Abstract
Mouse is a commonly used animal in life science studies and is classified as outbred if genetically diverse and inbred if genetically homogeneous. Outbred mouse stocks, are used in toxicology, oncology, infection and pharmacology research. The National Institute of Food and Drug Safety Evaluation (NIFDS; former the Korea National Institute of Health) have bred ICR mice for more than 50 years. We investigated to provide users with information and promote accountability to the Korl:ICR. To secure the indigenous data, biological characteristics of Korl:ICR were identified by comparing with other ICR stocks. This domestic ICR stock was denominated as ‘Korl:ICR’. Phylogenetic analysis using SNPs indicated that the population stratification of the Korl:ICR was allocated different area with other ICR. In addition, we measured litter size, body weight, body length, various organ weight, hematology and clinical blood chemistry of the Korl:ICR compared to other ICR. Otherwise, there are no significant differences among the biological phenotypes of Korl:ICR and other ICR. These results suggest that as a genetically indigenous source colony, the Korl:ICR is seperated (or independent) stock with other ICR. Also, we confirmed that there is no difference among the Korl:ICR and other ICR on biological phenotypes. Therefore, the Korl:ICR source colony might be a new stock in distinction from other ICR, it is a good milestone in securing ownership of the national laboratory animal resource. The NIFDS expects that the Korl:ICR mice will be useful animal resource for our domestic researchers.
Keywords: Outbred, Korl:ICR stock, Rodent, Breeder
Outbred mouse stocks, used in toxicology, oncology, infection and pharmacology research [1,2,3]. An outbred stock is defined as a restricted colony of animals with a limited increase of the inbreeding coefficient of <1% per generation and high degree of genetic variability [4,5]. Outbred stocks have been used extensively as a base population for selective breeding for phenotypes of interest. The best known examples are the many strains of mice selected for various aspects of response to alcohol, of which the long- (LS) and short-sleep (SS) mice are the best known example [6]. Mice have been also selected for immune responses to sheep red blood cells [7], high and low blood pressure [8], sensitivity to skin carcinogenesis [9], low antioxidant defenses [10], high activity [11], prolificacy and weight [12], obesity [13] and various traits related to drug abuse [14,15], among others. All these models have been used extensively to look for physiological, biochemical and genetic correlation with the traits of interest.
Most outbred stocks are derived from a small number of mice that were imported to the US by Clara J. Lynch in 1926 and are collectively known as Swiss mice [3]. Reports examining allelic variation affecting enzymatic activity in outbred CD-1 mice and its inbred derivatives concluded that random fixation, not inbreeding or population bottlenecks, accounted for slight losses in genetic variation among outbred mouse colonies [2,3,16]. Although outbred mice are commonly cited as models for outbred human populations [1,2], based on their histories, it is more likely that outbred mice reflect human founder populations rather than outbred human populations.
In the interpretation of animal toxicity testing data for potential adverse risk consequences to humans, there are a number confounding factors that must be considered. It is essential to consider the age, sex, physiologic status (e.g. pregnancy), microbiological status (e.g. whether ‘specific pathogen free’ SPF), nutrition, body weight, season and the genotype or strain of the test animals. Unlike human exposure, it should be borne in mind that the animal living conditions (temperature, light-dark cycle, humidity) are controlled and free of atmospheric pollutants, which minimizes potential interactions [17,18]. The choice of mouse can be further complicated by strain/stock as there are currently over different strains are carried out using a single strain; however, strain-related differences in responsiveness the chemicals have been well documented [19,20].
Currently, a number of worldwide breeders produce their own ICR stocks for commercial or academic purposes. Differences between ICR stocks have arisen in newly established stocks (founder effects) in different places worldwide and those that have been bred for a long time (drift effects). These differences have even been observed in identical stocks maintained by the same breeder.
The National Institute of Food and Drug Safety Evaluation (NIFDS) in Korea has an established ICR stock that has been used for the last 50 years, named “Korl:ICR” in the Guidelines for Nomenclature of Mouse and Rat Strains [21]. We investigated that the Korl:ICR is a genetically distinguished stock from other ICR. All ICR stocks are much alike in physiological phenotypes, but the Korl:ICR have a better breeding performance than commercial ICR. This study would serve as important momentum to turn the Korl:ICR into a new stock of global ICR stocks.
