Abstract
Objective
In this project we developed and evaluated a mobile health app to improve adherence to tobacco cessation medication.
Methods
The study was conducted in three phases: 1) Create app with input from our consultant, focus groups and user testing; 2) Test feasibility of the app; and 3) Develop and user-test the barcode scanner.
Results
Focus group feedback was instrumental in developing content and creating the user interface. User testing helped to identify problems and refine the app. The feasibility trial provided “real world” testing. We experienced challenges in recruitment due to the inclusion criteria. We had high attrition due to technical issues, medication side effects, enrollment procedures, and lack of personal contact. Among the five retained participants, use of the app was associated with good medication adherence and high consumer satisfaction.
Conclusion
The small sample size limits the generalizability of the findings and the conclusions that can be drawn from the study. However, the feasibility trial enabled the team to identify ways to improve the conduct of this and other mHealth studies.
Practical Implications
We should expand RxCoach to include all prescription and over-the-counter tobacco cessation medications, and re-test for feasibility using lessons learned to improve recruitment and retention.
Keywords: mHealth, mobile app, medication adherence, smoking, cessation
1. Introduction
Tobacco is the leading cause of preventable disease and death in the US [1]. Although effective tobacco cessation medications exist, their impact can be diminished by lack of adherence [2]. Studies assessing the effect of medication adherence for tobacco cessation suggest that lack of adherence negatively impacts treatment outcomes [3–6]. Several studies on the effect of varenicline show that good adherence is associated with better cessation outcomes [2, 7, 8]. Adherence to prescribed treatment is necessary to achieve desired health outcomes; however, studies have shown that 30% – 50% of patients do not take medications as prescribed [9–13]. Non-adherence to medication for chronic conditions has been associated with poor treatment outcomes, including increasing hospitalization rates and health care costs [11, 12, 14]. Conversely, research has found a dose-response between treatment adherence and positive health outcomes [3, 4, 12, 15, 16].
A number of factors are commonly associated with poor adherence to medication therapy. These include complicated dosing schedules, adverse effects from medications, and poor patient-provider communication [11, 17–20]. Instructions are often poorly understood, or there is no clear agreement between the patient and provider about the need for the medication or duration of treatment. Without some innovative approaches, such factors are likely to continue to impede optimal treatment of tobacco dependence. However, previous research also suggests that improved communication regarding the importance of and reasons for taking the medication may increase adherence [17–20].
The World Health Organization and the National Institutes of Health have acknowledged that medication adherence is a complex and difficult issue to solve [21, 22]. These two organizations recommend a multi-tiered approach that targets barriers at multiple levels, including the patient, the healthcare system, the support system, and their interactions. This type of approach needs to allow for multiple user input and interaction (eg, between patient, pharmacy, physician), link to external systems (eg, external electronic databases), eliminate patient need to enter medication information (eg, names and doses of medications), and offer tailored information and adherence strategies based on patient needs [21, 22].
A mobile health (mHealth) application (app) could provide a multi-tiered approach to overcoming these barriers for many patients who have been prescribed medication. The Pew Research Center reports that 90% of US adults use a mobile phone, and of these, 64% have smartphones [23]. Sixty-two percent of smart phone users have used a phone to look up information about a health condition [24]. African Americans and Latinos are more likely than other groups to use mobile health apps [25], and to be what Pew terms “smart phone dependent,” since the phone is their primary source for Internet connectivity [24].
There is a limited but growing body of evidence to suggest that mobile apps may improve patient adherence to medication [26, 27], with two studies providing preliminary evidence to support their use with tobacco cessation medication [28, 29]. Mobile apps have the potential to improve patient-provider communications and support greater accessibility to healthcare related to medication use [30, 31]. However, many apps rely on significant user input and fail to provide evidence-based interactivity aimed at engaging patients, tailoring adherence feedback, or facilitating communication between the patient, physician and/or pharmacist. A successful mobile app would need to address a number of intersecting barriers to medication adherence, including patient forgetfulness, lack of information, complex regimens that do not match the patient’s lifestyle, lack of perceived benefits, experience of side effects, poor patient-provider communication, and missed appointments [13]. Moreover, for smokers who want to quit, an effective mHealth app should provide evidence-based assistance, immediately available, at any time, on their mobile phone. This manuscript reports on the development and pilot feasibility evaluation of such an app: RxCoach™, designed to increase adherence to tobacco cessation medication.
2. Methods
2.1. Study Design
The study was conducted in three stages: 1. An app developmental stage consisting of program content development, interface design, prototype app programming, prototype app revisions with input from our consultant, iterative focus groups, and user testing; 2. A feasibility test of the prototype app; and 3. A supplemental stage in which a barcode scanning feature was developed and user tested. The study was conducted from May 18, 2012 – April 30, 2014. The study protocol and procedures were approved by the Oregon Research Institute and the University of Arizona Institutional Review Boards.
2.2. Prototype App Development
2.2.1. Overview
We designed and developed a fully functional prototype mobile health application for iOS (the Apple iPhone platform) to assist smokers who are taking varenicline for treatment of tobacco dependence to properly adhere to the medication regimen. We chose the Apple iPhone platform because it had the largest market share of smartphones at the time the study began. We decided to focus the prototype on varenicline because it was the most widely used prescription medication for tobacco cessation when we began the study, and it had a complex dosing schedule and several potential side effects that would require us to program the prototype to address these functions.
