Synopsis
Weight regain after bariatric surgery is common and can be managed with surgical interventions or less morbid endoscopic techniques. These endoscopic approaches target structural postoperative changes that are associated with weight regain, most notably dilation of the gastrojejunal anastomosis aperture. Purse string suture placement, as well as argon plasma coagulation application to the anastomosis, may result in significant and durable weight loss. Furthermore, various endoscopic approaches may be used to safely and effectively manage other complications of bariatric surgery that may result in poor weight loss or weight regain after surgery, including fistula formation.
Keywords: Bariatric endoscopy, therapeutic endoscopy, weight regain, endoscopic surgery, endoscopic suturing, gastric bypass, surgical complications, fistula
Introduction
Obesity is a lifelong condition of pandemic proportion that requires long-term multidisciplinary management leading up to and beyond any single intervention. Even after restrictive and metabolic surgeries like a Roux-en-Y gastric bypass (RYGB), patients have the potential to experience significant weight regain which is why a long-term care team is necessary for management of obesity. An emerging member of this care team is the bariatric endoscopist. The field of Endobariatrics includes revision procedures for patients who experience weight regain after bariatric surgery, as well as primary endoscopic procedures for the management of obesity.(1) This field also provides medical management of obesity as well as minimally invasive endoscopic treatments for various complications of bariatric surgery including perforations, leaks, stenosis and fistulas to name a few. This review will focus on the currently available endoscopic revision procedures for patients who experience weight regain after bariatric surgery, and will also touch on endoscopic techniques in the management of other complications of bariatric surgery that may contribute to weight regain including ulcerations and fistulae.
Patient Evaluation for Weight Regain after Bariatric Surgery
Prior to offering endoscopic revision procedures, an appropriate infrastructure must be in place. As a part of a multidisciplinary center offering care to the bariatric patient, the customary endoscopy suite will need to make some adjustments to provide safe, dignified and high quality care for this patient population. Common adaptations needed to safely and comfortably accommodate bariatric patients include:
Bariatric specialty furniture for the clinic and endoscopy suite including the waiting areas
Appropriately sized bathrooms, reinforced toilets and room structure including larger doorways
Bariatric rated stretchers and tables for the procedural arena
Anesthesia team attuned to and comfortable with bariatric patients
As part of the evaluation of the patient with weight regain after bariatric surgery, it is important to obtain a thorough medical history and physical examination. Comorbid conditions that may increase risk associated with procedural sedation are noted, especially since some endoscopic techniques may be safely performed with only conscious sedation, reducing the cost and time required by monitored anesthesiologist care. Prior operative reports should be reviewed to determine the patient's surgical anatomy including any post-operative complications that may have occurred which will aid in endoscopic procedural planning. The patient's pre-surgical weight, post-surgical nadir weight, and total weight regained should be recorded. It is important to discuss lifestyle issues related to weight regain, including diet and exercise habits, to determine other contributing factors to the patient's weight regain. In particular, dietary habits to avoid include grazing, rather than eating discrete meals at defined times and consumption of soft calories or “sliders”, rather than solid whole foods which require chewing and digestion. These two eating habits must be addressed prior to consideration of any endoscopic therapy. Appropriate referrals to a dietician, lifestyle coach and/or psychologist are made depending on the individual patient.
The cause of weight regain after bariatric surgery is generally multifactorial, but in some cases, reversible medical causes may be at play. Evaluation for medical conditions contributing to weight regain after gastric bypass include:
- Iron studies. Iron deficiency anemia must be corrected.
- TSH and free T4. Hypothyroidism and other relevant endocrinopathies should be addressed.
- Exercise and physical therapy. Movement limitations including arthritis should be addressed if possible.
Most patients with unresolved obesity, or those who have redevelop obesity (BMI >30kg/m2) and have had all of the above issues addressed will be considered candidates for endoscopic therapy. This is especially true with the presence of comorbid conditions related to their obesity (ie diabetes, hypertension, hyperlipidemia, fatty liver disease, obstructive sleep apnea, arthritis).
Endoscopic Bariatric Revision Procedures
Currently available endoscopic techniques for weight loss in the post-bariatric surgery patient are primarily aimed at patients who have undergone RYGB, and less commonly those with laparoscopic sleeve gastrectomy (LSG) anatomy. Cumulative numbers of patients who have undergone RYGB in the US are steadily increasing. Up to 20% of these patients fail to achieve therapeutic success defined as 50% excess weight loss at one year, and another 30% will experience some degree of weight regain, which has no consensus definition, but may be defined as 15% increase from nadir weight.(2-4) This phenomenon of weight regain can affect patient quality of life, lead to return or worsening of comorbid medical conditions, and increases healthcare expenditure. While maladaptive eating behaviors and sedentary lifestyle may contribute to weight regain, some reversible structural issues related to the patient's pouch and anatomy also contribute. Surgical revision, including limb-lengthening procedures, are effective and used in up to 13% of RYGB patients with weight regain. However, these are associated with complication rates of up to 50% and mortality rates more than double that of the original surgery, likely owing to the complexity of the non-native abdominal cavity with associated scars, adhesions and altered anatomy.(5-8) Out of this landscape, minimally invasive endoscopic methods of revision for weight regain after surgical bypass have emerged, targeting the dilated gastric pouch and gastrojejunal anastomosis through use of electrocautery and/or endoscopic suturing or plication techniques.
One landmark study has shown that dilation of the aperture of the gastrojejunal anastomosis (GJA) after surgery, is correlated with weight regain after RYGB.(9) In a multivariable logistic regression model, enlarged stomal size was the single greatest predictor of weight regain and a linear relationship between stomal aperture and weight regain was revealed. Based on this data, stomal diameter greater than or equal to 15mm may be defined as dilated and endoscopic revision of the anastomosis should be considered.
