 gene copy number is greater than 6.0 (single probe) and in case of HER2 2+ if either HER2/CEP17 ratio is ≥2.0 regardless gene copy number or if HER2/CEP17 ratio is <2.0 with an average HER2 copy number ≥6.0 (dual probe) | |||
| Gastric cancer | FISH amplified (positive): IHC/HER2 2+ tumor samples are considered FISH amplified if HER2/CEP17 ratio is ≥2 | |||
IHC immunohistochemistry, ISH in situ hybridization
Data from published series of HER2 and HER3 expression varies both in terms of methodology, reporting, and subsequent utility. We therefore set out to undertake a systematic review (i.e., pooled analysis of HER2 and HER3 expression in published BTC series), to provide a “summary estimate” of such expression, with a view to informing the design of future clinical trials.
Methods
Study selection criteria
Eligible studies were those which met the following inclusion criteria: (1) studies reporting membrane expression by IHC and/or amplification by ISH of HER2 and/or HER3 data in human BTC tissue; (2) studies in which data for invasive/infiltrating tumors was available; and (3) original article publications (or abstracts, in the absence of a full publication); studies reporting preclinical data, reviews, and case reports were excluded. Studies in which data for the subgroup of patients with BTC was not available (i.e., when only combined results were reported including non-BTC primary disease sites such as hepatocellular carcinoma, pancreatic carcinoma, or neuroendocrine tumors) were excluded. Other exclusion criteria were (1) studies reporting results which included mixed pathological entities [i.e., mixed hepato-cholangiocarcinoma, mixed adeno-neuroendocrine carcinomas (MANEC)]; (2) publications in which techniques other than IHC and ISH were employed (with no data for IHC or ISH available); and (3) studies in which HER2 pathway analysis was performed following successful anti-HER2 therapy were excluded due to patient selection bias. When studies reporting the same series of patients were identified (“duplicate data”), the study with the greater number of informative patients for the primary end point of this review was selected for inclusion.
Search strategy
A systematic search was conducted utilizing the PubMed/MEDLINE electronic data base (updated 20 November 2015); no dates of publication or language limits were applied. The following two search strategies were employed:
her2[All Fields] AND ((“cholangiocarcinoma”[MeSH Terms] OR “cholangiocarcinoma”[All Fields]) OR ((“biliary tract”[MeSH Terms] OR (“biliary”[All Fields] AND “tract”[All Fields]) OR “biliary tract”[All Fields]) AND (“carcinoma”[MeSH Terms] OR “carcinoma”[All Fields])) OR ((“gallbladder”[MeSH Terms] OR “gallbladder”[All Fields]) AND (“carcinoma”[MeSH Terms] OR “carcinoma”[All Fields])) OR (ampullary[All Fields] AND (“carcinoma”[MeSH Terms] OR “carcinoma”[All Fields])) OR ((“carcinoma”[MeSH Terms] OR “carcinoma”[All Fields]) AND (“ampulla of vater”[MeSH Terms] OR (“ampulla”[All Fields] AND “vater”[All Fields]) OR “ampulla of vater”[All Fields])));
her3[All Fields] AND ((“cholangiocarcinoma”[MeSH Terms] OR “cholangiocarcinoma”[All Fields]) OR ((“biliary tract”[MeSH Terms] OR (“biliary”[All Fields] AND “tract”[All Fields]) OR “biliary tract”[All Fields]) AND (“carcinoma”[MeSH Terms] OR “carcinoma”[All Fields])) OR ((“gallbladder”[MeSH Terms] OR “gallbladder”[All Fields]) AND (“carcinoma”[MeSH Terms] OR “carcinoma”[All Fields])) OR (ampullary[All Fields] AND (“carcinoma”[MeSH Terms] OR “carcinoma”[All Fields])) OR ((“carcinoma”[MeSH Terms] OR “carcinoma”[All Fields]) AND (“ampulla of vater”[MeSH Terms] OR (“ampulla”[All Fields] AND “vater”[All Fields]) OR “ampulla of vater”[All Fields]))) OR ((“receptor, erbb-2”[MeSH Terms] OR “genes, erbb-2”[MeSH Terms]) AND (((“cholangiocarcinoma”[MeSH Terms] OR “cholangiocarcinoma”[All Fields]) OR ((“biliary tract”[MeSH Terms] OR (“biliary”[All Fields] AND “tract”[All Fields]) OR “biliary tract”[All Fields]) AND (“carcinoma”[MeSH Terms] OR “carcinoma”[All Fields])) OR ((“gallbladder”[MeSH Terms] OR “gallbladder”[All Fields]) AND (“carcinoma”[MeSH Terms] OR “carcinoma”[All Fields])) OR (ampullary[All Fields] AND (“carcinoma”[MeSH Terms] OR “carcinoma”[All Fields]))) OR ((“carcinoma”[MeSH Terms] OR “carcinoma”[All Fields]) AND (“ampulla of vater”[MeSH Terms] OR (“ampulla”[All Fields] AND “vater”[All Fields]) OR “ampulla of vater”[All Fields])))).
Meeting abstracts from the American Society of Clinical Oncology (ASCO), European Society of Medical Oncology Congress (ESMO), and American Association for Cancer Research (AACR), presented over the last 5 years (2010–2015), were also reviewed using the following keywords: “her2” OR “her3” AND (“cholangiocarcinoma,” “biliary tract carcinoma,” “gallbladder carcinoma,” “ampullary carcinoma,” “carcinoma of ampulla of vater”).
Reference lists of eligible studies were cross-checked manually to identify potentially eligible articles.
Primary and secondary objectives
The primary objective of this systematic review and meta-analysis was to assess the prevalence of HER2 overexpression (measured by IHC) in patients with BTC, with the primary end point being mean HER2 expression rate.
Secondary objectives included HER2 amplification (measured by ISH) both in the whole population (“unselected population”) and in the population of patients with HER2 overexpression by IHC (“selected population”) and HER3 overexpression (measured by IHC) and amplification (measured by ISH). HER2 and HER3 expression and amplification were analyzed by primary tumor site; HER2 expression was also analyzed by quality of expression assessment (“high quality” vs. “low quality”) and by region (Western vs. Asian). Correlation between HER2 and HER3 expression and between HER2 expression and HER2 amplification (in “unselected population”) was also assessed.
Data collection
Eligibility for each of the studies was assessed by one of the authors (SG); queries were discussed with a second author (AL). Same process was followed for data collection. The total number of patients in each study together with numerator and denominator for each one of the reported rates were collected.
In order to perform the planned subgroup analyses, the following additional data were extracted from manuscripts (if available): primary site (CC, IHCC, EHCC, GBC, or AC) and ethnicity/region of patients involved in the study (Western vs. Asian). Tumor site was also subdivided into extrahepatic BTCs (EH-BTCs) which include EHCC, GBC, and AC and IHCC. In addition, eligible studies were classified according to the quality of HER expression assessment: studies were considered to be “high quality” (HQ) when moderate/strong HER2/HER3 overexpression was used to classify tumors, whereas studies were classified as “low quality” (LQ) when the HER2/HER3 overexpression threshold was not specified and/or not reported by authors or when “any” HER2/HER3 expression (including IHC 1+) was used.
For assessment of HER2 amplification rate, studies were classified according to the population in which ISH was performed: “unselected population” referred to studies in which the ISH was performed in the whole study population regardless of IHC results, while the term “selected population” was employed for those studies in which ISH was performed only in patients with overexpression of HER2 according to IHC.
Statistical analysis
The Stata/MP v.12 package was used for the statistical analysis. Mean and 95 % confidence intervals (95 % CI) were calculated for reported HER2/HER3 expression/amplification rate. Mean HER2/HER3 expression/amplification rates were calculated for each one of the prespecified subgroup analyses: primary tumor site, region, quality of HER2 expression assessment, and population in which ISH was performed. All these analyses were repeated for each one of the tumor sites. Shapiro-Wilks normality test was performed for continuous variables; based on these results, parametric/nonparametric tests were used for the statistical analysis of the results and comparison of expression/amplification rates between subgroups employing Student’s t test or Wilcoxon rank-sum test as appropriate. Correlation was assessed using Pearson or Spearman’s rho as appropriate, according to whether variables followed a normal distribution or not.