2.2.2. Description of RxCoach
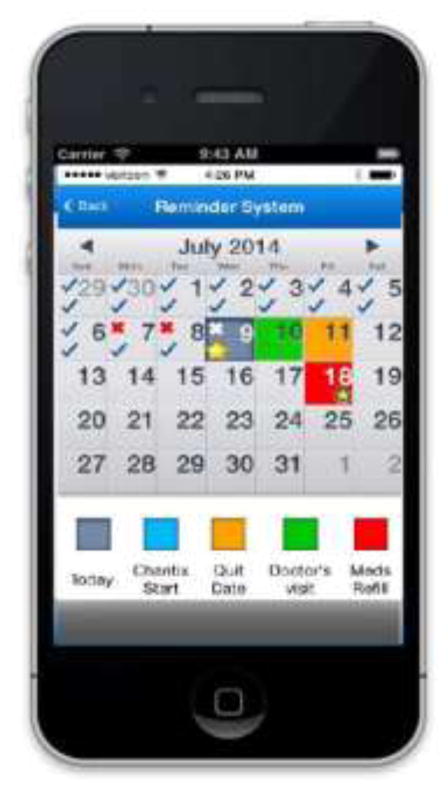
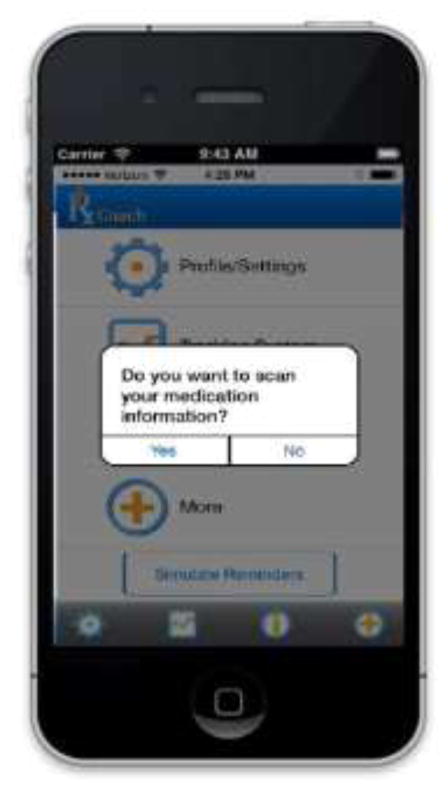
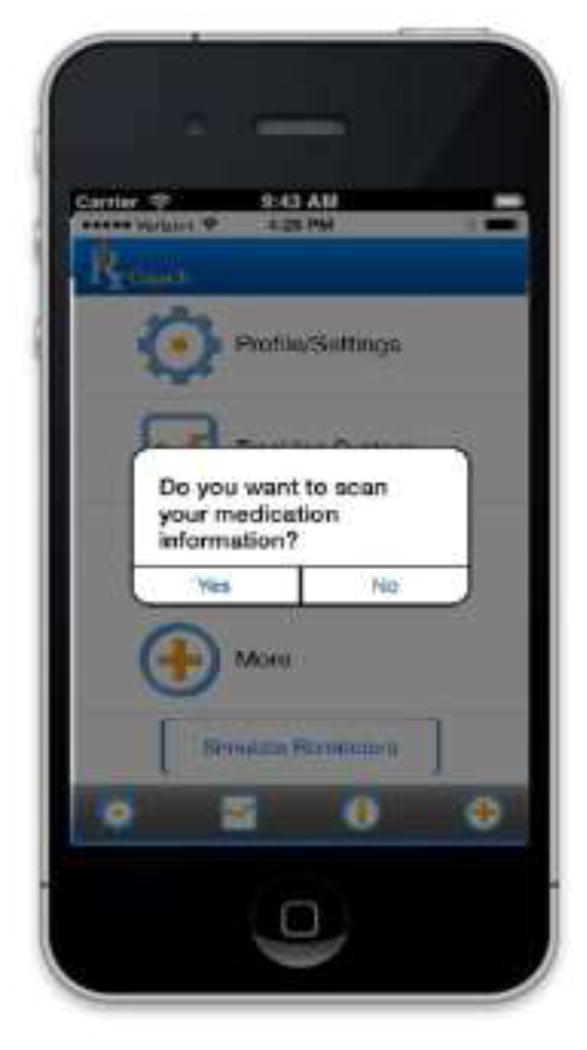
The prototype version of RxCoach gathers personal information and the specific medication regimen via user input and a barcode reader. The user can access the “home screen” at any time to change their profile settings, and display their tracking system and medication information (Figure 1). To overcome the barrier of entering complex dosing schedules, we allow participants to use a barcode reader to scan in their medication (Figure 2). All packaging for tobacco cessation mediations, including prescription bottles, includes a barcode. The app then presents the user with a choice to select the “standard dose” (which is used as the default for populating the reminder and tracking system) or enter a different dosing schedule, as prescribed by their physician (Figure 3). The app provides tailored adherence feedback and medication tracking information to improve motivation. Based on users’ responses, the app displays motivational messages, tips, or techniques for dealing with cravings, medication side effects, and/or smoking “slips.” Motivational messages were used to provide positive feedback to the user. For example, the message would display the number of days that the user had been taking the medication. Tips for dealing with cravings were displayed early in the quitting process when urges to smoke might be at their peak or if the user reported cravings. For example, the user might receive a message to take deep breaths, recognize that the craving will pass, or to engage in physical activity until the craving passes. Techniques for dealing with side effects were based on the users’ reports of side effects. For example, if a user reported “nausea” as a side effect, the app would recommend taking the medication with a meal and a full glass of water. Messages regarding “slips” included normalizing this as part of the quitting process, and instructing the user to continue to take the medication. A calendar displays medication usage, quit date, changes in dosing, scheduled prescription refills, and physician appointments (Figure 4). To address forgetfulness, the app provides the user with medication taking reminders, based on their dosing schedule, refill reminders, and physician appointment reminders (Figure 5). The user is prompted to scan their medication to improve accuracy of adherence reporting and earn virtual awards. RxCoach collects information about side effects and other barriers to taking the medication from the user and provides personalized solutions to problems reported (Figure 6). To facilitate communication between the user and their physician or pharmacist, the app requests that the user input the phone numbers for their prescribing physician and pharmacist, and displays a “one-touch,” direct connection (Figure 7) based on user input. For example, if the user reports a serious side effect or multiple minor side effects, the app displays a message encouraging the user to press the “one-touch” button to call their physician. The app provides consumer-oriented drug information and sets expectations regarding effectiveness and potential side effects.
