Abstract
Frontotemporal lobar degeneration (FTLD) is a neuropathological disorder that causes a variety of clinical syndromes including fronto-temporal dementia (FTD), progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS). FTD associated with parkinsonism occurs frequently as a result of mutations in the C9orf72 gene and also in the genes coding for the protein associated with microtubule tau (MAPT) and progranulin (GRN) on chromosome 17 (FTDP-17).
Herein we report an Argentinean family, of Basque ancestry, with an extensive family history of behavioral variant of FTD (bvFTD). Twenty one members over 6 generations composed the pedigree. An extensive neurological and neurocognitive examination was performed on 2 symptomatic individuals and 3 non-symptomatic individuals. Two different phenotypes were identified among affected members, CBS in the proband and FTD in his brother.
DNA was extracted from blood for these five individuals and whole-exome sequencing (WES) was performed on 3 of them followed by Sanger sequencing of candidate genes on the other 2. In both affected individuals a missense mutation (p.P301L; rs63751273) in exon 10 of the MAPT gene (chr17q21.3) was identified. Among MAPT mutations, p.P301L is the most frequently associated to different phenotypes: a) aggressive, symmetrical and early-onset Parkinsonism; b) late parkinsonism associated with FTD and c) PSP but only exceptionally it is reported associated to CBS. This is the first report of the occurrence of the p.P301L-MAPT mutation in South America and supports the marked phenotypic heterogeneity among members of the same family as previously reported.
Keywords: FTD, MAPT, P301L, Cognition, CBS
Introduction
Frontotemporal lobar degeneration (FTLD) refers to a spectrum of rare neurodegenerative disorders characterized by protein accumulation and degeneration of frontal and temporal lobes comprising: the behavioral variant of frontotemporal dementia (bvFTD), the semantic and non-fluent variant of primary progressive aphasia (svPPA and nfvPPA), FTD with motor neuron disease (FTD-MND), progressive supranuclear palsy (PSP), and corticobasal syndrome (CBS).
FTLD shows overlapping symptoms including behavioral and personality changes, language impairment, deficits in executive functioning, variable combinations of hyperkinetic or hypokinetic movement disorders (parkinsonism) and/or motor –neuron disease (Pottier et al. 2016; Mackenzie et al. 2016; Oeckl et al. 2016; Baizabal-Carvallo et al. 2016).
While PSP and CBS are classified as “tauopathies”, characterized by the presence of intracellular aggregates of microtubule-associated protein tau (MAPT), FTD may include underlying tau and TDP-43 pathologies. Nevertheless, PSP, CBD and Pick’s disease comprise by far the majority of cases of FTLD-tau
In the last few years a number of different mutations in the MAPT, progranulin (GRN) and C9orf72 have been shown to cause autosomal dominant forms of FTLD (FTD, PSP and CBS) (Pottier C et al. 2016; Mackenzie IR et al. 2016; Oeckl Pet al. 2016; Baizabal-Carvallo JF et al. 2016). Although no clear genotype-phenotype correlation has been established for all, more than 55 mutations in MAPT have classically been assigned as causative of autosomal dominant FTD, primary progressive aphasia (PPA), and PSP.
Here we report the results of a genetic study using whole-genome sequencing (WES) in an Argentinean family that includes clinically diagnosed CBS in one sibling and FTD in another, with an extensive family history of behavioral variant of FTD (bvFTD), originally diagnosed as “Pick’slike disease”, with an autosomal dominant pattern of inheritance.
2. Patients and Methods
2.1. Subjects
This study was approved by the institutional ethics committee. Each subject, from whom blood samples were obtained for genetic testing, provided a written informed consent.
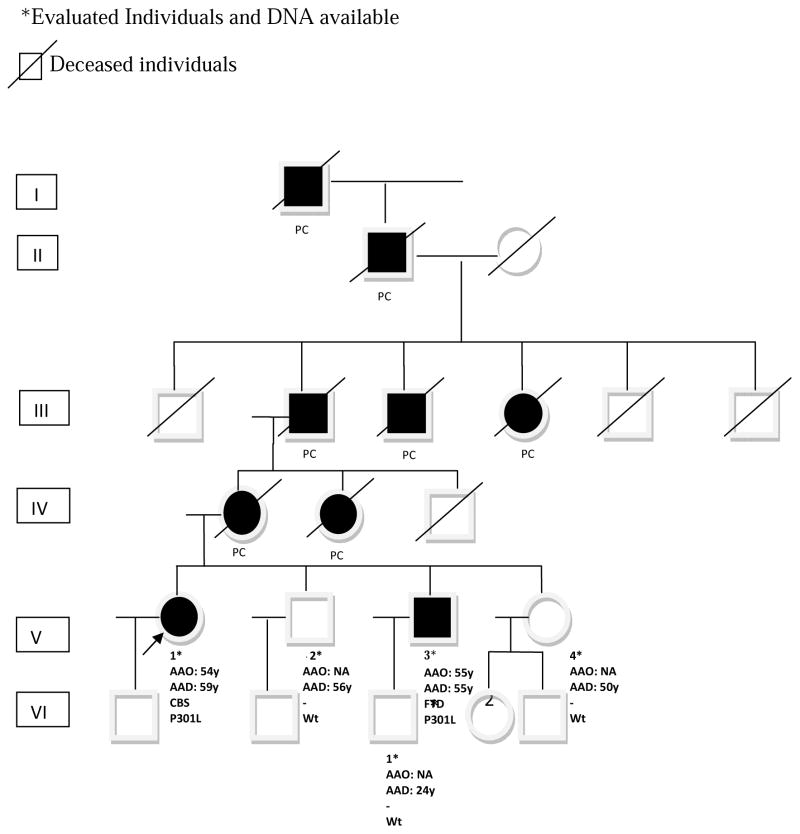
The pedigree consists of 26 family members over 6 generations with 9 affected individuals (Figure 1). A neurological examination was performed on both living symptomatic individuals (V-1, V-3) and 3 asymptomatic individuals (V-2, V-4 and VI-1).