Materials and Methods
Animals
The Korl:ICR mice were generated by 200 generations or more of crossbreeding. Korl:ICR is the resident stock of NIFDS. The A:ICR (Crl:CD1(ICR)) and B:ICR (Tac:ICR) mice were purchased from two different commercial laboratory animal breeding company in Korea. The mice were housed in a pathogene-free animal facility under a standard 12 h light/dark cycle, maintained in conventional housing conditions at 22±1℃ and 50±10% relative humidity. All mice were provided with ad libitum access to standard irradiated chow diet (Purina rodent chow 38057) consisting of moisture (9.03%), crude protein (21.52%), crude fat (5.03%), crude fiber (4.33%), crude ash (6.31%), calcium (1.18%), and phosphorus (0.35%) and drinking water. All animal procedures were conducted in accordance with guidelines of the Institutional Animal Care and Use Committee, National Institute of Food and Drug Safety Evaluation (NIFDS, Cheongju, Korea).
Phylogenetic analysis
Phylogenetic trees were constructed using the dnapars program of phylip (phylogeny inference package) Version 3.695 (http://evolution.genetics.washington.edu/phylip.html) based on alignment of SNPs. Seqboot and condense programs of the phylip package were used in bootstrap analysis of 100 resamplings of the data sets. The neighbor program of phylip was used to draw phylogenetic trees.
Biological characteristics
Measurement of body and organ weights was made based on the methods of the International Mouse Phenotyping Consortium [22]. Hematological analysis was determined using by ADVIA 2010i Hematology System with Autoslide (SIEMENS, Germany). Clinical chemical parameters were measured from a single serum sample using a HITACHI 7100 Automatic Analyzer (HITACHI, Japan).
Reproductive performance
Female mice, 6~12 weeks old, were mated with mature males. We noticed that Korl:ICR had a significant increase in the litter size of the second litter as compared with the first litter. Therefore, the number of offspring of the first litter was taken as to litter size. Time to delivery represents the time period from mating to birth and fertility rate represents a ration between number of female mice giving birth and those used for mating. Cages were observed three times per week and the number of pups born dead or alive and the number of pups weaned in each cage was recorded.
Statistical analysis
The results are presented as mean±standard deviation. Statistical changes in all data were determined using one-way analysis of variance (ANOVA). The significance level was limited to 5% (P<0.05). SPSS 22.0 was used as a tool for statistical analysis.
Results
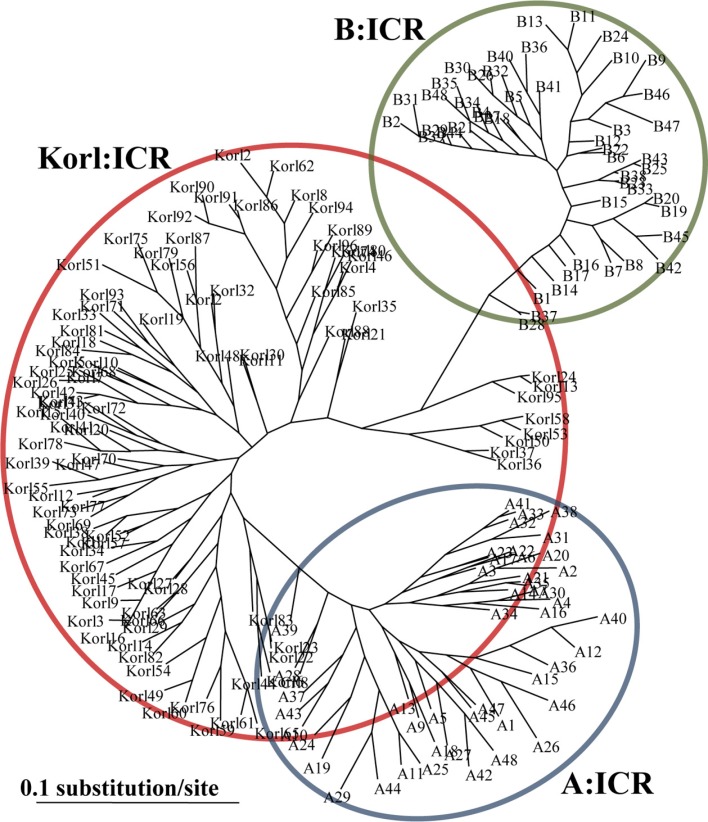
The phylogenetic trees based on distance, the minimum genetic distance of Korl:ICR and other ICR showed a genetic structure with three main clusters (Figure 1). From these distance matrices, one can infer the nature of the identified stock differences and the genetic relationships between them. This indicated that B stock has small genetic diversity compare with A stock and Korl stock, and there is large genetic diversity between A stock and B stock. In addition, Korl stock is genetically distinct form A and B stock.