Figure 1.
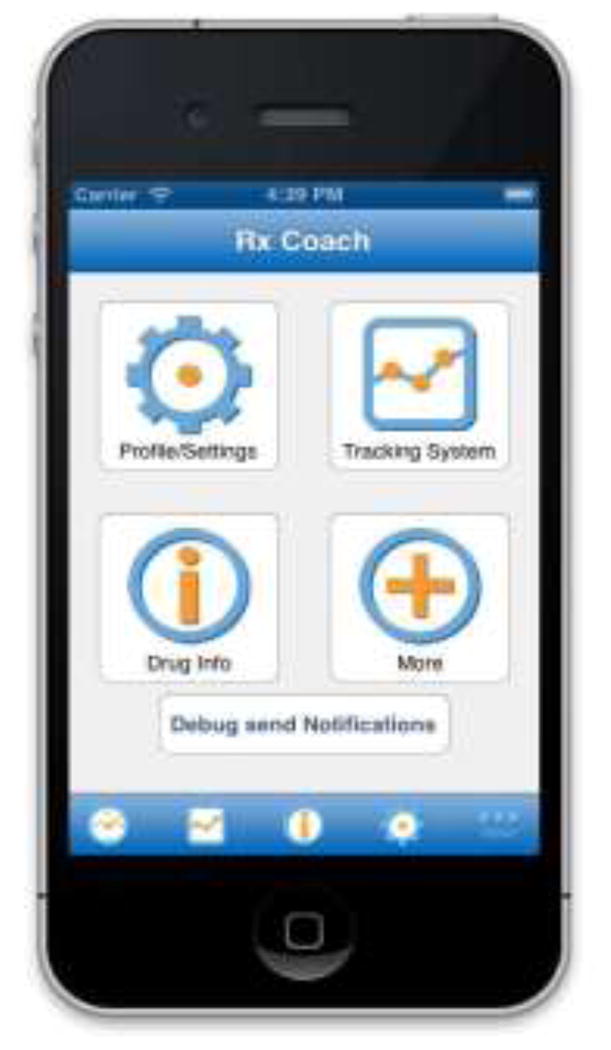
Home Screen
Figure 2.
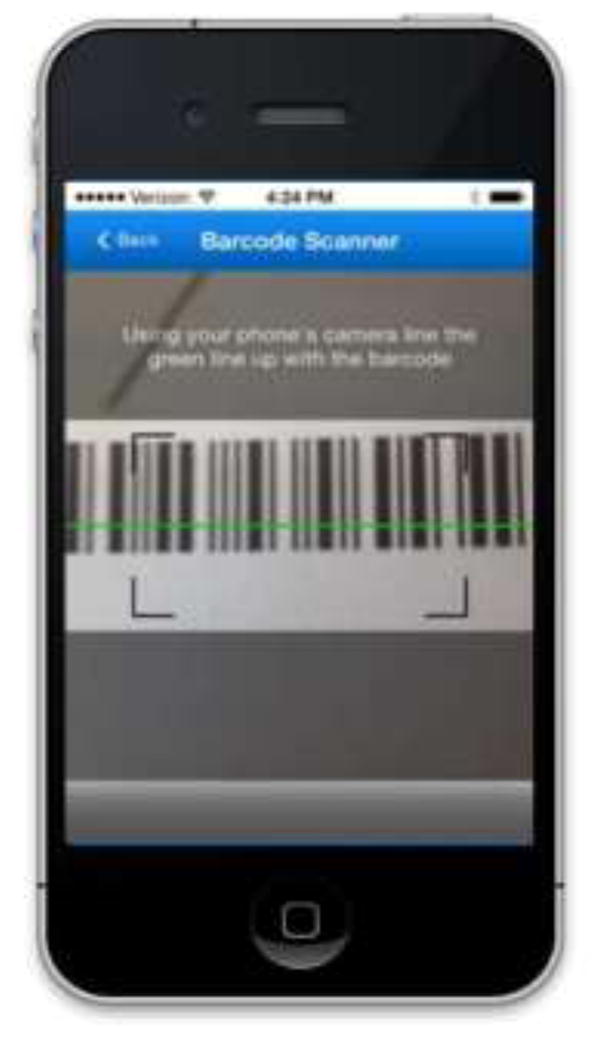
Barcode Scanner
Figure 3.
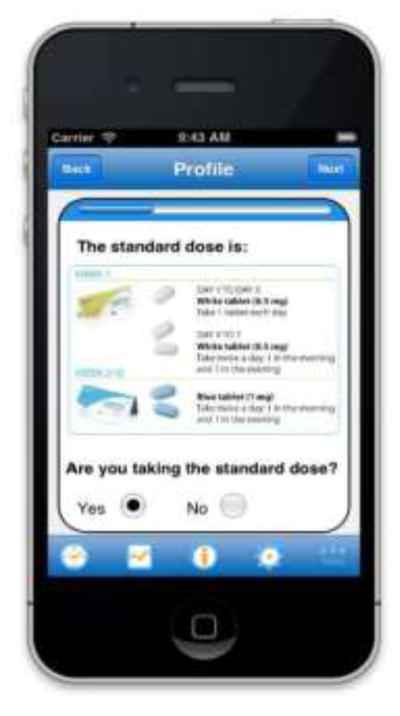
Profile
Figure 4.
Tracking System
Figure 5.
Medication Reminder
Figure 6.
Reporting Barriers
Figure 7.