Figure 1.
Pedigree of our family carrying a mutation (p.P301L; rs63751273) in exon 10 of the MAPT gene (chr17q21.3). Proband is indicated with an arrow. All affected members are represented in black. AO= Age at onset.P= proband. E+ = affected individuals with positive evaluation CBS= Corticobasal Syndrome; FTD= Frontotemporal Dementia; PC = Reported as a Pick’s Disease like (bvFTD). Mutation status is shown for all individuals that underwent genetic testing (Wt = wild type).
2.2. Clinical evaluation
Clinical evaluations were conducted at the neuroscience institute (INEBA) (V-1 and V-4) or at the Memory and Aging Center, part of the foundation for the fight against neurological diseases in children (FLENI) (V-2, V-3, VI-1) in Bueno Aires, Argentina.
Personal and family histories were analyzed. Neurologic examination was conducted by neurologists. Symptomatic patients underwent an extensive neuropsychological battery to evaluate the following areas of cognitive domains:
Orientation: from Mini Mental State Exam (MMSE)
Attention: digit span (forward and backward) and trail making test A
Memory: Logical memory from Wechsler Memory Scale, Rey Auditory Verbal Learning Test (RAVLT), and recall from Rey Complex Figure (RCF)
Language: Boston naming test (BNT), semantic and phonemic verbal fluency
Visuospatial: from MMSE and copy from Rey Complex Figure (RCF)
Executive functions: trail making B and verbal fluency
Ancillary test were performed in both symptomatic individuals (brain MRI, PET and SPECT).
2.3. Genetic analyses
First DNA was extracted from blood for the proband (V-1) and two asymptomatic siblings (V-2 and V-4). We performed WES on all 3 individuals following a previously described protocol (Mata et al., 2015). In summary the exome was captured using the Integrated DNA Technologies v1.0 kit (IDT, Coralville, IA) and sequenced with 100-base pair (bp) paired-end reads on a HiSeq2500 (Illumina, San Diego, CA) to achieve a mean coverage of 80–100X. Sequence reads were mapped using the Burrows-Wheeler Aligner and variants were called using GATK Haplotype Caller. We flagged variants failing to meet the quality thresholds described by the GATK “Best Practices” of QD. We excluded alleles that occurred at a frequency >1 % in the ExAC database (http://exac.broadinstitute.org/). Finally, we used custom software to analyze variants that passed all filters to identify alleles that segregated with disease.
To validate candidate variants and to examine segregation in the family we redrew blood from individuals (V-1, V-2) and added two new family members (V-3, affected and VI-1, healthy). In this case DNA was obtained from peripheral blood leukocytes using the Wizard Genomic DNA Purification Kit (PROMEGA) according to manufacturer’s instructions. Fifty nanograms of genomic DNA were used to PCR-amplify exon 10 of the MAPT gene (MIM: 157140) using the following forward and reverse primers, respectively: 5′-TGTCACTCATCGAAAGTGGAGG-3′ and 5′-TCCTGAGAGCCCAAGAAGGATT-3′. PCR product clean-up was performed by treatment with ExoSAP-IT reagent (AFFYMETRIX). Bi-directional automatic Sanger sequencing was performed. Electropherograms were analyzed using the Mutation Surveyor software (SOFTGENETICS) and FinchTV (GEOSPIZA).
3. Results
We studied a multigenerational Argentinean family of Basque origin in which 2 individuals (one female and one male) were affected with CBS and FTD, respectively (Figure 1).
Clinical diagnosis of bvFTD described like Pick’s disease was reported in 7 deceased patients over 4 generations in whom data was collected by interviews of relatives. All these cases were clinically described as early onset dementia where apathy and disinhibition were the prominent symptoms (Figure 1; cases: I-1, II-1, III-1; III-2, III-3; IV-1, IV-2).
