Abstract
Introduction
Chemotherapeutic drugs are used in combination to target multiple mechanisms involved in cancer cell survival and proliferation. Carriers are developed to deliver drug combinations to common target tissues in optimal ratios and desirable sequences. Nanoparticles (NP) have been a popular choice for this purpose due to their ability to increase the circulation half-life and tumor accumulation of a drug.
Areas covered
We review organic NP carriers based on polymers, proteins, peptides, and lipids for simultaneous delivery of multiple anticancer drugs, drug/sensitizer combinations, drug/photodynamic- or photothermal therapy combinations, and drug/gene therapeutics with examples in the past three years. Sequential delivery of drug combinations, based on either sequential administration or built-in release control, is introduced with an emphasis on the mechanistic understanding of such control.
Expert opinion
Recent studies demonstrate how a drug carrier can contribute to co-localizing drug combinations in optimal ratios and dosing sequences to maximize the synergistic effects. We identify several areas for improvement in future research, including the choice of drug combinations, circulation stability of carriers, spatiotemporal control of drug release, and the evaluation and clinical translation of combination delivery.
Keywords: Multimodal chemotherapy, drug synergism, nanoparticles, ratiometric delivery, sequential delivery, simultaneous delivery, spatiotemporal control
1. Introduction
The goal of chemotherapy is to inhibit tumor growth and metastatic dissemination using chemical compounds that target critical steps in cell division and signaling pathways. Paclitaxel (PTX) inhibits the division of tumor cells by interfering with the depolymerization of microtubules. Doxorubicin (Dox) binds to DNA-associated enzymes, intercalates with DNA base pairs, and affects various molecular targets to generate a range of cytotoxic effects [1]. However, chemotherapy based on a single drug will seldom bring satisfactory therapeutic outcomes, because tumor cells may acquire resistance to the drug and/or survive by activating alternative pathways [2]. Therefore, chemotherapy is often performed using combinations of multiple drugs with different biochemical targets and/or mechanisms of action. Combination therapy can increase the therapeutic effects, reduce the dose requirement, and attenuate the development of drug resistance [3].
Although combination therapy can bring about several benefits over monotherapy, dose regimens based on free drug cocktails have several limitations [4]. First of all, co-administering drugs at their maximum tolerated doses, typically done in clinical protocols, can result in dose-limiting toxicities due to concentration-dependent drug interactions [5]. In addition, distinct physicochemical properties of participating drugs, such as water solubility and molecular weight, may lead to inconsistent pharmacokinetics and biodistribution profiles. This makes it difficult to achieve timely and ratiometric delivery of a drug combination to target tissues, which are critical conditions for drug synergism [6]. For these reasons, drug delivery systems have gained an increasing interest as a way of unifying the pharmacokinetic behaviors of multiple drugs and concentrating the drugs to target tissues in the optimal ratio and desirable sequence [7]. In particular, nanoparticles (NP) have been a cornerstone of such efforts due to their potential to increase the circulation half-life and tumor accumulation of a drug [8].
Several NP systems have been developed to overcome the difference in pharmacokinetic and biodistribution profiles of multiple drugs and achieve their co-delivery to target tissues. While the initial efforts have focused on loading multiple drugs with distinct physicochemical properties in a single vehicle [9], there is an increasing need for carriers that can deliver the drugs to different cell populations and/or in specific sequences according to the cellular targets and mechanisms of action. This review will introduce recent drug delivery approaches for combination chemotherapy with an emphasis on organic NP systems, based on polymers, proteins, peptides, and lipids, and will discuss how recent approaches have attempted to deliver multiple drugs in a ratio and sequence optimal for drug synergism. For readers interested in the classification of NP or combination delivery based on inorganic systems, we recommend recent review articles that discuss each topic in detail [10–15].
2. Clinically used combination chemotherapy
Combination chemotherapy is used in treating a broad range of cancers. Traditional combinations include methotrexate-based combinations (methotrexate plus cyclophosphamide/5-fluorouracyl (5-FU)), anthracycline-based combinations (Dox plus 5-FU) and taxane-based combinations (PTX plus cisplatin/carboplatin) [16]. Platinum compounds are also used in combination with other chemotherapeutic drugs in a clinical setting. The choices of drug combinations and applications are continuously expanding. Recently, Dox has been combined with ifosfamide and tested in a Phase II study for the treatment of soft tissue sarcomas [17]. Albumin-bound PTX particles (Abraxane®) and gemcitabine (Gem) combination were approved by the US FDA as the first-line treatment for patients with metastatic pancreatic cancer [18]. Table 1 lists drug combinations used in a clinical setting or in various stages of clinical trials. In most cases, these combinations are intravenously administered as cocktails or sequential injections.
Table 1.
Drug combinations used in clinical settings
| Drug 1 | Drug 2 (and 3) | Cancer type | Ref. | |
|---|---|---|---|---|
| Anthracycline-based combinations | Liposomal Dox | Cyclophosphamide | Metastatic breast cancer | [19] |
| Liposomal Dox | Cyclophosphamide; 5-Fluorouracil (5-FU) | Metastatic breast cancer | [20] | |
| Dox | Ifosfamide | High grade soft tissue sarcomas | [17] | |
| Taxane-based combinations | PTX | Cisplatin or carboplatin | Stage IIB-IV ovarian cancer | [21] |
| PTX | Gemcitabine (Gem) | Metastatic triple-negative breast
cancer Advanced pancreatic cancer |
[22] [23, 24] |
|
| Docetaxel (DTX) | Capecitabine | Metastatic breast cancer | [25] | |
| DTX | Carboplatin | Advanced non-small cell lung cancer | [26] | |
| DTX | Cyclophosphamide | Early breast cancer | [27] | |
| DTX | Cisplatin, 5-FU | Advanced or locally recurrent gastric cancer | [28] | |
| Methotrexate (MTX)-based combinations | MTX | Mitomycin | Metastatic HER2-negative breast cancer | [29] |
| MTX | Cyclophosphamide, 5-FU | Node-positive breast cancer | [30] | |
| MTX | Vinblastine, Dox, Cisplatin | Muscle-invasive bladder cancer | [31] | |
| Platinum-based combinations | Carboplatin or Cisplatin | 5-FU | Metastatic and recurrent head and neck squamous cell carcinoma and nasopharyngeal carcinoma | [32] |
| Carboplatin or cisplatin | Etoposide | Small cell esophageal cancer Extensive stage small cell lung cancer |
[33] [34] |
|
| Cisplatin | Topotecan | Cervical cancer | [35] | |
| Cisplatin | Vinorelbine | Stage III non-small cell lung cancer | [36, 37] | |
| Cisplatin | Pemetrexed | Advanced non-small cell lung
cancer Advanced urothelial cancer |
[38, 39] [40] |
|
| Cisplatin | Gem | Metastatic triple-negative breast cancer | [22, 41] | |
| Oxaliplatin | Capecitabine | Metastatic colorectal cancer | [42] |
3. Simultaneous delivery
Multiple anticancer agents with different mechanisms of action are used together to address different molecular targets in the same tumor cells. Anticancer drugs are also combined with drug sensitizers, which do not have cytotoxic effects but can inhibit drug resistance mechanisms to make cells respond to anticancer drugs. The same applies to the combinations of anticancer drugs and agents to induce photothermal/photodynamic effects or gene therapeutics that target alternative survival pathways. In these cases, it is desirable to deliver the active agents to the same cells and have them to act simultaneously. This section introduces recent approaches to achieve simultaneous delivery of combination therapy to target tissues.
3.1 Combination of multiple anticancer drugs
Table 2 lists NP carriers used for simultaneous delivery of drug combinations. Liposomes, polymeric micelles, and polymeric NP are popular choices of carriers. Mitomycin C (MMC)-MTX combinations have been found to be effective as a follow-up treatment for patients with metastatic breast cancer who had been pretreated with anthracyclines and taxanes [58]. Chitosan NP modified with polyethylene glycol (PEG) was used for co-delivery of the MMC-MTX combination [43]. Here, chitosan NP was prepared by ionic gelation and chemical crosslinking, then PEGylated on the surface. MMC and MTX were covalently conjugated to PEGylated chitosan NP via amide bond, which would be hydrolyzed by the endo/lysosomal proteases. The (MTX+MMC)-PEG-chitosan-NP showed a greater tumor inhibition effect than a cocktail of MTX and MMC in mice bearing H22 tumors (inhibition rate: 59.2% vs. 8.2%). This result demonstrates the benefit of co-localizing two drugs with a single carrier. MTX played an additional role as a ligand to promote NP interaction with the folate receptor-expressing tumor cells [43].
Table 2.
NP for simultaneous delivery of multiple anti-cancer drugs
| Drug combinations | NP | Tumor model (in mice) | Dosing regimen* | Therapeutic outcomes | Ref. |
|---|---|---|---|---|---|
| MTX + MMC | PEGylated chitosan NP | Subcutaneous (s.c.) hepatoma-22 (H22) model | 4 mg/kg MMC eq. twice at 1 day interval | NP showed better tumor inhibition rate (tumor weight difference from control group) than MTX/MMC mixture (59.2% vs. 8.2%). | [43] |
| Dox + PTX | O-carboxymethyl-chitosan NP camouflaged with erythrocyte membrane | S.c. xenograft of Lewis lung carcinoma | 0.3 mg/kg Dox + 1 mg/kg PTX every day for 2 weeks | RGD-targeted NP showed the best tumor inhibition effect compared to the mixture of Dox and PTX and non-targeted NP. | [44] |
| PEG-polypeptide micelles | S.c. xenograft of A549 non-small cell lung cancer | 4 mg/kg Dox + 1 mg/kg PTX, three times at 4-day interval | Tumor volume of co-NP-treated group was 3.2-, 6.3-, and 2.4-fold smaller than those with free Dox, free PTX, and free DOX+PTX. | [45] | |
| Crosslinked multilamellar liposomes (cMLV) | S.c. xenograft model of 4T1 breast cancer | 3.33 mg/kg Dox + 0.67 mg/kg PTX (5:1), 2 mg/kg Dox + 2 mg/kg PTX (3:3), or 0.67 mg/kg Dox + 3.33 mg/kg PTX (1:5), every three days | cMLV with a Dox:PTX ratio of 5:1 or 3:3 showed greater attenuation of tumor growth than those with 1:5 ratio, while free drug cocktails showed minimal effects irrespective of the ratio. | [46] | |
| mPEG-b-PLGA polymeric micelles | Orthotopic xenograft of MCF-7 breast cancer | 5 mg/kg Dox + 16 mg/kg PTX, three times at 4-day interval | Co-NP showed the best antitumor effects compared to free DOX and PTX-only NP (Tumor suppression rate, TSR: 95.5%, 59.7%, and 67.7%, respectively). | [47] | |
| PTX + Cisplatin | PEG-PLGA NP | S.c. xenograft model of M109 non-small-cell lung cancer | 1.2 mg/kg PTX + 0.5 mg/kg cisplatin, three times at 4-day interval | Tumor volume of co-NP-treated group was 4.38 times smaller than free PTX+cisplatin-treated group. | [48, 49] |
| Polypeptide-based nanogels | Intraperitoneal xenograft model of A2780/luc ovarian cancer | 1 mg/kg PTX and 4 mg/kg cisplatin, four times at 4-day interval | Co-NP showed better antitumor effects than
free cisplatin or a mixture of single drug NP. Folate receptor-targeted Co-NP further increased the median survival time of tumor-bearing mice compared to nontargeted counterpart (41 vs. 31 days). |
[50] | |
| Telodendrimer nanocarriers (TM) | S.c. xenograft model of SKOV-3 ovarian cancer | 3 mg/kg PTX + 6 mg/kg cisplatin, three times at 4-day interval | TM(cisplatin/PTX) showed the best tumor growth inhibition with the median relative tumor volume (RTV) of 2.5 on day 28, compared to TM(cisplatin) at 4 mg/kg and 6 mg/kg cisplatin with RTV of 11.4 and 5.2, respectively. | [51] | |
| PEG-polypeptide micelles | S.c. xenograft model of A549 lung cancer | 3 mg/kg PTX + 10 mg/kg cisplatin, twice at 7-day interval | Co-NP showed better tumor growth inhibition than free PTX+cisplatin (TSR: 83.1 vs. 73.8%). Co-NP-treated group showed continued tumor regression and no obvious recrudescence in 10 days after the last drug administration. | [52] | |
| Polymeric micelles | Xenograft model of U14 cervical cancer | 40 mg/kg PTX + 6.5 mg/kg cisplatin, three times at 4-day interval | Co-NP-treated group showed better tumor growth inhibition than free PTX+cisplatin. | [53] | |
| PTX + Gem | Lipid-coated mesoporous silica NP | Orthotopic xenograft model of PANC-1 pancreatic cancer | 10 mg/kg PTX + 100 mg/kg Gem, four times over 3 weeks | Co-NP showed better antitumor effect than free Gem, free Gem+Abraxane, and Gem-only NP, with no evidence of metastases. | [54] |
| Polyelectrolyte complexes of drug-polymer conjugates | S.c. xenograft model of HuCCT1 biliary cancer | 54 μg PTX + 108.8 μg Gem per mouse, six times over 3 weeks | Co-NP showed the best antitumor effects compared to Gem-only NP and PTX+Gem GEM (Tumor volume: 9.8 mm3, 57.4 mm3 and 39.4 mm3, respectively). | [55] | |
| Gem + Cisplatin | PLGA NP | S.c. xenograft model of UMUC3 bladder cancer | 12 mg/kg Gem + 1.9 mg/kg PTX, three times at 3-day interval | Co-NP showed the best therapeutic efficacy compared to free drugs and the mixture of the two. | [7] |
| Dox + Irinotecan | HA/chitosan –PLGA/Pluronic F127 NP | Orthotopicxenograft model of human MDA-MB-231 breast cancer | 1.5 mg/kg Dox + 1.5 mg/kg Irinotecan, three times at 7-day interval | Co-NP showed better antitumor effect than a free drug mixture or Dox-only NP. | [56] |
| Dox + Dasatinib | PEGylated peptidic nanocarrier | S.c. xenograft model of 4T1.2 breast cancer | 5 mg/kg Dox + 5 mg/kg Dasatinib, three times at 3-day interval | Co-NP showed better antitumor effects with a tumor growth (95% less than control) than Dox-only NP (77.1%), Dasatinib-only NP (32.8%), and a mixture of free drugs (70.5%). | [57] |
IV injection.
Platinum drugs are frequently used in combination with PTX. Recently, a three-layered linear-dendritic telodendrimer micelle (TM) has been developed for the co-delivery of cisplatin and PTX [51]. The micelle was made of a linear-dendritic copolymer consisting of PEG, branches of 8 carboxyl groups, and 8 cholic acids conjugated to the distal peripheral. These components formed a hydrophilic surface layer, a middle layer for cisplatin complexation, and a hydrophobic core for PTX loading, respectively. Due to the high potency of PTX, TM(cisplatin/PTX) showed the best cytotoxic effect against ovarian cancer cells compared to free cisplatin and TM(cisplatin). Consistently, TM(cisplatin/PTX) (6 mg/kg cisplatin + 3 mg/kg PTX) exhibited greater anticancer efficacy than TM(cisplatin) (6 mg/kg cisplatin) (median relative tumor volume (RTV) of 2.5 vs 5.2). Comparison with a free drug cocktail was not reported [51].
