To the Editor
Features of patients with severe asthma include a greater frequency and severity of hospitalizations due to pneumonia, severe influenza and sinopulmonary infections.1 Viral infections are frequent triggers of asthma exacerbations.1 Impaired anti-viral responses in asthmatics have been noted.2 However, mechanisms of this phenomenon are not well understood. The asthmatic airway wall undergoes many alterations including increased and changed deposition of extracellular matrix (ECM). Hyaluronan (HA), a major component of ECM, accumulates in the asthmatic lung and serum, and correlates with disease severity.3 Low-molecular weight (LMW) forms of HA, generated during tissue injury or inflammation, have been linked to asthma 3, 4, but the mechanisms of that link are not well understood. We have recently described the mechanism by which LMW HA can activate cytosolic phospohlipase A2α (cPLA2α) and arachidonic acid (AA) production.5 We have previously reported an increased expression of cPLA2α in PBMCs of patients with severe asthma.6 cPLA2α is a rate-limiting enzyme in eicosanoid production, responsible for liberation of AA from cellular membranes.5 AA is the precursor of leukotrienes, prostaglandins, hydroxyeicosatetranoic acids, thromboxanes, lipoxins and epoxides, many of which are involved in asthma pathogenesis 7 and they are altered in viral infections.8 A thorough analysis of LMW HA effects on the lipidomic profile or global gene expression in asthmatic patients has never been performed and its possible influence on the disease progression has not been noted. Therefore, we performed a systemic analysis of LMW HA signaling in PBMCs of patients with mild-to-moderate and severe asthma using liquid chromatography and mass spectrometry combined with microarray, real-time PCR and multiplex protein analyses. The details of methods are provided in this article’s Online Repository. Thirteen asthmatics and six control subjects were enrolled under an NHLBI review board approved protocol (99-H-0076). Severe asthma was defined according to ERS/ATS guidelines.9 Participants’ demographic and phenotypic characteristics are presented in Table E1–E2.
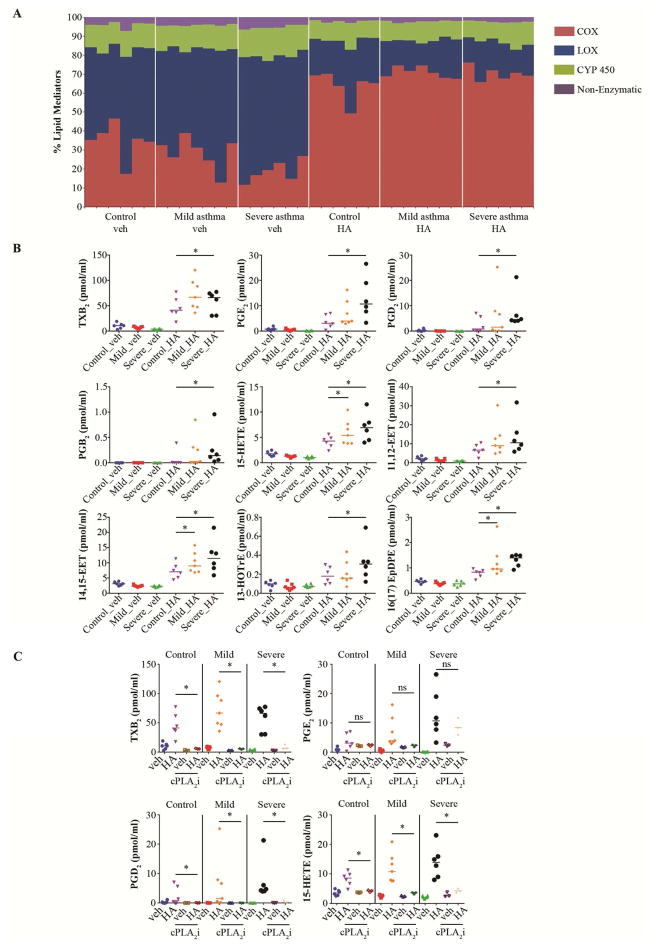
Of the 151 lipid species profiled, we detected 68 before or after LMW HA stimulation. At baseline, fewer metabolites were generated through the COX pathway compared with the combined LOX, CYP450 and non-enzymatic pathways in each group (Fig. 1, A; Fig E1, A). The relative percentage of all COX metabolites at baseline was significantly lower in severe asthmatics than in controls (Fig. 1, A; Fig E1, A). However, after LMW HA stimulation there was a significant increase in the relative percentage of COX-generated mediators and a corresponding decrease of metabolites derived from the other pathways in all groups (Fig. 1, A; Fig E1, A). Analysis of individual lipids revealed that 22 were significantly upregulated by LMW HA (Fig E1, B). Though at baseline several lipid species tended to have lower concentrations in severe asthmatics, the increase of 9 metabolites following LMW HA treatment was significantly more pronounced in those patients compared to controls (Fig 1, B). These included COX metabolites TxB2, PGE2, PGD2, and PGB2, and metabolites from other pathways including 15-HETE (via LOX), 11(12)-EET and 14(15)-EET (via CYP450), and non-arachidonic acid metabolites such as 13-HOTrE(y) and 16 (17) EpDPE. Only in severe asthmatics did the levels of most of these metabolites reach the range of activation of their cognate receptors, although 15-HETE, 14(15)-EET and 16 (17) EpDPE were significantly upregulated also in mild-to moderate asthmatics. Treatment with a specific cPLA2α inhibitor decreased the LMW HA-induced formation of eicosanoids (Fig 1, C), while PGB2, 13-HOTrE(y), 11(12)-EET, 14(15)-EET and 16(17) EpDPE were no longer detectable with or without LMW HA treatment, suggesting complete blockade of their production. These data reveal that in severe asthma there is an upregulation of several classes of cPLA2α-dependent lipids in response to LMW HA. It would be of interest to determine if subjects with aspirin sensitivity are different from other asthmatics in this regard (Figure E2).7
Figure 1. LMW HA causes cPLA2α-dependent changes in the specific lipidomic profile of severe asthmatics.