Argon Plasma Coagulation
Given the association between dilated gastrojejunal anastomosis and weight regain after RYGB, techniques to reduce the outlet diameter through formation of scar-tissue was originally studied using sclerotherapy and more recently through application of argon plasma coagulation (APC).(10-12) Endoscopic sclerotherapy, similar to sclerotherapy of esophageal varices, was accomplished using submucosal needle injection of sodium morrhuate around the gastrojejunal anastomosis to create edema, scarring and ideally reduction in aperture of the anastomosis. Because of safety concerns and decreasing availability of sodium morrhuate, as well as the availability of a safer and more easily applied technique using APC, sclerotherapy is no longer utilized. A study of 28 patients receiving sclerotherapy demonstrated that the majority (64%) of patients lost more than 75% of their regained weight after an average 2.3 procedures repeated every three to six months apart. Anastomotic diameters greater than 15mm are less likely to benefit from this technique and may benefit more from an endoscopic suturing revision procedure. A similar but newer and safer technique utilizing APC has gained popularity over sclerotherapy.(13, 14) In this technique, APC resurfacing of the gastrojejunal anastomosis is accomplished through application of cautery to the gastric side of the anastomosis by touching the tip of a straight-fire APC catheter to the target area. Unlike with most APC techniques, contact with the mucosa is made intentionally to allow for deeper, submucosal cautery. This creates a focal coagulation injury to the mucosa as well as the deeper submucosal layers. In one study of 30 patients who underwent 3 sessions of APC for weight regain of average 43.2lbs after their RYGB, an average of 34lbs were lost. The stomal diameter was reduced 66.9% after completion of these three sessions. We use pulsed APC with settings of flow 0.8 L/s, effect 2 and 55W. Circumferential resurfacing therapy is applied around the anastomosis in two to three rings of focal coagulation [Fig 1]. Edema, ulceration and scar tissue formation result in gradual aperture reduction. To allow for maximal healing and to prevent bleeding ulceration, patients are maintained on a twice daily PPI, as well as a liquid diet for 45 days after the procedure. Patients typically return for repeat therapy every 8-10 weeks for 3-4 sessions until the desired aperture size and satiety effect is reached. One international prospective nonrandomized study of 30 patients using APC at 90W revealed that after three treatment sessions every eight weeks, an average 15.5 kg of the average 19 kg regained after bariatric surgery was lost.(14)
Figure 1.
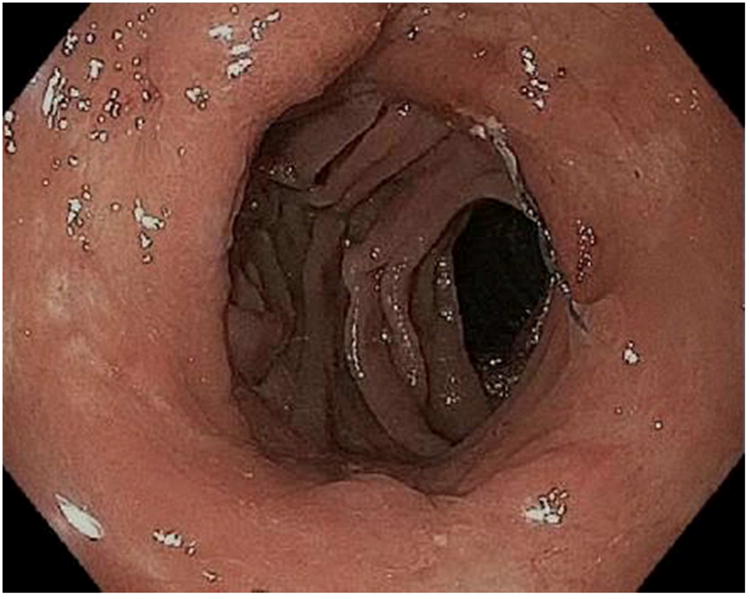
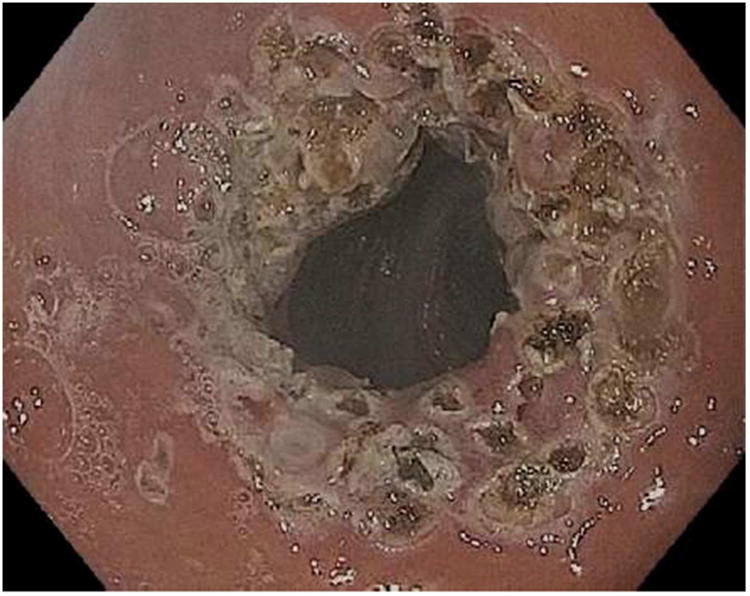
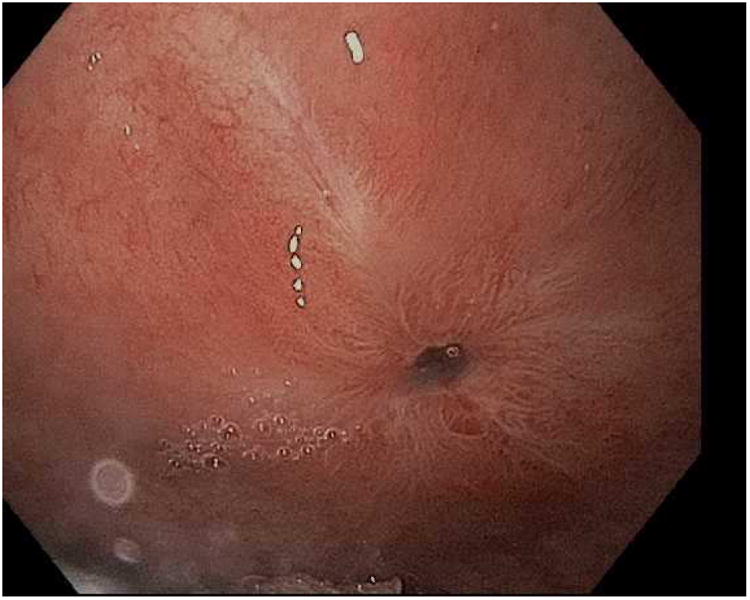
APC (argon plasma coagulation) resurfacing of the gastrojejunal anastomosis. A; Dilated gastrojejunal anastomosis with aperture of approximately 15mm, B; APC treatment applied to the gastric side of the stoma, C; In rare cases, overtreatment may result in stenosis requiring dilation.
