Abstract
Background
Erosive oral lichen planus and desquamative gingivitis are uncommon but severe debilitating variants of oral lichen planus. Treatment of these presentations is difficult and challenging.
Main observation
A 44-year-old woman was referred to the dermatology clinic with chronic painful lichen planus-related gingivitis and buccal erosions. She has failed multiple treatments including topical clobetasol and tacrolimus, intralesional corticosteroids and several systemic and immunosuppressive agents. Following completion of three months of treatment with oral apremilast at a dose of 30 mg twice daily, significant improvement was noted in her disease activity.
Conclusion
Oral apremilast may be a safe and effective treatment for erosive oral lichen planus.
Keywords: erosions, lichen planus, mucous membranes, oral cavity
Introduction
Oral lichen planus (OLP) affects approximately 2% of the population and has several overlapping morphological forms including reticular, erosive, papular, vesiculo-bullous, and atrophic/erythematous.[1,2] Erosive oral lichen planus (EOLP) is characterized by erythema and erosions that predominantly affect the buccal mucosae, but up to 25-30% of patients can have gingival involvement with diffuse pain, erythema and erosions resulting in desquamative gingivitis.[2,3]
Case Report
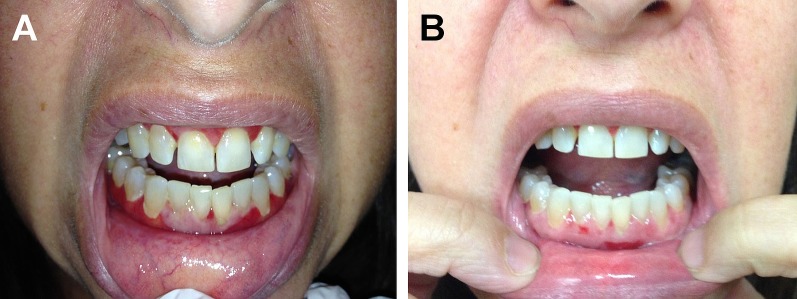
A 44-year-old female was referred to the dermatology clinic with a chief complain of pain and soreness of her gingivae progressing over a period of five years. Her past medical history was significant for cutaneous lichen planus (LP) treated to remission approximately 2 years prior to the onset of oral disease. Blood testing for hepatitis C was negative. Past treatments for her oral lesions included topical clobetasol and tacrolimus, intralesional triamcinolone, systemic prednisone, cyclosporine, acitretin, dapsone, hydroxychloroquine, azathioprine and mycophenolate mofetil. Skin examination was unremarkable. Intra-oral examination revealed bilateral buccal erosions and diffuse erythema and desquamation of the upper and lower gingivae [Fig. 1A]. The gingivae were tender to palpation and associated with bleeding on probing. Histopathological examination of oral mucosa was consistent with LP. Based on the clinical presentation and histopathological examination, she was diagnosed with EOLP and desquamative gingivitis. As the patient had failed many topical and systemic therapies, she was started on a trial of oral apremilast 30 mg twice daily. The patient developed nausea during the first 10 days and her dose was tapered down to 30 mg once daily. Following completion of 12-weeks treatment, marked improvement was observed in both the buccal and gingival lesions [Fig. 1B]. The patient also reported significant reduction in pain and discomfort with improvement of her quality of life with easier speaking, eating and chewing.
Figure 1.
Appearance of upper and lower gingivae before and after treatment with oral apremilast. (A) (Before): Diffuse glazed erythema of upper and lower gingivae, and small ulcerations on the mucosal side of the lower lip. (B) (After): Marked reduction in erythema and erosions on upper and lower gingivae after 12 weeks of 30 mg daily apremilast.
