Abstract
HemoHIM, herbal preparation has designed for immune system recovery. We investigated the anti-inflammatory effect of HemoHIM on cigarette smoke (CS) and lipopolysaccharide (LPS) induced chronic obstructive pulmonary disease (COPD) mouse model. To induce COPD, C57BL/6 mice were exposed to CS for 1 h per day (eight cigarettes per day) for 4 weeks and intranasally received LPS on day 26. HemoHIM was administrated to mice at a dose of 50 or 100 mg/kg 1h before CS exposure. HemoHIM reduced the inflammatory cell count and levels of tumor necrosis factor receptor (TNF)-α, interleukin (IL)-6 and IL-1β in the broncho-alveolar lavage fluid (BALF) induced by CS+LPS exposure. HemoHIM decreased the inflammatory cell infiltration in the airway and inhibited the expression of iNOS and MMP-9 and phosphorylation of Erk in lung tissue exposed to CS+LPS. In summary, our results indicate that HemoHIM inhibited a reduction in the lung inflammatory response on CS and LPS induced lung inflammation via the Erk pathway. Therefore, we suggest that HemoHIM has the potential to treat pulmonary inflammatory disease such as COPD.
Keywords: HemoHIM, cigarette smoke, inducible nnitric oxide synthase, matrix metalloproteinase-9, Erk
The prevalence of chronic obstructive pulmonary disease (COPD) has consistently increased owing to an elevation of smoking population and exposure to various chemicals [1]. As COPD increases, the cost of treatment is also increases, and the quality of life declines [2]. COPD is characterized by airway inflammation, mucus secretion and emphysema that resulted reduction of pulmonary function [3,4]. Therefore, many researchers have investigated the remedy to effectively suppress the development of COPD.
Cigarette smoke (CS) is well known to be the greatest risk factor related to the development of COPD [5]. CS continuously induces airway inflammation mediated by complex signaling pathways because it consists of thousands of toxic chemicals [6]. They produce reactive oxygen species (ROS), interleukines, chemokines, and proteases via direct or indirect stimulation of airway epithelial cells and macrophages [7]. The elevation in the inflammation index causes chronic airway inflammation and structural alterations exerting the loss of lung function [8]. Based on previous documents, the suppression of inflammatory responses is considered an important treatment strategy for inhibiting the development of COPD.
Extracellular signal-regulated kinases (ERKs) is one of mitogen-activated protein kinases (MAPKs) and an important mediator in cellular transcriptional activity including inflammatory responses [9]. ERK pathway is activated by various stimuli and CS is a powerful stimulus for ERK activation [10]. During the development of COPD, ERK activation produced pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 and matrix metalloproteinases (MMPs), which aggravates airway inflammation and destroy normal alveolar structure [11,12,13].
HemoHIM, a herbal preparation is designed to recover immune system and commercially used in South Korea. It consists of three herb; Angelica Radix, Cnidium Rhizoma and Paeonia Radix [14]. HemoHIM has been reported to improve the immune system in patients undergoing chemotherapy and have an anti-inflammatory effect in carrageenan-induced paw edema, downregulate Th1-like immune responses in fractionated γ-irradiated mice, and have an anti-diabetic effect in a streptozotocin-induced diabetic model [15,16,17,18]. However, there has not been a study on the protective effects of HemoHIM on lung inflammation induced by CS and LPS exposure.
Therefore, we examined the anti-inflammatory response of HemoHIM in the lung using a CS and LPS-induced model. To confirm the possible mechanism of HemoHIM, we investigated the expression and production of lung inflammatory mediators due to CS and LPS exposure.
Materials and Methods
Animals
Specific pathogen-free male C57BL/6N mice (20~25 g, six to eight weeks-old) were purchased from the Samtako Co. (Osan, Korea). They were housed in groups of nine under standard conditions (temperature 22±2℃, humidity 55±5%, 12-h-light/dark cycle) with food and water. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Chonnam National University.