The Transoral Outlet Reduction and Purse String
Using the OverStitch platform (Apollo Endosurgery, Austin, TX) the Transoral Outlet Reduction, or TORe procedure, is used internationally and at many centers across the US to reduce the aperture of the gastrojejunal anastomosis through use of a purse string suture of the anastomosis. The OverStitch device [Fig 2] is attached to the distal end of a double channel therapeutic endoscope, which allows for both use of the catheter-based actuating needle for driving and reloading suture, as well as deployment of a helix device to allow for tissue retraction and deeper suture placement.
Figure 2.
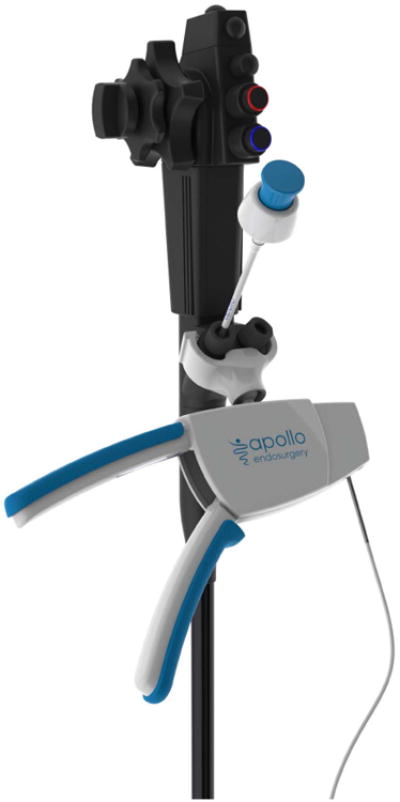
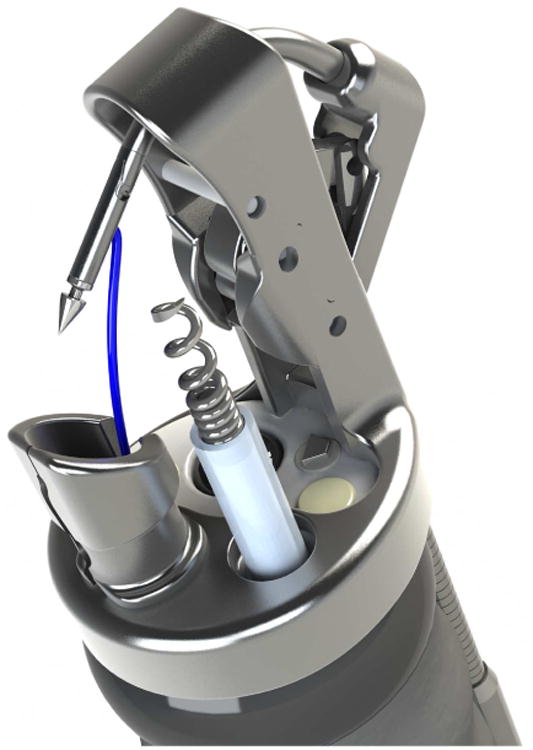
The Overstitch endoscopic suturing device. A; Handle to drive the needle and needle exchange catheter are attached to a double channel therapeutic endoscope, B; The distal attachement with needle and suture attached to the needle driver arm with helical tissue grabbing tool through the second working channel.
For the TORe procedure, an esophageal overtube is placed to protect the proximal esophagus from trauma that may occur with repeated intubations of the suturing device as it may be removed and replaced through the esophagus during the procedure. To prepare the outlet for suturing, the gastric mucosa adjacent to the anastomosis is treated with APC (forced coagulation, 0.8 L/min, 30 watts), and then full thickness sutures are placed with the needle driven from the jejunal to gastric side of the anastomosis to reduce the aperture [Fig 3]. One study of TORe that included 25 patients with dilated GJA resulted, on average, in an aperture reduction from 26.4mm to 6mm with weight loss of 11.7kg (69.5% of the regained weight was lost) at six months without adverse events.(15) In this study, smaller apertures resulted in increased nausea and vomiting and higher stitch loss with subsequent weight loss failure. As such, a modified technique using a purse string suture pattern was developed. Depending on the size of the outlet, 8 to 12 running stitches are placed using a single suture to create a purse string. Upon completion of the purse string, the suture is tightened over an 8mm through-the-scope esophageal balloon to size the final outlet diameter.
Figure 3.
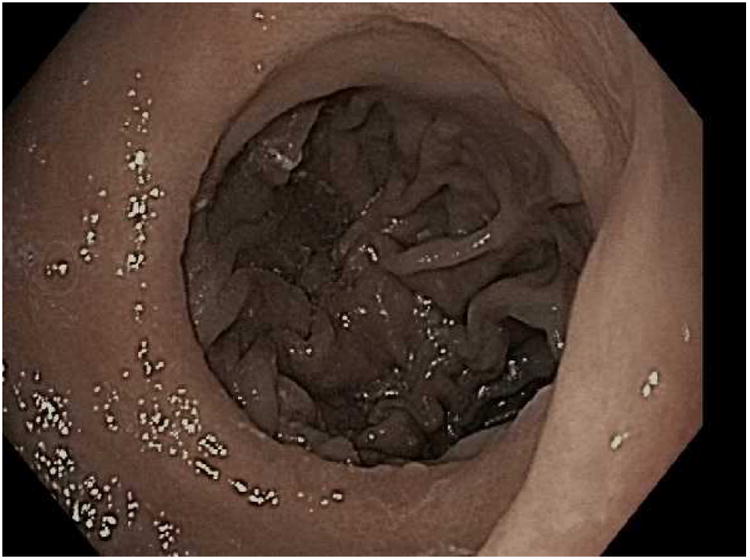
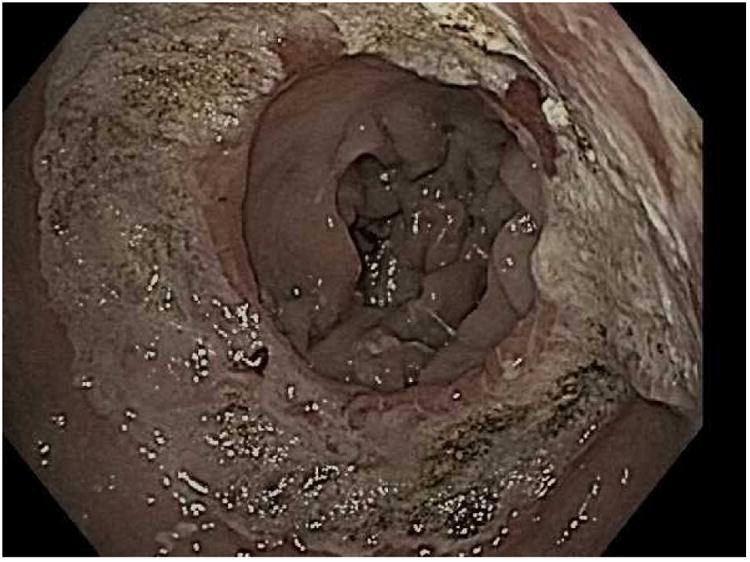
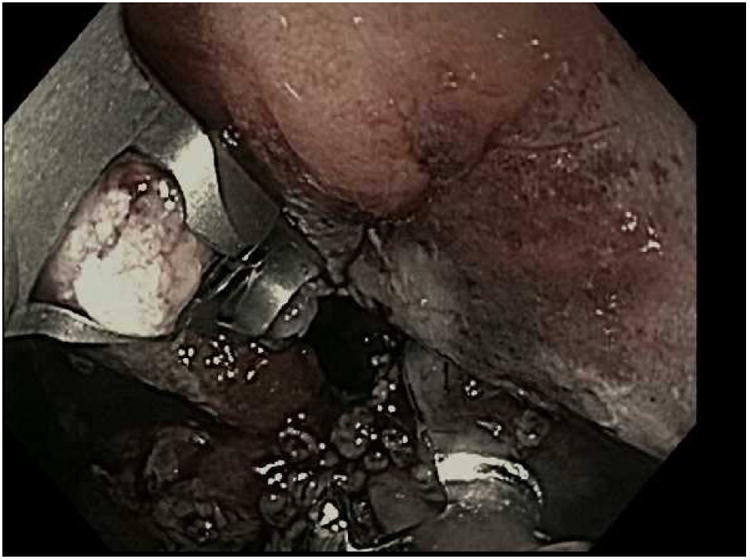
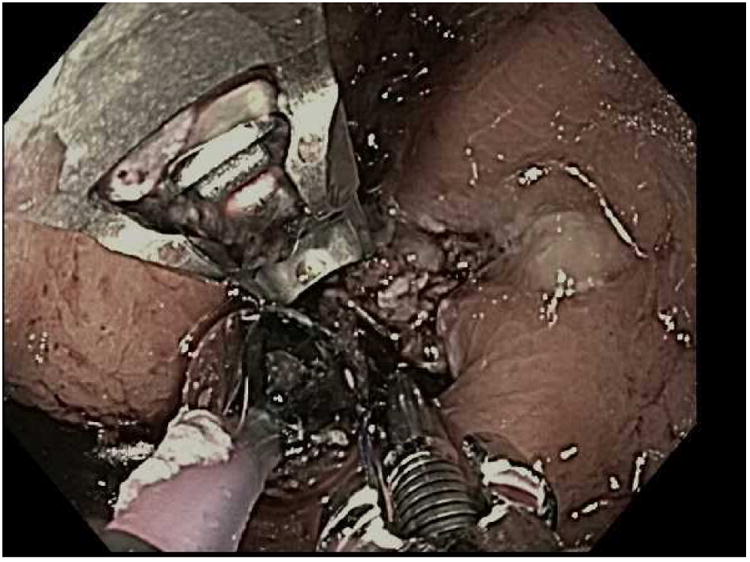
The TORe (transoral outlet revision) procedure. A; Dilated stoma of approximately 25mm is examined B; APC cautery is applied to the gastric musoca around the stoma, C; Full-thickness purse-string suture is then placed around the stoma, D; Final aperture is sized using an 8mm balloon.