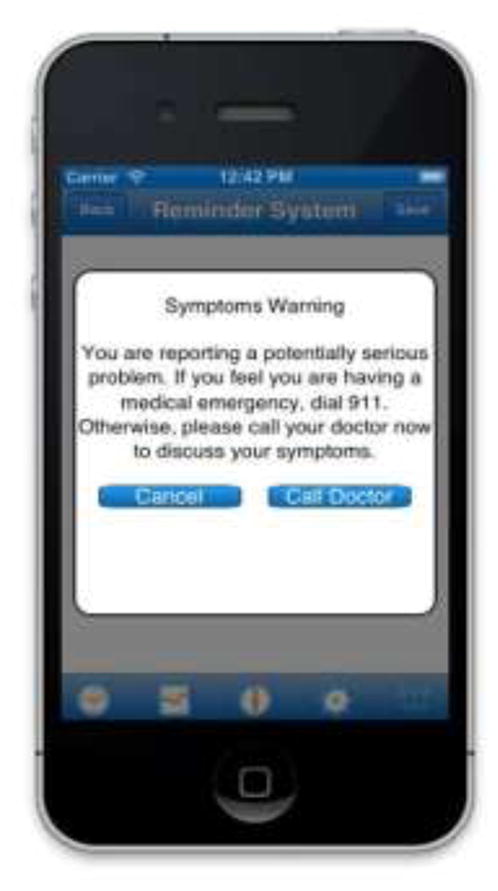
Warning
2.2.3. Focus and Usability Testing
Program content and design elements were developed by the project team and expert consultants. These concepts were refined through two iterative focus groups (n = 4) and two sets of 10 individual user tests. Mock-ups of the app were created to demonstrate the design and content of the app, and to simulate the functionality. These mock-ups were presented to two focus groups of smokers who had used varenicline and who also owned a smart phone, to provide feedback on the application’s interface, functions, and content. Focus groups were led by an experienced facilitator. The facilitator presented initial program content and design ideas. Following the presentation, participants completed surveys, and then the facilitator led a discussion. The facilitator audio-recorded each session, took notes, and cross-referenced the audio file with her notes. For user testing, the facilitator presented case scenarios and asked the participant to perform specific actions. The facilitator watched the participant use the app, and took notes on the process. At the end of each user session, participants completed a survey and were given the opportunity to discuss their experience using the app. Quantitative focus group data were analyzed following each group. Qualitative data were synthesized by the facilitator, organized according to app functionality, and reviewed by the project team after each group session. User testing data was summarized by the facilitator and reviewed by the project team after each individual test.
Participant suggestions led the team to adjust the home screen/menu design to make it more intuitive, to streamline app set-up, and to describe the need to collect particular user information, among other enhancements. For example, we added a status bar during the profile set-up process, displayed a quit date for users and gave them the option to change it. Moreover, participants identified a number of features that would be useful, such as medication auto-entry, which were added.
We conducted usability testing with 10 participants, fixing bugs and making content/user interface changes to the prototype based on user “think aloud” interactions with the app. Testing usability with five users per population of interest is generally deemed sufficient based on guidelines put forth by Nielsen [32]. As participants used the Alpha prototype, they were asked to think aloud, verbalizing their reactions as they viewed screens and describing their navigational choices as they made them. Participants performed each of the major tasks the application supports. We collected positive/negative reactions to the features and functions, and difficulty/facility using the application or interface. Usability testers also completed the 10-item adaptation of Brooke’s widely-used and validated System Usability Scale [33, 34]. Usability feedback informed revisions to the Alpha prototype.
User group results and feedback revealed some concerns about the app’s consistency and integration. As a result of these findings, we revised the functionality and text in the “dosing scheduler” screen at start-up and simplified our main menu. We found that participants struggled to use the medication reminder system to report a side effect. Therefore, we revised this system to contain simplified categories and lay language explanations. Moreover, we observed that participants did not read all of the content during set-up and in push notification messages. Therefore, we reduced the length of in-app text in these areas, as well as improved legibility by increasing the font size and adding more white space to text boxes.
A second set of user tests with 10 participants was conducted to gather information on the added auto-entry, barcode scanner feature. We used the same “think aloud” procedures as we did with earlier user group participants. In addition, we collected consumer satisfaction data from usability test participants to assess “likability” and perceived usefulness of the app. Based on the feedback received, modifications were incorporated into the design and operability of the scanner to make it easier to use.
2.2.4. Feasibility Testing
We conducted a feasibility study to gather information on app use, medication adherence, attitudes towards using varenicline, and consumer satisfaction with the app.
2.3. Participant Recruitment
Recruitment took place via multiple sources, including online advertising (eg Google AdWords, Facebook), in-app advertising (eg iAds), social media (eg Instagram, Tumblr, Twitter, Facebook), and through professional contacts at smoking cessation clinics nationwide. Participants were compensated $50 for their time. We found it difficult to recruit for this study, both locally and nationally. The study required participants to be current smokers, varenicline users, iPhone owners, with no current depression. This combination of inclusion and exclusion criteria created a challenging environment for recruitment.
2.4. Participant Screening, Eligibility, Enrollment, and Retention
Study personnel had little to no contact with study participants, since all screening, enrollment, and follow-up activities were conducted entirely through the app or online. To be enrolled in the study, eligible participants were required to complete an informed consent and baseline survey through a companion RxCoach website, and download the app. The process for downloading the app proved problematic at first, when only 50% of eligible participants were able to download the app. We subsequently changed the process, and 100% of eligible participants successfully downloaded RxCoach.
Fifty participants were screened for eligibility; 29 (58%) were excluded. Of those 21 who were eligible, 12 (51%) completed the consent and baseline survey, and 7 (33%) people downloaded the app. Of the seven enrolled participants, five (71%) participants completed the 1-month (30-day) survey, and 4 (57%) completed the 3-month (90-day) survey. These retention rates are consistent with web-based and other mHealth studies, which require no personal contact, and routinely report >50% attrition [35].