Dox and PTX are also frequently used in combination due to their distinct mechanisms of anti-proliferative action. The two drugs have vastly different pharmacokinetics profiles (PTX [59]: final elimination half-life (t1/2 γ) of 7.85 – 33.5 h, area under the curve (AUC) of 27.07 ± 8.61 μmol·h/L, and clearance (CL) of 10.25–27.03 L/h; Dox [60]: t1/2 γ of 25.8 ± 11.4 h, AUC of 2.98 ± 1.95 μmol·h/L, and CL of 60.40 ± 23.40 L/h). Therefore, many carriers have been explored for their co-delivery to target tumors. For example, polymeric micelles based on an amphiphilic triblock copolymer, methoxy poly(ethylene glycol)-b-poly(L-glutamic acid)-b-poly(L-lysine), decorated with deoxycholate (mPEG-b-PLG-b-PLL/DOCA) were used as a carrier of Dox and PTX [45]. The polymer spontaneously self-assembled into micelles, which contained three different domains: a hydrophobic PLL/DOCA core serving as a depot for PTX, a middle hydrophilic PLG shell as a container of DOX, and an outer shell of PEG protecting the NP in circulation [45]. The PTX and Dox co-delivered by NP (Co-NP) had a combination index (CI50) value lower than 1 (0.57) in an in-vitro cytotoxicity test, indicating that the two drugs show a synergistic effect. Interestingly, a free PTX+Dox mixture was antagonistic (CI50>1) at the same ratio, likely due to the differential uptake of the two drugs. This confirms the significance of a carrier in synchronizing cellular uptake of multiple drugs. The Co-NP also showed greater antitumor activity than free Dox, free PTX, free drug cocktails, or single drug NP in animals with A549 xenografts, with no obvious side effects during the treatment process [45].
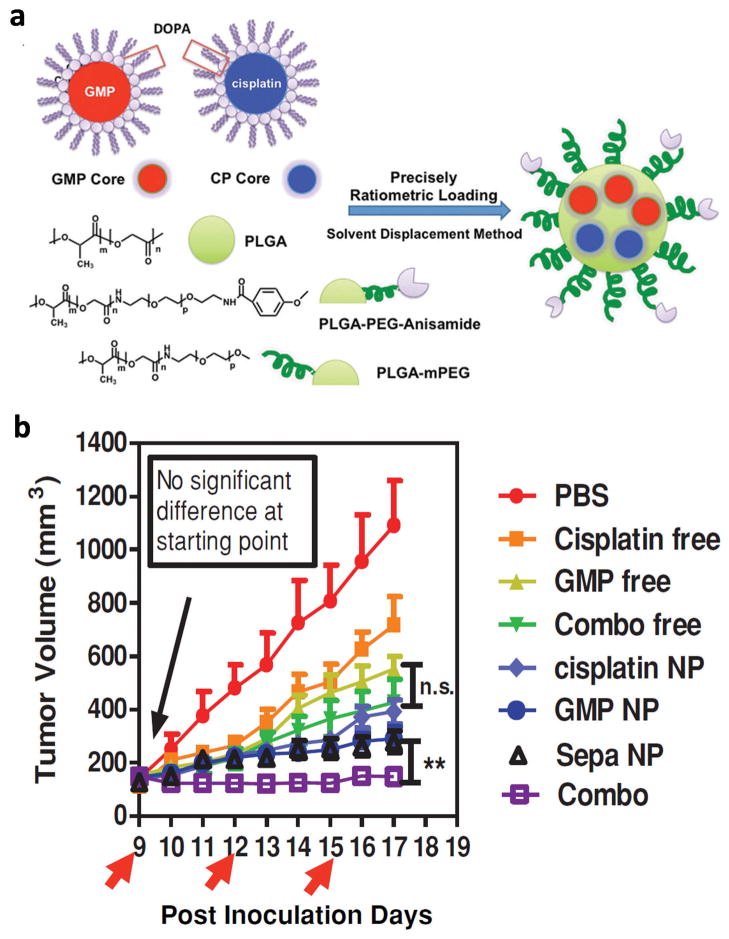
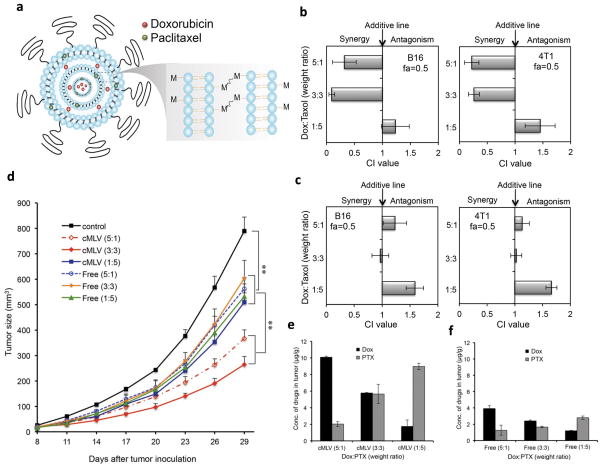
As implicated in the previous example, the ratio of combined agents dictates whether they will provide synergistic, additive, or antagonistic effects for certain drug combinations [6]. If the synergistic effect is achieved at a specific ratio, it is critical to ensure ratiometric delivery of the drugs to target tissues. NP are employed for this purpose. Miao et al. used poly(lactic-co-glycolic acid) (PLGA) NP for co-loading and co-delivery of gemcitabine monophosphate (GMP) and cisplatin which established the optimal synergistic effect at a molar ratio of 5:1 [7]. GMP and cisplatin were separately formulated as dioleoyl phosphatidic acid (DOPA)-coated core particles and co-loaded in the same PLGA NP at the optimal ratio (Fig. 1a). The Combo NP showed a significant cytotoxic effect on the UMUC3 Cells with an overall CI of <1. The in-vivo result with stroma-rich bladder xenograft tumor model was consistent with the cytotoxicity result: combo NP exhibited better anticancer effect compared to cocktails of free drugs or single drug NP (Fig. 1b) [7]. In another study, crosslinked multilamellar liposomes (cMLV) were used as a carrier for ratiometric delivery of Dox and PTX. Here, Dox and PTX were loaded in the aqueous space and in the lipid membranes of a liposomal vesicle, respectively (Fig. 2a) [46]. cMLV with Dox and PTX at a weight ratio of 5:1 or 3:3 exhibited synergistic effects in B16 and 4T1 tumor cells whereas those with the 1:5 ratio had an antagonistic effect (Fig. 2b). Free drug mixtures showed antagonistic or additive effects at all ratios (Fig. 2c). The in-vivo result showed a consistent trend: cMLV with a Dox:PTX ratio of 5:1 or 3:3 showed greater attenuation of tumor growth than those with the 1:5 ratio whereas free drug cocktails showed minimal effects irrespective of the ratio (Fig. 2d). cMLV maintained the original dose ratio at the tumor sites over 24h unlike the drug cocktail (Fig. 2e and 2f). This demonstrates how cMLV contributed to codelivering the two drugs in the optimal ratio.
Fig. 1.
(a) Schematic diagram of PLGA NP for ratiometric co-delivery of GMP and cisplatin. (b) Tumor inhibition effects of free drugs, Combo free (cocktails of free drugs), cisplatin NP, GMP NP, Sepa NP (cocktails of single drug NP), and Combo NP in a stroma-rich UMUC3 bladder cancer xenograft model. Arrows indicate time of injection. The tumors were treated with three IV injections at a dose of 1.9 mg/kg cisplatin and 12 mg/kg GMP in all the treatment groups. Reprinted with permission from [7]. Copyright (2014) John Wiley and Sons.
Fig. 2.
(a) Schematic diagram of cMLV for ratiometric co-delivery of PTX (Green) and Dox (Red). (b) Combination index (CI) histogram for cMLVs (different drug combinations) on cultured B16 and 4T1tumor cells. The histogram presents the CI values obtained at a fraction of 0.5 (fa=0.5). (c) CI histogram for free drug mixtures on culture B16 and 4T1 tumor cells. (d) Drug ratio-dependent tumor inhibition effects of cMLVs or free drug cocktails. (e–f) Concentrations of Dox and PTX in tumors 24 hours after intravenous injection of (e) cMLVs or (f) free drug mixtures, equivalent to 8.333 mg/kg Dox + 1.667 mg/kg PTX (5:1), 5 mg/kg Dox + 5 mg/kg PTX (1:1), or 1.667 mg/kg Dox + 8.33 mg/kg PTX (1:5). Reprinted with permission from [46].
These examples illustrate how NP can help co-deliver two or more anticancer drugs to a common cellular target in a synergistic ratio. In designing NP for ratiometric delivery of drug combinations, it is worthwhile to consider that the effectiveness of NP is predicated on the precise control of drug retention and release in vivo. If one drug is more rapidly released than the other in circulation, it is possible that they will reach the target in a suboptimal or even antagonistic ratio [61].
3.2 Combination of anticancer drugs and drug sensitizers
Over the course of chemotherapy, tumor cells may develop drug resistance by actively exporting a drug via efflux transporters or altering signaling/apoptosis pathways to promote the survival of tumor cells [4]. To maximize the therapeutic efficacy, anticancer drugs are used together with drug sensitizers that disable the molecular mechanism of drug resistance. Most drug sensitizers do not have their own anticancer activities, but they rather assist anticancer drugs to act more effectively; thus, drugs and sensitizers are typically co-delivered in anticipation that they will function simultaneously.
The drug resistance of cancer cells is often attributable to the overexpression of efflux transporters like P-glycoprotein (P-gp) [62] or breast cancer resistance protein (BCRP) [63]. Therefore, P-gp inhibitors such as verapamil and tariquidar have been co-delivered with anticancer drugs to restore the sensitivity of tumors to the drugs. PTX and tariquidar were co-delivered to the tumor site via PLGA NP [64]. Dual-drug loaded NP showed greater anti-tumor efficacy than PTX-loaded NP on drug-resistant tumors in Balb/C nude mice due to the drug-sensitizer effect of tariquidar. The dual-drug loaded NP with additional biotin modification showed even greater effect than its non-biotinylated counterpart due to the enhanced cellular uptake of the drugs [64]. Lonidamine (LND) is also used in combination with anticancer drugs as a sensitizer. LND interferes with ATP production by inhibiting mitochondrial hexokinase, thereby depleting energy source of P-gp [65]. PTX/LND combination was co-encapsulated in liposomes which were modified with hyaluronic acid (HA) and D-α-tocopheryl PEG succinate (TPGS). The dual-drug liposomes showed greater toxicity on drug-resistant MCF-7/ADR tumors than a mixture of single-drug liposomes in vitro and in vivo [65]. Here, TPGS also contributes to sensitizing the tumors to PTX by inhibiting P-gp. For this reason, TPGS is widely explored as a component of a drug carrier in the treatment of drug-resistant tumors. Polysorbates and poloxamers play a similar role [66].
Curcumin is a natural compound isolated from Curcuma longa. It downregulates nuclear factor-κB (NF-κB), a transcription factor that is overexpressed in many tumor cells and plays a key role in tumorigenesis, angiogenesis, and apoptosis. Curcumin has chemopreventive and therapeutic effects. In addition, it sensitizes cancer cells to chemotherapeutic agents [67]. PLGA NP stabilized with a monolayer of phospholipids were used to co-deliver DTX and curcumin to treat glioblastoma [68]. Animals treated with the dual-drug NP showed complete tumor regression in 32 days from the initiation of treatment with 100% survival up to 90 days. In contrast, tumors treated with DTX NP alone took one more week to show complete regression only to regrow a few weeks later [68]. Curcumin has also been combined with sorafenib in the treatment of hepatocellular carcinoma [67]. The two drugs were co-encapsulated in a micelle system made of TPGS and were delivered via oral administration. The dual drug-loaded micelles had stronger anti-proliferative activity with lower IC50 than free sorafenib or a mixture of sorafenib and curcumin. The dual drug micelles also inhibited proliferation and angiogenesis of tumors more effectively than free sorafenib alone in a mouse model of BEL-7402 xenograft [67]. Other natural compounds such as quercetin and chloroquine are also known to serve as a drug sensitizer in cancer cells [69] and have been co-delivered with anticancer drugs via polymeric NP [70] and liposomes [71], respectively.
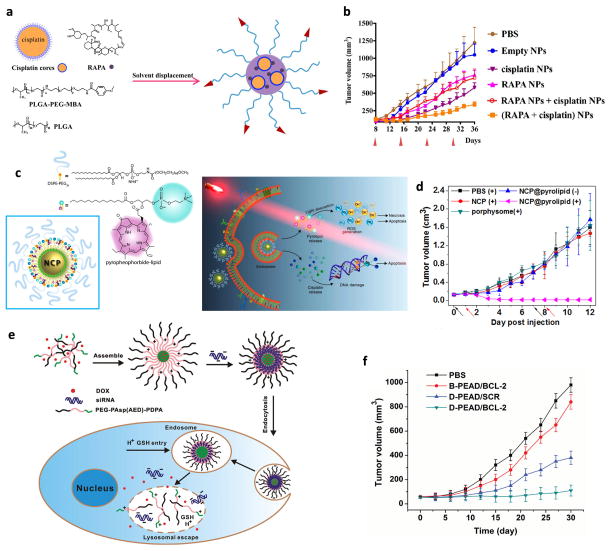
Chemotherapeutic agents have been co-delivered with sensitizers that modulate cells in the tumor microenvironment (TME) such as cancer stem cells (CSC) or stromal cells. All-trans-retinoic acid (ATRA) can differentiate CSC to non-CSC which has reduced self-renewal capacity and increased sensitivity to chemotherapy [72]. ATRA was co-delivered with Dox using PEGylated polylactic acid (PLA) NP [73]. The dual-drug loaded NP reduced the expression of stem cell-related genes and the frequency of CSC in a mammosphere model. It inhibited the tumor growth in a MDA-MB-231 orthotropic xenograft model to a greater extent than Dox-loaded NP, confirming the contribution of ATRA in sensitizing CSC to Dox [73]. It is worth noting that the dual-drug loaded NP was more effective than a mixture of single drug NP, which indicates the benefit of dual-drug loaded NP in co-localizing two drugs in target tissues. In another study, rapamycin (RAPA) was used in combination with cisplatin to modulate the TME and sensitize the tumors to chemotherapy [9]. RAPA has several biological activities including angiogenesis suppression, vascular normalization, and the downregulation of antiapoptotic proteins. The RAPA and cisplatin combination delivered by PLGA NP (Fig. 3a) eliminated tumor-associated fibroblasts (TAF) and collagen, a cellular product of TAF and a major component of extracellular matrix, thereby reducing the interstitial fluid pressure of tumors and enhancing the perfusion of NP into tumors. Consequently, the combination NP showed significantly enhanced tumor inhibitory effect as compared to RAPA-containing NP, cisplatin NP, or a mixture of the two (Fig. 3b) [9]. An initial challenge faced in this study was the inefficient encapsulation of the two drugs due to physicochemical differences (hydrophilic cisplatin and hydrophobic RAPA). This problem was addressed by converting the water soluble cisplatin into small particles stabilized with a lipid layer [74] and by co-loading them into the PLGA NP matrix together with RAPA. Interestingly, the cisplatin particles also improved the loading of RAPA in PLGA NP due to the increased hydrophobic interactions [9].