A randomized, sham-controlled, multicenter trial of transoral oulet reduction using the Bard Endocinch device was completed, and provided Level I evidence for the short-term safety and efficacy of TORe.(16) In this study, patients who underwent TORe experienced significantly greater weight loss than controls (3.5% versus 0.4%, p = 0.21). Systolic blood pressure was statistically improved and there was a trend toward improved metabolic parameters. This TORe study was performed with the EndoCinch suction-based superficial mucosal suturing device. Newer devices, as with the Apollo OverStitch, allow for full-thickness suturing and improved durability. A study comparing outlet reduction using the full-thickness OverStitch device to the superficial suturing with the EndoCinch device in 118 patients revealed superior weight loss in the OverStitch patients at both six and 12 months.(17) A follow up study showed enhanced durability with this system at three years, with an average 19.2% EWL at the three year mark post-TORe.(18) This summarizes the most complete body of work regarding revision of gastric bypass showing the procedure's effectiveness and durability within a multidisciplinary plan of care.
Post-procedure recommendations after completion of TORe include:
Twice daily PPI (ie omeprazole 40mg opened into applesauce) for 6 weeks
Staged diet: 48 hours clear liquids, 6 weeks full liquid diet, 2 weeks of soft solid foods followed by a solid calorie diet for maintenance
The Incisionless Operating Platform, or IOP (USGI, San Clemente, CA) used in the Primary Obesity Surgery, Endolumenal (POSE) procedure, has also been studied for endoscopic revision and management of weight regain after RYGB termed the Revision Obesity Surgery, Endolumenal, or ROSE procedure [Fig 4]. Specifically, this procedure is considered for patients with weight regain in the setting of an enlarged pouch as well as a dilated gastrojejunal anastomosis. Full thickness plications are placed with the disposable transoral platform with the goal of reducing both pouch size and anastomosis aperture. A study of 20 patients undergoing ROSE demonstrated technical success in 85%, with weight loss of 8.8kg at three months.(19) This study emphasized the importance of revising both the outlet and pouch, rather than the pouch alone. Particularly important to successful weight loss was reduction of the gastrojejunal anastomosis to less than one centimeter. A larger prospective multicenter study of 116 patients achieved technical success in 97%, with 32% of the weight regained after RYGB lost at six months and no significant adverse events.(20)
Figure 4.

The IOP (Incisionless Operating Platform). This disposable one-time use platform employs use of a slim gastroscope through one channel for visualization (not shown), and includes a tissue plication device (shown) through the main operating channel.