Induction of CS and LPS in C57BL/6 mice and drug administration
The CS was generated from 3R4F research cigarette (Kentuchy reference cigarette, University of Kentuchy, USA), containing 11.0 mg of total particulate matter, 9.4 mg of tar, and 0.76 mg of nicotine per cigarette. Exposure to CS (one puff/min, 35 mL puff volum over 2 seconds, every 60 seconds, 8 cigarettes per day) was conducted using cigarette smoke generator (Daehan Biolink, Republic of Korea). The mice were exposed to CS for 1 h in a chamber (50 cm×30 cm×30 cm) for 28 days. LPS were intranasally instilled (10 µg dissolved in 50 µL distilled water) under anesthesia on day 26. The HemoHIM was obtained from Korea Institute of Oriental Medicine (Daejeon, Republic of Korea). HemoHIM was administered to mice at doses of 50 or 100 mg/kg by oral gavage 1 h before CS exposure for 28 days. A positive control group was administered roflumilast (Sigma-Aldrich, St, Louis, MO, USA, 10 mg/kg) which is a PDE-4 inhibitor and manufactured for treatment COPD.
Collection of bronchoalveolar lavage fluid (BALF)
Forty-eight hours after the last intranasal LPS administration, the mice were sacrificed via an intraperitoneal injection of zoletil 50 (25 mg/kg; Virbac korea. Co., Seoul, Korea), and a tracheostomy was performed according to a previous study [19]. To obtain the broncho-alveolar lavage fluid (BALF), ice-cold PBS (0.7 mL) was infused into the lung and withdrawn via tracheal cannulation. This process was repeated once (total volume 1.4 mL). To determine the differential cell counts, 100 µL of BALF was centrifuged onto slides using a Cytospin (Hanil Science Industrial, Seoul, Korea). The slides were dried, and the cells were fixed and stained using Diff-Quik staining reagent (B4132-1A; IMEB Inc., Deerfield, IL) according to the manufacturer's instructions. The supernatant obtained from the BALF was stored at −70℃ for biochemical analysis.
Measurement of pro-inflammatory mediator in BALF
The pro-inflammatory mediators in the BALF were measured using ELISA kits (R&D System, Minneapolis, MN, USA) according to the manufacturer's protocols. The plates were incubated for 10 min in the dark, and the absorbance was measured at 450 nm using a microplate reader (Bio-Rad, Hercules, CA, Laboratories).
Immunoblotting
The lung tissue was homogenized (1/10 w/v) using a homogenizer in a Tissue Lysis/Extraction reagent (Sigma-Aldrich, St, Louis, MO, USA) that contained a protease inhibitor cocktail (Sigma-Aldrich). Protein concentrations were determined using Bradford reagent (Bio-Rad). Equal amounts of the total protein (30 µg) were resolved by 10% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were incubated with blocking solution (5% skim milk) followed by overnight incubation at 4℃ with the appropriate primary antibody. The following primary antibodies and dilutions were used: anti-β-actin (1:2000 dilution; Cell Signaling, Danvers, MA, USA), anti-pERK (1:1000 dilution; Cell Signaling), anti-ERK (1:1000 dilution; Cell Signaling) and anti-iNOS (1:1000 dilution; Santa Cruz Biotechnology, MA, USA). The blots were washed three times with Tris-buffered saline containing Tween 20 (TBST) and then incubated with a 1:10000 dilution of horseradish peroxidase (HRP)-conjugated secondary antibody (Jackson Immuno Research, West Grove, PA, USA) for 30 min at room temperature. The blots were then washed three times with TBST and then developed using an enhanced chemiluminescence (ECL) kit (Thermo Fisher Scientific, Carlsbad, CA, USA).
Gelatin zymography
SDSPAGE zymography was performed according to previous study (Shin et al., 2014) to determine gelatinase activitiy. Briefly, zymogram gels comprised of 10% SDS-PAGE containing 1% gelatin were used as the MMP substrate. The gels were washed in 2.5% Triton X-100 for 1 h to remove SDS and then incubated at 37℃ for 16 h in developing buffer (1M Tris-HCl, pH 7.5 with CaCl2). Thereafter, gels were stained with 25% methanol/8% acetic acid containing Coomassie Brilliant Blue. Gelatinase activity was visualized as white bands on a blue background that represented the areas of proteolysis.
Lung tissue histopathology
The lung tissue was fixed in 4% (v/v) paraformaldehyde, embedded in paraffin, sectioned at 4-µm thickness, and stained with hematoxylin and eosin (H&E_solution; Sigma-Aldrich) to estimate inflammation.
Immunohistochemical slides were deparaffinized, dehydrated, washed in PBS containing 0.05% tween 20 (PBS-T), and incubated for 20 min at room temperature with goat serum to block nonspecific staining. The slides were incubated for 2 h at room temperature with primary mouse anti-mouse MMP-9 antibody (diluted 1:100, Abcam). After incubation, they were washed three times, incubated for 1 h at room temperature with a biotinylated secondary antibody, and then incubated with an avidinbiotin-peroxidase complex (Vector Laboratories, Burlin-game, CA, USA) for 1 h at room temperature. Then, the slides were washed with PBS-T and incubated with diaminobenzidine (DAB, Abcam) for an additional 5min.