Results
Eligible studies
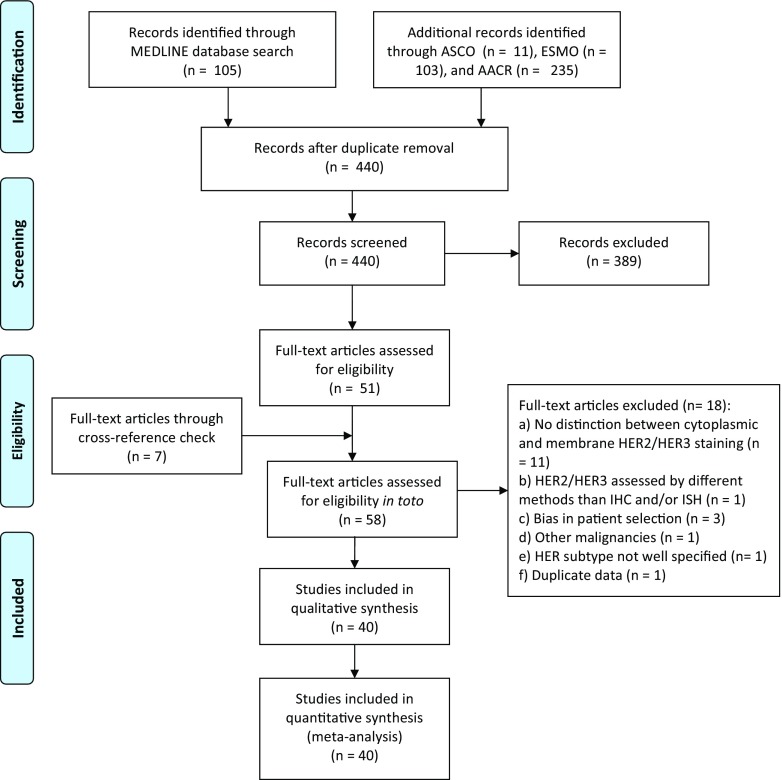
Figure 2 summarizes the PRISMA flow diagram for selection of eligible studies [28]; a total of 454 results were obtained from the searches in PubMed/MEDLINE (n = 105), ASCO (n = 11), ESMO (n = 103), and AACR (n = 235). Of these, 14 were duplicates and 389 did not meet the inclusion criteria and were therefore excluded. Of the 51 studies which appeared to be eligible after the initial screen, a full-text search was carried out. In addition, seven full-text records through cross-reference checking were identified for a total of 58 studies assessable for eligibility. Eighteen studies were excluded after the full-text review as per our inclusion/exclusion criteria: 11 studies did not report an optimal distinction between cytoplasmic and membrane HER2/HER3 staining [29–39]; one study employed a method other than IHC and/or ISH for evaluating HER2/HER3 expression with no IHC/ISH data reported [40]; three studies reported HER2 analysis following successful targeting therapy and were therefore excluded due to selection bias [10, 41, 42]; one study reported joined results for BTCs and pancreatic cancer with no specific data for BTC patients [43]; one study did not report which member of the HER family was being assessed [44]; and one study reported “duplicate data” [45].
Fig. 2.
PRISMA flow diagram
Patient population
Forty studies were included in the final analysis, reporting a total of 3839 patients with a diagnosis of BTC [46–85] (Table 2). All studies were retrospective series, with a median number of 53 patients per study (range 6–804).
Table 2.
HER2 and/or HER3 expression by immunohistochemistry (IHC) and/or amplification by in situ hybridization (ISH) in biliary tract carcinomas
| Study | Country | N | Primary | HER2/IHC | HER2/ISH | HER3/IHC | HER3/ISH | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | ||||
| Brunt EM | USA (Western) | 6 | CC | 4/6 | 66.7 | NR | NR | NR | |||
| Collier JD 1992 | UK (Western) | 10 | CC | 0/10 | 0 | NR | NR | NR | |||
| Lei S 1995 | USA (Western) | 6 | AC | 2/6 | 33.3 | NR | NR | NR | |||
| Chow NH 1995 | Taiwan (China) (Asian) | 18 | IHCC | 5/18 | 27.8 | NR | NR | NR | |||
| 18 | AC | 5/18 | 27.8 | NR | NR | NR | |||||
| 11 | GBC | 7/11 | 63.6 | NR | NR | NR | |||||
| Vaidya P 1996 | Japan (Asian) | 14 | EHCC | 10/14 | 71.4 | NR | 4/14 | 28.6 | NR | ||
| 13 | AC | 9/13 | 69.2 | NR | 4/13 | 30.8 | NR | ||||
| Terada T 1998 | Japan (Asian) | 47 | CC | 33/47 | 70 | NR | NR | NR | |||
| Kim YW 2001 | Not specified | 71 | GBC | 33/71 | 46.5 | NR | NR | NR | |||
| Ajiki T 2001 | Japan (Asian) | 30 | AC | 7/30 | 23 | NR | NR | NR | |||
| Ukita Y 2002 | Japan (Asian) | 22 | IHCC | 18/22 | 82 | 22/22 | 100 | NR | NR | ||
| Endo K 2002 | Japan, Thailand, USA (n/a) | 71 | CC | 21/71 | 29.6 | NR | NR | NR | |||
| Altimari A 2003 | Italy (Western) | 48 | IHCC | 2/48 | 4 | 2/48 | 4 | NR | NR | ||
| Matsuyama S 2004 | Japan (Asian) | 43 | GBC | 4/43 | 9.4 | NR | NR | NR | |||
| KIM HJ 2005 | South Korea (Asian) | 20 | CC | 5/20 | 25 | NR | NR | NR | |||
| Nakazawa K 2005 | Japan (Asian) | 28 | IHCC | 0/28 | 0 | NR | NR | NR | |||
| 78 | EHCC | 4/78 | 5.1 | NR | NR | NR | |||||
| 89 | GBC | 14/89 | 15.7 | NR | NR | NR | |||||
| 26 | AV | 3/26 | 11.5 | NR | NR | NR | |||||
| 71 | BTC | – | 15/71 | 21.1 | – | – | |||||
| 19 | BTC | – | 15/19 | 79 | – | – | |||||
| Settakorn J 2005 | Australia/Thailand (n/a) | 31 | IHCC | 10/31 | 32.3 | NR | NR | NR | |||
| Ogo Y 2006 | Japan (Asian) | 72 | BTCs | 47/72 | 65 | NR | NR | NR | |||
| Kim JH 2007 | South Korea (Asian) | 55 | EHCC | 16/55 | 29.1 | 10/55 | 18.1 | NR | NR | ||
| Kawamoto T 2007 | USA/Chile (Western) | 21 | IHCC | 7/21 | 33.3 | 0/14 | 0 | NR | NR | ||
| 16 | EHCC | 5/16 | 33.3 | 3/14 | 21.4 | NR | NR | ||||
| 77 | GBC | 24/77 | 31.2 | 14/67 | 20.9 | NR | NR | ||||
| Yoshikawa D 2008 | Japan (Asian) | 106 | IHCC | 1/106 | 0.9 | NR | NR | NR | |||
| 130 | EHCC | 11/130 | 8.5 | NR | NR | NR | |||||
| Miyahara n 2008 | Japan (Asian) | 51 | GBC | 16/51 | 31 | 4/16 | 25 | NR | NR | ||
| Joo HH 2007 | South Korea (Asian) | 112 | BTCs | 5/112 | 4.5 | NR | NR | NR | |||
| Puhalla H 2007 | Austria (Western) | 55 | GBC | 7/55 | 13 | NR | NR | NR | |||
| Kaufmann M 2008 | USA (Western) | 16 | GBC | 1/16 | 6.3 | NR | NR | NR | |||
| Baumhoer D 2008 | Switzerland, Germany, Italy (Western) | 82 | AV | NR | 5/82 | 6 | NR | NR | |||
| Choi HJ 2009 | Not specified | 50 | IHCC | 36/50 | 72 | NR | NR | NR | |||
| Aloysius MM 2009 | UK (Western) | 29 | EHCC | 0/29 | 0 | NR | NR | NR | |||
| 22 | AV | 0/22 | 0 | NR | NR | NR | |||||
| Harder J 2009 | Germany (Western) | 124 | BTCs | 25/124 | 20.2 | 6/25 | 24 | NR | NR | ||
| Shafizadeh N 2010 | USA (Western) | 26 | IHCC | 0/26 | 0 | NR | NR | NR | |||
| 19 | EHCC | 2/19 | 10.5 | NR | NR | NR | |||||
| 6 | GBC | 0/6 | 0 | NR | NR | NR | |||||
| Pignochino Y 2010 | Italy (Western) | 17 | IHCC | 0/10 | 0 | NR | NR | NR | |||
| 19 | EHCC | 4/19 | 21 | 2/4 | 50 | NR | NR | ||||
| 13 | GBC | 1/10 | 10 | 1/1 | 100 | NR | NR | ||||
| Toledo C 2012 | Chile (Western) | 12 | GBC | 4/12 | 33 | 0/12 | 0 | NR | NR | ||
| Kumari N 2012 | India (Asian) | 104 | GBC | 14/104 | 13.4 | NR | NR | NR | |||
| Lee HJ 2012 | South Korea (Asian) | 230 | EHCC | 13/224 | 6 | NR | 90/230 | 39 | NR | ||
| Roa Iván 2013 | Chile (Western) | 187 | GBC | 62/187 | 31.11 | NR | NR | NR | |||
| Wang W 2014 | China (Asian) | 58 | IHCC | 0/90 | 0 | 0/90 | 0 | NR | NR | ||
| 94 | EHCC | 4/90 | 4.4 | 3/94 | 3.5 | NR | NR | ||||
| Graham RP 2014 | USA (Western) | 100 | BTCs | 3/100 | 3 | 3/3 | 100 | NR | NR | ||
| Yang X 2014 | China (Asian) | 65 | IHCC | 0/65 | 0 | 0/65 | 0 | 8/65 | 12.3 | NR | |
| 110 | EHCC | 5/110 | 4.5 | 8/108 | 7.4 | 13/110 | 11.8 | NR | |||
| Kawamoto T 2015 | USA | 47 | GBC | 15/47 | 32 | 8/47 | 17 | 16/47 | 34 | 12/47 | 26 |
| Japan (n/a) | 66 | CC | 15/66 | 23 | 15/66 | 23 | 19/66 | 29 | 18/66 | 27 | |
| Hechtman J 2015 | USA (Western) | 106 | AC | 27/106 | 25.5 | 13/100 | 13 | NR | NR | ||
| Oliveira Fernandes VT 2015 | Brazil (Western) | 38 | CC | 11/38 | 30 | NR | NR | NR | |||
| Holcombe RF 2015 | USA (Western) | 126 | EHCC | NR | NR | 18 | NR | NR | |||
| 434 | IHCC | NR | NR | 1.5 | NR | NR | |||||
| 244 | GBC | NR | NR | 15 | NR | NR | |||||
CC cholangiocarcinoma, IHCC intrahepatic cholangiocarcinoma EHCC extrahepatic cholangiocarcinoma, GBC gallbladder carcinoma, AC carcinoma of ampulla of Vater, BTCs biliary tract carcinomas, NR not reported, n/a not applicable
According to the primary tumor site, the number of studies and number of patients reported were as follows: CC (24 studies; 2102 patients; 55 % of all patients reported), IHCCs (13 studies; 924 patients; 24 % of all patients reported; 44 % of all CC patients), EHCCs (12 studies; 920 patients; 24 % of all patients reported; 44 % of all CC patients), GBCs (15 studies; 1026 patients; 27 % of all patients reported), and ACs (8 studies; 303 patients; 8 % of all patients reported). In seven studies (258 patients; 7 % of all patients reported; 12 % of all CC patients), the type of CC (IHCC vs. EHCC) was not specified. In four studies (408 patients; 10 % of all patients reported), the type of BTC was not specified. Eighteen out of 40 (45 %) studies were conducted in Western countries and 17 (43 %) in Asian population, while the remaining 5 (12 %) studies were mixed or not specified (Table 2).
HER2 expression (IHC)