Other techniques of outlet revision have been reported, however these are limited to small series and mainstream adoption have not occurred. One technique used to address the pouch and gastrojejunal anastomosis dilation includes use of a large over-the-scope clip to reduce the rapid emptying that may occur with stomal dilation.(21) Another, using radiofrequency ablation to treat the pouch and stoma in 25 patients reported 18.4% excess weight loss at 12 months.(22) Reduction of the pouch and outlet using varied fasteners have also been reported, but are not commonly used today.(23)
Weight Regain after Sleeve Gastrectomy
Patients who have previously undergone LSG may re-gain weight if the sleeve is dilated from food bolus consumption over time. As in the case of a dilated RYGB pouch, the dilated sleeve may be managed with endoscopic suturing or tissue plication to reduce the sleeve diameter. Several reports exist detailing the feasibility and safety of this revision procedure using endoscopic suturing to reduce the sleeve volume, however more data will needed before this can be considered for mainstream use.(24)
Endoscopic Management of other Complications of Bariatric Surgery
Other complications of bariatric surgery may be implicated in weight regain. Gastrogastric fistula formation essentially results in metabolic reversal of a RYGB, as foodstuff may be permitted to enter the remnant stomach which may 1) serve as a reservoir for increased volume of consumption and 2) will negate the metabolic effects of the bypass surgery, allowing nutrient intake to be exposed to and absorbed by the previously excluded duodenum and proximal jejunum. Marginal ulceration, which may lead to iron deficiency anemia, is also often diagnosed and managed by the endoscopist. Marginal ulceration may lead to pain with eating resulting in conversion to soft calories. Both iron deficiency anemia and soft calorie consumption have been associated with weight regain. Endoscopic techniques for treating these two conditions are reviewed here.
Gastro-Gastric Fistula
Gastro-gastric fistula formation was a common complication of the non-divided RYGB surgical technique, previously reported in up to nearly 50% of cases.(25) Over the past decade, routine complete transection of the stomach and gastric pouch has significantly reduced this risk to a reported incidence of 0-6%.(26) Gastro-gastric fistulae often result in weight regain due to nutrients entering the gastric remnant and duodenum with reversal of the metabolic effects of the RYGB. In rare cases, complete reversal of the bypass anatomy may occur with stenosis of the gastrojejunal anastomosis due to recurrent ulceration and dilation of the fistula. Endoscopic therapies for gastro-gastric fistulae, including clips, glue, and endoscopic suturing have been reported and are increasingly considered as the first line approach given the substantial morbidity and mortality associated with repeat surgery. While the availability of various endoscopic approaches will depend on a center's expertise, endoscopic suturing of the fistula has been studied with the most success in durable fistula closure [Fig 7], however results are still limited. An initial study of 95 patients undergoing endoscopic gastrogastric fistula closure demonstrated initial success in 95% of patients, though 65% had recurrent fistula.(27) In this study, the only significant predictor of fistula recurrence was an initial fistula diameter greater than 20mm. A multicenter study of 20 patients using the OverStitch device achieved immediate closure in 100% of patients, with long term closure in six (30%).(28) Another study reported durable endoscopic closure in 4 out of 6 patients (66.7%) with gastrogastric fistula suggesting that a subset of patients with gastrogastric fistula may be best managed with endoscopic therapy, though more research on the ideal technique and patient selection is needed.(29)
Figure 7.
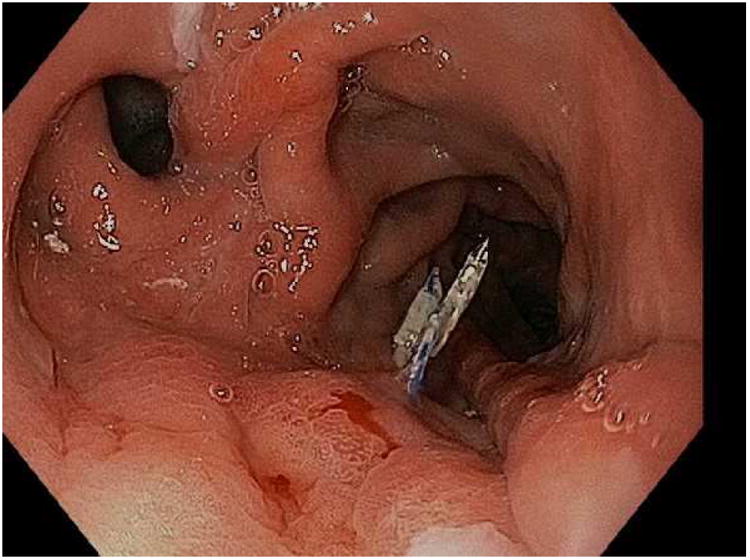
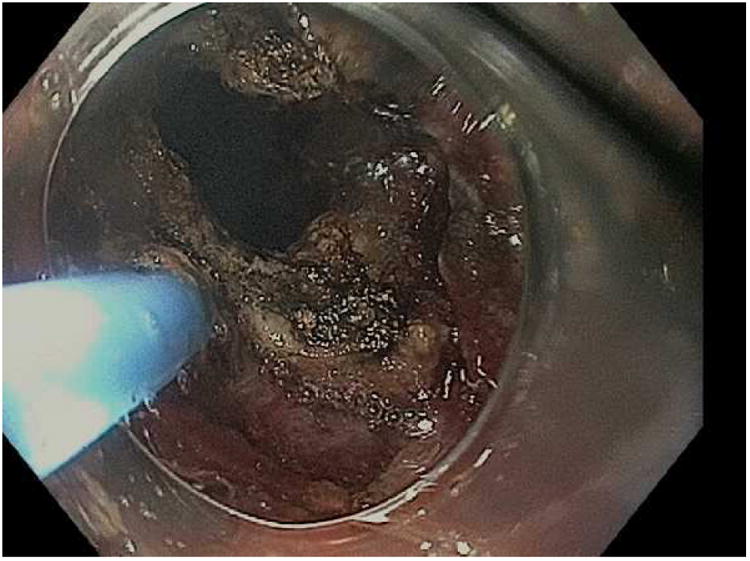
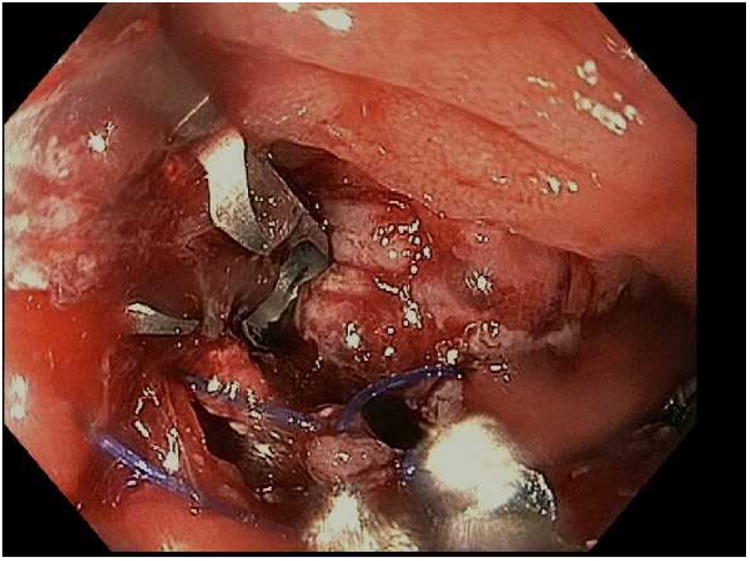
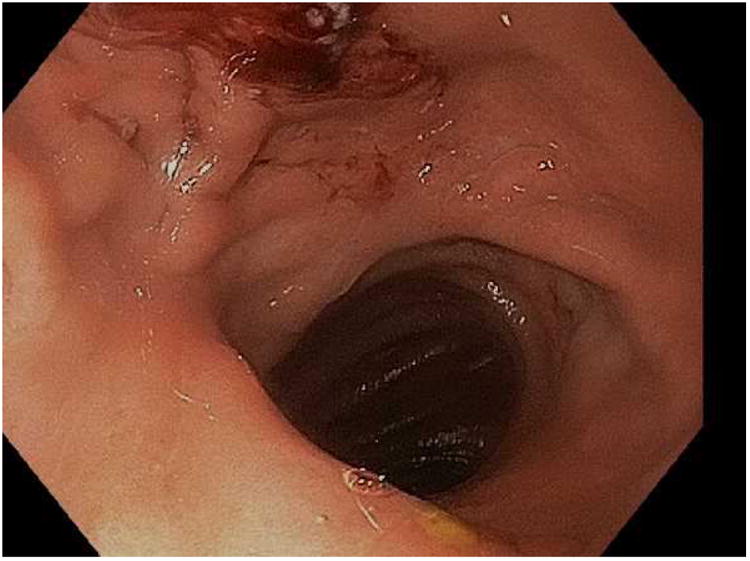
Closure of a gastro-gastric fistula through ESD and endoscopic suturing of the fistula. A; The fistula is shown here to the left of the gastrojejunal anastomosis, B; ESD is performed around the fistula opening to expose the muscular layer and then APC used to ablate any remaining mucosa, C; Running suture placed to close the defect, D; Fistula now fully closed on final inspection.
Marginal Ulceration and Bleeding