Figure 1. A phylogenetic tree showing the relationship between Korl:ICR and other ICR. The tree is unrooted. Values at nodes represent bootstrap support values (only values >50% are shown).
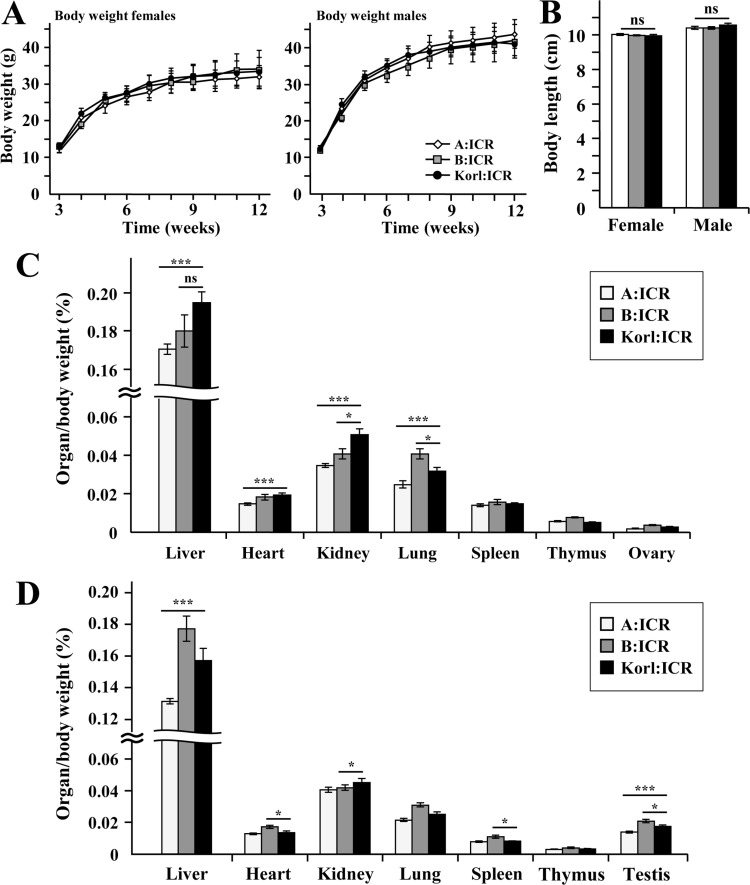
We measured litter size, body weight, body length, various organ weight, hematology and clinical blood chemistry of the Korl:ICR compared to other ICR. Mouse phenotype analysis, body weight of Korl:ICR, A:ICR and B:ICR has been measured during the designated developmental periods (3 weeks to 12 weeks). All ICR indicated a normal pattern of increasing in weight (Figure 2A). Korl:ICR and other ICR mice experienced similar in body length as well (Figure 2B). Organ weight of the mice with 12 weeks indicated as following. Female Korl:ICR mice were experiencing a significant increase in liver, heart and kidney weight compared to A:ICR, but no difference with B:ICR was observed (Figure 2C). Lung and ovary weight in all female mice were similar in pattern. Male Korl:ICR mice had a significant increase in liver, spleen and testis weight compared to A:ICR (Figure 2D).
Figure 2. Physiological characteristics of Korl:ICR mice. (A) Body weights of ICR mice during 12 weeks. (B) Body length of ICR mice at the 12 weeks. (C-D) Percentage organ weight to body weight ratio of ICR mice (ANOVA, *P<0.05, **P<0.01, ***P<0.001, ns: not significant).