Fig 3.
(a) Schematic diagram of the PLGA NP co-encapsulating cisplatin and RAPA. (b) Anti-tumor efficacy in A375-luc tumor bearing mice; RAPA NP + cisplatin NP: mixture of RAPA-containing NP and cisplatin NP; (RAPA + cisplatin) NP: combination NP. (a, b) Reprinted with permission from [9]. (c) Schematic diagram of the NCP@pyrolipid (pyrolipid/cisplatin prodrug assemblies) and the proposed cytotoxicity mechanism. (d) Antitumor activity in a human head and neck cancer SQ20B subcutaneous xenograft murine model; Cisplatin dose of 0.5 mg/kg or pyrolipid dose of 0.5 mg/kg followed by irradiation (670 nm, 100 mW/cm2) for 30 min 24 h post-injection. PBS (+): PBS with irradiation; NCP (+): single drug NP with irradiation; porphysome (+): mono-PDT with irradiation; NCP@pyrolipid (−): dual-loaded NP without irradiation; and NCP@pyrolipid (+): dual-loaded NP with irradiation. (c, d) Reprinted with permission from [76]. Copyright (2015) American Chemical Society. (e) Schematic diagram of a stimuli-responsive polymeric micelle system carrying Dox and Bcl-2 siRNA (siBcl-2). (f) Inhibition of the growth of SKOV-3 tumors in nude mice after tail-vein injection of different formulations. B-PEAD/BCL-2: micelles loaded with siBcl-2; D-PEAD/SCR: micelles loaded with Dox; D-PEAD/BCL-2: Dox/siBcl-2 dual-loaded micelles. (e, f) Reprinted with permission from [111]. Copyright (2014) John Wiley and Sons.
3.3 Combination of anticancer drugs and photodynamic therapy (PDT)
Photosensitizers have photodynamic effects. Upon light activation, they transfer photo energy to molecular oxygen in the surrounding environment to generate reactive oxygen species (ROS) which induce apoptosis of cancer cells and destroys tumor tissues [75]. Photodynamic therapy (PDT) is used in combination with chemotherapy to explore the synergism between ROS and chemotherapeutic agents. Photosensitizers are active only at the location irradiated with the proper wavelength; therefore, the therapy is highly specific as long as the light exposure can be controlled. Several photosensitizers such as porphyrins, chlorins and phthalocyanines have been combined with chemotherapeutic agents. Porphyrin and cisplatin were co-delivered in a self-assembled NP with a phospholipid-porphyrin (pyrolipid) in the shell and a mixture of Zn(NO3)2 and a cisplatin prodrug in the core, respectively (Fig. 3c) [76]. Because the pyrolipid molecules close to each other self-quench and generate little ROS, the NP had no photodynamic effect as long as pyrolipid layer is intact [77]. Once taken up by the cells, the NP let the pyrolipid shell gradually disintegrate and distribute in the cytoplasm and cell membrane, thus making them amenable to PDT. The dual-loaded NP combined with irradiation showed greater cytotoxicity and antitumor effect than single drug NP or mono-PDT (Fig. 3d). The antitumor effect of the dual-NP is attributed to multiple causes: stability in the extracellular environment, enhanced cancer cell uptake, negligible efflux of drug and photosensitizer, favorable biodistribution due to the enhanced permeability and retention (EPR) effect, and the synergistic induction of cell death [76].
Despite the promising preclinical outcomes, the clinical utility of combined chemotherapy and PDT is challenged by the limited penetration of the light source. Light needed for triggering most photosensitizers is in the UV-visible range; hence, it fails to reach tumors buried deep in the body. A new NP system called “upconversion nanoparticles” (UCNP) has been introduced to address this challenge. The NP convert a low-energy, deep penetrating NIR-light into higher-energy UV–visible light [78]. Yang et al designed NP based on a Mn2+-doped NaYF4:Yb/Er UCNP core coated by silica shell, which were modified with a long alkyl chain C18-chlorin e6 (Ce6) conjugate (photosensitizer) and an amphiphilic copolymer responsive to ROS [79]. The upconversion occurs when Yb and Er in the core NP are excited by 980 nm NIR light to a high energy state. The excited Yb (reservoir ion) non-radiatively transmits excess energy to Er (emitter ion) thereby exciting the Er to a higher excited state. Thus, when the Er returns to ground state, it emits a light with greater energy and a shorter emission wavelength within the visible green-blue region [80]. The doped Mn2+ switches the emission light to the red region that excites Ce6. Here, the NP was used as a carrier of Dox and provided dual effects: the core NP absorbed NIR light and converted it to visible red light, excited Ce6, and generated cytotoxic ROS. The ROS also triggered the polymer degradation and caused subsequent Dox release. Reflecting the combined effects of ROS and Dox, the NP combined with 980 nm irradiation induced shrinkage of KB tumors in athymic nude mice to a greater extent than drug-free NP (i.e., PDT only).
3.4 Combination of anticancer drugs and photothermal therapy (PTT)
Chemotherapy is also combined with a photothermal agent, a compound that generates cell-damaging hyperthermia in response to light stimulation [16], such as indocyanine dye [81], gold nanorods [82], and graphene [83]. For example, PTX was combined with indocyanine green (ICG) with human serum albumin (HSA) as a common carrier [84]. Both PTX and ICG have affinity for HSA; thus, three components mixed in the optimal ratio form stable NP in aqueous solution. The PTX/ICG co-loaded HSA NP accumulated at the tumor site via the EPR effect and induced rapid and mild heating (42°C) under 30 min NIR laser irradiation (808 nm) at 0.3 W/cm2. Upon laser irradiation, the NP induced apoptosis of cancer cells and showed complete elimination of subcutaneous 4T1 tumors without a single case of regrowth in 7 weeks. On the other hand, single component loaded NP and co-loaded NP without laser irradiation showed limited survival and gradual tumor growth [84]. Another study reports the delivery of an indocyanine dye IR825 and Dox combination [81]. Here, aggregates of IR825 were first formed in water, complexed with polyethyleneimine (PEI), and then coated with polyacrylic acid and PEG-amine for surface stabilization [81]. The IR825/PEI particles called J-aggregates efficiently absorbed red-shifted NIR light to show a photothermal effect. J-aggregates also served as a matrix to load Dox based on hydrophobic interactions. The combination NP with IR825 and Dox generated heat with a 915 nm NIR laser (0.8 W/cm2) and maintained high photostability after several cycles of laser-induced photothermal heating. In 4T1-tumor bearing mice, the NP increased the temperature at the site of the tumor to 45 °C with laser exposure and inhibited the tumor growth over 14 days after a single treatment, far surpassing drug-free NP + PTT or the NP with no laser irradiation [81].
3.5 Combination of anticancer drugs and gene therapeutics
Given multiple genetic abnormalities implicated in cancer, the effect of traditional chemotherapy is likely short-lived. Therefore, chemotherapy is combined with gene therapeutics that target various aspects of tumor progression [11]. Therapeutic genes are available as plasmid DNA (pDNA) [11], small interfering RNA (siRNA) [85], short hairpin RNA (shRNA), and micro RNA (miRNA) [86]. They are used to down-regulate genes, substitute or silence the mutated genes, or to induce protein expression. Combinations of chemotherapy and gene therapeutics have the potential to target multiple pathways involved in cancer progression simultaneously and eradicate tumors with high efficiency. However, the main challenge is that anticancer drugs and gene therapeutics exhibit vast differences in physiochemical properties, with the former typically being small molecules and the latter negatively charged macromolecules. A common carrier that can afford co-encapsulation of both agents is critical to the success of the drug/gene combination therapy. Accordingly, numerous efforts have been made to develop carriers of drug/gene combinations. These delivery systems include cationic liposomes, polysaccharides, and polymeric NP. This section will discuss recent examples of drug delivery systems used in delivering both chemo- and gene therapeutics, according to the roles of genes used in the combination therapy. Examples that are not discussed in the text are summarized in Table 3.
Table 3.
NP for co-delivery of genes and anti-cancer drugs
| Anticancer drug | Gene | Function of gene | NP | Tumor model in mice | Dosing regimen* | Therapeutic outcomes | Ref |
|---|---|---|---|---|---|---|---|
| PTX | Snail siRNA, Twist siRNA | Inhibit metastasis | Polymeric micelles | Pulmonary metastatic model of 4T1 breast cancer | 4 mg/kg PTX + 1 mg/kg siRNA, twice a week for two weeks | Co-NP had higher tumor inhibition ratio compared to gene-only NP and PTX-only NP. | [87] |
| PTX | AURKA siRNA | Sensitizes cancer cells to taxane | HA-based redox-sensitive polymeric micelles | S.c. xenograft model of MDA-MB-231 breast cancer | 5 mg/kg PTX + 1 mg/kg siRNA, five times every three days | Co-NP showed the strongest tumor growth inhibition compared to Taxol, PTX or gene-only NP and the mixture of gene and PTX. | [88] |
| PTX | MiR-34a | Induces cell apoptosis | Solid lipid NP | Lung metastasis model of B16F10 melanoma | 1 mg/kg PTX + 0.5 mg/kg miR-34a, 15 times once a day | Co-NP showed stronger tumor inhibition effect than miR-34a or PTX-only NP. | [89] |
| PTX | VEGF siRNA | Anti-angiogenesis | Protamine core and lipid shell NP | S.c. xenograft model of MCF-7 breast cancer | 15 mg/kg PTX + 1 mg/kg siRNA, 4 times every 2 days | Vapreotide modified co-NP showed the lowest tumor weight compared to siRNA-only NP, a mixture of Taxol and siRNA, and non-targeted co-NP. | [90] |
| Dox | VEGF siRNA | Anti-angiogenesis | PEI-hydrazone-Dox + PEI− PEG− folate siRNA nanocomplex | S.c. xenograft model of MCF-7 breast cancer | 0.75 mg/kg Dox + 1.33 mg/kg siRNA, on days 0, 2, 4, 7, 10, and 13. | Folate modified co-NP showed slower tumor growth than free Dox and non-folate modified co-NP. | [91] |
| Dox | shRNA-expressing pDNA | Promotes drug induced cell apoptosis | Poly (β-amino ester) NP | S.c. xenograft model of MCF-7/ADR breast cancer | 6 mg/kg Dox + 2 mg/kg RNA, three times at one week interval | Co-NP showed stronger tumor growth inhibition than free DOX and DOX or shRNA-only NP. | [92] |
| Dox | miR-21i | Suppresses Bcl-2 and blocks PI3K-AKT pathway | Polymeric micelles | S.c. xenograft model of LN229-luc glioblastoma | 5 mg/kg Dox, every two days for three weeks, intratumoral injection | Co-NP attenuated tumor growth better than Dox, miR-21i and Dox or miR-21i-only NP. | [93] |
| Dox | P53 plasmid | Induces apoptosis and inhibit proliferation | Crosslinked cationic polypeptide | S.c. xenograft model of H22 hepatocellular carcinoma | 9.5 μg Dox + 15 μg p53 per mouse, once every other day for 14 days, s.c. peritumoral injection | Co-NP showed greater tumor inhibition than free Dox, Dox-only NP and p53-only NP. | [94] |
| Dox | Beclin-1 si RNA | Overcomes antiapoptotic defense of cancer cells | Crosslinked polymeric NP | S.c. xenograft model of HeLa cervical cancer | Dox 500 μg/kg + Beclin1 si RNA 25 nmol/kg, on days 1, 4, 7, 10, 14 and 17, s.c. peritumoral injection | Co-NP showed smaller tumor volume than free Dox, siRNA and Dox-only NP. | [95] |
| Dox | Bcl-2 siRNA | Promotes apoptosis and inhibits drug resistance | bPEG- conjugated dendrimer NP | S.c. xenograft model of SMMC-7721 hepatocarcinoma | 6 μg Bcl-2 siRNA + 6 μg DOX per mouse, six times every three days, intraperitoneal injection | Co-NP showed stronger tumor growth inhibition than free Dox, siRNA, Dox-only NP, or siRNA-only NP | [96] |
| Cisplatin | siNotch1 | Suppresses CSC | Polymeric micelles | S.c. xenograft model of SMMC7721 hepatocellular carcinoma | 1 mg/kg siNotch1+ 1.5 mg/kg platinum, ten times every two days | Co-NP showed strongest tumor growth inhibition compared to free cisplatin, siNotch1, platinum-only NP or siNotch1-only NP. | [97] |
| Sorafenib | Survivin shRNA | Promotes drug induced cell apoptosis | Pluronic P85/PEI/PEG 1000 succinate complex NP | S.c. xenograft model of BEL-7402 or BEL-7402/5Fu hepatocellular carcinoma | 10 mg/kg sorafenib + 2 mg/kg shSur, four times every 4 days | Co-NP showed stronger anti-tumor efficacy than sorafenib, shRNA-only NP, or a mixture of the two. | [98] |
| Gem | miRNA-205 | Reverses chemo-resistance | Polymeric micelles | S.c. xenograft model of gemcitabine resistant MIA PaCa-2R pancreatic cancer | 40 mg/kg Gem + 1 mg/kg miR-205, thrice a week for two weeks, intratumoral injection | Co-NP had slower tumor growth than free Gem, Gem-only NP, co-NP containing Gem + control miRNA. | [99] |
| Gem | HIF1α siRNA | Reverses drug resistance | Lipid-polymer hybrid NP | S.c. xenograft model of panc-1 pancreatic cancer; orthotopic xenograft model of panc-1-luc pancreatic cancer | 4 mg/kg Gem + 1.33 μg/kg siRNA, four times on days 1, 3, 6 and 9 for s.c. model and three times on day 1, 4, and 7 for orthotopic model | Co-NP showed stronger tumor growth inhibition than free Gem, free Gem+siRNA, Gem-only NP or siRNA-only NP in s.c. tumor models. | [100] |
| Benzethonium chloride (BZT) | Bcl-2 siRNA | Promotes apoptosis and inhibits drug resistance | Pluronic F68-based nanocomplex | S.c. xenograft model of MDA-MB-231 breast cancer | 6 mg/kg co-NP, on days 1, 4, and 6, intratumoral injection | Co-NP showed greater tumor suppression than co-NP containing BZT+ control siRNA. | [101] |
| 7-ethyl-10-hydroxycamptothecin (SN-38) | VEGF siRNA | Anti-angiogenesis | Polymeric micelles | S.c. xenograft model of LS174T colorectal cancer | 10 mg/kg SN-38 + 20 μg of siRNA, five times every 2 days | Co-NP showed stronger tumor inhibition than siRNA alone or SN-38-only NP. | [102] |
| Obatoclax (Oba) | miR-124 mimic | suppresses proliferation, metastasis, and endothelial– mesenchymal transition | cholesterol-penetratin micelles | S.c. xenograft model of MCF-7 breast cancer | 2 mg/kg miR-124 mimic + obatoclax 1.5 mg/kg, once a day for 14 days | Co-NP showed stronger tumor growth inhibition than miR-124-only NP or Oba-only NP | [103] |
IV injection unless specified otherwise.