Marginal ulcerations may occur in up to 16% of patients after gastric bypass as a result of suture material, large gastric pouch, diabetes, use of tobacco cigarettes or H pylori infection, among other causes.(30) Ulceration may result in either weight loss because of post-prandial pain, or paradoxically may result in weight gain through the mechanism of transitioning the diet to soft foods and liquids which allows for increased calorie intake. Ulcerations may also result in occult blood loss and iron deficiency anemia, which can lead to appetite stimulation with weight gain and may also lead to perforation and peritonitis requiring emergency surgery with high mortality risk. As such, healing any ulcer(s) is of paramount importance to the patient. If the patient smokes tobacco, cessation counseling and pharmacotherapy aimed at cessation should be offered. NSAID medications should also be discontinued if possible. Additionally, we recommend the following aggressive medical therapy:
High dose proton pump inhibitor (PPI, ie Omeprazole 40mg) twice daily 15-30 minutes before meals, opening the capsule into apple sauce.
Sucralfate 1g up to four times daily one hour after meals to coat the ulcer and promote healing of the ulcer. These medications should be opened or crushed, respectively, or supplied in liquid form to ensure absorption and efficacy of the medications.
Some research shows that marginal ulceration will not heal as effectively without opening the PPI capsule or taking soluble formulation, as such the PPI and sucralfate must be opened, crushed or supplied in suspension.(31) Alterations in gastrointestinal pH, blood flow and absorptive surface area have been previously implicated in altered pharmaceutical concentrations after gastric bypass.(32)
If foreign material (suture or staples) from the prior surgery is present at the site of ulceration, we routinely remove this endoscopically to promote healing.(33) Tools including endoscopic scissors, loop cutters and simple biopsy forceps may be used to cut or tear the protruding suture material. Loop cutters may jam when used to cut braided or silk suture and should be used only for monofilament for this reason.
In the case of a bleeding ulcer, a standard approach using the Forrest classification should be taken, with care to avoid perforation of this weakened, sometimes ischemic area near or upon the surgical anastomosis site.(34) For active bleeding and visible vessel, dual endoscopic therapy with epinephrine injection and mechanical hemostasis (hemoclip placement) is advised, as with any gastric ulcer. In rare cases, non-healing ulcers have been managed with endoscopic oversewing with advancement of a mucosal flap using the OverStitch device.(35) Reoperation and surgical revision is reserved for the most recalcitrant ulcers.(36)
Summary/Discussion
The prevalence and societal cost of obesity and its complications have made it a primary public health concern. Surgical approaches to weight loss are effective, but fall short in achieving complete and long-term weight loss for some patients. As in other medical subspecialties like interventional cardiology and pulmonology, less invasive intraluminal endoscopic approaches are being developed to address the significant cost and morbidity associated with similar but more invasive surgical techniques. Endoscopic transoral outlet reduction of the gastrojejunal anastomosis is the only bariatric revision procedure with Level I evidence to support its use. In the obese patient population, endobariatric techniques aiming to reduce the diameter of the gastrojejunal anastomosis and pouch size in patients with prior RYGB, and sleeve diameter in patients with LSG, are safe and effective options that are becoming increasingly available. Similarly, many complications of bariatric surgical procedures, including ulceration and gastro-gastric fistula, which may contribute to weight regain, may be managed safely and effectively through the endoscopic approach.
Key to the success of these techniques, as with any modality targeting weight loss, is that they exist within a multidisciplinary approach. Gastroenterologists and other skilled endoscopists add a large number of physicians to the pool required to meet the demand of managing the obesity pandemic. Societies and organizations must continue working together to champion code development and reimbursement to increase availability of these and other emerging therapies.
Figure 5.
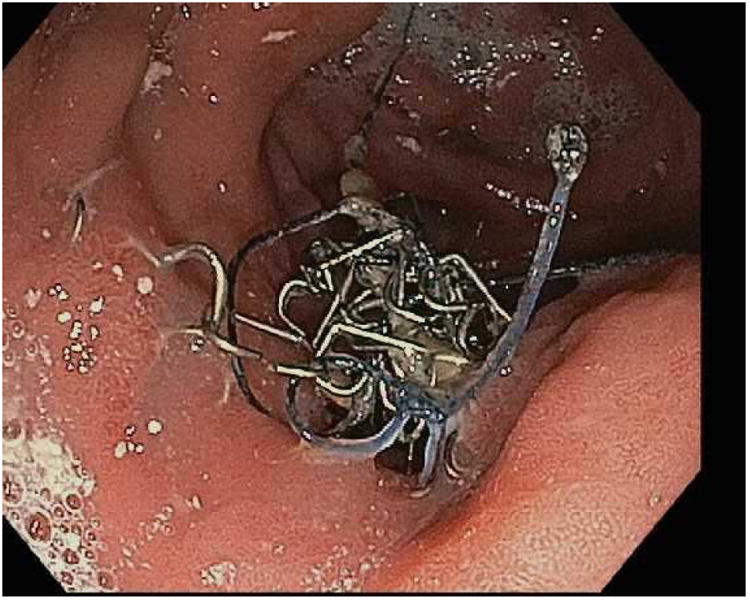
Extruded suture and staple material at the gastrojejunal anastomosis. This may lead to ulcers, pain and intermittent partial obstruction when foodstuff becomes impacted and tangled in the suture material.