3.1 Clinical description
Case V-1 (Proband)
This 59 year-old woman was referred for asymmetric parkinsonism with mild resting tremor and severe rigidity involving the upper right limb. She was born to a non-consanguineous couple and she has two brothers and one sister.
She was a physician; she first noticed unilateral resting tremor, severe bradykinesia and pain in her right upper limb at the age of 54 years. Micrographia was reported as one of the major complaints. She expressed some concerns about mild memory deficits and she was afraid to be a carrier of a possible genetic disorder since several members of her family, from the maternal side, were clinically diagnosed with Pick’s disease.
She was initially misdiagnosed as Parkinson s disease. Levodopa produced a moderated and transient benefit. An initial cognitive assessment, performed at age 56, showed some impairment in attention and executive functions (assessed by Trail Making Test (TMT) A and B). The other cognitive domains were normal
Symptoms progressed and during the next four years the patient exhibited gait disturbance, prominent postural instability (she was prone to fall), myoclonus, focal dystonia with flexor posture and alien limb phenomenon involving upper right limb, as well as, hyperreflexia involving predominantly the right hemibody, bilateral ankle clonus, and Hoffman’s reflex and apraxia. At age 59 she showed marked cognitive impairment involving memory, language, attention and executive domains.
Her family history was relevant as many of her relatives suffered from early onset dementia with prominent behavioral symptoms diagnosed as Pick’s disease (now referred as bvFTD), with a suggestive autosomal dominant pattern of inheritance (Figure 1).
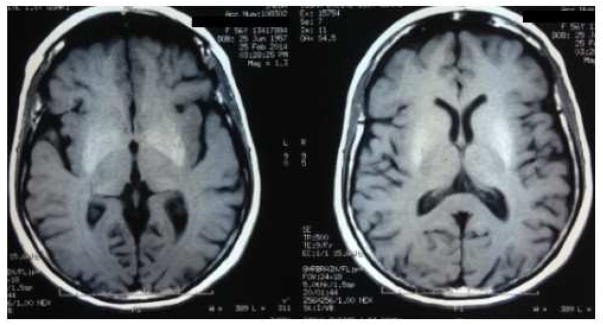
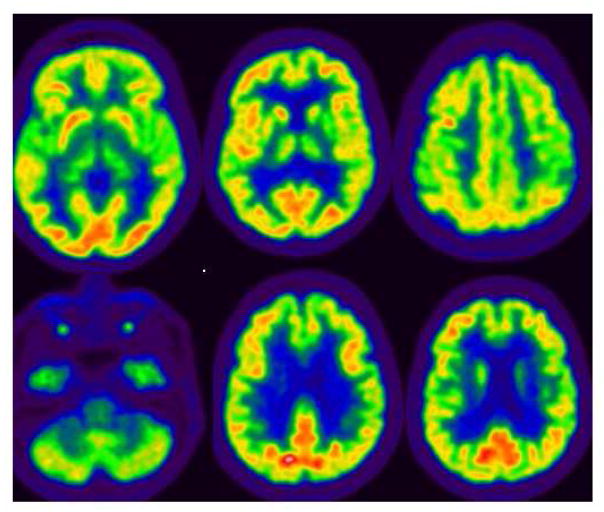
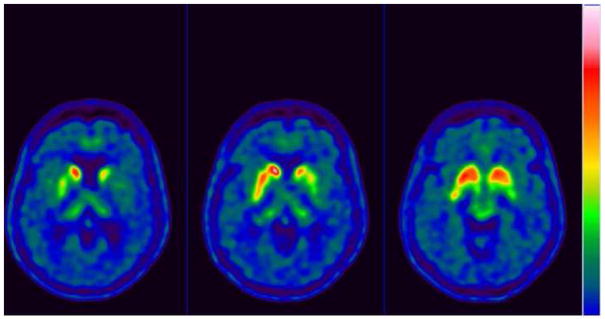
Ancillary test results : The thyroid functional analysis, urine and serum copper, vitamin B12 and vitamin E, manganese, ammonium, antiGAD antibody, antiVGKC antibody were all normal or negative. Brain MRI showed bilateral and symmetric putaminal hyperintense T1 signals (Figure 2). FDG PET: diffuse left hemisphere, thalamic, mesencephalic and basal ganglia hypometabolism as well as left motor cortex (Figure 3). 18F-DOPA PET scan left striatal dopaminergic degeneration (Figure 4). Cognitive assessment disclosed a non-amnesic multi-domain mild cognitive impairment. She fulfilled the proposed clinical criteria for possible corticobasal syndrome (CBS): asymmetrical rigidity and dystonia nonresponsive to L–dopa, insidious onset, progressive course and cortical dysfunction (Armstrong et al., 2013).
Figure 2.
Brain MRI from proband (V-I) showing bilateral and symmetric putaminal hyperintense T1 signals
Figure 3.
FDG PET from proband (V-I) showing diffuse left hemisphere, thalamic, mesencephalic and basal ganglia hypometabolism as well as left motor cortex
Figure 4.
18F-DOPA PET scan left striatal dopaminergic degeneration from proband (V-I).