2.5. Participant Characteristics
Participants in the feasibility trial who completed the baseline and at least one follow-up assessment were all female, White, and current smokers. At baseline, their mean age was 37.4. All had at least a college education. The participants had smoked for an average of 23.6 years, and smoked an average of 16.4 cigarettes per day. All reported smoking within 60 minutes of waking, indicating a high level of dependence. All of the participants had health insurance. The sample reported taking varenicline for an average of 10.4 days out of the past 30 days.
2.6. Assessment Procedures and Measures
Informed Consent Document and all assessments were completed online. Participants were assessed at baseline, and at 1-month and 3-months post-enrollment. The study measures included: demographics (collected at baseline), tobacco use, history of taking varenicline, medication use patterns, medication refills, medication adherence, use of other cessation aids, attitudes towards taking varenicline, perceived effectiveness of varenicline, consumer satisfaction (collected at 1-month); and app use (which was collected automatically throughout the participants’ use of the app).
Tobacco use status was assessed using a series of questions that have been standardized and employed in previous studies [36–38]. Tobacco dependence was measured via the Fagerström Test of Nicotine Dependence [39]. For measuring adherence, we adapted the widely-used 8-item Medication Adherence Questionnaire (MAQ) [40], which has been validated for use with tobacco cessation medication [41]. In addition, we included one item regarding medication-specific refill behavior. Users’ expectancies about taking tobacco cessation medication were assessed using the 10-item Beliefs about Medicines Questionnaire (BMQ) [42, 43].
Participants completed a consumer satisfaction survey that we have used in our previous research consisting of 8 items (using a 5-point Likert-type scale), measuring overall satisfaction with the program, perceived usefulness of the information, likeability, level of interest, and ease of use [36, 44]. Further, we used Tullis and Stetson’s 10-item adaptation of Brooke’s System Usability Scale to rate the usability of the app [33, 34]. Participants were compensated $50 for completing both assessments.
Finally, a variety of use and adherence data were collected via the app. For instance, participants reported medication side effects within the app through a checklist of varenicline’s common side effects (results not reported in this manuscript).
3. Results
3.1. Self-Reported Adherence to Varenicline at Follow Up
As depicted in Table 1, at the 1-month assessment, four out of five (80%) participants reported current use of varenicline. The average number of days reported for taking medication was 28 out of the past 30 days. All participants (100%) reported never forgetting to take their medication. Two (40%) participants reported refilling their prescription once, two (40%) reported refilling their prescription twice, and one (20%) did not refill her prescription, and did not intend to refill due to side effects. This participant discontinued her use of varenicline.
Table 1.
Medication Adherence at Follow-Up Assessments
| Variable | 1-month (N=5) | 3-month (N=4) |
|---|---|---|
| Are you currently taking varenicline? Yes | 4 | 1 |
|
| ||
| How many days did you take varenicline? | 28 | 64 |
|
| ||
| How often have you forgotten to take your varenicline? Never |
5 | 3 |
|
| ||
| How many times have you refilled your varenicline prescription? | ||
| None (I don’t intend to) | 1 | 0 |
| 1 | 2 | 1 |
| 2 | 2 | 0 |
| 3 or more | 0 | 3 |
Of the four participants completing the 3-month assessment, one reported continued use of varenicline, as three had finished their course of treatment. The mean number of days of use was 63.7 over the past 90 days (minimum = 30; maximum = 90). Three of the participants reported never forgetting to take their medication. One participant reported refilling her prescription once, and three participants reported filling their prescription three times (the maximum) over the past 90 days.
3.2. App Usage
Participants were instructed to use the app for the duration of their medication use, which could range from 30 to 90 days. The duration of use was dependent on when they started their medication and when they downloaded the app. Users who were just beginning to take their medication were prompted to record taking their medication once a day for the first 3 days, then twice a day for the following 87 days, for a maximum total of 177 prompts to open the app. However, if users were already taking varenicline, the number of prompts varied. Of the 5 participants, the average number of times the app was opened was 98 (minimum = 28; maximum = 186).
3.2. Consumer Satisfaction
We collected consumer satisfaction data from participants at the 1-month assessment using a 5-point Likert-type scale. As shown in Table 2, participants liked the program overall, thought it was easy to use, found it helpful in using varenicline, and would recommend it to others. A sample comment we received from a feasibility participant summarizes these findings: “This app is great & did remind me to take the pill when I had forgotten. I would recommend to my friends that are trying to quit.”
Table 2.
Consumer Satisfaction Ratings (N=5)
| Somewhat to Verya (N) | |
|---|---|
| Overall, how helpful is the app in using varenicline to quit smoking? | 4 |
| How organized was the app? | 5 |
| How much new information did the app provide? | 4 |
| How easy was the app to use? | 5 |
| How well did the app address issues with varenicline and smoking? | 4 |
| How much did you like the format/design of the app? | 5 |
| How helpful were the features of the app? | 5 |
| How likely would you be to recommend this app to others? | 5 |
Ratings were collected on a 5-point Likert-type scale with 1 = Not at all to 5 = very. Results are presented for the number of participants with ratings of 3–5 (somewhat to very)
3.3. Functionality
Participants were also asked to provide ratings of the apps usability and functionality. Results indicate that the app was easy to use, well integrated, easy to learn, and participants felt confident using the app (see Table 3).
Table 3.