Figure 6.
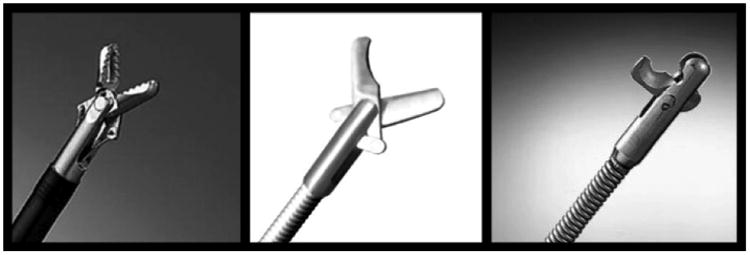
Tools used for removal of the suture and staple material. From left to right; biopsy forceps, reusable scissors, and loop cutters.
Key Points.
Weight regain after bariatric surgery is common and can be managed with less invasive endoscopic techniques.
Endoscopic techniques target structural postoperative changes that are associated with weight regain, most notably dilation of the gastrojejunal anastomosis aperture.
Purse string suture placement, as well as argon plasma coagulation application to the anastomosis, may result in significant and durable weight loss.
Various endoscopic approaches may be used to safely and effectively manage complications of bariatric surgery, including ulceration and fistula.
Acknowledgments
Dr. Thompson receives consulting fees from Boston Scientific, Covidien, Valentex, Olympus, and receives grants and consulting fees from USGI Medical, Apollo Endosurgery, Fractyl, GI Dynamics, Spatz, GI Windows and Aspire Bariatrics.
Footnotes
Andrew C Storm, MD, 75 Francis St., Boston, MA 02215, astorm@partners.org
Christopher C Thompson, MD, MHES, 75 Francis St., Boston, MA 02215
Disclosure Statement: Dr. Storm has nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew C. Storm, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women's Hospital, Harvard Medical School.
Christopher C. Thompson, Director of Therapeutic Endoscopy, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women's Hospital, Harvard Medical School.
References
- 1.Force ABET, Committee AT. Abu Dayyeh BK, Edmundowicz SA, Jonnalagadda S, Kumar N, et al. Endoscopic bariatric therapies. Gastrointest Endosc. 2015;81(5):1073–86. doi: 10.1016/j.gie.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288(22):2793–6. doi: 10.1001/jama.288.22.2793. [DOI] [PubMed] [Google Scholar]
- 3.McCormick JT, Papasavas PK, Caushaj PF, Gagne DJ. Laparoscopic revision of failed open bariatric procedures. Surg Endosc. 2003;17(3):413–5. doi: 10.1007/s00464-002-8533-3. [DOI] [PubMed] [Google Scholar]
- 4.Powers PS, Rosemurgy A, Boyd F, Perez A. Outcome of gastric restriction procedures: weight, psychiatric diagnoses, and satisfaction. Obes Surg. 1997;7(6):471–7. doi: 10.1381/096089297765555197. [DOI] [PubMed] [Google Scholar]
- 5.Behrns KE, Smith CD, Kelly KA, Sarr MG. Reoperative bariatric surgery. Lessons learned to improve patient selection and results. Ann Surg. 1993;218(5):646–53. doi: 10.1097/00000658-199321850-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coakley BA, Deveney CW, Spight DH, Thompson SK, Le D, Jobe BA, et al. Revisional bariatric surgery for failed restrictive procedures. Surg Obes Relat Dis. 2008;4(5):581–6. doi: 10.1016/j.soard.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Linner JH, Drew RL. Reoperative surgery--indications, efficacy, and long-term follow-up. Am J Clin Nutr. 1992;55(2 Suppl):606S–10S. doi: 10.1093/ajcn/55.2.606s. [DOI] [PubMed] [Google Scholar]
- 8.Buchwald H, Estok R, Fahrbach K, Banel D, Sledge I. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery. 2007;142(4):621–32. doi: 10.1016/j.surg.2007.07.018. discussion 32-5. [DOI] [PubMed] [Google Scholar]
- 9.Abu Dayyeh BK, Lautz DB, Thompson CC. Gastrojejunal stoma diameter predicts weight regain after Roux-en-Y gastric bypass. Clin Gastroenterol Hepatol. 2011;9(3):228–33. doi: 10.1016/j.cgh.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson CC, Slattery J, Bundga ME, Lautz DB. Peroral endoscopic reduction of dilated gastrojejunal anastomosis after Roux-en-Y gastric bypass: a possible new option for patients with weight regain. Surg Endosc. 2006;20(11):1744–8. doi: 10.1007/s00464-006-0045-0. [DOI] [PubMed] [Google Scholar]
- 11.Spaulding L, Osler T, Patlak J. Long-term results of sclerotherapy for dilated gastrojejunostomy after gastric bypass. Surg Obes Relat Dis. 2007;3(6):623–6. doi: 10.1016/j.soard.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Abidi WM, Schulman A, Thompson CC. 1137 A Large Case Series on the Use of Argon Plasma Coagulation for the Treatment of Weight Regain After Gastric Bypass. Gastroenterology. 2016;150(4):S231. [Google Scholar]
- 13.Aly A. Argon plasma coagulation and gastric bypass--a novel solution to stomal dilation. Obes Surg. 2009;19(6):788–90. doi: 10.1007/s11695-008-9763-9. [DOI] [PubMed] [Google Scholar]
- 14.Baretta GA, Alhinho HC, Matias JE, Marchesini JB, de Lima JH, Empinotti C, et al. Argon plasma coagulation of gastrojejunal anastomosis for weight regain after gastric bypass. Obes Surg. 2015;25(1):72–9. doi: 10.1007/s11695-014-1363-2. [DOI] [PubMed] [Google Scholar]
- 15.Jirapinyo P, Slattery J, Ryan MB, Abu Dayyeh BK, Lautz DB, Thompson CC. Evaluation of an endoscopic suturing device for transoral outlet reduction in patients with weight regain following Roux-en-Y gastric bypass. Endoscopy. 2013;45(7):532–6. doi: 10.1055/s-0032-1326638. [DOI] [PubMed] [Google Scholar]