3.5.1 Tumor suppressor genes
p53 is one of the tumor suppressor proteins. Its loss or mutation, as commonly found in various cancers, can lead to uncontrolled cell growth and resistance to chemotherapy. Therefore, co-delivery of pDNA encoding p53 can synergize the anticancer effect of Dox [104]. Li et al reported NP based on a star-shaped block co-polymer for the co-delivery of p53-pDNA and Dox [105]. The polymer was built upon hydrophobic polyhedral oligomeric silsesquioxane (POSS), which formed a core to entrap Dox, with branches of cationic poly[2-(dimethylamino)ethyl methacrylate] (PDMAEMA) and zwitterionic poly[N-(3-(methacryloylamino) propyl)-N,N-dimethyl-N-(3-sulfopropyl)ammonium hydroxide] (PMPDSAH), which formed a cationic shell for pDNA complexation and non-fouling corona for systemic application, respectively. The dual-loaded NP showed a greater p53 protein expression level in cancer cells and a greater anti-tumor effect in a MCF-7 xenograft model than single component NP due to the synergistic effect of p53-pDNA and Dox [105].
MicroRNAs (miRNAs) are a group of small non-coding RNAs that direct post-transcriptional regulation of gene expression, causing translational inhibition or destabilization of target messenger RNA [106]. MiRNA is co-delivered with chemotherapeutic drugs in similar ways as pDNA. A polyelectrolyte nanocomplex of HA and chitosan was used as a carrier of Dox as well as miR-34a, a tumor suppressor gene under-expressed in cancerous cells [107]. The NP were made by mixing cationic compounds (Dox, chitosan) and anionic counterparts (miRNA, HA). Here, chitosan and HA assembled into NP primary ionic complexes, in which Dox and miRNA were subsequently bound to their counter ions (Dox with HA and miRNA with chitosan). The miRNA/Dox co-loaded NP brought the two agents simultaneously into cancer cells via HA-mediated endocytosis and restored the expression of miR-34a. Then the miR-34a suppressed the expression of anti-apoptosis Bcl-2 and Notch 1 genes and thus sensitized the cancer cells to Dox-induced apoptosis. Consistently, the co-loaded NP inhibited the tumor growth completely in a MDA-MB-231 tumor model whereas NP with either Dox or miR-34a had limited effects.
3.5.2 Genes to reverse drug resistance
SiRNAs that reverse drug resistance are used in conjunction with anti-cancer drugs in order to sensitize cancer cells to the chemotherapy. Mainly two mechanisms are responsible for the multidrug resistance (MDR): upregulation of oncogenes encoding proteins to resist apoptosis (e.g., survivin, Bcl-2) [104] and expression of MDR genes encoding efflux transporters such as P-gp, multidrug resistance protein 1, 2 (MDR1 and MDR2) and BCRP [62].
Survivin is a protein overexpressed in various cancer cells, which inhibits cell apoptosis [108]. Genes to suppress survivin expression ultimately promote cell apoptosis and thus synergize chemotherapy. pDNA encoding survivin shRNA (iSur-pDNA) was co-encapsulated with PTX in a folate modified amphiphilic NP [109]. The NP consist of chitosan double-grafted with hydrophobic linoleic acid (LA) and anionic poly(β-malic acid) (PMLA) where chitosan permits iSur-pDNA binding, LA forms hydrophobic core for PTX loading and NP stabilizaiton, and PMLA counteracts chitosan to help release iSur-pDNA in the cells. Optimizing the degrees of LA and PMLA grafting was critical to balancing a stable retention of PTX and a timely unpacking of pDNA, two apparently conflicting features. In hepatoma bearing mice, the folate-modified dual-loaded NP showed greater tumor regression and a longer survival time than NP carrying PTX or pDNA alone [109]. This finding confirms that tumor growth could be effectively inhibited by taking advantage of the synergistic effect of PTX and iSur-pDNA.
P-gp is responsible for the efflux of multiple anti-cancer drugs and the resistance to chemotherapy. β-cyclodextrin-polyethylenimine (PEI-CyD) conjugated with cholesterol was used for dual delivery of P-gp siRNA (siP-gp) and Dox for the treatment of drug-resistant human breast cancer [110]. The PEI-CyD-cholesterol conjugate self-assembled into micelles, in which the polycationic PEI-CyD and cholesterol formed a hydrophilic surface and a hydrophobic core, respectively. siP-gp was attached to the PEI-CyD surface, and Dox was loaded in the cholesterol core. siP-gp and Dox were sequentially released due to the location in the NP: First, siRNA to knock down the P-gp expression and restore the chemosensitivity; then Dox to accumulate in the cells and provide its cytotoxic effects. The Dox/siP-gp dual-loaded micelles increased the intracellular retention of Dox within MCF-7/ADR cells and exhibited greater anti-tumor effect than free Dox in the MCF-7/ADR xenograft model. However, without comparison with simultaneous delivery, it is difficult to judge whether the sequential delivery was critical to achieving the synergistic effect.
Uncontrolled and incomplete release of anticancer drugs and genes at the target site may reduce the final therapeutic outcomes. To solve this problem, a stimuli-responsive polymeric micelle system was developed as a carrier of Dox and Bcl-2 siRNA (siBcl-2) [111]. This micelle was made of ternary block copolymer PEG-PAsp(AED)-PDPA. Here the PDPA (poly(2-(diisopropyl amino)ethyl methacrylate)) segment formed a pH-sensitive hydrophobic core to load Dox, and the PAsp(AED) (poly(N-(2,2′-dithiobis(ethylamine))aspartamide)) segment formed a redox-sensitive cationic interlayer to condense siBcl-2 (Fig. 3e). The Dox/siBcl-2 dual-loaded micelles showed fast release of Dox at pH 5 due to the protonation and dissolution of PDPA (pKa: 6.3) and the unpacking of siBcl-2 by 10 mM glutathione that reduced the disulfide bond to disintegrate PAsp(AED). The Dox/siBcl-2 dual-loaded micelles suppressed the expression of Bcl-2, an anti-apoptotic protein induced by Dox, and enhanced Dox-sensitivity in SKOV-3 ovarian cancer cells. Consistently, the Dox/siBcl-2 micelles inhibited the growth of s.c. SKOV-3 xenografts in mice to a greater extent than micelles containing each component (Fig. 3f) [111].
3.5.3 Genes to inhibit angiogenesis or regulate tumor microenvironment
Vascular endothelial growth factor (VEGF) promotes the growth of new blood vessels which supply tumors with nutrients and oxygen [112]. Therefore, VEGF has been considered an important target for cancer therapy. In anticipation of a synergistic effect of anti-angiogenesis and apoptosis, siRNA suppressing VEGF expression has been co-delivered with DTX by cationic liposomes modified with two targeting ligands [113]. Here, DTX was loaded in the hydrophobic tail part of the lipid bilayer and siVEGF were bound to the cationic surface. Two ligands - angiopep-2 and tLyP-1 - were used to target the blood brain barrier and promote tumor penetration of liposomes, respectively. The double-targeted liposomes showed greater cellular uptake and cytotoxicity than non-targeted ones. The DTX/siVEGF co-loaded liposomes showed a greater anti-tumor effect in a U87 MG xenograft model as compared to single agent-loaded counterparts, indicating an additive or synergistic effect of the two agents delivered simultaneously [113]. It is worth noting that siRNA and a hydrophobic compound representing DTX (Nile Red) underwent different intracellular trafficking according to their properties: while siRNA went through an endo/lysosomal escape, Nile Red was diffused throughout the cytoplasm. These trafficking modes support intracellular activity of siRNA and DTX that function in the cytoplasm. However, one may be cautioned that differential behaviors of the two agents may not be desirable if they occur prematurely (i.e., before they enter target cells), especially when their molecular targets are located in the cells.
The TME plays an important role in the tumor’s reaction towards chemotherapy and thus makes an attractive target for combination therapy. The cell portion in TME typically includes TAF, macrophages, and endothelial cells, which, together with the extracellular matrix (ECM), form a defensive layer around tumor cells thus creating an access barrier to the drug-loaded NP [114, 115]. Recently, Miao et al reported that chronic exposure to cisplatin induces TAF to secret Wnt16 which causes chemo-resistance of neighboring tumor cells and stroma reconstruction making it increasingly difficult to treat the cancer [116]. To address this challenge, they proposed to co-deliver siRNA targeting Wnt16 (siWnt) in addition to cisplatin. Due to the difference in the molecular properties, siWnt and cisplatin were encapsulated in separate NP with distinct cores but comparable size and surface properties, and these NP were delivered as a mixture. In a stroma-rich bladder cancer (SRBC) model, the mixture of the two types of NP effectively suppressed the expression of Wnt16 in tumors and showed greater anti-tumor effect than each NP, resulting in progression-free survival for 7 days after the last treatment. Even in large aggressive SRBC tumors (~700 mm3), the combination of siWnt NP and cisplatin NP had a superior tumor inhibitory effect compared to the single NP. This highlights the significance of addressing multiple targets of TME in treating cancer [116].
4. Sequential delivery
For drug combinations with the same cell targets (e.g., tumor cells), a carrier that encapsulates multiple drugs and unloads them in the target cells simultaneously may offer more benefits than drug cocktails or a mixture of the formulated drugs [7, 51]. However, the following cases require different delivery strategies. First, some drug combinations address different cell populations in target tissues. Consider a combination of anticancer drugs and anti-angiogenesis agents such as combretastatin. While anticancer drugs target tumor cells to inhibit their growth, combretastatin mainly affects the tumor vasculature by destroying the endothelial cells and causing a vascular shutdown [117, 118]. Long-term shutdown of tumor vasculature can prevent the subsequent delivery of anticancer drugs into tumors [118]. Therefore, one may reason that anticancer drugs need to be delivered before combretastatin may exert its effects. Secondly, drug combinations may show different therapeutic outcomes according to the sequence of drug administration. For example, a PTX/Gem combination shows different results depending on the dosing schedule [119]. Treatment with PTX followed by Gem (PTX→Gem) is more effective than the reverse sequence (Gem→PTX) and simultaneous exposure (PTX+Gem). A clinical regimen of PTX/Gem combination approved for the first-line treatment of metastatic pancreatic cancer recommends sequential administration of PTX followed by Gem [120]. In some cases, simultaneous delivery or reverse-order delivery may induce additive or even antagonistic effects [121–123]. Moreover, there have been clinical cases where simultaneous chemotherapy combination causes greater toxicity than sequentially-administered drugs with no significant advantages in overall survival time [124]. In these cases, simultaneous delivery may be unnecessary or even undesirable. Instead, they will benefit from carriers that can deliver multiple drugs in the optimal time frame, sequence, and location. This section discusses recent approaches to control temporal and spatial delivery of drug combinations.
4.1 Sequential administration
The simplest way of delivering multiple drugs to target tissues at different times is to administer them separately. For example, PTX and SU5416, a selective VEGF receptor-2 inhibitor, were individually formulated into PEGylated carriers and delivered sequentially to Colon-26 solid tumor-bearing mice [125]. Here, SU5416 was added first to normalize the tumor vasculature structurally and functionally and thus help transport the subsequent treatment (PTX) into the tumors. SU5416 and PTX were formulated as a PEGylated oil-in-water (O/W) emulsion (PE-SU5416) and a PEGylated liposome (PL-PTX), respectively [125]. The pretreatment of PE-SU5416 increased the distribution of PL-PTX in the core region of the tumors and thus improved the antitumor activity of PL-PTX [125]. It is worth noting that the dose of SU5416 and its timing of administration are critical to the success of this approach, because the excessive antiangiogenic effect could hinder the delivery of the subsequently administered treatment. In that regard, separate formulations have much greater flexibility than co-delivery systems in controlling the dose and regimen.
Another example of sequential delivery involves a combination of PTX and topoisomerase I inhibitor camptothecin (CPT). The two drugs were respectively encapsulated in peptide-functionalized “Nanosponges” made of poly(epoxyvalerolactone-α-allyl-δ-valerolactone) and were delivered intravenously to mice with Lewis lung carcinoma allografts [126]. In vitro studies presented a significant G2/M phase cell cycle arrest from sequential administration of PTX NP followed by CPT NP (67%), higher than simultaneous and reverse sequence treatment by 28% and 34%, respectively. Consistently, sequential PTX NP → CPT NP treatment caused more apoptosis than simultaneous treatment. The in vivo result was not as evident as in vitro in this study: Sequential PTX NP → CPT NP treatment was more efficient than single drug NP in delaying tumor growth; however, comparison with reverse sequence or simultaneous treatment is not available. This study also did not identify how the dosing schedule affected the cytotoxic effect. Other studies involving PTX suggest that PTX reduces the tumor stroma and facilitates the delivery of the subsequent treatment [23, 127].
A combination of PTX and a c-Myc inhibitor peptide H1-S6A F8A (H1) showed similar sequence-dependence in synergizing their antitumor effects and were delivered in sequence as individually-formulated drug-polymer conjugates [128]. Here, a N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer-DTX conjugate (P-DTX) administered first arrests tumor cells in the G2/M phase, making the nuclear membrane remain disassembled for a prolonged period of time. A subsequently-added HPMA-H1 conjugate (P-H1) takes advantage of the breach of the nuclear membrane to enter the nucleus and inhibit c-Myc. Accordingly, P-DTX → P-H1 treatment showed a synergistic effect (CI < 1) at the affected fraction (Fa) levels of 50% and 70% [128]. On the other hand, simultaneously added P-H1 and P-DTX (P-H1 + P-DTX) or reversely sequenced treatment (P-H1→ P-DTX) showed an antagonistic effect in killing HeLa cells with a CI of >1.1 at the same Fa levels. The P-DTX → P-H1 treatment also attenuated the growth of HeLa tumors in vivo better than free drugs delivered in the same sequence (DTX → H1), demonstrating the contribution of polymer carrier to colocalizing the two drugs in tumors. However, the comparison with reverse sequence or simultaneous treatment of polymer-drug conjugates was not reported [128].
There are many advantages of the sequential delivery of separately-prepared drug formulations. First of all, drugs with dramatically different physicochemical properties are challenging to load in a single carrier. Separate formulations allow one to choose appropriate carriers based on the drug properties and thus reduce technical burdens in formulation development. In addition, with sequential delivery clinicians can adjust the dose of each agent and dosing schedule according to the patient’s responses to ongoing treatments. On the other hand, a major shortcoming of sequential injections is that it does not warrant co-localization of multiple treatments due to the difference of carriers. Even the same type of carriers may not be co-localized if they are separately administered [129]. In this regard, it is worthwhile to consider carrier systems that co-encapsulate multiple drugs with a built-in control of each drug release.
4.2 Carriers with a built-in control of release sequence
Although technically challenging, a single carrier-based delivery has advantages over separate dosing in synchronizing pharmacokinetics and biodistribution of drug combinations. Therefore, increasing efforts have been made to design carriers that can load multiple drugs in specific ratios and release them in predetermined sequences.