Attitudes Towards Using the App/System Usability Scale (N=5)
| Agree to Strongly Agreea (N) | |
|---|---|
| I would use the app frequently | 5 |
| I found the app unnecessarily complex | 1 |
| I thought the app was easy to use | 5 |
| I think I would need support to use this app | 0 |
| I found the function well integrated | 5 |
| I thought there was too much inconsistency | 2 |
| Most people would learn to use this app very quickly | 5 |
| I found this app cumbersome | 0 |
| I felt confident using this app | 5 |
| I needed to learn a lot before I could get going with this app | 0 |
Ratings were collected on a 5-point Likert-type scale with 1 = Strongly Disagree to 5 = Strongly Agree. Results are presented for the number of participants with ratings of 3–5 (Agree to Strongly Agree)
3.3. Tobacco Use at Follow Up
Although this was not a smoking cessation app per se, we assessed tobacco use. At the 1-month assessment, 2 of the 5 participants reported abstinence (no smoking in the last 7 days). At the 3-month follow-up assessment, 1 of the 4 participants reported smoking abstinence. These results are lower than those observed in clinical trials, but consistent with the general decline seen when medications are used in “real world” settings [45, 46].
4. Discussion and Conclusion
4.1. Discussion
RxCoach stands apart from general medication management apps because it focuses on a tobacco cessation medication, reliance on evidence-based guidelines for quitting smoking, provision of tobacco cessation information and resources, and condition-specific support functions. RxCoach is unique among tobacco cessation apps in focusing on the complex needs of medication users, attempting to overcome barriers to adherence, and providing a mechanism to facilitate patient-provider (physician/pharmacist) communications. Unlike other studies [28], our app does not use text messaging, but rather resides on the phone so it can be used with or without cellular/internet connectivity. Our app was also designed to stand alone, rather than serve as an adjunct to a more extensive cessation program [29].
The use of RxCoach during the feasibility study was associated with good medication adherence, and the app received high consumer satisfaction ratings. These results suggest that point to RxCoach may be useful for addressing issues related to poor adherence, and facilitating medication taking. However, the small sample size and high attrition make it difficult to draw conclusions from the study results.
4.2. Limitations and Lessons Learned
We encountered several difficulties in recruiting participants due to the reasons outlined above under “Participant Recruitment.” We focused on smokers who were interested in quitting and had already obtained a prescription for varenicline. We chose varenicline because it was the first-line prescription smoking cessation medication at the time the study began. However, this may have inadvertently limited participant recruitment, as emerging after-market concerns (eg FDA-mandated “black box warning”) about varenicline greatly reduced its use in favor of nicotine replacement therapy and other prescription medications.
In addition, we developed the app for use only on the iPhone platform, which was surpassed by the Android operating system at the time of our study. Also at first, we used a cumbersome process for downloading the app wherein users had to find and report to us the unique device identifier (UDID) for their iPhone, a common approach for deploying prototype iPhone apps. However, 66% of potential participants who were screened for eligibility never downloaded the app. After discovering this gap, we deployed RxCoach to the App Store, and enrolled participants via a direct link from the screening survey. With this more streamlined process, we were able to convert 100% of participants.
We also discovered that in-app, Facebook, and Google paid advertising were not cost-effective methods of recruitment. Earned media (e.g., newspaper and radio interviews), social media (e.g., Facebook and Twitter), and professional contacts (e.g., tobacco cessation clinics) were the most effective methods for recruiting participants. We were able to revise our advertising strategy, but exhausted our limited advertising budget.
We also faced difficulties with retention. We began the study wanting to create a “real world” experiment. Therefore, research project staff had little to no contact with participants. In hindsight, this may not have been the optimal approach, as this lack of contact resulted in a high level of attrition. Other reasons for attrition included side effects of varenicline and technical issues (e.g., lost/upgraded phones).
The study design, in which we required participants to have already received a prescription for varenicline and the small sample size greatly limits the generalizability of our findings. Participants may have been at different stages in using their medication at the time of study enrollment. In addition, there was a lack of racial/ethnic diversity, and no males in our feasibility sample, further limiting generalizability.
Finally, we relied on self-report of medication adherence. Because this was a feasibility trial of a low-cost, easily disseminable mHealth app, we chose not to use expensive monitoring technology. In the second phase of the study, we programmed a barcode scanner that could be used in a future study to provide a more object method of measuring compliance with the treatment regimen. In the feasibility trial, the participants were instructed to use the barcode scanner each time they took their medication, and to take a picture of themselves taking each pill. They were also instructed to use the barcode scanner to scan each new box of medication when they received their prescription refill. The scanner automatically collected the use and refill data, and the participants could send the photos via text or email. We plan to integrate the photo function into a future version of the app, and use this as a cost-efficient method of measuring adherence.
4.3. Conclusion
We experienced challenges with recruitment and retention. Although the use of RxCoach was associated with good medication adherence, received high consumer satisfaction ratings, and demonstrated substantial feasibility and usability, the small sample size and high attrition greatly limit the generalizability of the findings. However, we gained valuable experience regarding the conduct of mHealth research, including the challenges and potential strategies for increasing recruitment and retention of participants. We learned the importance of diversification for both medications and technology. We developed the app on only one hardware/software platform, and focused on only one form of medication. To increase the potential “reach” of mHealth apps, developers and researchers should attempt to develop cross-platform solutions. In addition, apps targeting specific conditions should incorporate information regarding all the possible medications for treating those conditions. In addition, we have learned that a combination recruitment approach, including paid advertising, social media, and traditional media, can be more effective than relying on paid advertising alone. Also, researchers must maintain regular contact with participants to limit attrition in mHealth studies. To overcome these challenges, we plan to expand the app to include all other tobacco cessation medication, which would greatly expand the potential pool of participants. Furthermore, we plan to use new methods for recruitment, to verify participants, and to maintain contact with enrolled participants.
Highlights.
Focus and user testing of RxCoach was used to increase functionality and usability of the app.
Challenges were faced in recruitment and retention.
Use of the app was associated with good medication adherence.
The participants rated the app highly.
Due to study limitations, the results are not generalizable, and more research is needed.