- 16.Thompson CC, Chand B, Chen YK, Demarco DC, Miller L, Schweitzer M, et al. Endoscopic suturing for transoral outlet reduction increases weight loss after Roux-en-Y gastric bypass surgery. Gastroenterology. 2013;145(1):129–37 e3. doi: 10.1053/j.gastro.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Kumar N, Thompson CC. Comparison of a superficial suturing device with a full-thickness suturing device for transoral outlet reduction (with videos) Gastrointest Endosc. 2014;79(6):984–9. doi: 10.1016/j.gie.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar N, Thompson CC. Transoral outlet reduction for weight regain after gastric bypass: long-term follow-up. Gastrointest Endosc. 2016;83(4):776–9. doi: 10.1016/j.gie.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 19.Mullady DK, Lautz DB, Thompson CC. Treatment of weight regain after gastric bypass surgery when using a new endoscopic platform: initial experience and early outcomes (with video) Gastrointest Endosc. 2009;70(3):440–4. doi: 10.1016/j.gie.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 20.Horgan S, Jacobsen G, Weiss GD, Oldham JS, Jr, Denk PM, Borao F, et al. Incisionless revision of post-Roux-en-Y bypass stomal and pouch dilation: multicenter registry results. Surg Obes Relat Dis. 2010;6(3):290–5. doi: 10.1016/j.soard.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Heylen AM, Jacobs A, Lybeer M, Prosst RL. The OTSC(R)-clip in revisional endoscopy against weight gain after bariatric gastric bypass surgery. Obes Surg. 2011;21(10):1629–33. doi: 10.1007/s11695-010-0253-5. [DOI] [PubMed] [Google Scholar]
- 22.Abrams J, Komanduri S, Shaheen N, Wang Z, Rothstein R. Mo1947 Radiofrequency Ablation for the Treatment of Weight Regain After Roux-en-Y Gastric Bypass Surgery. Gastroenterology. 2016;150(4):S824. doi: 10.1016/j.gie.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 23.Eid GM, McCloskey CA, Eagleton JK, Lee LB, Courcoulas AP. StomaphyX vs a sham procedure for revisional surgery to reduce regained weight in Roux-en-Y gastric bypass patients : a randomized clinical trial. JAMA Surg. 2014;149(4):372–9. doi: 10.1001/jamasurg.2013.4051. [DOI] [PubMed] [Google Scholar]
- 24.Sharaiha RZ, Kedia P, Kumta N, Aronne LJ, Kahaleh M. Endoscopic sleeve plication for revision of sleeve gastrectomy. Gastrointest Endosc. 2015;81(4):1004. doi: 10.1016/j.gie.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Capella JF, Capella RF. Gastro-gastric fistulas and marginal ulcers in gastric bypass procedures for weight reduction. Obes Surg. 1999;9(1):22–7. doi: 10.1381/096089299765553674. discussion 8. [DOI] [PubMed] [Google Scholar]
- 26.Carrodeguas L, Szomstein S, Soto F, Whipple O, Simpfendorfer C, Gonzalvo JP, et al. Management of gastrogastric fistulas after divided Roux-en-Y gastric bypass surgery for morbid obesity: analysis of 1,292 consecutive patients and review of literature. Surg Obes Relat Dis. 2005;1(5):467–74. doi: 10.1016/j.soard.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Esparrach G, Lautz DB, Thompson CC. Endoscopic repair of gastrogastric fistula after Roux-en-Y gastric bypass: a less-invasive approach. Surg Obes Relat Dis. 2010;6(3):282–8. doi: 10.1016/j.soard.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 28.Mukewar S, Kumar N, Catalano M, Thompson C, Abidi W, Harmsen W, et al. Safety and efficacy of fistula closure by endoscopic suturing: a multi-center study. Endoscopy. 2016 doi: 10.1055/s-0042-114036. [DOI] [PubMed] [Google Scholar]
- 29.Campos JN, Manoel Galvão, Martins João, de Gordejuela Amador, Alhinho Helga, Pachu Eduardo, Ferraz Álvaro. Bariatric Surgical Practice and Patient Care. June. 2015;10(2):62–67. [Google Scholar]
- 30.Azagury DE, Abu Dayyeh BK, Greenwalt IT, Thompson CC. Marginal ulceration after Roux-en-Y gastric bypass surgery: characteristics, risk factors, treatment, and outcomes. Endoscopy. 2011;43(11):950–4. doi: 10.1055/s-0030-1256951. [DOI] [PubMed] [Google Scholar]
- 31.Schulman A, Devery A, Thompson CC. Su1060 Opening PPI Capsules Should be the New Standard of Care in the Treatment of Marginal Ulceration. Gastrointestinal Endoscopy. 83(5):AB314. [Google Scholar]
- 32.Benet LZ, K D, Sheiner LB. Phar- macokinetics: the dynamics of drug absorption, distribution and elimination. In: Hardman JG, Limbird LE, editors. Goodman and Gilman's the pharmacological basis of therapeutics. 9th. New York: McGraw-Hill; 1996. pp. 3–27. [Google Scholar]
- 33.Lee JK, Van Dam J, Morton JM, Curet M, Banerjee S. Endoscopy is accurate, safe, and effective in the assessment and management of complications following gastric bypass surgery. Am J Gastroenterol. 2009;104(3):575–82. doi: 10.1038/ajg.2008.102. quiz 83. [DOI] [PubMed] [Google Scholar]
- 34.Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974;2(7877):394–7. doi: 10.1016/s0140-6736(74)91770-x. [DOI] [PubMed] [Google Scholar]
- 35.Jirapinyo P, Watson RR, Thompson CC. Use of a novel endoscopic suturing device to treat recalcitrant marginal ulceration (with video) Gastrointest Endosc. 2012;76(2):435–9. doi: 10.1016/j.gie.2012.03.681. [DOI] [PubMed] [Google Scholar]
- 36.Fringeli Y, Worreth M, Langer I. Gastrojejunal Anastomosis Complications and Their Management after Laparoscopic Roux-en-Y Gastric Bypass. J Obes. 2015;2015:698425. doi: 10.1155/2015/698425. [DOI] [PMC free article] [PubMed] [Google Scholar]