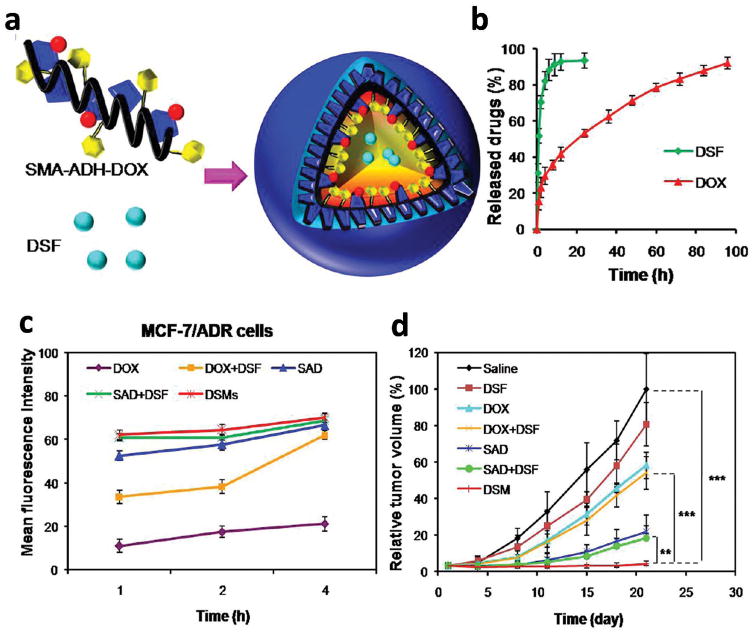
The release sequence of a drug combination may be determined empirically or designed according to the mechanism of action. An example of the latter is the combination of drug sensitizers and chemotherapeutic drugs where the sensitizers are released first to make cancer cells respond to the subsequently administered anticancer drugs. For example, Duan et al tried sequential delivery of Dox and disulfiram (DSF), a P-gp inhibitor and apoptosis inducer, using a polymeric micelle based on poly(styrene-co-maleic anhydride) (SMA) [130]. Here, Dox was conjugated to SMA via acid-cleavable adipic dihydrazide (ADH), and DSF was physically loaded into the micelles (Fig. 4a). Consequently, DSF was released first to inactivate P-gp and inhibit the efflux of Dox, which was released later in the acidity of endosomal environment (Fig. 4b). When compared to free Dox, the dual-drug micelles showed an increased intracellular Dox accumulation (Fig. 4c) and a stronger apoptosis-inducing effect to MCF-7/ADR cells. It also had greater inhibitory effect on the growth of drug-resistant breast cancer xenografts compared to Dox + DSF mixture or Dox-loaded micelles + DSF mixture (Fig. 4d) [130]. These results indicate the significance of the co-localization of drug/sensitizer combinations in achieving optimal synergistic effects. However, without comparing to NP simultaneously releasing both drugs, one would not be able to judge how critical it was to control the temporal release in achieving the chemosensitizing effect.
Fig 4.
(a) Schematic diagram of a polymeric micelle delivering DSF and Dox. (b) In vitro drug release. (c) Intracellular Dox accumulation in MCF-7/ADR cells after treatment of Dox, Dox+DSF, SAD (Dox-loaded micelles), SAD+DSF (Dox-loaded micelles + DSF mixture), or DSMs (dual-drug micelles). (d) Anti-tumor efficacy in a MCF-7/ADR xenograft model after different treatments. Reprinted with permission from [130]. Copyright (2013) American Chemical Society.
Sengupta et al reported a co-delivery system of Dox and combretastatin A4 (CA4), an anti-angiogenesis agent [118]. A carrier was designed to release CA4 first in order to shut down the tumor vasculature and isolate NP in tumors so that the subsequently-released Dox could induce cell apoptosis to maximize the effect on the tumors. For this purpose, Dox was conjugated to PLGA, made into NP cores, and CA4 was added to the phospholipid layer covering the surface. The surface-bound CA4 was released first whereas Dox was released slowly as the core degraded into smaller Dox-PLGA fragments and free Dox. In B16/F10 melanoma or Lewis lung carcinoma-bearing mice, the NP releasing CA4 and Dox sequentially showed significantly better anti-tumor effects than liposomes containing the two drugs with no temporal control over their release. Immunohistochemistry detected no difference in the vessel density between the two groups but found a greater number of apoptotic cells in the tumors treated with the sequential delivery system than those with co-loaded liposomes. This highlights how timely delivery of two drugs can make a difference in tumor responses to chemotherapy.
On the other hand, the above approach does not control the location at which each drug will be released. Spatial control of drug release is deemed necessary for the CA4/Dox combination due to the difference in cellular targets (CA4 targeting endothelial cells; Dox targeting tumor cells). A dual-pH response polypeptide system was thus constructed as a carrier of the CA4/Dox combination [131]. Dox was first conjugated with polyaspartate (pAsp) via a hydrazone bond to form a macromolecular prodrug (pAsp-Dox), which was self-assembled with CA4 and polyethylene glycol-polyhistidine (PEG-pHis). The dual pH-sensitivity came from the hydrazone bond and pHis, cleaved at endo/lysosomal pH 5–6 and protonated at pH 6–7 of the tumor ECM, respectively. Therefore, the NP was expected to swell and release CA4 in the tumor ECM and release Dox in endosomes and lysosomes in the cells thus providing more intimate access to each cellular target. The in vitro release assay supported the pH sensitivity: Both drugs were released very little at pH 7.4 (CA4: 8.7% in 12h, Dox: 8.1% in 2 weeks), but the release increased substantially at acidic pHs (CA4: 64.1% at pH 6.5 in12h; Dox: 57.1% at pH 5.8 in 2 weeks). In a MCF-7/ADR tumor xenograft model, the pH-sensitive dual-drug NP showed a greater tumor inhibition effect than a dual-drug NP with no hydrazone bond (thus likely to release Dox before CA4) or a mixture of pAsp-Dox and CA4 (which does not warrant co-delivery of two drugs) with no obvious toxicity to normal tissues [131].
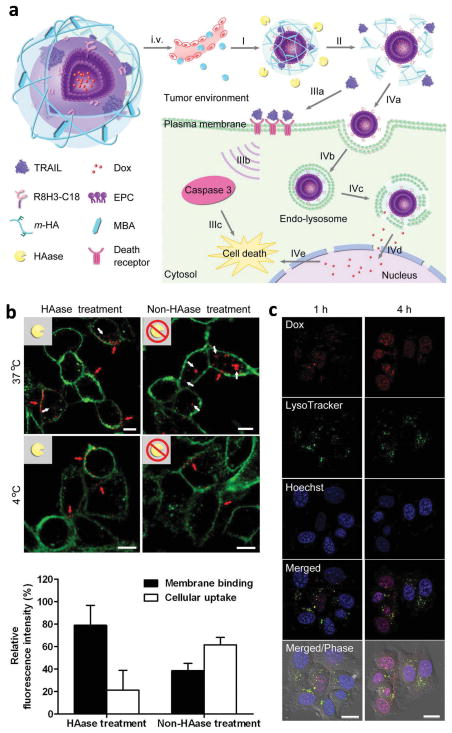
Drugs acting on different subcellular structures also require a carrier capable of differential drug release. For example, Dox is used in combination with a tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), but the two drugs address distinct subcellular targets (nucleus vs. cell membrane) [132]. A core-shell type NP vehicle was constructed for the co-delivery of the two: Dox was loaded into liposomes modified with cell-penetrating peptides (CPP, R8H3) serving as a core, and TRAIL was loaded into the outer shell consisting of a UV-crosslinked HA (Fig. 5a). The outer HA layer is supposed to be degraded by hyaluronidase (HAase), highly expressed in the TME, so that TRAIL may be released and interact with a death receptor on the cell membrane to induce the programmed cell death. In the meantime, the exposed liposomal cores enter cells via peptide ligands and release Dox, which then accumulates in the nuclei to trigger apoptosis and cytotoxicity. Confocal microscopy with MDA-MB-231 cells supported the hypothesis. Rhodamine-labeled TRAIL (r-TRAIL) entered the cells in the absence of HAase due to the endocytosis of NP via HA-CD44 interactions, whereas r-TRAIL stayed on the cellular membrane when added after a 30 min HAase treatment (Fig. 5b). In addition, the HAase-treated NP entered the cells, underwent endo-lysosomal escape, and released Dox in the cells to allow their nuclear accumulation (Fig. 5c). Consistently, the HAase-treated NP caused apoptosis in a greater population of cells (80.6%) than each treatment alone (free TRAIL: 36.5%, Core liposome with Dox: 38.5%). In a MDA-MB-231 tumor xenograft model, the dual drug-loaded NP attenuated the tumor growth significantly better than free Dox or Dox NP (without TRAIL). A comparison with a mixture of single component NP would have made a stronger case for both the sequence- and the site-specific delivery, but it was not addressed in this study.
Fig. 5.
(a) Schematic representation of a core-shell type NP for sequential and site-specific delivery TRAIL and Dox. (b) Binding of r-TRAIL (red) loaded in NP to plasma membrane (green) with and without 30 min of HAase pre-treatment (Top). Quantitative analysis of r-TRAIL in the plasma membrane and in the cells (Bottom). (c) Intracellular delivery of HAase-treated NP on MDA-MB-231 cells at different times observed by confocal microscopy. Late endosomes/lysosomes: green; nuclei: blue; Dox: red. Reprinted with permission from [132]. Copyright (2014) John Wiley and Sons.
This example demonstrates a reasonable way of co-encapsulating two drugs with different molecular weights and cellular targets, but it has a limitation with respect to the drug loading capacity and the stability in circulation. To overcome these challenges, the same group designed a new graphene-based co-delivery nanosystem for the sequence- and the site-specific delivery of TRAIL and Dox [133]. Dox was loaded in graphene oxide (GO) via supramolecular π–π stacking interaction, and GO was coupled to a TRAIL-conjugated furin-cleavable peptide via a hetero-bifunctional PEG linker. Furin is a protease highly expressed on the cell membrane and Golgi complex of many cancer cells. Serving as a covalent bond between TRAIL and GO, a furin-cleavable peptide reduces premature leakage of TRAIL during circulation. When the nanosystem reaches the tumor membrane, the peptide linker is cleaved by furin and release TRAIL to facilitate its binding to the membrane target. In the meantime, the exposed Dox-loaded GO nanosheet becomes available for cellular uptake. This way, the furin-cleavable peptide supports the balance between circulation stability and target-specific drug release. In support of this hypothesis, the furin-sensitive GO loaded with TRAIL and Dox (TRAIL/DOX-fGO) showed the lowest IC50 on A549 cells (14 ng/mL as TRAIL and 140 ng/mL as Dox) compared to TRAIL-fGO (119 ng/mL as TRAIL), DOX-fGO (509 ng/mL as Dox), and furin-insensitive TRAIL/DOX-nGO (33 ng/mL as TRAIL and 329 ng/mL as Dox) in A549 cells. Consistently, animals treated with TRAIL/DOX-fGO showed slower A549 tumor growth than those treated with TRAIL-fGO, DOX-fGO, or TRAIL/DOX-nGO. In particular, the comparison between TRAIL/DOX-fGO and TRAIL/DOX-nGO confirmed the significance of the timely release of each drug component [133].
As illustrated in Section 3.5.1, miRNA can sensitize cancer cells to chemotherapy; thus, combinations of miRNA and chemotherapeutic drugs are widely pursued [15, 93]. Increasing evidence supports that the sequence and the timing of the administration of the agents are critical to the outcome of the miRNA/drug combination therapy [121, 134]. A recent study employs lasers to control the sequence of the combination’s release [122]. MiRNA-21 is an antiapoptotic factor [135] and inhibited by another miRNA called miR-21i [122]. A combination of miR-21i and Dox is expected to have a synergistic anticancer effect, especially when the miR-21i is treated prior to Dox. A dual-delivery system of Dox and miR-21i was prepared with a hollow gold NP (HGNP) conjugated with PAMAM dendrimers, which accommodated Dox and miR-21i, respectively [122]. First, the dual NP enter cells; miR-21i is released from the PAMAM dendrimer into the cytoplasm to sensitize the cells to the subsequent chemotherapy; then the Dox release is triggered by near-infrared (NIR) irradiation which causes the collapse of HGNP. This way, the sequence of drug release can be precisely controlled based on the timing of laser application. Confocal microscopy provided the proof of concept: in 1h after the NP treatment, the miR-21i signal was readily seen in the cytoplasm, but the Dox signal was visible in the nuclei only after the NIR exposure. MiR-21i/Dox dual-NP showed the lowest IC50 value of Dox (0.13 μg/mL) in MDA-MB-231 cells when the laser was applied 4h after the treatment, as compared to free Dox (1.1 μg/mL) or the dual-NP applied with simultaneous NIR activation (0.27 μg/mL). A similar effect was seen in MDA-MB-231-derived stem cells (CSC): CSC were more sensitive to the sequence therapy with an IC50 value of 0.39 μg/mL, but the IC50 values for free Dox and the co-administration were 27 μg/mL and 1.3 μg/mL, respectively. In MDA-MB-231 tumor xenograft models, animals receiving miR-21i/Dox dual-NP and NIR irradiation with 4h intervals (i.e., sequential delivery) showed slower tumor growth than those receiving the NIR treatment immediately after NP injection (simultaneous delivery). Examples of sequential delivery of nucleic acids and small molecule drugs are currently limited. Given the benefit demonstrated in this study, future investigation is warranted for additional nucleic acids/drug combinations [10].
5. Conclusion
Combination chemotherapy has been the mainstay of cancer treatment for several decades. The effectiveness of combination therapy depends largely on the drug ratio, timing and sequence of dosing, which are difficult to control with simple cocktails of free drugs. Various efforts have been made to develop drug carriers to achieve ratiometric dosing and precise control over the drug release kinetics and sequences, with a couple of dual drug formulations currently in clinical trials [4, 136, 137]. Given the increasing number of newly identified drug combinations and the breadth of therapeutic agents used in combination, the role of drug carriers will be even more critical in the future. In particular, NP have gained significant interest for this purpose due to the small size amenable to systemic applications and the variable physicochemical features that may be modified to control their pharmacokinetic and biodistribution profiles. Several NP systems demonstrate how the spatiotemporal control of drug combination delivery can improve the therapeutic outcomes in preclinical animal models. Future work should focus on expanding the current efforts to different drug combinations and dosing schedules as well as translating their potential to clinically useful products.
6. Expert opinion
Ideally, a carrier for combination therapy should be able to accommodate multiple drugs with different chemical properties in an optimal ratio for synergistic effects, maintain the ratio during circulation, and release the drugs in the location and time according to their mechanisms of action. The recent examples discussed in this article show how these requirements can be met. However, several issues remain to be considered in order to further improve their translational potential.
First, rigorous tests should be done to identify synergistic drug combinations and ratios as well as the optimal dosing schedule. The term “synergistic effects” is often abused and misused. It is not simply two drugs used together to perform better than each individual drug (A+B>A or A+B>B), which is a common misconception in the literature. Rather, the term drug synergism refers to the interaction of two or more drugs which leads to a combined effect greater than the sum of the effects seen when each drug is given alone [138]. This should be determined by mathematical analysis of dose-effect data of single drugs and combinations in different ratios [3].