A, Percentage of all cyclooxygenase (COX), lipoxygenase (LOX), cytochrome P450 (CYP 450) and non-enzymatic metabolites before and after LMW HA treatment. Each vertical line represents data from a single subject. B, LMW HA-induced lipid mediators, significantly upregulated in severe asthmatics. C, LMW HA lipidomic profile is cPLA2α-dependent. * P<.05 as indicated, cPLA2i; cPLA2 inhibitor.
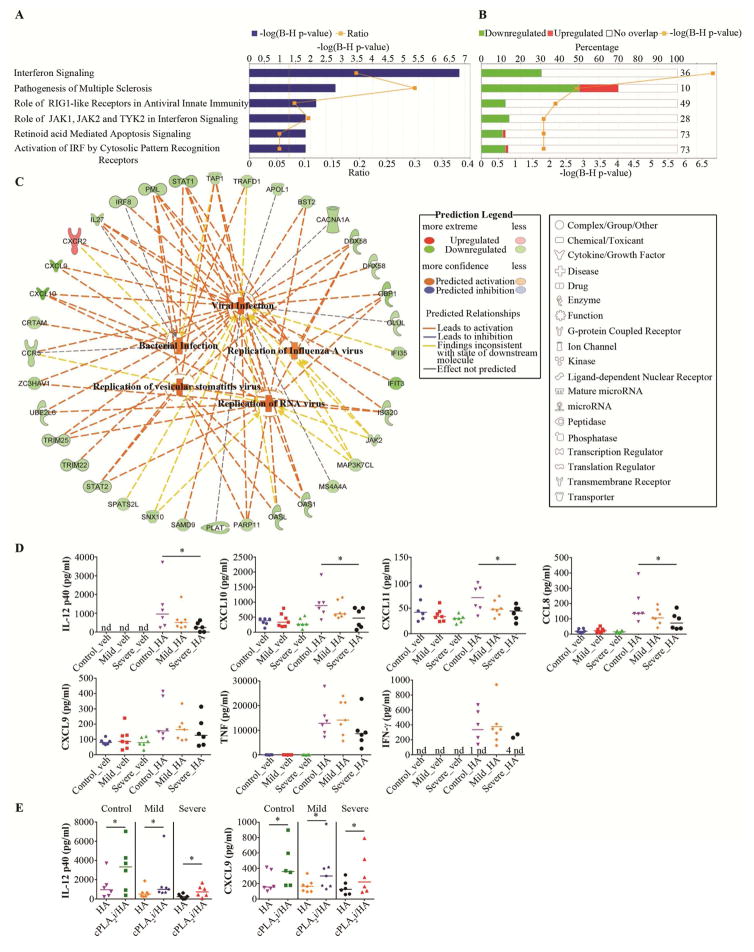
Next, we found that LMW HA caused profound changes in global gene expression in all subjects (Table E3). It led to a significant enrichment of genes in 177 canonical pathways (Table E4). In these pathways, HA stimulation led to an overall increase of gene expression (Fig. E3; Table E5–9). We confirmed the LMW HA-induced changes in the expression of selected genes by real-time PCR (Table E11) and by their translation to protein by Multiplex (Fig. E4). We found 6 canonical pathways that were significantly changed in severe asthma vs controls (Fig. 2, A), while there were no changed pathways in mild-to-moderate asthma vs controls. These pathways were created from significantly less upregulated genes compared to their expression after HA treatment in control subjects (Fig. 2, B). This further corresponded to the significantly decreased Antimicrobial Response category, containing a predicted decrease of antiviral response (p=2.94E-7; z-sore=−1.94) and antibacterial response (p=1.66E-6; z-score=−1.94), and a decrease in cell movement of lymphocytes (6.12E-3; z-score=−2.02) and homing T lymphocytes (5.11E-3; z-score=−1.99). Detailed analysis led to building the model of LMW HA-dependent signaling impairment in severe asthma (Fig. 2, C). This model predicts that less upregulated expression of genes by LMW HA in the Interferon Signaling and other canonical pathways in severe asthmatics might lead to impaired activation of antiviral and antimicrobial responses and allow for an increase in Viral Infection probability (Fig. 2, C; Table E12). We further confirmed the direction of changes in the array expression on the protein level (Fig. 2, D). We indeed found that IL12p40, CXCL10, CXCL11 and CCL8 were significantly less activated by LMW HA in severe asthmatics, compared to control subjects (Fig. 2, D). There was a similar trend in CXCL9, TNF and IFNγ release (Fig. 2, D). Proteins not involved in this model, such as IL-6, IL-10, CCL20, IL-1α and IL-1β were upregulated by LMW HA but they were not different between phenotypes (Fig. E4). Most importantly, we noted that treatment of PBMCs with cPLA2α inhibitor, which blocked lipid mediator production (Fig. 1, C) significantly increased IL-12p40 and CXCL9, in each phenotype (Fig. 2, D). The increase of these specific proteins in the presence of lipid mediator blockade is a functional link between lipidomics and proteomics, pointing out that lipid production alters gene and protein expression in these pathways. It has been previously noted that PGE2 decreases IFN type I production and increases influenza susceptibility in vivo.10 However, it is not established whether the treatment effects of LMW HA reported here would be reproduced in vivo.