Thirty-eight studies reported HER2 positivity assessed by IHC (Table 3); two studies did not perform HER2 IHC analysis [66, 85]. Technical details regarding this assessment were available for 37 of the 38 studies: in the remaining study, this data was not available [71]. The most commonly used (23 of 37 studies; 62 %) anti-HER2 antibody was polyclonal (Dako®, Dakopatts®, Nichirei®, or Zymed Lab®), followed by monoclonal antibody in 13 studies (35 %) (Triton Biosciences Inc.®, Immunotech®, DAKO®, Oncogene®, Zymed Lab®, Carpinteria®, Ventana®, or Novocastra®); this information was not available in one study (3 %). HER2 expression was qualitatively analyzed in 5 out of 37 (14 %) studies, while a semiquantitative score, estimating the fraction of positive cells, was used in 32 studies (86 %) (Table 3).
Table 3.
Descriptive features of immunohistochemistry (IHC) and in situ hybridization (ISH) for HER2 and HER3 in biliary tract carcinomas
| Study | HER2 | HER3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Platform | IHC scoring | Qualitative/semiquantitative | LQ/HQ assessment | ISH | Selection of patients for ISH | Platform | IHC scoring | Qualitative/semiquantitative | LQ/HQ assessment | ISH | |
| Brunt EM 1992 | MAb (Triton Biosciences Inc., USA) | Weak (1+), moderate (2+) or strong (3+), and focal or diffuse | Semiquantitative | LQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Collier JD 1992 | MAb NCL-CB11 | Negative and positive | Qualitative | LQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Lei S 1995 | MAb CB11 | Negative, weak, moderate, or strong | Semiquantitative | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Chow NH 1995 | MAb-1 (Triton Biosciences Inc., USA) | Focal staining (+) and diffuse staining (++) | Qualitative | LQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Vaidya P 1996 | PolyAb (Dakopatts, Denmark) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | n/a | n/a | Novocastra Lab. Ltd., UK | A 4r-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | n/a |
| Terada T 1998 | MAb 3B5 (Immunotech, France) | A 5-point scale: –, +, ++, +++, and ++++ | Semiquantitative | LQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Kim YW 2001 | PolyAb (Dako, USA) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | LQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Ajiki T 2001 | PolyAb (Dako, Denmark) | Immunoreactivity present in more than 10 % of tumor cells | Semiquantitative | LQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Ukita Y 2002 | MAb 3B5 (Immunotech, France) | A 5-point scale: –, +, ++, +++, and ++++ | Semiquantitative | LQ | FISH | Unselected | n/a | n/a | n/a | n/a | n/a |
| Endo K 2002 | Mab F-11 (Dako, USA) | A 5-point scale: 0, ±, +, 2+, and 3+ | Semiquantitative | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Altimari A 2003 | PolyAb HercepTest (Dako, Denmark) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | CISH | Unselected | n/a | n/a | n/a | n/a | n/a |
| Matsuyama S 2004 | PolyAb HercepTest (Dako, Denmark) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Kim HJ 2005 | MAb (Oncogene, USA) | Negative or positive | Qualitative | LQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Nakazawa 2005 | PolyAb (Nichirei, Japan) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | FISH | Unselected and selected | n/a | n/a | n/a | n/a | n/a |
| Settakorn J 2005 | PolyAb (HercepTest, Dako) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Ogo Y 2006 | PolyAb (HercepTest Dako, A0485) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Kim HJ 2007 | PolyAb (Zymed Lab, USA) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | CISH | Unselected | n/a | n/a | n/a | n/a | n/a |
| Kawamoto T 2007 | PolyAb HercepTest (Dako, Denmark) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | FISH | Unselected | n/a | n/a | n/a | n/a | n/a |
| Yoshikawa D 2008 | Polyab HercepTest (Dako, Denmark) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Baumhoer D 2008 | n/a | n/a | n/a | LQ | FISH | Unselected | n/a | n/a | n/a | n/a | n/a |
| Miyahara N 2008 | PolyAb HercepTest (Dako) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | FISH | Selected | n/a | n/a | n/a | n/a | n/a |
| Joo HH 2007 | PolyAb (Zymed) | A distinctive membrane staining was referred as positive | Qualitative | LQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Puhalla H 2007 | PolyAb HercepTest (Dako) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Kaufmann M 2008 | MAb CB11 (Carpinteria, USA) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Aloysius MM 2009 | PolyAb HercepTest (Dako) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Harder J 2009 | PolyAb (Dako REAL™, Denmark) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | FISH | Selected | n/a | n/a | n/a | n/a | n/a |
| Choi 2009 | NR | NR | NR | LQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Shafizadeh N 2010 | MAb CB11, (Ventana, USA) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Pignochino Y 2010 | PolyAb HercepTest (Dako) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | FISH | Selected | n/a | n/a | n/a | n/a | n/a |
| Toledo C 2012 | MAb NCL-CBE-356 (Novocastra) | Positive or negative | Qualitative | LQ | FISH | Unselected | n/a | n/a | n/a | n/a | n/a |
| Kumari N 2012 | PolyAb (Dako) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Lee HJ 2012 | PolyAb A0485 (Dako, Denmark) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | n/a | n/a | MAb RTJ.2 (Santa Cruz, USA) | Staining intensity and percentage of positive cellsa | Semiquantitative | HQ | n/a |
| Roa I 2013 | MAb NCL-CB11 (Novocastra) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Wang W 2014 | PolyAb (Dako, USA) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | FISH | Unselected | n/a | n/a | n/a | n/a | n/a |
| Graham 2014 | PolyAb HercepTest (Dako, USA) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | FISH | Selected | n/a | n/a | n/a | n/a | n/a |
| Yang X 2014 | PolyAb (Dako) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | FISH | Unselected | sc-415 (Santa Cruz Biotechnology, USA) | Rajikumar scoreb | Semiquantitative | HQ | n/a |
| Kawamoto T 2015 | PolyAb HercepTest II (Dako A/S, Denmark) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | FISH | Unselected | Spring Bioscience, USA | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | FISH |
| Hechtman J 2015 | MAb 4B5 (Ventana Medical Systems, USA) | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | CISH | Unselected | n/a | n/a | n/a | n/a | n/a |
| Oliveira Fernandes VT 2015 | NR | A 4-point scale: 0, 1+, 2+, and 3+ | Semiquantitative | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Holcombe RF 2015 | n/a | n/a | n/a | LQ | FISH | Unselected | n/a | n/a | n/a | n/a | n/a |
NR not reported, n/a not applicable, LQ low quality, HQ high quality, FISH fluorescence in situ hybridization, CISH chromogenic in situ hybridization
aHER3 was scored based on the intensity of staining as 0 (negative), 1 (weak), or 2 (strong) and the percentage of positive epithelial cells as 0 (<5 %), 1 (6–25 %), 2 (26–50 %), 3 (51–75 %), or 4 (>76 %). A Histoscore was generated as the product of intensity and area. The Histoscore was then dichotomized into no/lower expression (Histoscore, 0–6) and overexpression (Histoscore, 8)
bRajikumar score based on two parameters: staining intensity (range, 0–3) and percentage of positive cells [range, 0–4 = 0 (0–10 %); 1 (11–25 %), 2 (26–50 %), 3 (51–75 %), 4 (>76 %)]. Slides with scores of ≥8 were classified as overexpression and slides with scores <8 as nonoverexpression
Globally, the mean HER2 expression rate was 26.5 % (95 % CI, 18.9–34.1 %; Table 4). There were no statistically significant differences between regions (Asian mean HER2 expression rate 28.4 % (95 % CI 14.5–42.3 %) vs. Western 19.7 % (95 % CI 10.1–29.2 %); p value 0.4936; Table 4). With respect to the quality of HER2 expression assessment, LQ studies (11 studies; 27 % of all studies reporting HER2-IHC data) had a significantly higher mean HER2 expression rate compared to HQ studies (27 studies; 68 % of all studies reporting HER2-IHC data): 41.7 % (95 % CI 22.9–60.5 %) vs. 20.3 % (95 % CI 13.3–27.4 %), respectively, p value 0.0336; Table 4.