Proband’s brothers (V-2 and V-3) were evaluated at the Memory and Aging Center, (FLENI, Buenos Aires) seeking genetic counseling since they were concerned about their family history.
Case V-2
He was 56 years old at the time of consultation and had come from another Latin American country where he is still living. He did not report any cognitive complaint at the moment he was interviewed and he was worried about his own risk of suffering from the same disease. He added data about the family history and he was willing to undergo cognitive testing, MRI and genetic testing if necessary. Also he confessed he had come to convince his brother (V-3) to seek medical attention. From his point of view, his brother (V-3) seemed a little more distracted and his nephew (VI-1) had reported to him that his father exhibited behavioral problems. Therefore, his main intention was to assist his nephew (subject VI-1) with his brother’s medical attention.
He was asked for a blood sample for DNA banking. In addition, a brain MRI and neuropsychological battery test were performed.
The MRI of subject V-2 did not show any overt findings. The cognitive assessment showed some impairments on Rey complex figure (copy and recognition) and TMT A and B. The other cognitive domains were normal. Despite the findings on the cognitive testing, no conclusive diagnosis was made because of the lack of correlation between the clinical exam, the brain MRI and cognitive assessment. Therefore he was cataloged as non-amnestic mild cognitive impairment (MCI). It was suggested to him that annual cognitive assessment should be made and the subject returned to his country of residence.
Case V-3
He was 55 years old at the time of consultation. He is a physician and in the past few years had been working in the International Health Policies but he is currently unemployed. At the time he was interviewed he was living with his son (subject VI-1) and he was not married. From the initial visit, he seemed an extroverted person. He did not respect the conventional interviewing timeframes. Although it was the first time he had met the physicians from the Memory and Aging Center, he talked to them in a very casual way and sometimes using foul language or even switching between Spanish and English language. He had logorrhea and a tangential speech. Despite his extroverted attitude, he was fully aware of the family circumstances and also reported his own concern on having a neurological disease. However, he did not report any issues regarding his cognitive abilities. In fact, he said he was writing stories and seemed very satisfied and content with his life. Even though he was unemployed and was receiving monthly payments from his brother (V-2), he did not report any financial issues and he looked very comfortable with this situation. He said he was expecting to collect an important amount of money from an old investment he had made in Europe a long time ago. When he was asked about medical condition, he referred he was in a good physical condition but he used to go to a psychiatrist occasionally due to mild anxiety and for that he was taking pregabalin 75 mg once a day. When his brother (V-2) was asked about personality traits of this patient, he responded that his brother had always been a very extroverted person and this was his normal way of behavior. However, he acknowledged he visits his brother very occasionally.
When his son (VI-1) was asked about his father’s behavioral changes he added a lot more information. He said his father had lost contact with the rest of the family, that he stays at home almost every day sleeping and was awake during the nights writing. The writing level had decreased substantially and tales became very puerile from his point of view. Also, he added that the story of the money to be collected was not real and although he was financially supported by his brother, he did not account for domestic money issues. Importantly, he reported that his father was abusing alcohol and he always had the same meals. Sometimes his father would wake him up in middle of the night just simply to talk. According to the symptoms reported by his son, subject V-3 was suffering from misjudgments in his financial and social situation, lack of empathy with his son a rest of the family, confabulation, some compulsive behavior (alcoholism) and changes in his daily diet and grooming. Otherwise no hyperorality, hypersexuality or other perseverative behavior was reported nor evidenced.
The cognitive assessment of subject V-3 showed impairments in Logical Memory (immediate and delayed) and total and delayed scores of the Rey auditory learning verbal test (RALVT) with preservation of the recognition phase. Also language, attention and executive domains were impaired. The cognitive profile was compatible with attention and executive disorder. In regard to the brain MRI, there was significant slightly asymmetrical frontal atrophy focally distributed to the frontal lobes whereas the other lobes were preserved. Taken together, these findings were compatible with probable behavioral variant of FTD.
Case VI-1
He was 24 years old at the time of the genetic counseling. He works as a chef in a restaurant and he did not report any cognitive complain about himself. He was convinced that his father was suffering from a neurological disease and he was well-aware of his own risk so he also wanted to undergo genetic testing.
3.2 Genetic studies
We performed WES on the proband (V-1) and two unaffected family members (V-2 and V-4). After filtering out all variants with frequency >1% in the ExAC database or those that failed to meet the quality thresholds of the Genome Analysis ToolKit (GATK) “Best Practices” we ended up with 151 variants not shared between the affected proband and the unaffected family members. We then filtered out those variants with a Combined Annotation Dependent Depletion (CADD; http://cadd.gs.washington.edu/) score < 20 (N=131), thus selecting those included in the 1% more deleterious substitutions in the human genome. Two variants stuck out above the rest, with CADD scores of 38 and 29.
The first one was a G to A substitution in GRIN3B (chr19 p13.3) causing an early stop codon, resulting in premature termination of the protein (Trp575*; rs112116006). This variant, although rare in Europeans, is a common SNP in Latinos (MAF >6%).