Statistical analysis
The data are expressed as the means±standard deviation (SD). Statistical significance was determined using an analysis of variance (ANOVA) followed by a multiple comparison test with Dunnet's adjustment. P values <0.05 were considered significant.
Results
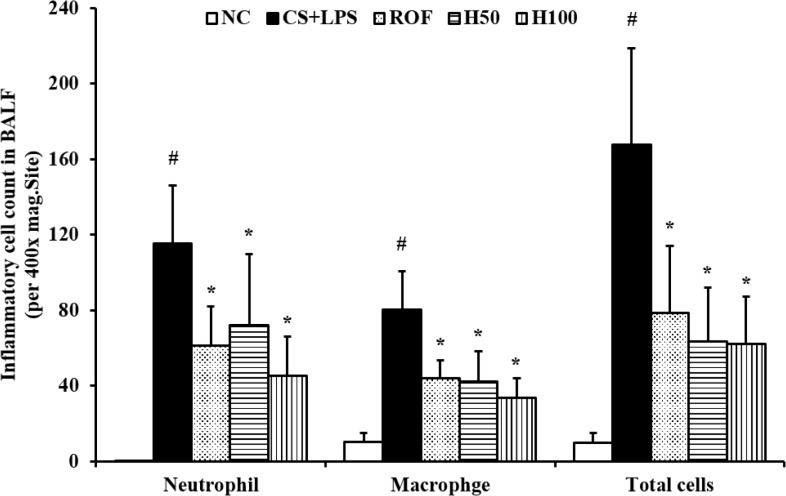
HemoHim reduce the number of inflammatory cells in BALF induced by CS and LPS exposure
The number of inflammatory cells in BALF was increased in CS and LPS exposed mice compared with vehicle control mice. Specifically, CS and LPS exposure markedly increased the number of neutrophils in BALF compared to control. In HemoHim treated mice, however, the number of neutrophils in BALF decreased in a dose-dependent manner compared to CS and LPS exposed mice (Figure 1).
Figure 1. HemoHIM reduced the number of inflammatory cells in the BALF. NC: Non-induced mice; CS+LPS: cigarette smoke (CS) and lipopolysaccharides (LPS) induced mice; ROF: roflumilast (10 mg/kg) and CS and LPS induced mice; H50: HemoHIM (50 mg/kg) and CS and LPS induced mice; H100 (100 mg/kg) and CS and LPS induced mice. The values are expressed as the means±SD. #Significantly different from the control mice, P<0.05; *Significantly different from the CS mice, P<0.05.
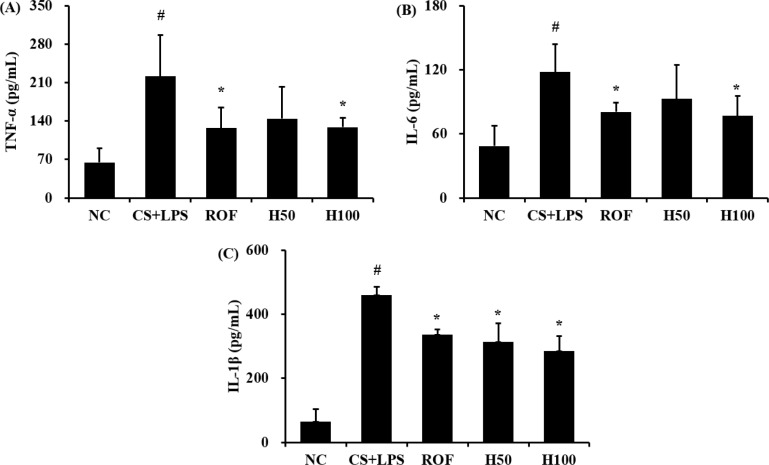
HemoHim decrease pro-inflammatory cytokines induced by CS and LPS exposure
In CS and LPS exposed mice, the levels of TNF-α in BALF were significantly increased compared to the vehicle control mice (Figure 2A). Roflumilast significantly reduced TNF-α in CS and LPS exposed mice. In addition, HemoHim treated mice showed a dose-dependent decrease TNF-α compared to CS and LPS exposed mice. The results of IL-6 and IL-1β in BALF were similar to those of TNF-α (Figure 2A, B). CS and LPS exposure mice significantly increased level of IL-6 and IL-1β in BALF compared with the vehicle control mice, and HemoHim treated mice significantly decreased the level of IL-6 and IL-1β in BALF in CS and LPS exposed mice.