Table 4.
HER2 expression and amplification results in biliary tract carcinomas
| HER2 status | No. of studies | Expression rate mean (95 % CI, %) | p value | |
|---|---|---|---|---|
| Overall expression by IHC | All | 38 | 26.5 % (18.9–34.1 %) | |
| By ethnicity | Asian | 17 | 28.4 % (14.5–42.3 %) | Ref |
| Western | 16 | 19.7 % (10.1–29.2 %) | 0.4936 | |
| By IHC assessment (quality) | Low quality (LQ) | 11 | 41.7 % (22.9–60.5 %) | Ref |
| High quality (HQ) | 27 | 20.3 % (13.2–27.5 %) | 0.0336 | |
| By site of primary (HQ studies only) | IHCC | 8 | 4.8 % (0–14.5 %) | Ref |
| EH-BTC | 28 | 19.9 % (12.8–27.1 %) | 0.0049 | |
| EHCC | 11 | 17.4 % (3.4–31.4 %) | 0.0134 | |
| GBC | 12 | 19.1 % (11.2–26.8 %) | 0.0123 | |
| AC | 5 | 27.9 % (0–60.7 %) | 0.0642 | |
| Overall amplification by ISH | All | 16 | 30.1 % (11.7–48.5 %) | |
| By site of primary | IHCC | 6 | 17.6 % (0–60.1 %) | Ref |
| EH-BTC | 14 | 22.5 % (7.9 %–37.2 %) | 0.0468 | |
| By patient selection | Unselected | 12 | 17.9 % (0.1–35.4 %) | Ref |
| Selected | 5 | 57.6 % (16.2–99 %) | 0.0072 |
LQ low quality, HQ high quality, ISH in situ hybridization, IHCC intrahepatic cholangiocarcinoma, EHCC extrahepatic cholangiocarcinoma, EH-BTCs extrahepatic biliary tract cancers, GBC gallbladder carcinoma, AC ampulla of Vater carcinoma, Ref category used as reference for comparisons
Bold-italics represent statistically significant results
In all 38 studies, no differences in HER2/IHC expression rates were found between tumor sites when considering all studies, regardless of the quality of HER2 expression assessment (Table 4). In contrast, when only HQ studies were considered, the mean HER2 overexpression rate in EH-BTCs was statistically significantly higher to IHCCs (Table 4). Moreover, mean HER2 overexpression rate was statistically significantly higher in EHCCs compared to IHCCs and in GBCs compared to IHCCs, whereas there was only a marginal difference between ACs and IHCCs (Table 4).
HER2 amplification (ISH)
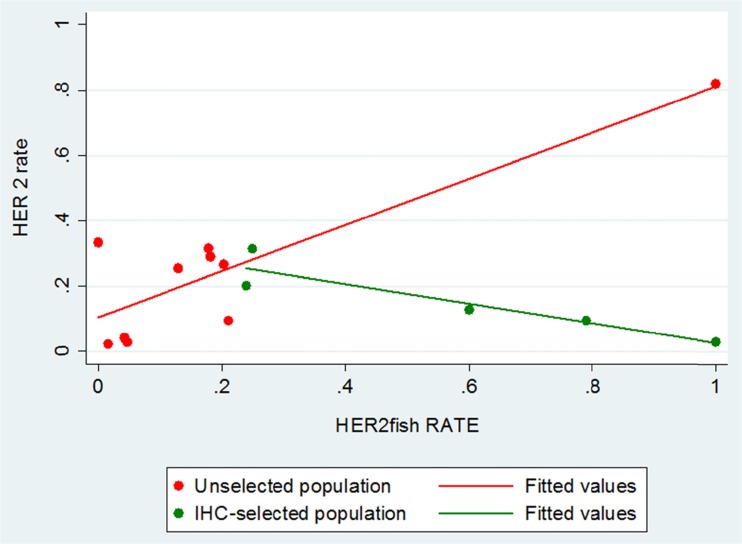
HER2 amplification analysis was performed in 16 studies: applying FISH and CISH in 13 (81 %) and 3 (19 %) studies, respectively (Table 3). Mean HER2 amplification rate was 30.1 % (95 % CI 11.7–48.5 %) when all BTCs were analyzed together (Table 4). When all studies were included (regardless of applying ISH for “selected” or “unselected” population), mean HER2 amplification rate was statistically significantly higher in patients with EH-BTCs compared to IHCCs (Table 4). Interestingly, the mean HER2 amplification rate was higher in the five studies [59, 68, 72, 73, 78] in which ISH test was performed in “selected” population when compared to the 12 studies in which ISH test was applied to “unselected population” only [17.9 % (95 % CI 0.1–35.4 %) vs. 57.6 % (95 % CI 16.2–99 %), p value 0.0072] [55, 56, 59, 63, 64, 66, 77, 80–83, 85] (Table 4). Nakazawa et al. reported data from 221 patients, 71 of whom had FISH testing performed: meaningful differences in HER2 amplification rate were shown between “unselected” [15/71 (21 %)] and “selected” [15/19 (79 %)] populations [59] (Table 2).
Correlation between HER2 expression and amplification
Ten studies [55, 56, 59, 63, 64, 77, 80, 82, 83, 85] and five studies [59, 68, 72, 73, 78] had data for both HER2 expression and amplification in the “unselected” and “selected” population, respectively. While no statistically significant correlation was observed in studies with the “selected” population (five studies; Spearman rho = −0.9; p value 0.037), a better correlation (although not statistically significant) was shown in “unselected” patients (10 studies; Spearman rho 0.38; p value 0.2763) (Fig. 3).
Fig. 3.
Correlation between HER2 expression and amplification
HER3 expression and amplification
HER3 expression rate was reported in four studies in which different commercially available antibodies were used (Novocastra®, Santa Cruz®, or Spring Bioscience®). All four studies had a HQ HER3 expression assessment (Table 3). The pooled mean overall HER3 overexpression rate was 27.9 % (95 % CI 9.7–46.1 %) [51, 76, 81, 83]; only one study reported HER3 amplification rate (26.5 %) [83] (Table 2).
Further subgroup analyses for HER3 expression and amplification were not possible due to limited number of studies.