The second variant, with a CADD score of 29, was a C to T transition in exon 10 in the MAPT gene (chr17q21.3, MIM: 157140), which causes a nonsynonymous proline to leucine change at codon 301 (p.P301L; rs63751273). Mutations in this gene has been previously shown to cause several neurodegenerative disorders including FTD, Parkinson’s disease, PSP and multiple system atrophy (Hodges et al., 2016 ; Tang et al., 2016). MAPT-p.P301L is located in a highly conserved region of the gene, and affects only the 4-repeat tau isoforms since exon 10 is spliced out in the 3-repeat isoform. This mutation is considered pathogenic and has been found in approximately 32 FTD families worldwide.
We then genotyped this variant in all remaining available family members, including the proband (to confirm the variant) using Sanger sequencing. The same variant was also found in the other affected sibling (V-3).
Together, these data support co-segregation of this mutation with the disease
Discussion
We report the first Argentinean family with a pathogenic variant (p.P301L; rs63751273) in exon 10 of the MAPT gene causing a heterogeneous neurological phenotype including both CBS and bvFTD. Positive family history in FTLD has been identified in 30–50% of cases (Gasca-Salas et al., 2016), with an autosomal dominant pattern of inheritance in ~10– 20% of them (Sieben A, et al, 2012; Chow et al., 1999). Among all forms of FTLD, mutations in the GRN, MAPT and C9orf72 genes account for approximately 17% of the cases (Majounie et al., 2012), with GRN and MAPT accounting for approximately 5–20% of all familial FTLD (Rademakers et al., 2012). In all these cases a great clinical heterogeneity is reported including bvFTD (in some cases associated with motor neuron disease); primary progressive aphasias (PPAs, with three variants nonfluent agrammatic, logopenic and semantic); PSP-variant Steele-Richardson Olszewski syndrome; and the CBS (Coyle-Gilchrist et al., 2016). However, CBS and PSP combined were present in only 8.6% of FTLD (Ioannidis et al., 2012; Ghetti et al., 2015; Gasca-Salas et al., 2016).
To date, more than 55 mutations have been identified in the MAPT gene causing autosomal dominant forms of FTD and parkinsonism, with a wide clinical heterogeneity mainly consistent of typical bvFTD (Ghetti et al., 2015; Irwin et al., 2016). Despite having some risk associations with MAPT variants (Jung et al., 2012; Rossi et al. 2008; Wischik et al., 2015) pathogenic MAPT mutations are an unusual cause of CBS thus making it hard to define a clear genotype-phenotype correlation.
In 1998 a mutation in the MAPT gene, p.P301L, was described in several families with FTD and parkinsonism (Hutton et al., 1998; Dumanchin et al., 1998). In 1999, Bugiani et al., reported an Italian family with a different mutation in the same amino acid, p.P301S, that presented with the same phenotype of our family, CBS in some siblings and FTD in others highlighting the intrafamilial variability (Bugiani et al., 1999). Moreover, as in our proband case, brain MRI showed Ti weight hyperintensity in caudate, thalami and putamen (Buggiani et al., 1999).
Kouri et al. reported a new MAPT causing mutation in exon 13 (p.N410H), in a family with not only clinical diagnosed but also neuropathological findings meeting the diagnostic criteria for CBD (Kouri et al., 2014; Donker Kaat et al., 2009; Irwin et al., 2015; Baizabal-Carvallo et al., 2016). Recently, Marshall et al reported a novel heterozygous mutation, p.C291R, with an unusual clinical presentation characterized by progressive apraxia of speech and CBS (Marshall et al., 2015). Although in this family apraxia of speech was the dominant sign, CBS is also known to overlap PPA and FTD and word finding difficulties can be a presenting symptom in CBS (Jung et al., 2012).
Adding to the complex heterogeneity, a p.G389R amino acid substitution in MAPT gene has been reported in a clinically sporadic patient with CBS (Rossi et al., 2008).
Some authors mention some concerns about the true MAPT-CBS association because in many cases a neuropathological confirmation is not available or failed to meet pathologic criteria for CBD (Kouri et al., 2014; Wszolek et al., 2003). An illustrative case showed a MAPT mutation (p.I260V) in exon 9 with differential FTD phenotype and CBD neuropathology (Grover et al. 2003).
Among different MAPT mutations, p.N279K and p.P301L, not only can cause specific phenotypes but also can show different pathophysiological mechanisms. The p.N279K mutation shows abnormally premature developmental 4R tau expression, including changes in the 3R:4R isoform ratio while the p.P301L MAPT mutation shows thicker processes which contain 4R tau and alpha-synuclein and abnormal mitochondrial retrograde transport (Iovino M et al., 2015).
On the other hand, newly performed CBS genome-wide association studies (GWAS) showed that genes other than MAPT increase the risk of CBS, among them MOBP appears as an interesting gene to explore the link between neuronal and oligodendrocyte pathology in CBS (Kouri et al., 2015).