The mean litter size of Korl:ICR mice was 14.5±2.9 which was higher than commercial ICRs' (Table 1). Otherwise, there are no significant differences among the biological phenotypes of Korl:ICR and other ICR. As summarized in Table 2, 3, some hematological and biochemical parameters have been observed with sporadically significant differences.
Table 1. Reproductive performance of the Korl:ICRs.
| Litters | Pups | Litter size | |
|---|---|---|---|
| Korl:ICR | 1,347 | 19,575 | 14.5±2.9 |
| C:ICR1) | - | - | 11.5 |
The data shown represent the mean±SD.
1)The average litter size of commercial ICR.
Table 2. Hematology and serum chemistry values for female of Korl:ICR and other ICR mice at the age of 12 weeks.
| A:ICR | B:ICR | Korl:ICR | P - value | |||
|---|---|---|---|---|---|---|
| n=8 | n=8 | n=8 | A-B | A-Korl | B-Korl | |
| WBC (K/µL) | 3.98±2.39 | 4.42±1.26 | 3.51±2.09 | |||
| RBC (M/µL) | 9.69±0.78 | 11.17±0.4 | 8.88±1.68 | ** | * | |
| HCT (%) | 55.01±4.42 | 63.87±3.33 | 50.38±9.59 | *** | ** | |
| MCV (fL) | 56.83±2.58 | 57.13±1.12 | 56.74±2.58 | |||
| MCH (pg) | 16.26±0.38 | 15.93±0.35 | 16.59±0.68 | |||
| MCHC (g/dL) | 28.63±0.8 | 27.97±0.15 | 29.26±1.07 | |||
| PLT (K/µL) | 1234±502.7 | 964±127.4 | 864±384 | |||
| MPV (fL) | 8.06±1.04 | 7.7±0.1 | 7.71±1.94 | |||
| n=10 | n=10 | n=14 | ||||
| ALP (U/L) | 472.1±103.1 | 398.9±82.08 | 371.6±70.34 | ** | ||
| ALT (U/L) | 35.2±9.93 | 34.8±12.54 | 29.86±8.2 | |||
| AST (U/L) | 106.7±27.75 | 114.2±43.12 | 98.57±38.17 | |||
| TP (g/dL) | 6.33±0.43 | 7.07±0.48 | 6.33±0.15 | ** | *** | |
| GLU (mg/dL) | 188.5±49.55 | 181.1±104.5 | 235.9±75.5 | |||
| HDL (mg/dL) | 74.52±16.25 | 80.19±10.68 | 70.11±8.34 | * | ||
| LDL (mg/dL) | 18.98±7.37 | 15.82±4.57 | 11.78±2.86 | ** | * | |
| BUN (mg/dL) | 21.66±2.71 | 22.57±2.33 | 20.02±4.48 | |||
| CRE (mg/dL) | 0.44±0.03 | 0.49±0.05 | 0.45±0.02 | ** | ** | |
| UA (mg/dL) | 6.46±1.71 | 5.68±2.36 | 6.64±1.4 | |||
| ALB (g/dL) | 2.16±0.14 | 2.39±0.14 | 2.19±0.05 | ** | *** | |
| Ca2+ (mg/dL) | 11.98±0.64 | 12.3±1.7 | 12.72±0.79 | * | ||
The data shown represent the mean±SD (Student t-test, *P<0.05, **P<0.01, ***P<0.001).
Table 3. Hematology and serum chemistry values for male of Korl:ICR and other ICR mice at the age of 12 weeks.