Figure 2. HemoHIM decreased pro-inflammatory cytokines. (A) TNF-α, (B) IL-6, and (C) IL-1β. NC: Non-induced mice; CS+LPS: CS and LPS induced mice; ROF: roflumilast (10 mg/kg) and CS and LPS induced mice; H50: HemoHIM (50 mg/kg) and CS and LPS induced mice; H100 (100 mg/kg) and CS and LPS induced mice. The values are expressed as the means±SD. #Significantly different from the control mice, P<0.05; *Significantly different from the CS mice, P<0.05.
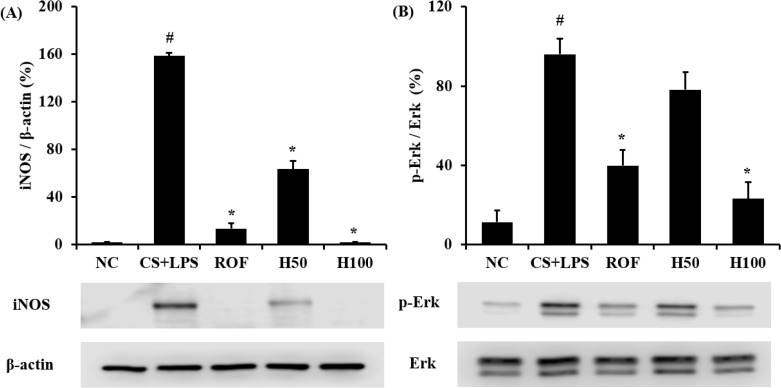
HemoHim reduce the expression of iNOS and phosphorylation of Erk in lung tissue induced by CS and LPS exposure
iNOS expression increased in the lung tissue of CS and LPS exposed mice compared to vehicle control mice. Roflumilast treated mice markedly decreased iNOS expression in the lung tissue compared with CS and LPS exposed mice. In addition, HemoHIM treated mice showed a dose-dependent decrease of iNOS expression in lung tissue compared to CS and LPS exposed mice (Figure 3A).
Figure 3. HemoHIM inhibited the iNOS and phosphorylation of ERK expression in lung tissue. (A) Expression of iNOS. (B) Phosphorylation of ERK. (C and D) Quantitative analysis of iNOS expression and phosphorylation of ERK expression. NC: Non-induced mice; CS+LPS: CS and LPS induced mice; ROF: roflumilast (10 mg/kg) and CS and LPS induced mice; H50: HemoHIM (50 mg/kg) and CS and LPS induced mice; H100 (100 mg/kg) and CS and LPS induced mice. The values are expressed as the means±SD. #Significantly different from the control mice, P<0.05; *Significantly different from the CS mice, P<0.05.
In comparison to vehicle control mice, the phosphorylation of Erk was significantly increased in CS and LPS exposed mice. HemoHIM markedly and dose-dependently decreased phosphorylation of Erk in CS and LPS exposed mice (Figure 3B).
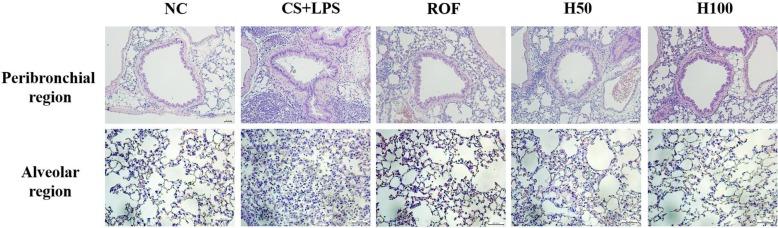
HemoHim decrease inflammatory responses in lung tissue induced by CS and LPS exposure
CS and LPS exposed mice exhibited extensive inflammatory cell infiltration into the lung tissue (Figure 4). Inflammatory cells mainly accumulating in peribronchial and alveolar lesions. In contrast, roflumilast treated mice decreased inflammatory cell infiltration into lung tissue induced by CS and LPS exposure. Similarly, inflammatory cell infiltration was significantly reduced in a dose-dependent manner in HemoHim treated mice compared to CS and LPS exposed mice.