In contrast, intronic and exonic mutations that affect exon 10 splicing and lead to an overproduction of four-repeat tau tend to be associated with a parkinsonism plus predominant phenotype (Ghetti et al., 2015).
As was mentioned above, FTD, PPA, CBS and PSP should be regarded as a clinically and biologically cohesive spectrum and some authors continue to validate the historically eponymic term of Pick’s complex, although the neuronal inclusions exhibit distinct differences. These observation could explain the clinical diagnosis of Pick’s disease that was mentioned for deceased siblings in our family (Kertesz A., 2003; Ikeda et al. 2002)
This report has several strengths defined by the fact that is a first family in our country, with a pathogenic variant in MAPT and variable phenotype in several members of the same kindred. Moreover, a rare phenotype (CBS) contributes to support the variable spectrum associated with MAPT mutations. The identification and report of families with a MAPT mutation give us the opportunity to investigate potential biomarkers, also in very early stage of the disease. Interestingly, the analysis of carriers and non-carriers from a four-generation French-Canadian family segregating p.P310L showed a frontal executive and attentional tasks dysfunction even in presymptomatic individuals (Rademakers et al., 2004). This neuropsychological dysfunction was early described in the proband and his affected brother.
We cannot ignore some weakness such as the lack of pathological confirmation of CBS diagnosis and the scarce objective information of all generations with a recall bias and different historical classification of previously called Pick’s.
In summary, our findings contribute to support the high phenotypic variability among families and also, within the same family with the same MAPT mutation. This observation underscores the concept that other environmental or genetic factors could modify the phenotype.
Highlights.
We found the p.P301L-MAPT mutation in a family with behavioral variant of FTD (bvFTD)
This family included clinically diagnosed CBS in one sibling and FTD in another
First report of p.P301L mutation in the Tau gene in South America
This supports the high phenotypic heterogeneity in p.P301L-MAPT mutation carriers
Acknowledgments
Authors would like to acknowledge all the individuals in this family for their collaboration and doctors Marcelo Kauffman and Sergio Rodriguez, Laboratorio de Neurogenetica, Hospital Ramos Mejía, Buenos aires Argentina for their scientific collaboration.
This work was supported by grants from the Parkinson’s Disease Foundation, the Department of Veterans Affairs (1I01BX000531) and the National Institutes of Health (R01 NS065070, P50 NS062684).
Footnotes
Disclosure Statement
The authors have not actual or potential conflicts of interest
Disclamer
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M, Josephs KA, Kertesz A, Lee SE, Miller BL, Reich SG, Riley DE, Tolosa E, Tröster AI, Vidailhet M, Weiner WJ. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baizabal-Carvallo JF, Jankovic J. Parkinsonism, movement disorders and genetics in frontotemporal dementia. Nat Rev Neurol. 2016;12:175–185. doi: 10.1038/nrneurol.2016.14. [DOI] [PubMed] [Google Scholar]
- Bugiani O, Murrell JR, Giaccone G, Hasegawa M, Ghigo G, Tabaton M, Morbin M, Primavera A, Carella F, Solaro C, Grisoli M, Savoiardo M, Spillantini MG, Tagliavini F, Goedert M, Ghetti B. Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. J Neuropathol Exp Neurol. 1999;58:667–677. doi: 10.1097/00005072-199906000-00011. [DOI] [PubMed] [Google Scholar]
- Chow TW, Miller BL, Hayashi VN, Geschwind DH. Inheritance of frontotemporal dementia. Arch Neurol. 1999;56:817–822. doi: 10.1001/archneur.56.7.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle-Gilchrist IT, Dick KM, Patterson K, Vázquez Rodríquez P, Wehmann E, Wilcox A, Lansdall CJ, Dawson KE, Wiggins J, Mead S, Brayne C, Rowe JB. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86:1736–1743. doi: 10.1212/WNL.0000000000002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donker Kaat L, Boon AJ, Azmani A, Kamphorst W, Breteler MM, Anar B, Heutink P, van Swieten JC. Familial aggregation of parkinsonism in progressive supranuclear palsy. Neurology. 2009;73:98–105. doi: 10.1212/WNL.0b013e3181a92bcc. [DOI] [PubMed] [Google Scholar]