Correlation between HER2 and HER3 overexpression
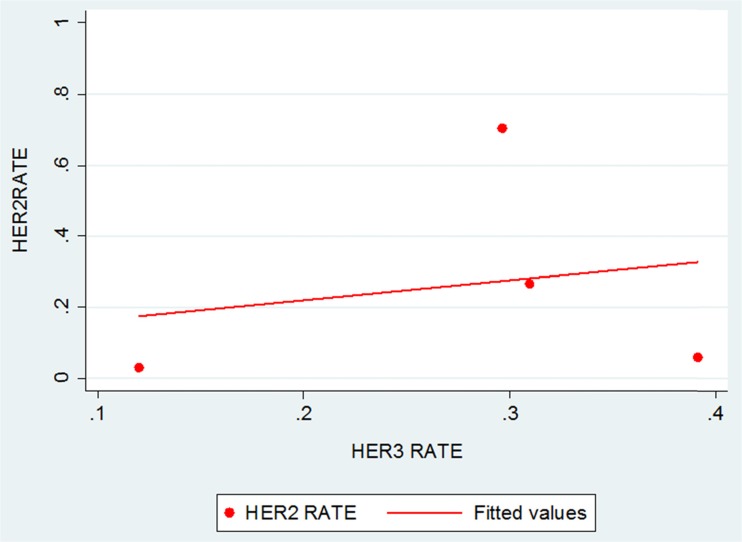
All of the four studies with HER3 expression data had HER2 expression data available for correlation analysis [51, 76, 81, 83]. No statistically significant correlation was identified between HER2 and HER3 overexpression (four studies; Spearman rho = 0.2; p value 0.8) (Fig. 4).
Fig. 4.
Correlation between HER2 and HER3 expression
Discussion
The present systematic review and meta-analysis found that there is a higher moderate/strong HER2 expression rate (~20 %) in extrahepatic biliary tract carcinomas than in IHCC (<5 %). In a previous meta-analysis, Wiggers et al. also reported a statistically significant higher expression of HER2 in EHCC [risk ratio 0.22 (95 % CI, 0.07–0.65)] than in IHCC [86]. However, this work was based on a smaller number of studies (five) detecting HER2 expression in only IHCC and EHCC, excluding GBC and AC, and did not provide any information about ISH testing. Subgroup analysis by site of primary in the current study suggested that HER2 overexpression was higher in EHCC, GBC, and AC tumors than in IHCCs [of note, this difference was not statistically significant between the group of IHCC and ACs, probably due to a smaller sample size of this subgroup (303 patients)].
Interestingly, in the IHC “selected population” (patients with moderate/strong expression by IHC), HER2 amplification rate was found to be ~60 %. Therefore, these findings (moderate/strong HER2 expression rates in EH-BTCs and FISH rates in IHC “selected” patients) suggest around 10–20 % of EH-BTCs can be virtually considered HER2 upregulated. In a recent case series of 211 consecutive GBC tumors, 16.6 % of tumors were globally found to be HER2 positive when IHC3+ and IHC 2+/FISH-amplified tumors were considered altogether [87]. According to international HER2 assessment criteria used for breast and gastric cancer [6, 26, 27], it may be assumed that BTCs scoring 3+ on immunohistochemistry should be interpreted as positive, while the application of in situ hybridization (fluorescence or chromogenic) could be carried out only in tumors with an ambiguous (IHC 2+) score.
These results, in addition to some preclinical data demonstrating that constitutive overexpression of activated HER2 can result in cholangiocarcinoma development [88], provide some support that the HER2 protein may play an important role in extrahepatic biliary carcinogenesis. Consequently, the HER2 pathway may be considered as a potential actionable target in EH-BTCs. Inhibition of HER2-mediated signaling is an established therapeutic strategy in HER2-positive breast and gastric cancer in which HER2 overexpression rates (up to 20 %) are similar to that found in EH-BTCs [26, 89]. Anti-HER2 therapy options might include the antibodies trastuzumab, pertuzumab, or trastuzumab emtansine (T-DM1) or the small-molecule, orally active, TKI, lapatinib [6, 90, 91].
Beyond HER2, in BTC, other biomarkers might be involved in cancer pathogenesis, prognosis, and resistance to therapy. In the current meta-analysis, approximately one in four patients had moderate/strong expression of HER3 or HER3 gene amplification. Most interestingly, HER2/HER3 co-expression in BTCs ranges from 9 to 53 % [76, 83] and has been demonstrated to be frequently associated with phosphorylation (activation) of HER2 and AKT [83]. HER3 is often correlated with poorly differentiated biliary tumors [81] and appears to be a poor prognostic factor in EHCCs [76], whereas the prognostic meaning of HER2 has not been completely clarified [79, 87]. Interestingly, the combination of pertuzumab and trastuzumab has been reported to induce a synergistic inhibition of in vivo tumor growth in BTCs, likely because of a more comprehensive blockade of HER2/HER3 signaling [83]. Moreover, HER4 was found to be overexpressed in 63.1 % of IHCCs and in 56.4 % of EHCCs, respectively, demonstrating to be a significant poor prognostic factor in EGFR-negative IHCC cases [81]. KRAS/NRAS mutations occur in 6.1–6.5 % of BTCs [73, 82, 85] and they appear to be mutually exclusive with HER2 amplification, at least in ACs [82]. Less frequently, BTCs harbor BRAF mutations (0–8.1 %) or PI3K mutations (7.3–10.2 %) [73, 82], while MET expression measured by IHC ranges from 5.6 to 44.1 % [54, 59, 62]. Importantly, investigational research should mainly define magnitude and prognostic impact of these biomarkers in BTCs and their correlation with HER2/HER3 pathway.
Therefore, due to inherent anatomical and molecular features, BTCs should no longer be classified as a singular entity and, in the future, differences in tumor location or tumor biology as well as an accurate distinction from other neoplastic entities should be carefully considered so as to minimize disappointing results in both clinical practice and scientific research.
This systematic review and meta-analysis has limitations, mainly linked to inter-study heterogeneity. In several studies, a clear definition of the primary tumor site was not available or results were not reported separately for each subgroup, thus limiting the eligible data for inclusion in subgroup analyses. Since no standardized techniques and scores to assess HER2 amplification and expression are available in BTCs, and because there are no internationally accepted and validated methods for HER3 testing established in any tumor, inconsistency in methodology may be an issue. Furthermore, the articles included in this meta-analysis covered a long period of time (1992 to 2015), and thus various laboratory assays were likely utilized to determine HER2 protein expression and gene amplification with different cutoff values for positivity employed. Differences in methodology, disease stage (early vs. advanced), tumor specimen (resection specimen vs. biopsy), site of tumor specimen (primary vs. metastases), IHC scoring system (qualitative vs. semiquantitative), threshold definition of IHC overexpression (provided vs. not), and/or choice of tumors in which the ISH test was applied (HER2 overexpression vs. no overexpression) may explain the wide range of both HER2 expression (0 to 82 %) and amplification (0 to 100 %) positivity reported in this review. Moreover, available literature indicates a certain variability between polyclonal and monoclonal antibodies in the ability to detect membranous HER2 protein, a higher level of concordance between IHC and ISH for polyclonal antibodies, and the possibility of influencing antigen retrieval through utilization of various application methods on tissue samples [92]. Due to the characteristics of the data reported, it was not possible to perform analysis of co-expression rate between HER2 and HER3 or concordance between IHC and ISH. Finally, no survival data was available, making it impossible to assess the prognostic implications of HER2/HER3 expression/amplification.
Despite the abovementioned limitations, this meta-analysis is the first study to systematically estimate the prevalence of HER2 and HER3 in all BTCs. Approximately one fifth of EH-BTCs are HER2 overexpressed, suggesting that the development of strategies against this receptor could be a reasonable therapeutic approach. Further data is required regarding the impact of co-expression of both HER2 and HER3. Standardization of ISH and IHC techniques, validation of scoring criteria for HER2 and HER3 immunohistochemistry, and assessment of concordance between IHC and ISH, focusing on the high intra-tumoral heterogeneity of HER2 membranous protein [87], are needed if the techniques are to be adopted to clinical practice. Assuming that an overexpression of HER2 of 5 % or less could be considered “un-interesting,” in this era of personalized medicine and spending review, our data may be particularly pertinent for the most cost-effective selection of patients with BTCs who may benefit from anti-HER2-targeted therapy.
Well-designed prospective clinical trials, for patients rigorously selected by HER2-positive tumors and, possibly, stratified by tumor location, are warranted to confirm the benefit of adding anti-HER2-targeted agents to chemotherapy in advanced disease. Given the lack of benefit reported for lapatinib in previous phase II trials in BTCs [93, 94] as well as in phase III trials in HER2-positive advanced gastric cancer [95, 96], alternative anti-HER2 therapies such as monoclonal antibodies trastuzumab and pertuzumab seem to be more promising.
Acknowledgements
We would like to express our gratitude to Mr. Russell Edu Samuel William for his help with literature research and Mr. Daniele Maffeis for scientific figure designing (applicable to Fig. 1).
Contribution of each author
SG and JV formulated the research question. SG, AL, and JV were responsible for the study design. SG collected the data. AL analyzed the data. All authors interpreted and wrote the manuscript.
Compliance with ethical standards
Conflict of interest
Authors declare no conflict of interest.
Funding
Salvatore Galdy is part-funded by “Clinical Unit Visit” European Society of Medical Oncology (ESMO) Fellowship.
Angela Lamarca is part-funded by Pancreatic Cancer Research Fund (PCRF) Grant and Spanish Society of Medical Oncology (SEOM) Translational Fellowship Grant.