Figure 4. HemoHIM reduced inflammatory cell infiltration induced by CS and LPS exposure. Hematoxylin and eosin (H&E) staining showed inflammatory infiltration in the peribronchial region and alveolar region. NC: Non-induced mice; CS+LPS: CS and LPS induced mice; ROF: roflumilast (10 mg/kg) and CS and LPS induced mice; H50: HemoHIM (50 mg/kg) and CS and LPS induced mice; H100 (100 mg/kg) and CS and LPS induced mice. The values are expressed as the means±SD. #Significantly different from the control mice, P<0.05; *Significantly different from the CS mice, P<0.05.
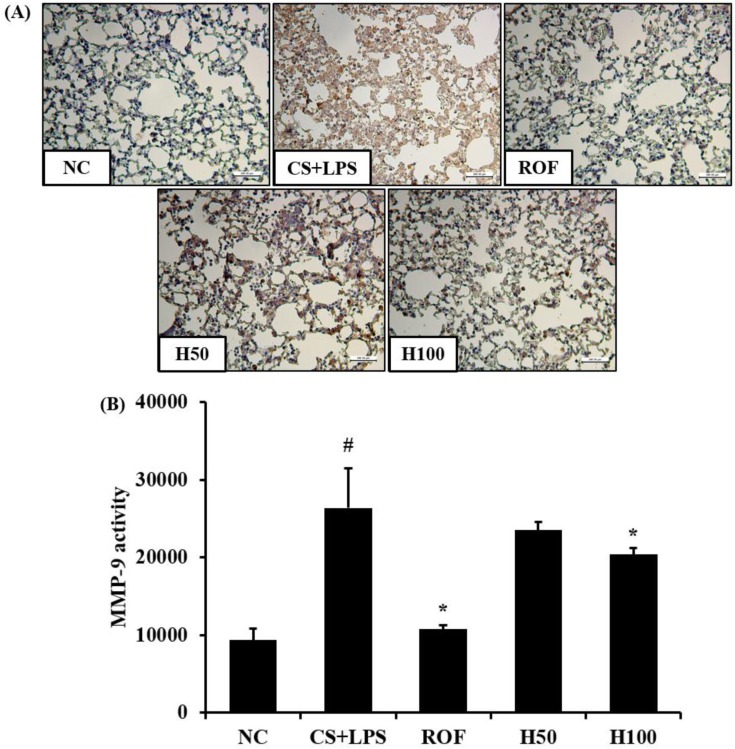
HemoHim reduce the expression and activity of MMP-9 in lung tissue induced by CS and LPS exposure
MMP-9 expression in lung tissue was markedly increased in CS and LPS exposed mice compared to vehicle control mice (Figure 5A). HemoHim treated mice, however, reduced this increased expression of MMP-9 in lung tissue induced by CS and LPS exposure. In zymographs, CS and LPS exposed mice showed a marked increase in MMP-9 activity compared with the vehicle control mice, whereas HemoHim treated mice exhibited a marked and dose-dependent reduction in MMP-9 activity compared with CS and LPS exposed mice (Figure 5B).
Figure 5. HemoHIM decreased the expression and activity of matrix metalloproteinase (MMP)-9 in lung tissue induced by CS and LPS exposure. (A) Representative figure of MMP-9 expression in lung tissue. (B) MMP-9 activity using zymography. NC: Non-induced mice; CS+LPS: CS and LPS induced mice; ROF: roflumilast (10 mg/kg) and CS and LPS induced mice; H50: HemoHIM (50 mg/kg) and CS and LPS induced mice; H100 (100 mg/kg) and CS and LPS induced mice. The values are expressed as the means±SD. #Significantly different from the control mice, P<0.05; *Significantly different from the CS mice, P < 0.05.
Discussion
HemoHIM is used to overcome side effects of chemotherapy in patients with cancer. Recent studies have reported that HemoHIM possesses anti-inflammatory, antioxidative, and antidiabetic effects. In this study, we investigated the effects of HemoHIM on CS and LPS exposed airway inflammation models. HemoHIM markedly suppresses the increased inflammatory cell count and pro-inflammatory cytokines in BALF induced by CS and LPS exposure, which was accompanied by a reduction of inflammatory cell infiltration into lung tissue as seen in the histopathology. Furthermore, HemoHIM profoundly decreased the phosphorylation of Erk and the expression of MMP-9 and iNOS in the lung tissue of CS and LPS exposed mice.