- Dumanchin C, Camuzat A, Campion D, Verpillat P, Hannequin D, Dubois B, Saugier-Veber P, Martin C, Penet C, Charbonnier F, Agid Y, Frebourg T, Brice A. Segregation of a missense mutation in the microtubule-associated protein tau gene with familial frontotemporal dementia and parkinsonism. Hum Mol Genet. 1998;7:1825–9. doi: 10.1093/hmg/7.11.1825. [DOI] [PubMed] [Google Scholar]
- Gasca-Salas C, Masellis M, Khoo E, Shah BB, Fisman D, Lang AE, Kleiner-Fisman G. Characterization of Movement Disorder Phenomenology in Genetically Proven, Familial Frontotemporal Lobar Degeneration: A Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0153852. doi: 10.1371/journal.pone.0153852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti B, Oblak AL, Boeve BF, Johnson KA, Dickerson BC, Goedert M. Invited review: Frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: a chameleon for neuropathology and neuroimaging. Neuropathol Appl Neurobiol. 2015;41:24–46. doi: 10.1111/nan.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover A, England E, Baker M, Sahara N, Adamson J, Granger B, Houlden H, Passant U, Yen SH, DeTure M, Hutton M. A novel tau mutation in exon 9 (1260V) causes a four-repeat tauopathy. Exp Neurol. 2003;184:131–40. doi: 10.1016/s0014-4886(03)00393-5. [DOI] [PubMed] [Google Scholar]
- Hodges K, Brewer SS, Labbé C, Soto-Ortolaza AI, Walton RL, Strongosky AJ, Uitti RJ, van Gerpen JA, Ertekin-Taner N, Kantarci K, Lowe VJ, Parisi JE, Savica R, Graff-Radford J, Jones DT, Knopman DS, Petersen RC, Murray ME2, Graff-Radford NR, Ferman TJ, Dickson DW, Wszolek ZK, Boeve BF, Ross OA, Lorenzo-Betancor O. RAB39B gene mutations are not a common cause of Parkinson’s disease or dementia with Lewy bodies. Neurobiol Aging. 2016;45:107–108. doi: 10.1016/j.neurobiolaging.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–5. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Akiyama H, Arai T, Tsuchiya K. Pick-body-like inclusions in corticobasal degeneration differ from Pick bodies in Pick’s disease. Acta Neuropathol. 2002;103:115–118. doi: 10.1007/s004010100440. [DOI] [PubMed] [Google Scholar]
- Ioannidis P, Konstantinopoulou E, Maiovis P, Karacostas D. The frontotemporal dementias in a tertiary referral center: classification and demographic characteristics in a series of 232 cases. J Neurol Sci. 2012;318:171–173. doi: 10.1016/j.jns.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Iovino M, Agathou S, Gonzalez-Rueda A, Del Castillo Velasco-Herrera M, Borroni B, Alberici A, Lynch T, O’Dowd S, Geti I, Gaffney D, Vallier L, Paulsen O, Káradóttir RT, Spillantini MG. Early maturation and distinct tau pathology in induced pluripotent stem cell-derived neurons from patients with MAPT mutations. Brain. 2015;138:3345–3359. doi: 10.1093/brain/awv222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DJ, Cairns NJ, Grossman M, McMillan CT, Lee EB, Van Deerlin VM, Lee VM, Trojanowski JQ. Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta Neuropathol. 2015;129:469–491. doi: 10.1007/s00401-014-1380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DJ, Brettschneider J, McMillan CT, Cooper F, Olm C, Arnold SE, Van Deerlin VM, Seeley WW, Miller BL, Lee EB, Lee VM, Grossman M, Trojanowski JQ. Deep clinical and neuropathological phenotyping of Pick disease. Ann Neurol. 2016;79:272–287. doi: 10.1002/ana.24559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HH, Bremer J, Streffer J, Virdee K, Spillantini MG, Crowther RA, Brugger P, Van Broeckhoven C, Aguzzi A, Tolnay M. Phenotypic variation of autosomal-dominant corticobasal degeneration. Eur Neurol. 2012;67:142–150. doi: 10.1159/000334731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. Pick’s complex and FTDP-17. Mov Disord Suppl. 2003;6:S57–S62. doi: 10.1002/mds.10564. [DOI] [PubMed] [Google Scholar]