Figure 2. LMW HA-dependent antimicrobial and antiviral signaling impairment in severe asthma.
A, B Significantly enriched canonical pathways after LMW HA treatment in severe asthma in comparison with non-asthmatics. A, −log(B-H p-value), a Benjamini Hochberg false discovery rate. B, Percentage of genes in the data set creating enrichment, with the number on right stating the entire number of genes assigned to this pathway. C, Prediction model of the influence of changes in the experimental data set on the downstream antiviral and antimicrobial immunity. D, LMW HA-induced chemokines and cytokines following the trend of the prediction model. E, LMW HA-induced protein expression profile is partially dependent on cPLA2α. * P<.05 as indicated.
We demonstrated here that fragmented hyaluronan increased production of several unique lipid species in severe asthmatics. Analysis of the whole genome profile, captured simultaneously with the lipidomic profile, revealed decreases in antiviral gene and protein expression upon LMW HA treatment in severe asthmatics, which was partially reversed upon inhibition of lipid production. Our current findings provide new evidence on the connection between extracellular matrix, global lipid mediator production and decreased antiviral responses in severe asthma.
Supplementary Material
Acknowledgments
Source of funding: NIH intramural program (Critical Care Medicine Department, Clinical Center and Division of Intramural Research, NHLBI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, et al. Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med. 2012;185:356–62. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gielen V, Sykes A, Zhu J, Chan B, Macintyre J, Regamey N, et al. Increased nuclear suppressor of cytokine signaling 1 in asthmatic bronchial epithelium suppresses rhinovirus induction of innate interferons. J Allergy Clin Immunol. 2015;136:177–88. e11. doi: 10.1016/j.jaci.2014.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauer ME, Majors AK, Comhair S, Ruple LM, Matuska B, Subramanian A, et al. Hyaluronan and its heavy chain modification in asthma severity and experimental asthma exacerbation. J Biol Chem. 2015 Sep 18;290:23124–34. doi: 10.1074/jbc.M115.663823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang J, Jiang D, Jung Y, Xie T, Ingram J, Church T, et al. Role of hyaluronan and hyaluronan-binding proteins in human asthma. J Allergy Clin Immunol. 2011;128:403–11. e3. doi: 10.1016/j.jaci.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokolowska M, Chen LY, Eberlein M, Martinez-Anton A, Liu Y, Alsaaty S, et al. Low molecular weight hyaluronan activates cytosolic phospholipase A2 alpha and eicosanoid production in monocytes and macrophages. J Biol Chem. 2014;289:4470–88. doi: 10.1074/jbc.M113.515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokolowska M, Stefanska J, Wodz-Naskiewicz K, Cieslak M, Pawliczak R. Cytosolic phospholipase A2 group IVA is overexpressed in patients with persistent asthma and regulated by the promoter microsatellites. J Allergy Clin Immunol. 2010;125:1393–5. doi: 10.1016/j.jaci.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Liu T, Laidlaw TM, Katz HR, Boyce JA. Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proc Natl Acad Sci U S A. 2013;110:16987–92. doi: 10.1073/pnas.1313185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tam VC, Quehenberger O, Oshansky CM, Suen R, Armando AM, Treuting PM, et al. Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell. 2013;154:213–27. doi: 10.1016/j.cell.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–73. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 10.Coulombe F, Jaworska J, Verway M, Tzelepis F, Massoud A, Gillard J, et al. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity. 2014;40:554–68. doi: 10.1016/j.immuni.2014.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.