Cigarette smoke (CS) is a major risk factor for the development of COPD, which leads to airway inflammation associated with neutrophils and macrophages in the airway [20,21]. These cells produced pro-inflammatory cytokines, chemokines, and proteases exhibiting aggravation of airway inflammation, mucus secretion, and structural alteration [22]. Pro-inflammatory cytokines, TNF-α, IL-6, and IL-1β were involved in the destruction of the parenchyma by proteinase release and required airway remodeling via the upregulation of MMP-9 in CS induced in vitro and in vivo models [23,24,25]. Therefore, inhibition of pro-inflammatory cytokines is important for attenuation of CS and LPS induced airway inflammation.
In this study, CS and LPS exposed mice showed marked increases in inflammatory cell counts, TNF-α, IL-6, and IL-1β in BALF compared to the controls. However, HemoHIM treated mice exhibited a significant reduction in these pathophysiological factors in comparison to CS and LPS exposed mice. In addition, these events were accompanied by the reduction in histopathological alteration of lung tissue. CS- and LPS-exposed mice showed the extensive infiltration of inflammatory cells into the lung tissue, whereas HemoHim-treated mice exhibited a reduction in the histopathological alteration induced by CS and LPS exposure. Based on these results, HemoHIM may have an anti-inflammatory effect on airway inflammation mediated by CS exposure.
ERK is a MAPK transcription factor and plays a key role in the expression of various inflammatory genes such as MMP-9 and iNOS [19,26]. Previous studies have shown an increase in ERK with MMP-9 in CS and LPS induced mice models and CS condensate-stimulated cells [19]. CS stimulated the phosphorylation of Erk in airway epithelial cells, macrophages, and neutrophils, which eventually elevates the MMP-9 and iNOS expression [19,27].
MMP-9 is involved in airway inflammatory responses and the destruction of normal lung tissue via degradation of collagen and gelatin. iNOS is associated with the initiation and aggravation of airway inflammation via the elevation of nitric oxide production in CS induced airway inflammation [10,28]. This signaling was observed in COPD clinical trials. Patients with COPD increased the phosphorylation of Erk, MMP-9, and iNOS expression in their sputum and lavage [28,29,30,31]. Our results show that CS and LPS exposed mice increased phosphorylation of ERK with elevated MMP-9 and iNOS expression in their lung tissue compared to the controls. However, HemoHim treated mice exhibited a marked reduction in the phosphorylation of Erk with decreases in MMP-9 and iNOS expression in the lung tissue in comparison to CS and LPS exposed mice. These results suggest that the therapeutic effects of HemoHIM on CS and LPS exposed airway inflammation are closely associated with a reduction in MMP-9 and iNOS expression via the suppression of Erk phosphorylation in CS and LPS exposed lung tissue.
In conclusion, we evaluated the anti-inflammatory effects of HemoHIM on airway inflammation induced by CS and LPS exposure. HemoHIM significantly reduced the inflammatory cells and pro-inflammatory cytokines in BALF induced by CS and LPS exposure. HemoHIM decreased the elevated expression of iNOS and MMP-9 induced by CS and LPS exposure in lung tissue. These effects may be linked to the inhibition of ERK phosphorylation. Therefore, our study suggests that HemoHIM has the potential to treat airway inflammatory diseases, such as COPD induced by CS exposure.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT & Future Planning (grant number: NRF-016R1C1B2008818).
Footnotes
Conflict of interests: The authors declare that there is no financial conflict of interests to publish these results.