Discussion
Patients with EOLP and LP desquamative gingivitis are often debilitated with severe discomfort and pain which interferes with speaking and eating.[3,4] Treatment options include potent topical corticosteroids, topical tacrolimus, intralesional corticosteroids, systemic corticosteroids, systemic retinoids, dapsone, mycophenolate mophetil, intravenous immunoglobulins, cyclosporin, and azathioprine.[1-4] However, these modalities are not always effective or associated with adverse effects when used for sustained periods of time. Our decision to use apremilast for EOLP was based on the mechanism of action of apremilast and the pathogenesis of OLP. Apremilast is a phosphodiesterase-4 inhibitor that has been successfully used in the treatment of psoriasis and oral ulceration in Behcet's disease.[5,6] Apremilast effectively inhibits the production of TNF-alpha, IFN-gamma, IL-2, IL-5, IL-8, and IL-12, all of which contribute to the pathogenesis of OLP by activation of cytotoxic T-cells, and mediating basal keratinocytes apoptosis in OLP.[4,5,7]
In a recent study, ten patients with biopsy-proven cutaneous LP received 20 mg of apremilast orally twice daily for 12 weeks with 4 weeks of treatment-free follow-up. Thirty percent of the patients achieved a 2-grade or more improvement in the Physician Global Assessment after 12 weeks of treatment and all patients demonstrated clinical improvement with lesions count at the end of treatment. One of these 10 patients had oral involvement which improved significantly by the end of the study.[8]
Apremilast appears to be safe as a long term treatment. There were no serious adverse effects reported in large studies of apremilast when used for psoriasis and psoriatic arthritis.[5,6,9] Apremilast therefore would be an ideal systemic agent for this chronic disease given its good safety profile. Up to now cases reports of patients with oral lichen planus treated with apremilast were published, both in 2016.[10,11]
In conclusion, our case suggests that apremilast may be a new, safe and effective treatment for EOLP. Large studies are needed to better assess efficacy, proper dose, long term efficacy and safety of this treatment option.
References
- De Rossi SS, Ciarrocca K. Oral lichen planus and lichenoid mucositis. Dent Clin North Am. 2014;58:299–313. doi: 10.1016/j.cden.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Au J, Patel D, Campbell JH. Oral lichen planus. Oral Maxillofac Surg Clin North Am. 2013;25:93–100. doi: 10.1016/j.coms.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Camacho-Alonso F, López-Jornet P, Bermejo-Fenoll A. Gingival involvement of oral lichen planus. J Periodontol. 2007;78:640–644. doi: 10.1902/jop.2007.060303. [DOI] [PubMed] [Google Scholar]
- Lavanya N, Jayanthi P, Rao UK, Ranganathan K. Oral lichen planus: An update on pathogenesis and treatment. J Oral Maxillofac Pathol. 2011;15:127–132. doi: 10.4103/0973-029X.84474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks ED. Apremilast: A Review in Psoriasis and Psoriatic Arthritis. Drugs. 2015;75:1393–1403. doi: 10.1007/s40265-015-0439-1. [DOI] [PubMed] [Google Scholar]
- Hatemi G, Melikoglu M, Tunc R, Korkmaz C, Turgut Ozturk B, Mat C, Merkel PA, Calamia KT, Liu Z, Pineda L, Stevens RM, Yazici H, Yazici Y. Apremilast for Behçet's syndrome--a phase 2, placebo-controlled study. N Engl J Med. 2015;372:1510–1518. doi: 10.1056/NEJMoa1408684. [DOI] [PubMed] [Google Scholar]
- Roopashree MR, Gondhalekar RV, Shashikanth MC, George J, Thippeswamy SH, Shukla A. Pathogenesis of oral lichen planus--a review. J Oral Pathol Med. 2010;39:729–734. doi: 10.1111/j.1600-0714.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- Paul J, Foss CE, Hirano SA, Cunningham TD, Pariser DM. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68:255–261. doi: 10.1016/j.jaad.2012.07.014. [DOI] [PubMed] [Google Scholar]
- AbuHilal M, Walsh S, Shear N. Use of Apremilast in Combination With Other Therapies for Treatment of Chronic Plaque Psoriasis: A Retrospective Study. J Cutan Med Surg. 2016;20:313–316. doi: 10.1177/1203475416631328. [DOI] [PubMed] [Google Scholar]
- Hafner J, Gubler C, Kaufmann K, Nobbe S, Navarini AA, French LE. Apremilast Is Effective in Lichen Planus Mucosae-Associated Stenotic Esophagitis. Case Rep Dermatol. 2016;8:224–226. doi: 10.1159/000447051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt M. Oral Lichen Planus Treated With Apremilast. J Drugs Dermatol. 2016;15:1026–1028. [PubMed] [Google Scholar]