| A:ICR | B:ICR | Korl:ICR | P - value | |||
|---|---|---|---|---|---|---|
| n=8 | n=8 | n=12 | A-B | A-Korl | B-Korl | |
| WBC (K/µL) | 5.47±2.48 | 4.65±3.95 | 4.97±1.4 | |||
| RBC (M/µL) | 9.24±0.54 | 10.03±0.82 | 9±0.79 | * | * | |
| HCT (%) | 55.34±4.08 | 62.13±6.42 | 50.76±3.9 | * | * | *** |
| MCV (fL) | 59.95±3.99 | 61.9±1.91 | 56.47±2.31 | * | *** | |
| MCH (pg) | 16.53±0.41 | 16.83±0.56 | 16.53±0.88 | |||
| MCHC (g/dL) | 27.66±1.84 | 27.24±1.69 | 29.26±1.38 | * | ** | |
| PLT (K/µL) | 1275±361.4 | 2147±539 | 979.7±299.8 | ** | *** | |
| MPV (fL) | 7.86±0.59 | 8.09±0.99 | 6.75±0.93 | ** | ** | |
| n=9 | n=9 | n=9 | ||||
| ALP (U/L) | 294.6±104.7 | 261.2±63.77 | 317.3±47.89 | |||
| ALT (U/L) | 29.78±7.26 | 29.22±4.94 | 33.33±10.97 | |||
| AST (U/L) | 100.7±57.22 | 70.11±21.35 | 88.11±25.39 | |||
| TP (g/dL) | 5.72±0.29 | 5.91±0.45 | 6.67±0.35 | *** | ** | |
| GLU (mg/dL) | 231.5±51.14 | 215.6±67 | 202.5±52.74 | |||
| HDL (mg/dL) | 101.2±15.61 | 102.8±12.88 | 116.3±20.38 | |||
| LDL (mg/dL) | 10.59±7.37 | 10.06±3.88 | 13.79±3.67 | |||
| BUN (mg/dL) | 22.32±2 | 26.74±4.82 | 21.71±3.58 | * | * | |
| CRE (mg/dL) | 0.42±0.05 | 0.37±0.05 | 0.44±0.03 | * | ||
| UA (mg/dL) | 6.68±1.46 | 6.54±1.9 | 7.73±2.13 | ** | ||
| ALB (g/dL) | 1.91±0.14 | 1.91±0.18 | 2.23±0.11 | *** | *** | |
| Ca2+ (mg/dL) | 11.44±0.79 | 12.35±0.61 | 13.41±0.85 | * | *** | ** |
The data shown represent the mean±SD (Student t-test, *P<0.05, **P<0.01, ***P<0.001).
Discussion
Laboratory mice are the essential resource to identify the potency and safety of the drugs and estimate the potency and adverse effects of drugs during the developing stage. According to the effectuation of the Nagoya Protocol, laboratory mice are not only indispensable for life science studies but also are being considered as important resource. Among laboratory mice, ICR mice are most popular outbred mice broadly used in various fields like toxicology, oncology, pharmacology, pharmaceutical products safety test, and etc. [1,3,17,23,24]. ICR mice derive from two males and seven females, known as ‘Swiss’ mice, that were crossed in 1926 and then adopted by the Institute of Cancer Research in 1974 (subsequently merged with “Fox Chase” in 1974). Commercial production of ICR by companies began in 1959, and the strain was made available to academic and commercial breeders [2,3,16]. Currently, many breeders worldwide have possessed their own ICR stocks and the ICR stocks have been reproduced for their academic or commercial purpose. The opinions that there might be possible to have differences among ICR mice already ensured in various regions of world which have been raised for long term and among mice of the identical stock bred by different breeders have been proposed in some point of view, but there are not sufficient evidences to support these yet.
Even though outbred mice are hard to realize the reliability and the repeatability, they are consider to be worked as better resource when the test result is applied to human due to the genetic diversity if compared to inbred mice. In addition, the advantage of using the ICR stocks is extremely high in reproduction rate than the other outbred mice so that it may help the study cost effective. Every ICR breeder has adopted and being bred these following common protocols: maintain ICR stocks as long as possible, maintain genetic diversity as possible by composing multiple colonies, and minimize the genetic drift. This study is carried out to establish scientific supports how different in aspect of normal growth curve, breed grad, and clinical blood chemical analysis to ensure the characterization data of ICR stock mouse which have been bred by circulation breed approach for more than 50 years. The breed capacity of both Korl:ICR mice and commercial ICR mice were identified. The mean litter size of the Korl:ICR mice were higher than other ICR mice.