References
- 1.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. The New England Journal of Medicine. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 2.Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Annals of Oncology: Official Journal of the European Society for Medical Oncology / ESMO. 2014;25(2):391–398. doi: 10.1093/annonc/mdt540. [DOI] [PubMed] [Google Scholar]
- 3.Merla A, Liu KG, Rajdev L. Targeted therapy in biliary tract cancers. Current Treatment Options in Oncology. 2015;16(10):48. doi: 10.1007/s11864-015-0366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valle JW, Wasan H, Lopes A, Backen AC, Palmer DH, Morris K, et al. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): a randomised phase 2 trial. The Lancet.Oncology. 2015;16(8):967–978. doi: 10.1016/S1470-2045(15)00139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. The New England Journal of Medicine. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 6.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet (London, England) 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 7.Law LY. Dramatic response to trastuzumab and paclitaxel in a patient with human epidermal growth factor receptor 2-positive metastatic cholangiocarcinoma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2012;30(27):e271–e273. doi: 10.1200/JCO.2012.42.3061. [DOI] [PubMed] [Google Scholar]
- 8.Sorscher S. Marked radiographic response of a HER-2-overexpressing biliary cancer to trastuzumab. Cancer Management and Research. 2013;9:1–3. doi: 10.2147/CMAR.S55091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subbiah IM, Subbiah V, Tsimberidou AM, Naing A, Kaseb AO, Javle M, et al. Targeted therapy of advanced gallbladder cancer and cholangiocarcinoma with aggressive biology: eliciting early response signals from phase 1 trials. Oncotarget. 2013;4(1):153–162. doi: 10.18632/oncotarget.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Javle M, Churi C, Kang HC, Shroff R, Janku F, Surapaneni R, et al. HER2/neu-directed therapy for biliary tract cancer. Journal of Hematology & Oncology. 2015;8:58. doi: 10.1186/s13045-015-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J, Park SH, Chang HM, Kim JS, Choi HJ, Lee MA, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. The Lancet.Oncology. 2012;13(2):181–188. doi: 10.1016/S1470-2045(11)70301-1. [DOI] [PubMed] [Google Scholar]
- 12.Chen JS, Hsu C, Chiang NJ, Tsai CS, Tsou HH, Huang SF, et al. A KRAS mutation status-stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer. Annals of Oncology: Official Journal of the European Society for Medical Oncology / ESMO. 2015;26(5):943–949. doi: 10.1093/annonc/mdv035. [DOI] [PubMed] [Google Scholar]
- 13.Malka D, Cervera P, Foulon S, Trarbach T, de la Fouchardiere C, Boucher E, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. The Lancet.Oncology. 2014;15(8):819–828. doi: 10.1016/S1470-2045(14)70212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leone F, Marino D, Cereda S, Filippi R, Belli C, Spadi R, et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-type KRAS advanced biliary tract cancer: a randomized phase 2 trial (vecti-BIL study) Cancer. 2016;122(4):574–581. doi: 10.1002/cncr.29778. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, et al. ErbB receptors: from oncogenes to targeted cancer therapies. The Journal of Clinical Investigation. 2007;117(8):2051–2058. doi: 10.1172/JCI32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Molecular Cell. 2003;11(2):495–505. doi: 10.1016/S1097-2765(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 17.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. The New England Journal of Medicine. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 18.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nature Reviews Cancer. 2005;5(5):341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 19.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. The EMBO Journal. 1997;16(7):1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nature Reviews Cancer. 2009;9(7):463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 21.Sierke SL, Cheng K, Kim HH, Koland JG. Biochemical characterization of the protein tyrosine kinase homology domain of the ErbB3 (HER3) receptor protein. The Biochemical Journal. 1997;322(Pt 3):757–763. doi: 10.1042/bj3220757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, et al. A hierarchical network of interreceptor interactions determines signal transduction by neu differentiation factor/neuregulin and epidermal growth factor. Molecular and Cellular Biology. 1996;16(10):5276–5287. doi: 10.1128/MCB.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amin DN, Sergina N, Ahuja D, McMahon M, Blair JA, Wang D, et al. Resiliency and vulnerability in the HER2-HER3 tumorigenic driver. Science Translational Medicine. 2010;2(16):16ra7. doi: 10.1126/scitranslmed.3000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science (New York, N.Y.) 2007;316(5827):1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 25.Choi BK, Fan X, Deng H, Zhang N, An Z. ERBB3 (HER3) is a key sensor in the regulation of ERBB-mediated signaling in both low and high ERBB2 (HER2) expressing cancer cells. Cancer Medicine. 2012;1(1):28–38. doi: 10.1002/cam4.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann M, Stoss O, Shi D, Buttner R, van de Vijver M, Kim W, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52(7):797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Medicine: A Peer-Reviewed, Independent, Open-Access Journal. 2009;3(3):e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 29.Voravud N, Foster CS, Gilbertson JA, Sikora K, Waxman J. Oncogene expression in cholangiocarcinoma and in normal hepatic development. Human Pathology. 1989;20(12):1163–1168. doi: 10.1016/S0046-8177(89)80006-1. [DOI] [PubMed] [Google Scholar]
- 30.Vaidya P, Yosida T, Sakakura T, Yatani R, Noguchi T, Kawarada Y. Combined analysis of expression of c-erbB-2, ki-67 antigen, and tenascin provides a better prognostic indicator of carcinoma of the papilla of vater. Pancreas. 1996;12(2):196–201. doi: 10.1097/00006676-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Kamel D, Paakko P, Nuorva K, Vahakangas K, Soini Y. p53 and c-erbB-2 protein expression in adenocarcinomas and epithelial dysplasias of the gall bladder. The Journal of Pathology. 1993;170(1):67–72. doi: 10.1002/path.1711700111. [DOI] [PubMed] [Google Scholar]
- 32.Yukawa M, Fujimori T, Hirayama D, Idei Y, Ajiki T, Kawai K, et al. Expression of oncogene products and growth factors in early gallbladder cancer, advanced gallbladder cancer, and chronic cholecystitis. Human Pathology. 1993;24(1):37–40. doi: 10.1016/0046-8177(93)90060-T. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki H, Isaji S, Pairojkul C, Uttaravichien T. Comparative clinicopathological study of resected intrahepatic cholangiocarcinoma in northeast Thailand and Japan. Journal of Hepato-Biliary-Pancreatic Surgery. 2000;7(2):206–211. doi: 10.1007/s005340050177. [DOI] [PubMed] [Google Scholar]
- 34.Aishima SI, Taguchi KI, Sugimachi K, Shimada M, Sugimachi K, Tsuneyoshi M. c-erbB-2 and c-met expression relates to cholangiocarcinogenesis and progression of intrahepatic cholangiocarcinoma. Histopathology. 2002;40(3):269–278. doi: 10.1046/j.1365-2559.2002.00353.x. [DOI] [PubMed] [Google Scholar]
- 35.Kalekou H, Miliaras D. Immunohistochemical study of microvessel density, CD44 (standard form), p53 protein and c-erbB2 in gallbladder carcinoma. Journal of Gastroenterology and Hepatology. 2004;19(7):812–818. doi: 10.1111/j.1440-1746.2004.03357.x. [DOI] [PubMed] [Google Scholar]
- 36.Chaube A, Tewari M, Garbyal RS, Singh U, Shukla HS. Preliminary study of p53 and c-erbB-2 expression in gallbladder cancer in indian patients manuscript id: 8962091628764582. BMC Cancer. 2006;6:126. doi: 10.1186/1471-2407-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng J, Zhu YM. Expression of c-erbB-2 proto-oncogene in extrahepatic cholangiocarcinoma and its clinical significance. Hepatobiliary & Pancreatic Diseases International: HBPD INT. 2007;6(4):412–415. [PubMed] [Google Scholar]