References
- 1.Tang W, Shen Z, Guo J, Sun S. Screening of long non-coding RNA and TUG1 inhibits proliferation with TGF-induction in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2951–2964. doi: 10.2147/COPD.S109570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korpershoek Y, Vervoort S, Nijssen L, Trappenburg J, Schuurmans MJ. Factors influencing exacerbation-related self-management in patients with COPD: a qualitative study. Int J Chron Obstruct Pulmon Dis. 2016;11:2977–2990. doi: 10.2147/COPD.S116196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehme SA, Franz-Bacon K, Ludka J, DiTirro DN, Ly TW, Bacon KB. MAP3K19 Is Overexpressed in COPD and Is a Central Mediator of Cigarette Smoke-Induced Pulmonary Inflammation and Lower Airway Destruction. PLoS One. 2016;11(12):e0167169. doi: 10.1371/journal.pone.0167169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang J, Yu HM, Zhou XD, Huang HP, Han Zh, Kolosov VP, Perelman JM. Cigarette smoke induces mucin hypersecretion and inflammatory response through the p66shc adaptor protein-mediated mechanism in human bronchial epithelial cells. Mol Immunol. 2016;69:86–98. doi: 10.1016/j.molimm.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Lin K, Liu S, Shen Y, Li Q. Berberine attenuates cigarette smoke-induced acute lung inflammation. Inflammation. 2013;36(5):1079–1086. doi: 10.1007/s10753-013-9640-0. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res. 2012;91(2):142–149. doi: 10.1177/0022034511421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue WH, Shi XQ, Liang SH, Zhou L, Liu KF, Zhao J. Emodin Attenuates Cigarette Smoke Induced Lung Injury in a Mouse Model via Suppression of Reactive Oxygen Species Production. J Biochem Mol Toxicol. 2015;29(11):526–532. doi: 10.1002/jbt.21723. [DOI] [PubMed] [Google Scholar]
- 8.Ge LT, Liu YN, Lin XX, Shen HJ, Jia YL, Dong XW, Sun Y, Xie QM. Inhalation of ambroxol inhibits cigarette smoke-induced acute lung injury in a mouse model by inhibiting the Erk pathway. Int Immunopharmacol. 2016;33:90–98. doi: 10.1016/j.intimp.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt KR, Diestel A, Lehnardt S, Schwartlander R, Lange PE, Berger F, Ullrich O, Abdul-Khaliq H. Hypothermia suppresses inflammation via ERK signaling pathway in stimulated microglial cells. J Neuroimmunol. 2007;189(1-2):7–16. doi: 10.1016/j.jneuroim.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Shin IS, Ahn KS, Shin NR, Jeon CM, Kwon OK, Chin YW, Lee K, Oh SR. Homoegonol attenuates the asthmatic responses induced by ovalbumin challenge. Arch Pharm Res. 2014;37(9):1201–1210. doi: 10.1007/s12272-013-0327-8. [DOI] [PubMed] [Google Scholar]
- 11.Cho A, Graves J, Reidy MA. Mitogen-activated protein kinases mediate matrix metalloproteinase-9 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20(12):2527–2532. doi: 10.1161/01.atv.20.12.2527. [DOI] [PubMed] [Google Scholar]
- 12.Lee IT, Yang CM. Inflammatory signalings involved in airway and pulmonary diseases. Mediators Inflamm. 2013;2013:791231. doi: 10.1155/2013/791231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doz E, Noulin N, Boichot E, Guénon I, Fick L, Le Bert M, Lagente V, Ryffel B, Schnyder B, Quesniaux VF, Couillin I. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol. 2008;180(2):1169–1178. doi: 10.4049/jimmunol.180.2.1169. [DOI] [PubMed] [Google Scholar]
- 14.Park HR, Jo SK, Jung U, Yee ST. Restoration of the immune functions in aged mice by supplementation with a new herbal composition, HemoHIM. Phytother Res. 2008;22(1):36–42. doi: 10.1002/ptr.2255. [DOI] [PubMed] [Google Scholar]
- 15.Jo SK, Lee HJ, Kim SR, Kim JC, Bae CS, Jung U, Park HR, Jang JS, Kim SH. Antiinflammatory activity of an herbal preparation (HemoHIM) in rats. Phytother Res. 2007;21(7):625–628. doi: 10.1002/ptr.2068. [DOI] [PubMed] [Google Scholar]
- 16.Park HR, Jo SK, Choi NH, Jung U. HemoHIM ameliorates the persistent down-regulation of Th1-like immune responses in fractionated γ-irradiated mice by modulating the IL-12p70-STAT4 signaling pathway. Radiat Res. 2012;177(5):676–684. doi: 10.1667/rr2768.1. [DOI] [PubMed] [Google Scholar]