Second, once the synergistic drug combination and ratio are identified, it is critical to ensure that a carrier delivers multiple drugs to the common target in the optimal ratio. Loading multiple drugs in a fixed ratio does not necessarily guarantee the ratiometric delivery if one of the components is preferentially leached out during circulation. This may be avoided by increasing the carrier stability in circulation or by accelerating its distribution to target tissues. Both approaches have challenges. The former bears the risk of making the system too stable and thus unable to release drugs within a critical time frame. This would be equally undesirable for therapeutic activities. Finding a fine balance between the stability and timely drug release is an ongoing challenge, which is frequently overlooked in nanomedicine research due to inappropriate characterization methods [139]. The latter approach, often involving external stimuli such as ultrasound or magnetic forces, is found effective in counteracting premature drug release during circulation, at least in preclinical studies [140]. However, the need for the use of external stimuli and corresponding materials may complicate the product design and application, which may hamper clinical translation of the technology.
Third, a multi-drug loaded carrier is ideal for co-localization of drug combinations in tissue/cell targets, but it can be technically challenging to load the drugs in a specific weight ratio, especially when the two drugs have very different physicochemical properties. Moreover, if the drugs are to act on different cells in target tissues and/or show a synergistic effect only when provided in a specific order, it is even more complicated to include a trigger mechanism to induce drug release accordingly. In this case, co-encapsulation is not necessary and can even be undesirable. Instead, it is conceivable to formulate each drug separately according to their properties and desired release profiles with a post-hoc control of size and surface chemistry and administer them as a mixture or sequentially.
Fourth, in the development and evaluation of combination delivery systems, it is necessary to use the right set of control groups corresponding to the factor of interest. Ideally, the combination delivery systems need to be compared with a ratiometric mixture of free drugs to evaluate their contribution to the biodistribution and the co-localization of multiple drugs. For evaluation of synergistic effects between drugs delivered with the carrier system, control groups should include the component drugs individually formulated in comparable carriers, preferably over a range of doses. However, some studies neglect to compare with appropriate control groups and make an incomplete conclusion based on the comparison with a single drug.
Fifth, the complexity of delivery systems should be considered a necessary evil, which may be justified only when the benefits outweigh (greatly) the negative consequences. While complex material systems are considered almost inevitable choices for encapsulating multiple compounds with different properties, such complexity is not well received from the industry and regulatory perspectives and thus becomes a significant hurdle to the product development. Moreover, the carrier materials can cause unintended biological effects with therapeutic consequences [141]; thus, a greater caution needs to be taken when a complex material system is employed.
Finally, one must be aware of the expanding horizons of combination therapy and the increasing opportunities for the controlled delivery of drug combinations. Recently, adaptive resistance has emerged as a key driver of chemotherapy failure and has gained interest as a new target for combination therapy. Here, cancer cells acquire drug-resistant phenotypes during chemotherapy and show a narrow time frame in which they become highly sensitive to the inhibitor of the acquired phenotype [142]. A carrier that can deliver the drug pair in the right sequence can take advantage of the phenotypic transition and greatly improve the outcome of chemotherapy. Moreover, recent advances in cancer immunotherapy open up new opportunities in combination therapy. For example, prominent immune checkpoint antagonists are inactivated by a chemokine produced by TAF in pancreatic cancer. AMD3100, an inhibitor of the chemokine receptor, sensitizes the tumors to the immune checkpoint inhibitors by unleashing cytotoxic T-cells in the tumors. AMD3100 and immune checkpoint inhibitors make a promising combination for cancer immunotherapy, and we expect to see many more combinations follow. A carrier to co-deliver diverse therapeutic agents will play a significant role in advancing cancer immunotherapy.
Article highlights.
The main goal of NP systems for combination therapy is to deliver the desired drugs in a ratio and sequence optimal for drug synergism.
There is a great demand for multi-drug carriers due to the increase in the number of newly identified drug combinations and the diversity of therapeutic agents used in combination.
Early studies have focused on loading multiple drugs with different physicochemical properties into a single vehicle to help co-localize them to target tissues.
Increasing efforts have been made to develop carriers and strategies to deliver drug combinations to distinct cell populations and/or in specific sequences according to the mechanisms of action.
Several challenges persist and impede the clinical translation of combination delivery.
Acknowledgments
The authors thank Andrew N. Wakefield for proofreading the manuscript.
Abbreviations
- BCRP
Breast cancer resistance protein
- CPT
Camptothecin
- CSC
Cancer stem cells
- CPP
Cell-penetrating peptides
- CA4
Combretastatin A4
- DTX
Docetaxel
- Dox
Doxorubicin
- EPR
Enhanced permeability and retention effect
- ECM
Extracellular matrix
- 5-FU
5-fluorouracyl
- Gem
Gemcitabine
- GO
Graphene oxide
- HA
Hyaluronic acid
- ICG
Indocyanine green
- MTX
Methotrexate
- miRNA
Micro RNA
- MMC
Mitomycin C
- MDR
Multidrug resistance
- NP
Nanoparticles
- NIR
Near infrared
- PTX
Paclitaxel
- PDT
Photodynamic therapy
- PTT
Photothermal therapy
- P-gp
P-glycoprotein
- pDNA
Plasmid DNA
- PEI
Polyethyleneimine
- PEG
Polyethylene glycol
- PLGA
Poly(lactic-co-glycolic acid)
- RAPA
Rapamycin
- ROS
Reactive oxygen species
- shRNA
Short hairpin RNA
- siRNA
Small interfering RNA
- s.c
Subcutaneous
- TAF
Tumor-associated fibroblasts
- TME
Tumor microenvironment
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
- VEGF
Vascular endothelial growth factor
Footnotes
Declaration of interest
This work was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number R01EB017791 and China Scholarship Council Fellowship (F.M., N.H.). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Tacar O, Sriamornsak P, Dass CR. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65(2):157–70. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg Robert A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3**.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–81. doi: 10.1124/pr.58.3.10. A comprehensive overview of drug synergism, experimental designs, and analysis of the effect of drug combinations. [DOI] [PubMed] [Google Scholar]
- 4**.Liboiron BD, Mayer LD. Nanoscale particulate systems for multidrug delivery: Towards improved combination chemotherapy. Ther Deliv. 2014;5(2):149–71. doi: 10.4155/tde.13.149. A comprehensive review of recent progress of nanoscale particulate systems for multidrug delivery. [DOI] [PubMed] [Google Scholar]
- 5.Mayer LD, Harasym TO, Tardi PG, Harasym NL, Shew CR, Johnstone SA, et al. Ratiometric dosing of anticancer drug combinations: Controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5(7):1854–63. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- 6.Tardi P, Johnstone S, Harasym N, Xie S, Harasym T, Zisman N, et al. In vivo maintenance of synergistic cytarabine:Daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33(1):129–39. doi: 10.1016/j.leukres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 7*.Miao L, Guo S, Zhang J, Kim WY, Huang L. Nanoparticles with precise ratiometric co-loading and co-delivery of gemcitabine monophosphate and cisplatin for treatment of bladder cancer. Adv Funct Mater. 2014;24(42):6601–11. doi: 10.1002/adfm.201401076. Reports a nano-delivery system with precise ratiometric co-loading and co-delivery of two hydrophilic drugs for synergistic anti-tumor effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 9.Guo S, Lin CM, Xu Z, Miao L, Wang Y, Huang L. Co-delivery of cisplatin and rapamycin for enhanced anticancer therapy through synergistic effects and microenvironment modulation. ACS Nano. 2014;8(5):4996–5009. doi: 10.1021/nn5010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai X, Tan C. Combination of microrna therapeutics with small-molecule anticancer drugs: Mechanism of action and co-delivery nanocarriers. Adv Drug Deliv Rev. 2015:81184–97. doi: 10.1016/j.addr.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 11**.Teo PY, Cheng W, Hedrick JL, Yang YY. Co-delivery of drugs and plasmid DNA for cancer therapy. Adv Drug Deliv Rev. 2016:9841–63. doi: 10.1016/j.addr.2015.10.014. A good summary of drug delivery systems for co-delivery of pDNA and drugs. [DOI] [PubMed] [Google Scholar]
- 12.He C, Tang Z, Tian H, Chen X. Co-delivery of chemotherapeutics and proteins for synergistic therapy. Adv Drug Deliv Rev. 2016:9864–76. doi: 10.1016/j.addr.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Chia-Wei Su C-SC, Li Wei-Ming, Hu Shang-Hsiu, Chen San-Yuan. Multifunctional nanocarriers for simultaneous encapsulation of hydrophobic and hydrophilic drugs in cancer treatment. Nanomedicine (Lond) 2014;9(10):1499–515. doi: 10.2217/nnm.14.97. [DOI] [PubMed] [Google Scholar]
- 14.He C, Lu J, Lin W. Hybrid nanoparticles for combination therapy of cancer. J Control Release. 2015:219224–36. doi: 10.1016/j.jconrel.2015.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi NS, Tekade RK, Chougule MB. Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: Current progress and advances. J Control Release. 2014;194:238–56. doi: 10.1016/j.jconrel.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Q, Sun W, Wang C, Gu Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv Drug Deliv Rev. 2016;98:19–34. doi: 10.1016/j.addr.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka K, Mizusawa J, Fukuda H, Araki N, Chuman H, Takahashi M, et al. Perioperative chemotherapy with ifosfamide and doxorubicin for high-grade soft tissue sarcomas in the extremities (JCOG0304) Jpn J Clin Oncol. 2015;45(6):555–61. doi: 10.1093/jjco/hyv042. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Food and Drug Administration. [Last accessed: April 25, 2016];Paclitaxel. 2013 Available at: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm367613.htm.
- 19.Lorusso V, Giotta F, Bordonaro R, Maiello E, Del Prete S, Gebbia V, et al. Non-pegylated liposome-encapsulated doxorubicin citrate plus cyclophosphamide or vinorelbine in metastatic breast cancer not previously treated with chemotherapy: a multicenter phase III study. Int J Oncol. 2014;45(5):2137–42. doi: 10.3892/ijo.2014.2604. [DOI] [PubMed] [Google Scholar]
- 20.Rau KM, Lin YC, Chen YY, Chen JS, Lee KD, Wang CH, et al. Pegylated liposomal doxorubicin (Lipo-DOX®) combined with cyclophosphamide and 5-fluorouracil is effective and safe as salvage chemotherapy in taxane-treated metastatic breast cancer: an open-label, multi-center, non-comparative phase II study. BMC Cancer. 2015;15(423):015–1433. doi: 10.1186/s12885-015-1433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greimel ER, Bjelic-Radisic V, Pfisterer J, Hilpert F, Daghofer F, du Bois A. Randomized study of the arbeitsgemeinschaft gynaekologische onkologie ovarian cancer study group comparing quality of life in patients with ovarian cancer treated with cisplatin/paclitaxel versus carboplatin/paclitaxel. J Clin Oncol. 2006;24(4):579–86. doi: 10.1200/JCO.2005.02.4067. [DOI] [PubMed] [Google Scholar]
- 22.Hu XC, Zhang J, Xu BH, Cai L, Ragaz J, Wang ZH, et al. Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015;16(4):436–46. doi: 10.1016/S1470-2045(15)70064-1. [DOI] [PubMed] [Google Scholar]
- 23.Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: A phase I/II trial. J Clin Oncol. 2011;29(34):4548–54. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surmeli ZG, Varol U, Cakar B, Degirmenci M, Arslan C, Piskin GD, et al. Capecitabine maintenance therapy following docetaxel/capecitabine combination treatment in patients with metastatic breast cancer. Oncol Lett. 2015;10(4):2598–602. doi: 10.3892/ol.2015.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang H, Zhang X, Chen J, Zhang L, Xiong J, Zhong L, et al. A study of weekly docetaxel and carboplatin as first-line chemotherapy for advanced non-small cell lung cancer. J Thorac Dis. 2014;6(2):79–85. doi: 10.3978/j.issn.2072-1439.2014.01.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones S, Holmes FA, O’Shaughnessy J, Blum JL, Vukelja SJ, McIntyre KJ, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US oncology research trial 9735. J Clin Oncol. 2009;27(8):1177–83. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Xu R, Li J, Bai Y, Liu T, Jiao S, et al. Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer. 2016;19(1):234–44. doi: 10.1007/s10120-015-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukuda T, Tanabe M, Kobayashi K, Fukada I, Takahashi S, Iwase T, et al. Combination chemotherapy with mitomycin C and methotrexate is active against metastatic HER2-negative breast cancer even after treatment with anthracycline, taxane, capecitabine, and vinorelbine. Springerplus. 2015;4(376):015–1159. doi: 10.1186/s40064-015-1159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira Filho AF, Di Leo A, Paesmans M, Beauduin M, Vindevoghel A, Michel J, et al. The feasibility of classical cyclophosphamide, methotrexate, 5-fluorouracil (CMF) for pre- and post-menopausal node-positive breast cancer patients in a belgian multicentric trial: a study of consistency in relative dose intensity (RDI) and cumulative doses across institutions. Ann Oncol. 2002;13(3):416–21. doi: 10.1093/annonc/mdf051. [DOI] [PubMed] [Google Scholar]
- 31.Plimack ER, Hoffman-Censits JH, Viterbo R, Trabulsi EJ, Ross EA, Greenberg RE, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol. 2014;32(18):1895–901. doi: 10.1200/JCO.2013.53.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kua VF, Ismail F, Chee Ee Phua V, Aslan NM. Carboplatin/5-fluorouracil as an alternative to cisplatin/5-fluorouracil for metastatic and recurrent head and neck squamous cell carcinoma and nasopharyngeal carcinoma. Asian Pac J Cancer Prev. 2013;14(2):1121–6. doi: 10.7314/apjcp.2013.14.2.1121. [DOI] [PubMed] [Google Scholar]