- 38.Schlitter AM, Jang KT, Kloppel G, Saka B, Hong SM, Choi H, et al. Intraductal tubulopapillary neoplasms of the bile ducts: clinicopathologic, immunohistochemical, and molecular analysis of 20 cases. Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc. 2015;28(9):1249–1264. doi: 10.1038/modpathol.2015.61. [DOI] [PubMed] [Google Scholar]
- 39.Ito Y, Takeda T, Sasaki Y, Sakon M, Yamada T, Ishiguro S, et al. Expression and clinical significance of the erbB family in intrahepatic cholangiocellular carcinoma. Pathology, Research and Practice. 2001;197(2):95–100. doi: 10.1078/0344-0338-00016. [DOI] [PubMed] [Google Scholar]
- 40.Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142(4):1021–1031. doi: 10.1053/j.gastro.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maurya SK, Tewari M, Sharma B, Shukla HS. Expression of procaspase 3 and activated caspase 3 and its relevance in hormone-responsive gallbladder carcinoma chemotherapy. The Korean Journal of Internal Medicine. 2013;28(5):573–578. doi: 10.3904/kjim.2013.28.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suder A, Ang JE, Kyle F, Harris D, Rudman S, Kristeleit R, et al. A phase I study of daily afatinib, an irreversible ErbB family blocker, in combination with weekly paclitaxel in patients with advanced solid tumours. European Journal of Cancer (Oxford, England: 1990) 2015;51(16):2275–2284. doi: 10.1016/j.ejca.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 43.Euscher ED, Marsh WL, Jr, Lucas JG, Frankel WL. Histologic and immunohistochemical changes in the stented common bile duct. Applied Immunohistochemistry & Molecular Morphology: AIMM / Official Publication of the Society for Applied Immunohistochemistry. 2007;15(3):299–304. doi: 10.1097/01.pai.0000213104.10945.a7. [DOI] [PubMed] [Google Scholar]
- 44.Zhu L, Kim K, Domenico DR, Appert HE, Howard JM. Adenocarcinoma of duodenum and ampulla of vater: clinicopathology study and expression of p53, c-neu, TGF-alpha, CEA, and EMA. Journal of Surgical Oncology. 1996;61(2):100–105. doi: 10.1002/(SICI)1096-9098(199602)61:2<100::AID-JSO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 45.Ooi A, Suzuki S, Nakazawa K, Itakura J, Imoto I, Nakamura H, et al. Gene amplification of myc and its coamplification with ERBB2 and EGFR in gallbladder adenocarcinoma. Anticancer Research. 2009;29(1):19–26. [PubMed] [Google Scholar]
- 46.Ajiki T, Kamigaki T, Hasegawa Y, Fujino Y, Suzuki Y, Takeyama Y, et al. Proliferating cell nuclear antigen, p53, and c-erbB-2 expression in relation to clinicopathological variables and prognosis in cancer of the ampulla of Vater. Hepato-Gastroenterology. 2001;48(41):1266–1270. [PubMed] [Google Scholar]
- 47.Brunt EM, Swanson PE. Immunoreactivity for c-erbB-2 oncopeptide in benign and malignant diseases of the liver. American Journal of Clinical Pathology. 1992;97(5 Suppl 1):S53–S61. [PubMed] [Google Scholar]
- 48.Collier JD, Guo K, Mathew J, May FE, Bennett MK, Corbett IP, et al. c-erbB-2 oncogene expression in hepatocellular carcinoma and cholangiocarcinoma. Journal of Hepatology. 1992;14(2–3):377–380. doi: 10.1016/0168-8278(92)90186-S. [DOI] [PubMed] [Google Scholar]
- 49.Chow NH, Huang SM, Chan SH, Mo LR, Hwang MH, Su WC. Significance of c-erbB-2 expression in normal and neoplastic epithelium of biliary tract. Anticancer Research. 1995;15(3):1055–1059. [PubMed] [Google Scholar]
- 50.Lei S, Appert HE, Nakata B, Domenico DR, Kim K, Howard JM. Overexpression of HER2/neu oncogene in pancreatic cancer correlates with shortened survival. International Journal of Pancreatology: Official Journal of the International Association of Pancreatology. 1995;17(1):15–21. doi: 10.1007/BF02788354. [DOI] [PubMed] [Google Scholar]
- 51.Vaidya P, Kawarada Y, Higashiguchi T, Yoshida T, Sakakura T, Yatani R. Overexpression of different members of the type 1 growth factor receptor family and their association with cell proliferation in periampullary carcinoma. The Journal of Pathology. 1996;178(2):140–145. doi: 10.1002/(SICI)1096-9896(199602)178:2<140::AID-PATH450>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 52.Terada T, Ashida K, Endo K, Horie S, Maeta H, Matsunaga Y, et al. c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology. 1998;33(4):325–331. doi: 10.1046/j.1365-2559.1998.00496.x. [DOI] [PubMed] [Google Scholar]
- 53.Kim YW, Huh SH, Park YK, Yoon TY, Lee SM, Hong SH. Expression of the c-erb-B2 and p53 protein in gallbladder carcinomas. Oncology Reports. 2001;8(5):1127–1132. doi: 10.3892/or.8.5.1127. [DOI] [PubMed] [Google Scholar]
- 54.Endo K, Yoon BI, Pairojkul C, Demetris AJ, Sirica AE. ERBB-2 overexpression and cyclooxygenase-2 up-regulation in human cholangiocarcinoma and risk conditions. Hepatology (Baltimore, Md.) 2002;36(2):439–450. doi: 10.1053/jhep.2002.34435. [DOI] [PubMed] [Google Scholar]
- 55.Ukita Y, Kato M, Terada T. Gene amplification and mRNA and protein overexpression of c-erbB-2 (HER-2/neu) in human intrahepatic cholangiocarcinoma as detected by fluorescence in situ hybridization, in situ hybridization, and immunohistochemistry. Journal of Hepatology. 2002;36(6):780–785. doi: 10.1016/S0168-8278(02)00057-0. [DOI] [PubMed] [Google Scholar]
- 56.Altimari A, Fiorentino M, Gabusi E, Gruppioni E, Corti B, D'Errico A, et al. Investigation of ErbB1 and ErbB2 expression for therapeutic targeting in primary liver tumours. Digestive and Liver Disease: Official Journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2003;35(5):332–338. doi: 10.1016/S1590-8658(03)00077-X. [DOI] [PubMed] [Google Scholar]
- 57.Matsuyama S, Kitajima Y, Sumi K, Mori D, Satoh T, Miyazaki K. Gallbladder cancers rarely overexpress HER-2/neu, demonstrated by Hercep test. Oncology Reports. 2004;11(4):815–819. [PubMed] [Google Scholar]
- 58.Kim HJ, Kim JS, Kang CD, Lee SJ, Kim JY, Yeon JE, et al. Expression of epidermal growth factor receptor, ErbB2 and matrix metalloproteinase-9 in hepatolithiasis and cholangiocarcinoma. The Korean Journal of Gastroenterology = Taehan Sohwagi Hakhoe Chi. 2005;45(1):52–59. [PubMed] [Google Scholar]
- 59.Nakazawa K, Dobashi Y, Suzuki S, Fujii H, Takeda Y, Ooi A. Amplification and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancers. The Journal of Pathology. 2005;206(3):356–365. doi: 10.1002/path.1779. [DOI] [PubMed] [Google Scholar]
- 60.Settakorn J, Kaewpila N, Burns GF, Leong AS. FAT, E-cadherin, beta catenin, HER 2/neu, Ki67 immuno-expression, and histological grade in intrahepatic cholangiocarcinoma. Journal of Clinical Pathology. 2005;58(12):1249–1254. doi: 10.1136/jcp.2005.026575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ogo Y, Nio Y, Yano S, Toga T, Koike M, Hashimoto K, et al. Immunohistochemical expression of HER-1 and HER-2 in extrahepatic biliary carcinoma. Anticancer Research. 2006;26(1B):763–770. [PubMed] [Google Scholar]
- 62.Joo HH, Song EY, Jin SH, Oh SH, Choi YK. Expressions and clinical significances of c-met, c-erbB-2, COX-2, and IL-6 in the biliary tract cancers. The Korean Journal of Gastroenterology = Taehan Sohwagi Hakhoe Chi. 2007;50(6):370–378. [PubMed] [Google Scholar]
- 63.Kawamoto T, Krishnamurthy S, Tarco E, Trivedi S, Wistuba II, Li D, et al. HER receptor family: novel candidate for targeted therapy for gallbladder and extrahepatic bile duct cancer. Gastrointestinal Cancer Research: GCR. 2007;1(6):221–227. [PMC free article] [PubMed] [Google Scholar]
- 64.Kim HJ, Yoo TW, Park DI, Park JH, Cho YK, Sohn CI, et al. Gene amplification and protein overexpression of HER-2/neu in human extrahepatic cholangiocarcinoma as detected by chromogenic in situ hybridization and immunohistochemistry: its prognostic implication in node-positive patients. Annals of Oncology: Official Journal of the European Society for Medical Oncology / ESMO. 2007;18(5):892–897. doi: 10.1093/annonc/mdm006. [DOI] [PubMed] [Google Scholar]
- 65.Puhalla H, Wrba F, Kandioler D, Lehnert M, Huynh A, Gruenberger T, et al. Expression of p21(Wafl/Cip1), p57(Kip2) and HER2/neu in patients with gallbladder cancer. Anticancer Research. 2007;27(3B):1679–1684. [PubMed] [Google Scholar]
- 66.Baumhoer D, Zlobec I, Tornillo L, Dietmaier W, Wuensch PH, Hartmann A, et al. Immunophenotyping and oncogene amplifications in tumors of the papilla of Vater. Virchows Archiv: An International Journal of Pathology. 2008;453(6):579–588. doi: 10.1007/s00428-008-0669-7. [DOI] [PubMed] [Google Scholar]