Between monthly litter size and litter size per number of pregnancy of the Korl:ICR mice did not experience a significant difference, but the size of second and third litter were higher than first and fourth litter (data not shown). The Korl:ICR mice were having median value of between A:ICR and B:ICR in a basic phenotype analysis. To tests evaluation of reactivity drugs, their behavioral changes were observed after administering caffeine and chlorpromazine which activates the central nervous system; It was possible to identify that the recovery rate of the Korl:ICR was similar to other ICR mice (data not shown). These results are biological characteristics and other factors. Korl:ICR was deduced that there was no significant difference with commercial ICRs, and some weight gaps of the organs might be dependent the breeding condition (feeds, the breeding place). Since the comparison studies on laboratory mice have not been remarkably activated, we have faced the difficulty of comparing the study result at this moment. The physiological and hematological data values had consistency in the study. Song et al. (2016) suggested that three ICR groups, the Korl:ICR, A:ICR and B:ICR derived from different sources have an overall similar response of body weight, histopathological structure and inflammation to gastric ulcer inducers [25]. Alcohol induced ulcer model comparison assessment for reviewing the characteristics of the Korl:ICR were having similar result. However, the result would be more appropriate to compare with control group rather than the comparison of the absolute value because of a significant difference in certain enzymes.
Another implication of the study is that references data (breeding rate and basic phenotype) regarding the Korl:ICR are secured and can be shared with the other researchers. The Korl:ICR stocks have been used for decades in terms of conducting Lot release project and more, in the National Institute of Food and Drug Safety Evaluation. In conclusion, we will study secure general documents needed to have as mice resource through this accumulation of various experimental data.
Acknowledgments
This study was supported by a grant (14181MFDS622) from Ministry of Food and Drug Safety in 2014.
Footnotes
Conflict of interests: The authors declare that there is no financial conflict of interests to publish these results.
References
- 1.Cui S, Chesson C, Hope R. Genetic variation within and between strains of outbred Swiss mice. Lab Anim. 1993;27(2):116–123. doi: 10.1258/002367793780810397. [DOI] [PubMed] [Google Scholar]
- 2.Rice MC, O'Brien SJ. Genetic variance of laboratory outbred Swiss mice. Nature. 1980;283(5743):157–161. doi: 10.1038/283157a0. [DOI] [PubMed] [Google Scholar]
- 3.Chia R, Achilli F, Festing MF, Fisher EM. The origins and uses of mouse outbred stocks. Nat Genet. 2005;37(11):1181–1186. doi: 10.1038/ng1665. [DOI] [PubMed] [Google Scholar]
- 4.Yamada J, Nikaido H, Matsumoto S. Genetic variability within and between outbred Wistar strains of rats. Jikken Dobutsu. 1979;28(2):259–265. doi: 10.1538/expanim1978.28.2_259. [DOI] [PubMed] [Google Scholar]
- 5.Phelan JP. Genetic variability and rodent models of human aging. Exp Gerontol. 1992;27(2):147–159. doi: 10.1016/0531-5565(92)90039-3. [DOI] [PubMed] [Google Scholar]
- 6.DeFries JC, Wilson JR, Erwin VG, Petersen DR. LS X SS recombinant inbred strains of mice: initial characterization. Alcohol Clin Exp Res. 1989;13(2):196–200. doi: 10.1111/j.1530-0277.1989.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 7.Feingold N, Feingold J, Mouton D, Bouthillier Y, Stiffel C, Biozzi G. Polygenic regulation of antibody synthesis to sheep erythrocytes in the mouse: a genetic analysis. Eur J Immunol. 1976;6(1):43–51. doi: 10.1002/eji.1830060110. [DOI] [PubMed] [Google Scholar]