- Kouri N, Carlomagno Y, Baker M, Liesinger AM, Caselli RJ, Wszolek ZK, Petrucelli L, Boeve BF, Parisi JE, Josephs KA, Uitti RJ, Ross OA, Graff-Radford NR, DeTure MA, Dickson DW, Rademakers R. Novel mutation in MAPT exon 13 (p.N410H) causes corticobasal degeneration. Acta Neuropathol. 2014;127:271–282. doi: 10.1007/s00401-013-1193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouri N, Ross OA, Dombroski B, Younkin CS, Serie DJ, Soto-Ortolaza A, Baker M, Finch NC, Yoon H, Kim J, Fujioka S, McLean CA, Ghetti B, Spina S, Cantwell LB, Farlow MR, Grafman J, Huey ED, Ryung Han M, Beecher S, Geller ET, Kretzschmar HA, Roeber S, Gearing M, Juncos JL, Vonsattel JP, Van Deerlin VM, Grossman M, Hurtig HI, Gross RG, Arnold SE, Trojanowski JQ, Lee VM, Wenning GK, White CL, Höglinger GU, Müller U, Devlin B, Golbe LI, Crook J, Parisi JE, Boeve BF, Josephs KA, Wszolek ZK, Uitti RJ, Graff-Radford NR, Litvan I, Younkin SG, Wang LS, Ertekin-Taner N, Rademakers R, Hakonarsen H, Schellenberg GD, Dickson DW1. Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy. Nat Commun. 2015;6:7247. doi: 10.1038/ncomms8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M. Molecular neuropathology of frontotemporal dementia: insights into disease mechanisms from postmortem studies. J Neurochem. 2016;138(Suppl 1):54–70. doi: 10.1111/jnc.13588. [DOI] [PubMed] [Google Scholar]
- Majounie E, Renton AE, Mok K, Dopper EG, Waite A, Rollinson S, Chiò A, Restagno G, Nicolaou N, Simon-Sanchez J, van Swieten JC, Abramzon Y, Johnson JO, Sendtner M, Pamphlett R, Orrell RW, Mead S, Sidle KC, Houlden H, Rohrer JD, Morrison KE, Pall H, Talbot K, Ansorge O, Hernandez DG, Arepalli S, Sabatelli M, Mora G, Corbo M, Giannini F, Calvo A, Englund E, Borghero G, Floris GL, Remes AM, Laaksovirta H, McCluskey L, Trojanowski JQ, Van Deerlin VM, Schellenberg GD, Nalls MA, Drory VE, Lu CS, Yeh TH, Ishiura H, Takahashi Y, Tsuji S, Le Ber I, Brice A, Drepper C, Williams N, Kirby J, Shaw P, Hardy J, Tienari PJ, Heutink P, Morris HR, Pickering-Brown S, Traynor BJ. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol. 2012;11:323–330. doi: 10.1016/S1474-4422(12)70043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Guerreiro R, Thust S, Fletcher P, Rohrer JD, Fox NC. A Novel MAPT Mutation Causing Corticobasal Syndrome Led by Progressive Apraxia of Speech. J Alzheimer Dis. 2015;48:923–926. doi: 10.3233/JAD-150477. [DOI] [PubMed] [Google Scholar]
- Mata I, Yongwoo J, Chun-Hyung K, Hanna DS, Dorschner M, Samii A, Agarwal P, Roberts JW, Klepitskaya O, Shprecher DR, Chung KA, Factor SA, Espay AJ, Revilla FJ, Higgins DS, Litvan I, Leverenz JB, Yearout D, Inca-Martinez M, Martinez E, Thompson TR, Cholerton BA, Hu SC, Edwards KL, Kim KS, Zabetian CP. The RAB39B p.G192R mutation causes X-linked dominant Parkinson’s disease. Mol Neurodegener. 2015;24(10):50. doi: 10.1186/s13024-015-0045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeckl P, Steinacker P, Feneberg E, Otto M Chromosome 9-ALS/FTD Consortium; French research network on FTLD/FTLD/ALS; ITALSGEN Consortium. Neurochemical biomarkers in the diagnosis of frontotemporal lobar degeneration: an update. J Neurochem. 2016;138(Suppl 1):184–92. doi: 10.1111/jnc.13669. [DOI] [PubMed] [Google Scholar]
- Pottier C, Ravenscroft TA, Sanchez-Contreras M, Rademakers R. Genetics of FTLD: overview and what else we can expect from genetic studies. J Neurochem. 2016;138(Suppl 1):32–53. doi: 10.1111/jnc.13622. [DOI] [PubMed] [Google Scholar]
- Rademakers R, Cruts M, van Broeckhoven C. The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum Mutat. 2004;24:277–295. doi: 10.1002/humu.20086. [DOI] [PubMed] [Google Scholar]
- Rademakers R, Neumann M, Mackenzie IR. Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol. 2012;8:423–434. doi: 10.1038/nrneurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G, Marelli C, Farina L, Laurà M, Maria Basile A, Ciano C, Tagliavini F, Pareyson D. The G389R mutation in the MAPT gene presenting as sporadic corticobasal syndrome. Mov Disord. 2008;23:892–895. doi: 10.1002/mds.21970. [DOI] [PubMed] [Google Scholar]
- Sieben A, Van Langenhove T, Engelborghs S, Martin JJ, Boon P, Cras P, De Deyn PP, Santens P, Van Broeckhoven C, Cruts M. The genetics and neuropathology of frontotemporal lobar degeneration. Acta Neuropathol. 2012;124:353–372. doi: 10.1007/s00401-012-1029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SS, Li J, Tan L, Yu JT. Genetics of Frontotemporal Lobar Degeneration: From the Bench to the Clinic. J Alzheimers Dis. 2016;52:1157–1176. doi: 10.3233/JAD-160236. [DOI] [PubMed] [Google Scholar]
- Wischik CM, Staff RT, Wischik DJ, Bentham P, Murray AD, Storey JM, Kook KA, Harrington CR. Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer’s disease. J Alzheimers Dis. 2015;44:705–720. doi: 10.3233/JAD-142874. [DOI] [PubMed] [Google Scholar]
- Wszolek ZK, Tsuboi Y, Farrer M, Uitti RJ, Hutton ML. Hereditary tauopathies and parkinsonism. Adv Neurol. 2003;91:153–163. [PubMed] [Google Scholar]