- 33.Gao R, Zhang Y, Wen XP, Fu J, Zhang GJ. Chemotherapy with cisplatin or carboplatin in combination with etoposide for small-cell esophageal cancer: a systemic analysis of case series. Dis Esophagus. 2014;27(8):764–9. doi: 10.1111/dote.12149. [DOI] [PubMed] [Google Scholar]
- 34.Tiseo M, Boni L, Ambrosio F, Camerini A, Vitale MG, Baldini E, et al. Italian multicenter phase III randomized study of cisplatin-etoposide with or without bevacizumab as first-line treatment in extensive stage small cell lung cancer: Treatment rationale and protocol design of the GOIRC-AIFA FARM6PMF JM trial. Clin Lung Cancer. 2015;16(1):67–70. doi: 10.1016/j.cllc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Sun W, Wang T, Shi F, Wang J, Hui B, Zhang Y, et al. Randomized phase III trial of radiotherapy or chemoradiotherapy with topotecan and cisplatin in intermediate-risk cervical cancer patients after radical hysterectomy. BMC Cancer. 2015;15(353):015–1355. doi: 10.1186/s12885-015-1355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mertsoylu H, Kose F, Sumbul AT, Sedef AM, Dogan O, Besen AA, et al. Concurrent chemoradiotherapy with vinorelbine plus split-dose cisplatin may be an option in inoperable stage III non-small cell lung cancer: a single-center experience. Med Sci Monit. 2015;21:661–6. doi: 10.12659/MSM.892730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katsaounis P, Kotsakis A, Agelaki S, Kontopodis E, Agelidou A, Kentepozidis N, et al. Cisplatin in combination with metronomic vinorelbine as front-line treatment in advanced non-small cell lung cancer: a multicenter phase II study of the hellenic oncology research group (HORG) Cancer Chemother Pharmacol. 2015;75(4):821–7. doi: 10.1007/s00280-015-2707-x. [DOI] [PubMed] [Google Scholar]
- 38.Scagliotti GV, Gridelli C, de Marinis F, Thomas M, Dediu M, Pujol JL, et al. Efficacy and safety of maintenance pemetrexed in patients with advanced nonsquamous non-small cell lung cancer following pemetrexed plus cisplatin induction treatment: a cross-trial comparison of two phase III trials. Lung Cancer. 2014;85(3):408–14. doi: 10.1016/j.lungcan.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Cohen MH, Justice R, Pazdur R. Approval summary: pemetrexed in the initial treatment of advanced/metastatic non-small cell lung cancer. Oncologist. 2009;14(9):930–35. doi: 10.1634/theoncologist.2009-0092. [DOI] [PubMed] [Google Scholar]
- 40.Choi YJ, Lee SH, Lee JL, Ahn JH, Lee KH, You D, et al. Phase II study of pemetrexed in combination with cisplatin in patients with advanced urothelial cancer: The PECULIAR study (KCSG 10–17) Br J Cancer. 2015;112(2):260–5. doi: 10.1038/bjc.2014.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Wang Z, Hu X, Wang B, Wang L, Yang W, et al. Cisplatin and gemcitabine as the first line therapy in metastatic triple negative breast cancer. Int J Cancer. 2015;136(1):204–11. doi: 10.1002/ijc.28966. [DOI] [PubMed] [Google Scholar]
- 42.Cassidy J, Tabernero J, Twelves C, Brunet R, Butts C, Conroy T, et al. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol. 2004;22(11):2084–91. doi: 10.1200/JCO.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 43.Jia M, Li Y, Yang X, Huang Y, Wu H, Huang Y, et al. Development of both methotrexate and mitomycin C loaded PEGylated chitosan nanoparticles for targeted drug codelivery and synergistic anticancer effect. ACS Appl Mater Interfaces. 2014;6(14):11413–23. doi: 10.1021/am501932s. [DOI] [PubMed] [Google Scholar]
- 44.Fu Q, Lv P, Chen Z, Ni D, Zhang L, Yue H, et al. Programmed co-delivery of paclitaxel and doxorubicin boosted by camouflaging with erythrocyte membrane. Nanoscale. 2015;7(9):4020–30. doi: 10.1039/c4nr07027e. [DOI] [PubMed] [Google Scholar]
- 45.Lv S, Tang Z, Li M, Lin J, Song W, Liu H, et al. Co-delivery of doxorubicin and paclitaxel by PEG-polypeptide nanovehicle for the treatment of non-small cell lung cancer. Biomaterials. 2014;35(23):6118–29. doi: 10.1016/j.biomaterials.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Fang J, Kim Y-J, Wong MK, Wang P. Codelivery of doxorubicin and paclitaxel by cross-linked multilamellar liposome enables synergistic antitumor activity. Mol Pharm. 2014;11(5):1651–61. doi: 10.1021/mp5000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Q, Lv S, Tang Z, Liu M, Zhang D, Yang Y, et al. A co-delivery system based on paclitaxel grafted mPEG-b-PLG loaded with doxorubicin: preparation, in vitro and in vivo evaluation. Int J Pharm. 2014;471(1–2):412–20. doi: 10.1016/j.ijpharm.2014.05.065. [DOI] [PubMed] [Google Scholar]
- 48.He Z, Shi Z, Sun W, Ma J, Xia J, Zhang X, et al. Hemocompatibility of folic-acid-conjugated amphiphilic PEG-PLGA copolymer nanoparticles for co-delivery of cisplatin and paclitaxel: treatment effects for non-small-cell lung cancer. Tumour Biol. 2015:1–13. doi: 10.1007/s13277-015-4634-1. [DOI] [PubMed] [Google Scholar]
- 49.He Z, Huang J, Xu Y, Zhang X, Teng Y, Huang C, et al. Co-delivery of cisplatin and paclitaxel by folic acid conjugated amphiphilic PEG-PLGA copolymer nanoparticles for the treatment of non-small lung cancer. Oncotarget. 2015;6(39):42150–68. doi: 10.18632/oncotarget.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desale SS, Soni KS, Romanova S, Cohen SM, Bronich TK. Targeted delivery of platinum-taxane combination therapy in ovarian cancer. J Control Release. 2015;220(Pt B):651–9. doi: 10.1016/j.jconrel.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai L, Xu G, Shi C, Guo D, Wang X, Luo J. Telodendrimer nanocarrier for co-delivery of paclitaxel and cisplatin: a synergistic combination nanotherapy for ovarian cancer treatment. Biomaterials. 2015;37:456–688. doi: 10.1016/j.biomaterials.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song W, Tang Z, Li M, Lv S, Sun H, Deng M, et al. Polypeptide-based combination of paclitaxel and cisplatin for enhanced chemotherapy efficacy and reduced side-effects. Acta Biomater. 2014;10(3):1392–402. doi: 10.1016/j.actbio.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 53.Xiao H, Song H, Yang Q, Cai H, Qi R, Yan L, et al. A prodrug strategy to deliver cisplatin(IV) and paclitaxel in nanomicelles to improve efficacy and tolerance. Biomaterials. 2012;33(27):6507–19. doi: 10.1016/j.biomaterials.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 54.Meng H, Wang M, Liu H, Liu X, Situ A, Wu B, et al. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano. 2015;9(4):3540–57. doi: 10.1021/acsnano.5b00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noh I, Kim HO, Choi J, Choi Y, Lee DK, Huh YM, et al. Co-delivery of paclitaxel and gemcitabine via CD44-targeting nanocarriers as a prodrug with synergistic antitumor activity against human biliary cancer. Biomaterials. 2015;53:763–74. doi: 10.1016/j.biomaterials.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 56.Wang H, Agarwal P, Zhao S, Xu RX, Yu J, Lu X, et al. Hyaluronic acid-decorated dual responsive nanoparticles of pluronic F127, PLGA, and chitosan for targeted co-delivery of doxorubicin and irinotecan to eliminate cancer stem-like cells. Biomaterials. 2015:7274–89. doi: 10.1016/j.biomaterials.2015.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang P, Li J, Ghazwani M, Zhao W, Huang Y, Zhang X, et al. Effective co-delivery of doxorubicin and dasatinib using a PEG-Fmoc nanocarrier for combination cancer chemotherapy. Biomaterials. 2015:67104–14. doi: 10.1016/j.biomaterials.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanabe M, Ito Y, Tokudome N, Sugihara T, Miura H, Takahashi S, et al. Possible use of combination chemotherapy with mitomycin C and methotrexate for metastatic breast cancer pretreated with anthracycline and taxanes. Breast Cancer. 2009;16(4):301–06. doi: 10.1007/s12282-009-0093-0. [DOI] [PubMed] [Google Scholar]
- 59.Huizing MT, Vermorken JB, Rosing H, ten Bokkel Huinink WW, Mandjes I, Pinedo HM, et al. Pharmacokinetics of paclitaxel and three major metabolites in patients with advanced breast carcinoma refractory to anthracycline therapy treated with a 3-hour paclitaxel infusion: A european cancer centre (ECC) trial. Ann Oncol. 1995;6(7):699–704. doi: 10.1093/oxfordjournals.annonc.a059287. [DOI] [PubMed] [Google Scholar]
- 60.Mross K, Maessen P, van der Vijgh WJ, Gall H, Boven E, Pinedo HM. Pharmacokinetics and metabolism of epidoxorubicin and doxorubicin in humans. J Clin Oncol. 1988;6(3):517–26. doi: 10.1200/JCO.1988.6.3.517. [DOI] [PubMed] [Google Scholar]
- 61.Abraham SA, McKenzie C, Masin D, Ng R, Harasym TO, Mayer LD, et al. In vitro and in vivo characterization of doxorubicin and vincristine coencapsulated within liposomes through use of transition metal ion complexation and pH gradient loading. Clin Cancer Res. 2004;10(2):728–38. doi: 10.1158/1078-0432.ccr-1131-03. [DOI] [PubMed] [Google Scholar]
- 62**.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP–dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. A comprehensive rewiew on ABC transportors in normal and cancer cells and their effects on drug resistance. [DOI] [PubMed] [Google Scholar]
- 63.Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2) Oncogene. 2003;22(47):7340–58. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- 64.Patil Y, Sadhukha T, Ma L, Panyam J. Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tariquidar overcomes tumor drug resistance. J Control Release. 2009;136(1):21–29. doi: 10.1016/j.jconrel.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 65.Assanhou AG, Li W, Zhang L, Xue L, Kong L, Sun H, et al. Reversal of multidrug resistance by co-delivery of paclitaxel and lonidamine using a TPGS and hyaluronic acid dual-functionalized liposome for cancer treatment. Biomaterials. 2015:73284–95. doi: 10.1016/j.biomaterials.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 66.Werle M. Natural and synthetic polymers as inhibitors of drug efflux pumps. Pharm Res. 2008;25(3):500–11. doi: 10.1007/s11095-007-9347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cao H, Wang Y, He X, Zhang Z, Yin Q, Chen Y, et al. Codelivery of sorafenib and curcumin by directed self-assembled nanoparticles enhances therapeutic effect on hepatocellular carcinoma. Mol Pharm. 2015;12(3):922–31. doi: 10.1021/mp500755j. [DOI] [PubMed] [Google Scholar]
- 68.Stigliano C, Key J, Ramirez M, Aryal S, Decuzzi P. Radiolabeled polymeric nanoconstructs loaded with docetaxel and curcumin for cancer combinatorial therapy and nuclear imaging. Adv Funct Mater. 2015;25(22):3371–79. [Google Scholar]
- 69.Abdallah HM, Al-Abd AM, El-Dine RS, El-Halawany AM. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: a Review. J Adv Res. 2015;6(1):45–62. doi: 10.1016/j.jare.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jain AK, Thanki K, Jain S. Co-encapsulation of tamoxifen and quercetin in polymeric nanoparticles: implications on oral bioavailability, antitumor efficacy, and drug-induced toxicity. Mol Pharm. 2013;10(9):3459–74. doi: 10.1021/mp400311j. [DOI] [PubMed] [Google Scholar]
- 71.Gao M, Xu Y, Qiu L. Enhanced combination therapy effect on paclitaxel-resistant carcinoma by chloroquine co-delivery via liposomes. Int J Nanomedicine. 2015;10:6615–32. doi: 10.2147/IJN.S91463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gudas LJ, Wagner JA. Retinoids regulate stem cell differentiation. J Cell Physiol. 2011;226(2):322–30. doi: 10.1002/jcp.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun R, Liu Y, Li SY, Shen S, Du XJ, Xu CF, et al. Co-delivery of all-trans-retinoic acid and doxorubicin for cancer therapy with synergistic inhibition of cancer stem cells. Biomaterials. 2015;37:405–14. doi: 10.1016/j.biomaterials.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 74.Guo S, Miao L, Wang Y, Huang L. Unmodified drug used as a material to construct nanoparticles: Delivery of cisplatin for enhanced anti-cancer therapy. J Control Release. 2014;174:137–42. doi: 10.1016/j.jconrel.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61(4):250–81. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He C, Liu D, Lin W. Self-assembled core–shell nanoparticles for combined chemotherapy and photodynamic therapy of resistant head and neck cancers. ACS Nano. 2015;9(1):991–1003. doi: 10.1021/nn506963h. [DOI] [PubMed] [Google Scholar]
- 77.Master A, Livingston M, Sen Gupta A. Photodynamic nanomedicine in the treatment of solid tumors: perspectives and challenges. J Control Release. 2013;168(1):88–102. doi: 10.1016/j.jconrel.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tian G, Ren W, Yan L, Jian S, Gu Z, Zhou L, et al. Red-emitting upconverting nanoparticles for photodynamic therapy in cancer cells under near-infrared excitation. Small. 2013;9(11):1929–38. doi: 10.1002/smll.201201437. [DOI] [PubMed] [Google Scholar]
- 79.Yang S, Li N, Liu Z, Sha W, Chen D, Xu Q, et al. Amphiphilic copolymer coated upconversion nanoparticles for near-infrared light-triggered dual anticancer treatment. Nanoscale. 2014;6(24):14903–10. doi: 10.1039/c4nr05305b. [DOI] [PubMed] [Google Scholar]
- 80.Chatterjee DK, Gnanasammandhan MK, Zhang Y. Small upconverting fluorescent nanoparticles for biomedical applications. Small. 2010;6(24):2781–95. doi: 10.1002/smll.201000418. [DOI] [PubMed] [Google Scholar]
- 81.Song X, Zhang R, Liang C, Chen Q, Gong H, Liu Z. Nano-assemblies of J-aggregates based on a NIR dye as a multifunctional drug carrier for combination cancer therapy. Biomaterials. 2015;57:84–92. doi: 10.1016/j.biomaterials.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 82.Li X, Takashima M, Yuba E, Harada A, Kono K. Pegylated PAMAM dendrimer-doxorubicin conjugate-hybridized gold nanorod for combined photothermal-chemotherapy. Biomaterials. 2014;35(24):6576–84. doi: 10.1016/j.biomaterials.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 83.Chen YW, Su YL, Hu SH, Chen SY. Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv Drug Deliv Rev. 2016 doi: 10.1016/j.addr.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 84.Chen Q, Liang C, Wang C, Liu Z. An imagable and photothermal “Abraxane-like “ nanodrug for combination cancer therapy to treat subcutaneous and metastatic breast tumors. Adv Mater. 2015;27(5):903–10. doi: 10.1002/adma.201404308. [DOI] [PubMed] [Google Scholar]
- 85**.Oh YK, Park TG. siRNA delivery systems for cancer treatment. Adv Drug Deliv Rev. 2009;61(10):850–62. doi: 10.1016/j.addr.2009.04.018. A detailed overview of siRNA delivery, challenges and applications in cancer treatment. [DOI] [PubMed] [Google Scholar]
- 86**.Chen Y, Gao DY, Huang L. In vivo delivery of miRNAs for cancer therapy: Challenges and strategies. Adv Drug Deliv Rev. 2015;81:128–41. doi: 10.1016/j.addr.2014.05.009. Introduction to the mechanism, challenges and applications of miRNA in cancer therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang S, Yin Q, Su J, Sun H, Meng Q, Chen Y, et al. Inhibition of metastasis and growth of breast cancer by pH-sensitive poly (β-amino ester) nanoparticles co-delivering two siRNA and paclitaxel. Biomaterials. 2015;48:1–15. doi: 10.1016/j.biomaterials.2015.01.049. [DOI] [PubMed] [Google Scholar]