Acknowledgments
This study was funded by a grant from the National Cancer Institute, Grant #1R41CA162502-1A1, Judith Gordon, PI.
Footnotes
Conflict of Interest
The authors report no conflicts of interest in this work. The following posters have been presented on this project:
Gordon JS, Muramoto MS, & Armin J. Mobile Phone Application to Improve Adherence to Tobacco Cessation Medication. Poster presented at the Arizona Health and Sciences Center 2012 Frontiers in Biomedical Research Poster Forum. Tucson, AZ, November 7, 2012.
Gordon JS, Muramoto M, Cunningham J, Armin J, Christiansen S, & Jacobs T. RxCoach: Development and Evaluation of a Mobile app to increase tobacco cessation medication adherence. Poster presented at the annual meeting of the Society for Research on Nicotine and Tobacco, Philadelphia, PA, February 24–28, 2015.
Gordon JS, Muramoto M, Cunningham J, Armin J, Christiansen S, & Jacobs T. Development and evaluation of the RxCoach mHealth app to increase tobacco cessation medication adherence. Poster presented at the annual meeting of the Society for Behavioral Medicine, San Antonio, TX, April 22–25, 2015.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Public Health Service, Office of the Surgeon General; Rockville, MD: 2014. [Google Scholar]
- 2.Hays T, Leischow SJ, Lawrence D, Lee TC. Adherence to treatment for tobacco dependence: Association with smoking abstinence and predictors of adherence. Nicotine & Tobacco Research. 2010;12:574–581. doi: 10.1093/ntr/ntq047. [DOI] [PubMed] [Google Scholar]
- 3.Alterman AI, Gariti P, Cook TG, Canaan A. Nicodermal patch adherence and its correlates. Drug and Alcohol Dependence. 1999;53:159–165. doi: 10.1016/s0376-8716(98)00124-0. [DOI] [PubMed] [Google Scholar]
- 4.Fish LJ, Peterson BL, Namenek Brouwer RJ, Lyna P, Oncken CA, Swamy GK. Adherence to nicotine replacement therapy among pregnant smokers. Nicotine & Tobacco Research. 2009;11:514–518. doi: 10.1093/ntr/ntp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leischow S, Ranger-Moore J, Muramoto ML, Matthews E. Effectiveness of the Nicotine Inhaler for Smoking Cessation in an OTC Setting. American Journal of Health Behavior. 2004;28:291–301. doi: 10.5993/ajhb.28.4.1. [DOI] [PubMed] [Google Scholar]
- 6.Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: Secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clinical Therapeutics. 2008;30:1852–1858. doi: 10.1016/j.clinthera.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Catz SL, Jack LM, McClure JB, Javitz HS, Deprey M, Zbikowski SM, … Swan GE. Adherence to varenicline in the COMPASS smoking cessation intervention trial. Nicotine & Tobacco Research. 2011;13(5):361–368. doi: 10.1093/ntr/ntr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liberman JN, Lichtenfeld MJ, Galaznik A, Mastey V, Harnett J, Zou KH, … Kirchner HL. Adherence to varenicline and associated smoking cessation in a community-based patient setting. Journal of Managed Care Pharmacy. 2013;19(2):125–131. doi: 10.18553/jmcp.2013.19.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costano PM, Stockwell MS, Malbon KM. Using digital technologies to improve treatment adherence. Clinical Obstetrics and Gynecology. 2013;56:434–445. doi: 10.1097/GRF.0b013e3182988a3b. [DOI] [PubMed] [Google Scholar]
- 10.DiMatteo MR. Variations in patients’ adherence to medical recommendations: A quantitative review of 50 years of research. Medical Care. 2004;42:200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 11.Kelly M, McCarthy S, Sahm LJ. Knowledge, attitudes, and beliefs of patients and careers regarding medication adherence: A review of qualitative literature [Supplemental material] European Journal of Clinical Pharmacology. 2014 doi: 10.1007/s00228-014-1761-3. Retrieved from http://link.springer.com/article/10.1007/s00228-014-1761-3/fulltext.html. [DOI] [PubMed]
- 12.Lakshminarayana R, Burn D, Chaudhuri R, Cummins G, Galtrey C, Hellman B, Pal S, Stamford J, Steiger M, Wang D, Williams A. Smartphone- and internet- assisted self-management and adherence tools to manage Parkinson’s disease(SMART-PD): Study protocol for a randomised controlled trial [Supplemental material] Trials. 2014 doi: 10.1186/1745-6215-15-374. Retrieved from http://www.trialsjournal.com/content/pdf/1745-6215-15-374.pdf. [DOI] [PMC free article] [PubMed]
- 13.Osterberg L, Blaschke T. Adherence to medication. New England Journal of Medicine. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. Journal of the American Medical Association. 2007;297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 15.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews. 2008:CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: A randomized controlled trial. Journal of the American Medical Association. 2006;296:2563–2571. doi: 10.1001/jama.296.21.joc60162. [DOI] [PubMed] [Google Scholar]
- 17.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: A systematic review. Archives of Internal Medicine. 2007;167:540–550. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 18.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: Scientific review. Journal of the American Medical Association. 2002;288:2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 19.Schedlbauer A, Schroeder K, Peters TJ, Fahey T. Interventions to improve adherence to lipid lowering medication. Cochrane Database of Systematic Reviews. 2004:CD004371. doi: 10.1002/14651858.CD004371.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroeder K, Fahey T, Ebrahim S. Interventions for improving adherence to treatment in patients with high blood pressure in ambulatory settings. Cochrane Database of Systematic Reviews. 2004:CD004804. doi: 10.1002/14651858.CD004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. WHO Library Cataloguing-in-Publication Data. Geneva, Switzerland: 2003. Adherence to Long-Term Therapies: Evidence for action. [Google Scholar]
- 22.National Institutes of Health. Office of Behavioral and Social Science Research. Adherence Research Network. 2016 Apr 11; Downloaded from: https://obssr-archive.od.nih.gov/scientific_areas/health_behaviour/adherence/index.aspx.