- 67.Kaufman M, Mehrotra B, Limaye S, White S, Fuchs A, Lebowicz Y, et al. EGFR expression in gallbladder carcinoma in North America. International Journal of Medical Sciences. 2008;5(5):285–291. doi: 10.7150/ijms.5.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miyahara N, Shoda J, Ishige K, Kawamoto T, Ueda T, Taki R, et al. MUC4 interacts with ErbB2 in human gallbladder carcinoma: potential pathobiological implications. European Journal of Cancer (Oxford, England: 1990) 2008;44(7):1048–1056. doi: 10.1016/j.ejca.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 69.Yoshikawa D, Ojima H, Iwasaki M, Hiraoka N, Kosuge T, Kasai S, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. British Journal of Cancer. 2008;98(2):418–425. doi: 10.1038/sj.bjc.6604129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aloysius MM, Lobo DN, Rowlands BJ, Madhusudan S, Ilyas M, Zaitoun AM. HER-2/neu overexpression is a rare event in peri-ampullary cancer: assessment using the HercepTest. Histopathology. 2009;55(2):236–237. doi: 10.1111/j.1365-2559.2009.03351.x. [DOI] [PubMed] [Google Scholar]
- 71.Choi HJ, Kim HJ, Choi JH. Expression of c-erbB-2 and cyclooxygenase-2 in intrahepatic cholangiocarcinoma. Hepato-Gastroenterology. 2009;56(91–92):606–609. [PubMed] [Google Scholar]
- 72.Harder J, Waiz O, Otto F, Geissler M, Olschewski M, Weinhold B, et al. EGFR and HER2 expression in advanced biliary tract cancer. World Journal of Gastroenterology. 2009;15(36):4511–4517. doi: 10.3748/wjg.15.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pignochino Y, Sarotto I, Peraldo-Neia C, Penachioni JY, Cavalloni G, Migliardi G, et al. Targeting EGFR/HER2 pathways enhances the antiproliferative effect of gemcitabine in biliary tract and gallbladder carcinomas. BMC Cancer. 2010;10:631. doi: 10.1186/1471-2407-10-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shafizadeh N, Grenert JP, Sahai V, Kakar S. Epidermal growth factor receptor and HER-2/neu status by immunohistochemistry and fluorescence in situ hybridization in adenocarcinomas of the biliary tree and gallbladder. Human Pathology. 2010;41(4):485–492. doi: 10.1016/j.humpath.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Kumari N, Kapoor VK, Krishnani N, Kumar K, Baitha DK. Role of C-erbB2 expression in gallbladder cancer. Indian Journal of Pathology & Microbiology. 2012;55(1):75–79. doi: 10.4103/0377-4929.94862. [DOI] [PubMed] [Google Scholar]
- 76.Lee HJ, Chung JY, Hewitt SM, Yu E, Hong SM. HER3 overexpression is a prognostic indicator of extrahepatic cholangiocarcinoma. Virchows Archiv: An International Journal of Pathology. 2012;461(5):521–530. doi: 10.1007/s00428-012-1321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Toledo C, Matus CE, Barraza X, Arroyo P, Ehrenfeld P, Figueroa CD, et al. Expression of HER2 and bradykinin B(1) receptors in precursor lesions of gallbladder carcinoma. World Journal of Gastroenterology. 2012;18(11):1208–1215. doi: 10.3748/wjg.v18.i11.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Graham RP, Barr Fritcher EG, Pestova E, Schulz J, Sitailo LA, Vasmatzis G, et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Human Pathology. 2014;45(8):1630–1638. doi: 10.1016/j.humpath.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 79.Roa I, de Toro G, Schalper K, de Aretxabala X, Churi C, Javle M. Overexpression of the HER2/neu gene: a new therapeutic possibility for patients with advanced gallbladder cancer. Gastrointestinal Cancer Research: GCR. 2014;7(2):42–48. [PMC free article] [PubMed] [Google Scholar]
- 80.Wang W, Zhang J, Zhan X, Lin T, Yang M, Hu J, et al. SOX4 is associated with poor prognosis in cholangiocarcinoma. Biochemical and Biophysical Research Communications. 2014;452(3):614–621. doi: 10.1016/j.bbrc.2014.08.124. [DOI] [PubMed] [Google Scholar]
- 81.Yang X, Wang W, Wang C, Wang L, Yang M, Qi M, et al. Characterization of EGFR family gene aberrations in cholangiocarcinoma. Oncology Reports. 2014;32(2):700–708. doi: 10.3892/or.2014.3261. [DOI] [PubMed] [Google Scholar]
- 82.Hechtman JF, Liu W, Sadowska J, Zhen L, Borsu L, Arcila ME, et al. Sequencing of 279 cancer genes in ampullary carcinoma reveals trends relating to histologic subtypes and frequent amplification and overexpression of ERBB2 (HER2) Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc. 2015;28(8):1123–1129. doi: 10.1038/modpathol.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kawamoto T, Ishige K, Thomas M, Yamashita-Kashima Y, Shu S, Ishikura N, et al. Overexpression and gene amplification of EGFR, HER2, and HER3 in biliary tract carcinomas, and the possibility for therapy with the HER2-targeting antibody pertuzumab. Journal of Gastroenterology. 2015;50(4):467–479. doi: 10.1007/s00535-014-0984-5. [DOI] [PubMed] [Google Scholar]
- 84.Oliveira Fernandes, V.T., De Barros E, Silva, M.J., Begnami, M.D., Saito, A. (2015). Prognosis of HER2 expression in cholangiocarcinoma when evaluated using gastric cancer methodology of immunohistochemistry. Journal of Clinical Oncology, 33, 2015 (suppl; abstr e15203).
- 85.Holcombe, R.F, Xiu, J., Pishvaian, M.J., Millis, S.Z., Gatalica, Z., Reddy, S.K., et al. (2015). Tumor profiling of biliary tract carcinomas to reveal distinct molecular alterations and potential therapeutic targets. Journal of Clinical Oncology,33, 2015 (suppl 3; abstr 285).
- 86.Wiggers JK, Ruys AT, Groot Koerkamp B, Beuers U, ten Kate FJ, van Gulik TM. Differences in immunohistochemical biomarkers between intra- and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. Journal of Gastroenterology and Hepatology. 2014;29(8):1582–1594. doi: 10.1111/jgh.12620. [DOI] [PubMed] [Google Scholar]
- 87.Yoshida H, Shimada K, Kosuge T, Hiraoka N. A significant subgroup of resectable gallbladder cancer patients has an HER2 positive status. Virchows Archiv: An International Journal of Pathology. 2016;468(4):431–439. doi: 10.1007/s00428-015-1898-1. [DOI] [PubMed] [Google Scholar]
- 88.Kiguchi K, Carbajal S, Chan K, Beltran L, Ruffino L, Shen J, et al. Constitutive expression of ErbB-2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Research. 2001;61(19):6971–6976. [PubMed] [Google Scholar]
- 89.Wadhwa R, Song S, Lee JS, Yao Y, Wei Q, Ajani JA. Gastric cancer-molecular and clinical dimensions. Nature Reviews Clinical Oncology. 2013;10(11):643–655. doi: 10.1038/nrclinonc.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology: Official Journal of the European Society for Medical Oncology/ESMO. 2015;26(Suppl 5):v8–30. doi: 10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 91.Cardoso F, Costa A, Norton L, Senkus E, Aapro M, Andre F, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2)dagger. Annals of Oncology: Official Journal of the European Society for Medical Oncology / ESMO. 2014;25(10):1871–1888. doi: 10.1093/annonc/mdu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Press MF, Hung G, Godolphin W, Slamon DJ. Sensitivity of HER-2/neu antibodies in archival tissue samples: potential source of error in immunohistochemical studies of oncogene expression. Cancer Research. 1994;54(10):2771–2777. [PubMed] [Google Scholar]
- 93.Ramanathan RK, Belani CP, Singh DA, Tanaka M, Lenz HJ, Yen Y, et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemotherapy and Pharmacology. 2009;64(4):777–783. doi: 10.1007/s00280-009-0927-7. [DOI] [PubMed] [Google Scholar]
- 94.Peck J, Wei L, Zalupski M, O'Neil B, Villalona Calero M, Bekaii-Saab T. HER2/neu may not be an interesting target in biliary cancers: results of an early phase II study with lapatinib. Oncology. 2012;82(3):175–179. doi: 10.1159/000336488. [DOI] [PubMed] [Google Scholar]
- 95.Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC—a randomized phase III trial. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2016;34(5):443–451. doi: 10.1200/JCO.2015.62.6598. [DOI] [PubMed] [Google Scholar]
- 96.Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN—a randomized, phase III study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2014;32(19):2039–2049. doi: 10.1200/JCO.2013.53.6136. [DOI] [PubMed] [Google Scholar]