- 17.Kim JJ, Cho HW, Park HR, Jung U, Jo SK, Yee ST. Preventative effect of an herbal preparation (HemoHIM) on development of airway inflammation in mice via modulation of Th1/2 cells differentiation. PLoS One. 2013;8(7):e68552. doi: 10.1371/journal.pone.0068552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JJ, Choi J, Lee MK, Kang KY, Paik MJ, Jo SK, Jung U, Park HR, Yee ST. Immunomodulatory and Antidiabetic Effects of a New Herbal Preparation (HemoHIM) on Streptozotocin-Induced Diabetic Mice. Evid Based Complement Alternat Med. 2014;2014:461685. doi: 10.1155/2014/461685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin IS, Shin NR, Park JW, Jeon CM, Hong JM, Kwon OK, Kim JS, Lee IC, Kim JC, Oh SR, Ahn KS. Melatonin attenuates neutrophil inflammation and mucus secretion in cigarette smoke-induced chronic obstructive pulmonary diseases via the suppression of Erk-Sp1 signaling. J Pineal Res. 2015;58(1):50–60. doi: 10.1111/jpi.12192. [DOI] [PubMed] [Google Scholar]
- 20.Lee H, Park JR, Kim EJ, Kim WJ, Hong SH, Park SM, Yang SR. Cigarette smoke-mediated oxidative stress induces apoptosis via the MAPKs/STAT1 pathway in mouse lung fibroblasts. Toxicol Lett. 2016;240(1):140–148. doi: 10.1016/j.toxlet.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 21.Lee KH, Lee CH, Jeong J, Jang AH, Yoo CG. Neutrophil Elastase Differentially Regulates Interleukin 8 (IL-8) and Vascular Endothelial Growth Factor (VEGF) Production by Cigarette Smoke Extract. J Biol Chem. 2015;290(47):28438–28445. doi: 10.1074/jbc.M115.663567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gernez Y, Tirouvanziam R, Chanez P. Neutrophils in chronic inflammatory airway diseases: can we target them and how? Eur Respir J. 2010;35(3):467–469. doi: 10.1183/09031936.00186109. [DOI] [PubMed] [Google Scholar]
- 23.Jeong SH, Park JH, Kim JN, Park YH, Shin SY, Lee YH, Kye YC, Son SW. Up-regulation of TNF-alpha secretion by cigarette smoke is mediated by Egr-1 in HaCaT human keratinocytes. Exp Dermatol. 2010;19(8):e206–e212. doi: 10.1111/j.1600-0625.2009.01050.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Yang T, Shen Y, Wan C, Li X, Li D, Liu Y, Wang T, Xu D, Wen F, Ying B. Ghrelin Inhibits Interleukin-6 Production Induced by Cigarette Smoke Extract in the Bronchial Epithelial Cell Via NF-κB Pathway. Inflammation. 2016;39(1):190–198. doi: 10.1007/s10753-015-0238-6. [DOI] [PubMed] [Google Scholar]
- 25.Markovics JA, Araya J, Cambier S, Somanath S, Gline S, Jablons D, Hill A, Wolters PJ, Nishimura SL. Interleukin-1beta induces increased transcriptional activation of the transforming growth factor-beta-activating integrin subunit beta8 through altering chromatin architecture. J Biol Chem. 2011;286(42):36864–36874. doi: 10.1074/jbc.M111.276790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cha SM, Cha JD, Jang EJ, Kim GU, Lee KY. Sophoraflavanone G prevents Streptococcus mutans surface antigen I/II-induced production of NO and PGE2 by inhibiting MAPK-mediated pathways in RAW 264.7 macrophages. Arch Oral Biol. 2016;68:97–104. doi: 10.1016/j.archoralbio.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Grzela K, Litwiniuk M, Zagorska W, Grzela T. Airway Remodeling in Chronic Obstructive Pulmonary Disease and Asthma: the Role of Matrix Metalloproteinase-9. Arch Immunol Ther Exp (Warsz) 2016;64(1):47–55. doi: 10.1007/s00005-015-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang WT, Liu XS, Xu YJ, Ni W, Chen SX. Expression of Nitric Oxide Synthase Isoenzyme in Lung Tissue of Smokers with and without Chronic Obstructive Pulmonary Disease. Chin Med J (Engl) 2015;128(12):1584–1589. doi: 10.4103/0366-6999.158309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mercer PF, Shute JK, Bhowmik A, Donaldson GC, Wedzicha JA, Warner JA. MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation. Respir Res. 2005;6:151. doi: 10.1186/1465-9921-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roh GS, Yi CO, Cho YJ, Jeon BT, Nizamudtinova IT, Kim HJ, Kim JH, Oh YM, Huh JW, Lee JH, Hwang YS, Lee SD, Lee JD. Anti-inflammatory effects of celecoxib in rat lungs with smoke-induced emphysema. Am J Physiol Lung Cell Mol Physiol. 2010;299(2):L184–L191. doi: 10.1152/ajplung.00303.2009. [DOI] [PubMed] [Google Scholar]
- 31.Hesslinger C, Strub A, Boer R, Ulrich WR, Lehner MD, Braun C. Inhibition of inducible nitric oxide synthase in respiratory diseases. Biochem Soc Trans. 2009;37(Pt 4):886–891. doi: 10.1042/BST0370886. [DOI] [PubMed] [Google Scholar]