- 23.Pew. Mobile Technology Fact Sheet: Highlights of the Pew Internet Project’s research related to mobile technology. 2015 Apr 21; Downloaded from: http://www.pewinternet.org/fact-sheets/mobile-technology-fact-sheet/
- 24.Pew. US Smartphone Use in 2015. 2015 Apr 21; Downloaded from: http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015/
- 25.Pew. Mobile health 2012. 2014 Nov 3; Downloaded from www.pewinternet.org/files/old-media//Files/Reports/2012/PIP_MobileHealth2012_FINAL.pdf.
- 26.Cole-Lewis H, Kershaw T. Text messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev. 2010;32:56–69. doi: 10.1093/epirev/mxq004. Epub 2010 Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishna S, Boren SA, Balas EA. Healthcare via cell phones: a systematic review. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews. doi: 10.1089/tmj.2008.0099. [Internet]. http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0027335/. Review published: 2009. [DOI] [PubMed]
- 28.Krebs P, Tseng TY, Pham H, Wong S, Sherman SE, Shelley D, … Wolfe H. Formative Evaluation of a Text Messaging Intervention to Promote Varenicline Adherence Among Tobacco-Dependent Persons with HIV. Journal of health communication. 2015;20(9):1021–1025. doi: 10.1080/10810730.2015.1018595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClure JB, Anderson ML, Bradley K, An LC, Catz SL. Evaluating an Adaptive and Interactive mHealth Smoking Cessation and Medication Adherence Program: A Randomized Pilot Feasibility Study. JMIR mHealth and uHealth. 2016;4(3):e94. doi: 10.2196/mhealth.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charani E, Castro-Sanchez E, Moore LSP, Holmes A. Do smartphone applications in healthcare require a governance and legal framework? It depends on the application! BioMed Central. 2014;12(29) doi: 10.1186/1741-7015-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nanjappa S, Chambers S, Marcenes W, et al. A theory led narrative review of one-to-one health interventions: the influence of attachment style and client-provider relationship on client adherence. Health Educ Res. 2014 Jun 3; doi: 10.1093/her/cyu029. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen J. [accessed November 4, 2014];Why You Only Need to Test with 5 Users. 2000 Mar 19; http://www.nngroup.com/articles/why-you-only-need-to-test-with-5-users/
- 33.Brooke J. SUS: A quick and dirty usability scale. In: Jordon PW, Thomas B, Weerdmeester BA, McClelland IL, editors. Usability evaluation in industry. London: Taylor & Francis; 1996. pp. 189–194. [Google Scholar]
- 34.Tullis TS, Albert B. Measuring the user experience: Collecting, analyzing, and presenting usability metrics. New York: Morgan Kaufmann Publishers; 2008. [Google Scholar]
- 35.Eysenbach G. The law of attrition. J Med Internet Res. 2005;7(1):e11. doi: 10.2196/jmir.7.1.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Severson HH, Gordon JS, Danaher BG, Akers L. ChewFree.com: Evaluation of a Web-based cessation program for smokeless tobacco users. Nicotine & Tobacco Research. 2008;10:381–391. doi: 10.1080/14622200701824984. [DOI] [PubMed] [Google Scholar]
- 37.Gordon JS, Andrews JA, Crews KM, Payne TJ, Severson HH. The 5A’s vs 3A’s plus proactive quitline referral in private practice dental offices: Preliminary results. Tob Control. 2007;16:285–288. doi: 10.1136/tc.2007.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Severson HH, Andrews JA, Lichtenstein E, Gordon JS, Barckley M, Akers L. A self-help cessation program for smokeless tobacco users: Comparison of two interventions. Nicotine Tobacco Res. 2000;2:363–370. doi: 10.1080/713688152. [DOI] [PubMed] [Google Scholar]
- 39.Fagerstrom KO, Schneider NG. Measuring nicotine dependence: A review of the Fagerstrom tolerance questionnaire. J Behav Med. 1989;12(2):159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- 40.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive Validity of a Medication Adherence Measure in an Outpatient Setting. Journal of Clinical Hypertension. 2008;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Toll BA, McKee SA, Marti DJ, Jatlow P, O’Malley S. Factor structure and validity of the Medication Adherence Questionnaire (MAQ) with cigarette smokers trying to quit. Nicotine & Tobacco Research. 2007;9:597–605. doi: 10.1080/14622200701239662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: The development and evaluation of a new method for assessing the cognitive representation of medication. Psych Health. 1999;14:1–24. [Google Scholar]
- 43.Bender BG, Apter A, Bogen DK, Dickinson P, Fisher L, Wamboldt FS, Westfall JM. Controlled test of an interactive voice response intervention to improve adherence to controller medications in adults with asthma. Journal of the American Board of Family Medicine. 2010;23:159–165. doi: 10.3122/jabfm.2010.02.090112. [DOI] [PubMed] [Google Scholar]
- 44.Gordon JS, Mahabee-Gittens EM, Andrews JA, Christiansen SM, Byron DM. A Randomized Clinical Trial of a Web-Based Tobacco Cessation Education Program. Pediatrics. 2013;131:e455–e462. doi: 10.1542/peds.2012-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin K, Bégaud B, Latry P, Miremont-Salamé G, Fourrier A, Moore N. Differences between clinical trials and postmarketing use. Br J Clin Pharmacol. 2004 Jan;57(1):86–92. doi: 10.1046/j.1365-2125.2003.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson MH. Differences between clinical trial efficacy and real-world effectiveness. Am J Manag Care. 2006 Nov;12(15 Suppl):S405–11. [PubMed] [Google Scholar]