- 88.Yin T, Wang L, Yin L, Zhou J, Huo M. Co-delivery of hydrophobic paclitaxel and hydrophilic AURKA specific siRNA by redox-sensitive micelles for effective treatment of breast cancer. Biomaterials. 2015;61:10–25. doi: 10.1016/j.biomaterials.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 89.Shi S, Han L, Deng L, Zhang Y, Shen H, Gong T, et al. Dual drugs (microRNA-34a and paclitaxel)-loaded functional solid lipid nanoparticles for synergistic cancer cell suppression. J Control Release. 2014;194:228–37. doi: 10.1016/j.jconrel.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 90.Feng Q, Yu MZ, Wang JC, Hou WJ, Gao LY, Ma XF, et al. Synergistic inhibition of breast cancer by co-delivery of VEGF siRNA and paclitaxel via vapreotide-modified core-shell nanoparticles. Biomaterials. 2014;35(18):5028–38. doi: 10.1016/j.biomaterials.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 91.Dong D, Gao W, Liu Y, Qi XR. Therapeutic potential of targeted multifunctional nanocomplex co-delivery of siRNA and low-dose doxorubicin in breast cancer. Cancer Lett. 2015;359(2):178–86. doi: 10.1016/j.canlet.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 92.Tang S, Yin Q, Zhang Z, Gu W, Chen L, Yu H, et al. Co-delivery of doxorubicin and RNA using pH-sensitive poly (β-amino ester) nanoparticles for reversal of multidrug resistance of breast cancer. Biomaterials. 2014;35(23):6047–59. doi: 10.1016/j.biomaterials.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 93.Qian X, Long L, Shi Z, Liu C, Qiu M, Sheng J, et al. Star-branched amphiphilic PLA-b-PDMAEMA copolymers for co-delivery of miR-21 inhibitor and doxorubicin to treat glioma. Biomaterials. 2014;35(7):2322–35. doi: 10.1016/j.biomaterials.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 94.Chen S, Lei Q, Li SY, Qin SY, Jia HZ, Cheng YJ, et al. Fabrication of dual responsive co-delivery system based on three-armed peptides for tumor therapy. Biomaterials. 2016;92:25–35. doi: 10.1016/j.biomaterials.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 95.Jia HZ, Zhang W, Zhu JY, Yang B, Chen S, Chen G, et al. Hyperbranched-hyperbranched polymeric nanoassembly to mediate controllable co-delivery of siRNA and drug for synergistic tumor therapy. J Control Release. 2015;216:9–17. doi: 10.1016/j.jconrel.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 96.Wang K, Hu Q, Zhu W, Zhao M, Ping Y, Tang G. Structure-invertible nanoparticles for triggered co-delivery of nucleic acids and hydrophobic drugs for combination cancer therapy. Adv Funct Mater. 2015;25(22):3380–92. [Google Scholar]
- 97.Shen S, Sun CY, Du XJ, Li HJ, Liu Y, Xia JX, et al. Co-delivery of platinum drug and siNotch1 with micelleplex for enhanced hepatocellular carcinoma therapy. Biomaterials. 2015;70:71–83. doi: 10.1016/j.biomaterials.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 98.Shen J, Sun H, Meng Q, Yin Q, Zhang Z, Yu H, et al. Simultaneous inhibition of tumor growth and angiogenesis for resistant hepatocellular carcinoma by co-delivery of sorafenib and survivin small hairpin RNA. Mol Pharm. 2014;11(10):3342–51. doi: 10.1021/mp4006408. [DOI] [PubMed] [Google Scholar]
- 99.Mittal A, Chitkara D, Behrman SW, Mahato RI. Efficacy of gemcitabine conjugated and miRNA-205 complexed micelles for treatment of advanced pancreatic cancer. Biomaterials. 2014;35(25):7077–87. doi: 10.1016/j.biomaterials.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 100.Zhao X, Li F, Li Y, Wang H, Ren H, Chen J, et al. Co-delivery of HIF1α siRNA and gemcitabine via biocompatible lipid-polymer hybrid nanoparticles for effective treatment of pancreatic cancer. Biomaterials. 2015;46:13–25. doi: 10.1016/j.biomaterials.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 101.Lee E, Oh C, Kim IS, Kwon IC, Kim S. Co-delivery of chemosensitizing siRNA and an anticancer agent via multiple monocomplexation-induced hydrophobic association. J Control Release. 2015;210:105–14. doi: 10.1016/j.jconrel.2015.05.262. [DOI] [PubMed] [Google Scholar]
- 102.Lee SY, Yang CY, Peng CL, Wei MF, Chen KC, Yao CJ, et al. A theranostic micelleplex co-delivering SN-38 and VEGF siRNA for colorectal cancer therapy. Biomaterials. 2016;86:92–105. doi: 10.1016/j.biomaterials.2016.01.068. [DOI] [PubMed] [Google Scholar]
- 103.Zhang N, Huang Y, Wu F, Zhao Y, Li X, Shen P, et al. Co-delivery of miR-124 mimic and obatoclax by cholesterol-penetratin micelles simultaneously inducing apoptosis and inhibiting autophagic flux against breast cancer in vitro and in vivo. Mol Pharm. 2016;13(7):2466–83. doi: 10.1021/acs.molpharmaceut.6b00211. [DOI] [PubMed] [Google Scholar]
- 104**.Kemp JA, Shim MS, Heo CY, Kwon YJ. “Combo” nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv Drug Deliv Rev. 2016;98:3–18. doi: 10.1016/j.addr.2015.10.019. A summary of rationales, challenges, principles and examples of combination therapy in cancer treatment. [DOI] [PubMed] [Google Scholar]
- 105.Li Y, Xu B, Bai T, Liu W. Co-delivery of doxorubicin and tumor-suppressing p53 gene using a POSS-based star-shaped polymer for cancer therapy. Biomaterials. 2015;55:12–23. doi: 10.1016/j.biomaterials.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. J Control Release. 2013;172(3):962–74. doi: 10.1016/j.jconrel.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deng X, Cao M, Zhang J, Hu K, Yin Z, Zhou Z, et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials. 2014;35(14):4333–44. doi: 10.1016/j.biomaterials.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 108.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3(8):917–21. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 109.Yu B, Tang C, Yin C. Enhanced antitumor efficacy of folate modified amphiphilic nanoparticles through co-delivery of chemotherapeutic drugs and genes. Biomaterials. 2014;35(24):6369–78. doi: 10.1016/j.biomaterials.2014.04.095. [DOI] [PubMed] [Google Scholar]
- 110.Shen J, Wang Q, Hu Q, Li Y, Tang G, Chu PK. Restoration of chemosensitivity by multifunctional micelles mediated by P-gp siRNA to reverse MDR. Biomaterials. 2014;35(30):8621–34. doi: 10.1016/j.biomaterials.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 111.Chen W, Yuan Y, Cheng D, Chen J, Wang L, Shuai X. Co-delivery of doxorubicin and siRNA with reduction and pH dually sensitive nanocarrier for synergistic cancer therapy. Small. 2014;10(13):2678–87. doi: 10.1002/smll.201303951. [DOI] [PubMed] [Google Scholar]
- 112.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl 3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 113.Yang ZZ, Li JQ, Wang ZZ, Dong DW, Qi XR. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials. 2014;35(19):5226–39. doi: 10.1016/j.biomaterials.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 114**.Ernsting MJ, Murakami M, Roy A, Li SD. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J Control Release. 2013;172(3):782–94. doi: 10.1016/j.jconrel.2013.09.013. Summary of formulation and biological factors to be considerend for designing tumor-targeted drug delivery systems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meng H, Zhao Y, Dong J, Xue M, Lin Y-S, Ji Z, et al. Two-wave nanotherapy to target the stroma and optimize gemcitabine delivery to a human pancreatic cancer model in mice. ACS Nano. 2013;7(11):10048–65. doi: 10.1021/nn404083m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miao L, Wang Y, Lin CM, Xiong Y, Chen N, Zhang L, et al. Nanoparticle modulation of the tumor microenvironment enhances therapeutic efficacy of cisplatin. J Control Release. 2015;217:27–41. doi: 10.1016/j.jconrel.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tozer GM, Kanthou C, Parkins CS, Hill SA. The biology of the combretastatins as tumour vascular targeting agents. Int J Exp Pathol. 2002;83(1):21–38. doi: 10.1046/j.1365-2613.2002.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118**.Sengupta S, Eavarone D, Capila I, Zhao G, Watson N, Kiziltepe T, et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436(7050):568–72. doi: 10.1038/nature03794. A landmark paper regarding sequential delivery of multiple agents using a single particle. [DOI] [PubMed] [Google Scholar]
- 119**.Zhang R, Yang J, Sima M, Zhou Y, Kopecek J. Sequential combination therapy of ovarian cancer with degradable N-(2-hydroxypropyl)methacrylamide copolymer paclitaxel and gemcitabine conjugates. Proc Natl Acad Sci U S A. 2014;111(33):12181–6. doi: 10.1073/pnas.1406233111. Reports sequential delivery of paclitaxel and gemcitabine for the therapy of ovarian cancer using drug-polymer conjugates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saif MW. U.S. Food and drug administration approves paclitaxel protein-bound particles (Abraxane®) in combination with gemcitabine as first-line treatment of patients with metastatic pancreatic cancer. J Pancreas. 2013;14(6):686–8. doi: 10.6092/1590-8577/2028. [DOI] [PubMed] [Google Scholar]
- 121.Qian X, Ren Y, Shi Z, Long L, Pu P, Sheng J, et al. Sequence-dependent synergistic inhibition of human glioma cell lines by combined temozolomide and miR-21 inhibitor gene therapy. Mol Pharm. 2012;9(9):2636–45. doi: 10.1021/mp3002039. [DOI] [PubMed] [Google Scholar]
- 122.Ren Y, Wang R, Gao L, Li K, Zhou X, Guo H, et al. Sequential co-delivery of miR-21 inhibitor followed by burst release doxorubicin using NIR-responsive hollow gold nanoparticle to enhance anticancer efficacy. J Control Release. 2016;228:74–86. doi: 10.1016/j.jconrel.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 123.Damaraju VL, Kuzma M, Mowles D, Cass CE, Sawyer MB. Interactions of multitargeted kinase inhibitors and nucleoside drugs: Achilles heel of combination therapy? Mol Cancer Ther. 2015;14(1):236–45. doi: 10.1158/1535-7163.MCT-14-0337. [DOI] [PubMed] [Google Scholar]
- 124.Dear RF, McGeechan K, Jenkins MC, Barratt A, Tattersall MH, Wilcken N. Combination versus sequential single agent chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev. 2013;(12):CD008792. doi: 10.1002/14651858.CD008792.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yoshizawa Y, Ogawara K, Fushimi A, Abe S, Ishikawa K, Araki T, et al. Deeper penetration into tumor tissues and enhanced in vivo antitumor activity of liposomal paclitaxel by pretreatment with angiogenesis inhibitor SU5416. Mol Pharm. 2012;9(12):3486–94. doi: 10.1021/mp300318q. [DOI] [PubMed] [Google Scholar]
- 126.Hariri G, Edwards AD, Merrill TB, Greenbaum JM, van der Ende AE, Harth E. Sequential targeted delivery of paclitaxel and camptothecin using a cross-linked “nanosponge” network for lung cancer chemotherapy. Mol Pharm. 2014;11(1):265–75. doi: 10.1021/mp400432b. [DOI] [PubMed] [Google Scholar]
- 127.Lu Z, Tsai M, Lu D, Wang J, Wientjes MG, Au JL. Tumor-penetrating microparticles for intraperitoneal therapy of ovarian cancer. J Pharmacol Exp Ther. 2008;327(3):673–82. doi: 10.1124/jpet.108.140095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li L, Sun W, Zhang Z, Huang Y. Time-staggered delivery of docetaxel and H1-S6A,F8A peptide for sequential dual-strike chemotherapy through tumor priming and nuclear targeting. J Control Release. 2016;232:62–74. doi: 10.1016/j.jconrel.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 129.Stirland DL, Matsumoto Y, Toh K, Kataoka K, Bae YH. Analyzing spatiotemporal distribution of uniquely fluorescent nanoparticles in xenograft tumors. J Control Release. 2016;227:38–44. doi: 10.1016/j.jconrel.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Duan X, Xiao J, Yin Q, Zhang Z, Yu H, Mao S, et al. Smart pH-sensitive and temporal-controlled polymeric micelles for effective combination therapy of doxorubicin and disulfiram. ACS Nano. 2013;7(7):5858–69. doi: 10.1021/nn4010796. [DOI] [PubMed] [Google Scholar]
- 131*.Dong Y, Yang J, Liu H, Wang T, Tang S, Zhang J, et al. Site-specific drug-releasing polypeptide nanocarriers based on dual-pH response for enhanced therapeutic efficacy against drug-resistant tumors. Theranostics. 2015;5(8):890–904. doi: 10.7150/thno.11821. Reports a polypeptide-based nanocarrier system for pH-controlled, site-specific drug release in drug-resistant tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132*.Jiang T, Mo R, Bellotti A, Zhou J, Gu Z. Gel–liposome-mediated co-delivery of anticancer membrane-associated proteins and small-molecule drugs for enhanced therapeutic efficacy. Adv Funct Mater. 2014;24(16):2295–304. Reports a core-shell type NP for sequential and site-specific delivery of a protein and a small-molecule drug. [Google Scholar]
- 133.Jiang T, Sun W, Zhu Q, Burns NA, Khan SA, Mo R, et al. Furin-mediated sequential delivery of anticancer cytokine and small-molecule drug shuttled by graphene. Adv Mater. 2015;27(6):1021–28. doi: 10.1002/adma.201404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee MJ, Ye AS, Gardino AK, Heijink AM, Sorger PK, MacBeath G, et al. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell. 2012;149(4):780–94. doi: 10.1016/j.cell.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65(14):6029–33. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 136.Pacardo DB, Ligler FS, Gu Z. Programmable nanomedicine: synergistic and sequential drug delivery systems. Nanoscale. 2015;7(8):3381–91. doi: 10.1039/c4nr07677j. [DOI] [PubMed] [Google Scholar]
- 137.Zhang HB, Wang G, Yang H. Drug delivery systems for differential release in combination therapy. Expert Opin Drug Deliv. 2011;8(2):171–90. doi: 10.1517/17425247.2011.547470. [DOI] [PubMed] [Google Scholar]
- 138. [Last accessed: May 14, 2016];NCI Dictionary of Cancer Terms. Available at: http://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=330184.
- 139.Abouelmagd SA, Sun B, Chang AC, Ku YJ, Yeo Y. Release kinetics study of poorly water-soluble drugs from nanoparticles: Are we doing it right? Mol Pharm. 2015;12(3):997–1003. doi: 10.1021/mp500817h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Park J, Kadasala NR, Abouelmagd SA, Castanares MA, Collins DS, Wei A, et al. Polymer–iron oxide composite nanoparticles for EPR-independent drug delivery. Biomaterials. 2016;101:285–95. doi: 10.1016/j.biomaterials.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yeo Y, Kim B-K. Drug carriers: not an innocent delivery man. AAPS J. 2015;17(5):1096–104. doi: 10.1208/s12248-015-9789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142**.Goldman A, Majumder B, Dhawan A, Ravi S, Goldman D, Kohandel M, et al. Temporally sequenced anticancer drugs overcome adaptive resistance by targeting a vulnerable chemotherapy-induced phenotypic transition. Nat Commun. 2015:66139. doi: 10.1038/ncomms7139. Reports sequential delivery of anticancer drugs to overcome drug-resistance induced by taxane. [DOI] [PMC free article] [PubMed] [Google Scholar]