- 8.Schlager G. Genetic Hypertension in the Mouse. Amsterdam: Elsevier; 1994. pp. 158–172. [Google Scholar]
- 9.Boutwell RK. Some Biological Aspects of Skin Carcinogenisis. Prog Exp Tumor Res. 1964;4:207–250. doi: 10.1159/000385978. [DOI] [PubMed] [Google Scholar]
- 10.Mathews CE, Bagley R, Leiter EH. ALS/Lt: a new type 2 diabetes mouse model associated with low free radical scavenging potential. Diabetes. 2004;53(1 suppl):125–129. doi: 10.2337/diabetes.53.2007.s125. [DOI] [PubMed] [Google Scholar]
- 11.Garland T, Jr, Morgan MT, Swallow JG, Rhodes JS, Girard I, Belter JG, Carter PA. Evolution of a small-muscle polymorphism in lines of house mice selected for high activity levels. Evolution. 2002;56(6):1267–1275. doi: 10.1111/j.0014-3820.2002.tb01437.x. [DOI] [PubMed] [Google Scholar]
- 12.Kirkpatrick BW, Mengelt A, Schulman N, Martin IC. Identification of quantitative trait loci for prolificacy and growth in mice. Mamm Genome. 1998;9(2):97–102. doi: 10.1007/s003359900696. [DOI] [PubMed] [Google Scholar]
- 13.Horvat S, Bünger L, Falconer VM, Mackay P, Law A, Bulfield G, Keightley PD. Mapping of obesity QTLs in a cross between mouse lines divergently selected on fat content. Mamm Genome. 2000;11(1):2–7. doi: 10.1007/s003350010002. [DOI] [PubMed] [Google Scholar]
- 14.Crabbe JC, Belknap JK, Buck KJ. Genetic animal models of alcohol and drug abuse. Science. 1994;264(5166):1715–1723. doi: 10.1126/science.8209252. [DOI] [PubMed] [Google Scholar]
- 15.Grahame NJ, Li TK, Lumeng L. Selective breeding for high and low alcohol preference in mice. Behav Genet. 1999;29(1):47–57. doi: 10.1023/a:1021489922751. [DOI] [PubMed] [Google Scholar]
- 16.Lynch CJ. The so-called Swiss mouse. Lab Anim Care. 1969;19(2):214–220. [PubMed] [Google Scholar]
- 17.Keenan KP, Smith PF, Hertzog P, Soper K, Ballam GC, Clark RL. The effects of overfeeding and dietary restriction on Sprague-Dawley rat survival and early pathology biomarkers of aging. Toxicol Pathol. 1994;22(3):300–315. doi: 10.1177/019262339402200308. [DOI] [PubMed] [Google Scholar]
- 18.Keenan KP, Ballam GC, Dixit R, Soper KA, Laroque P, Mattson BA, Adams SP, Coleman JB. The effects of diet, overfeeding and moderate dietary restriction on Sprague-Dawley rat survival, disease and toxicology. J Nutr. 1997;127(5 suppl):851S–856S. doi: 10.1093/jn/127.5.851S. [DOI] [PubMed] [Google Scholar]
- 19.Kacew S, Ruben Z, McConnell RF. Strain as a determinant factor in the differential responsiveness of rats to chemicals. Toxicol Pathol. 1995;23(6):701–714. doi: 10.1177/019262339502300608. [DOI] [PubMed] [Google Scholar]
- 20.Kacew S, Ruben Z. Importance of confounding factors including strain, sex and diet in modeling chemically-induced toxicity. Toxicol Ecotoxicol News. 1997;4:158–160. [Google Scholar]
- 21.Snell GD. Biology of the Laboratory Mouse. 1st Edn. McGraw-Hill: New York, MA, US; 1941. [Google Scholar]
- 22.Brown SD, Moore MW. The International Mouse Phenotyping Consortium: past and future perspectives on mouse phenotyping. Mamm Genome. 2012;23(9-10):632–640. doi: 10.1007/s00335-012-9427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14(5):511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong SZ, Ge QH, Qu R, Li Q, Ma SP. Paeonol attenuates neurotoxicity and ameliorates cognitive impairment induced by d-galactose in ICR mice. J Neurol Sci. 2009;277(1-2):58–64. doi: 10.1016/j.jns.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Song SH, Kim JE, Go J, Koh EK, Sung JE, Lee HA, Choi KM, Kim HD, Jung YS, Kim KS, Hwang DY. Comparison of the response using ICR mice derived from three different sources to ethanol/hydrochloric acid-induced gastric injury. Lab Anim Res. 2016;32(1):56–64. doi: 10.5625/lar.2016.32.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]