Introduction
Asthma is a complex inflammatory disease of the respiratory system with strong genetic and environmental components. (1) Approximately 7.1 million U.S. children have asthma with African American children experiencing higher asthma morbidity and mortality than non-Hispanic white children. (2,3) Poverty is considered one marker for developing asthma (4) and ongoing symptoms, (5) primarily due to increased exposure to indoor allergens and medication non-adherence. Exposure and sensitization to indoor pests, i.e. cockroach and rodents, and exposure to second hand smoke (SHS) is high in inner city children. Positive allergen sensitization is found in most children hospitalized for asthma (6) and atopy is a major contributor to asthma morbidity.
Anti-inflammatory or controller medications are the mainstay of preventive asthma therapy. (7) In particular, inhaled corticosteroid (ICS), and combination ICS-Long-acting beta-agonists (ICS/LABAs) improved lung function with leukotriene modifiers (LTM) having a secondary role.(7) Despite national guidelines recommending daily use of controller medication for all children with persistent asthma, ICS use ranges from 40–70% and tends to be lowest among poor minority children.(8–10) One reason for lack of guideline based care in inner city children with persistent asthma may be low rate of referral to asthma specialty care. Asthma specialists are more likely to prescribe guideline based controller medication therapy as compared to primary care physicians, (11) yet few minority children with asthma receive specialty care potentially resulting in poor asthma control, (12) and higher costs from increased reliance on hospitals and EDs for episodic care. (13, 14)
One measure of guideline based care is the Asthma Medication Ratio (AMR), or the ratio of controller medication fills to total asthma medication fills over the past 12 months. (14) Ratios of 0.50 or higher have been associated with decreased asthma morbidity and ED utilization and increased adult quality of life. (14) Understanding factors associated with controller medication use in children with high asthma ED visits may inform clinical interventions aimed at improving asthma control and receipt of guideline based asthma care among high ED utilizers. The objectives of this study were (1) to examine the level of controller medication fills in children with frequent asthma ED visits and (2) to identify health and social factors associated with guideline-based controller medication use.
Methods
Design and study setting
This was a cross sectional study examining baseline data obtained from 222 children with persistent asthma who enrolled in a randomized controlled trial testing the efficacy of an ED and home-based environmental control intervention for children with frequent ED visits for asthma. (10) All children were recruited during an asthma ED visit and received serum allergen-specific IgE serologic tests measured by fluorescent enzyme immunoassay (FEIA) to identify allergen sensitization and salivary cotinine measurement to screen for exposure to environmental tobacco smoke during the ED visit. Survey and saliva data collection was performed by three trained research assistants (RA) and blood was drawn from the child by trained ED nurses during the enrollment ED visit. Fidelity checks were performed with monthly examination of all item frequencies in REDCap©. When irregularities were detected, RA retraining occurred. QC of cotinine samples included reanalyzing samples with outlier values (top 10th percentile).
Allergen sensitization and cotinine results were provided to the caregiver and or primary care provider (PCP) for all children. Caregivers assigned to the intervention arm received timely, targeted environmental control education and provision of environmental control remediation supplies for any positive indoor IgE tests (i.e. cockroach bait for positive cockroach IgE allergen). Caregivers of children with positive cotinine levels received a brief motivational interview intervention to implement a total home smoking ban. All families received $30.00 for completing the baseline interview survey and no additional incentives were provided to intervention families. The study is registered with Clinical Trials.gov with number NCT01981564. The Johns Hopkins Medical Institutional and the University of Maryland Institutional Review Boards approved the study protocol. Baseline data is presented in this manuscript.
Data collection
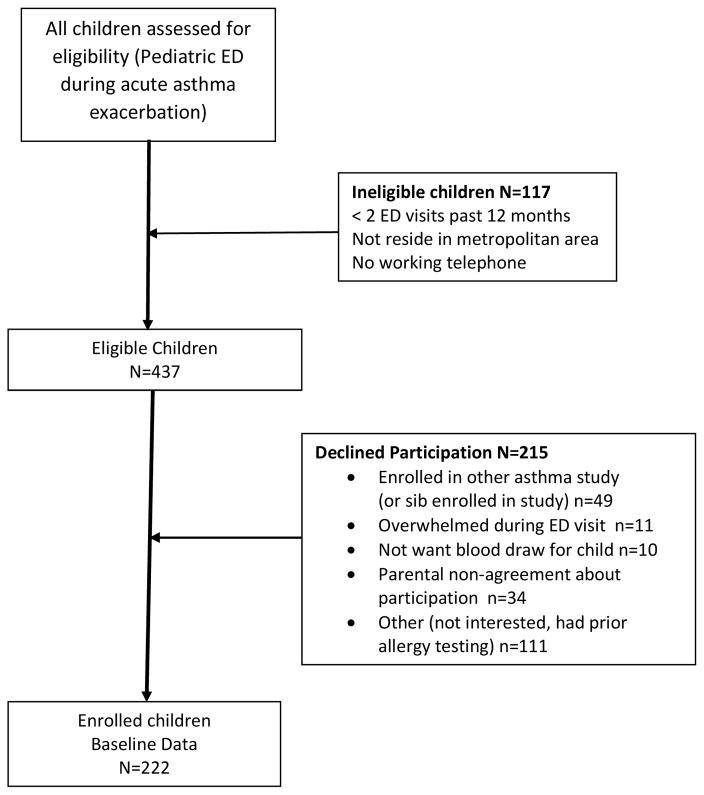
Families of children aged 3–12 years were recruited and enrolled during an asthma ED visit throughout August 2013 to February 2016. Inclusion criteria were physician diagnosed persistent and uncontrolled asthma based on current National Asthma Education Prevention Program (NAEPP) guidelines,(7) and having 2 or more ED asthma visits or ≥ 1 hospitalization over the past 12 months and residing in the Baltimore metropolitan area. Children were excluded if they had significant other non-asthma respiratory conditions, i.e., cystic fibrosis. Written informed consent was obtained from each child’s primary caregiver/legal guardian and all children over age 8 years provided verbal assent to participate. Saliva was collected from the child for cotinine measurement to determine level of second hand smoke (SHS) exposure (15) and blood was collected to test for child sensitization to common environmental allergens. Additionally, the caregiver completed a 45 minute survey interview ascertaining sociodemographic and health information. All eligible children were approached and screened for study participation. Seen in Figure 1, a total of 554 children and caregivers were screened for study enrollment in the ED. Out of the 554 child/caregivers, 215 caregivers declined to participate, another 117 children were ineligible for enrollment, resulting in 222 children enrolled in the study. No significant differences were noted in child age, race/ethnicity or neighborhood zip codes between enrolled and non-enrolled children.
Figure 1.
Recruitment and Enrollment Flow Diagram
Measures
Asthma Medication Fills and Caregiver Worry about Medication Side Effects
The primary outcome was the Asthma Medication Ratio (AMR) measure based on data obtained from pharmacy dispensing records. All asthma medications dispensed over the prior 12 months were obtained for each child. Pharmacy records for child asthma medications were obtained from all pharmacies used as reported by the caregiver over a 12-month period. Each pharmacy used and identified at baseline was contacted via fax with copy of the signed consent and HIPAA forms requesting a complete list of all asthma medications dispensed over the prior 12 months. Pharmacies not responding within one week were contacted by the study nurse to retrieve records over the phone. Pharmacy records were considered complete if every pharmacy identified at baseline responded with pharmacy data for the specified period or indicated that they had no record or prescriptions for the subject during the specific time period requested. (16) Pharmacy records included the dispensing date, product name, strength, dosage form, and quantity dispensed. Rescue medications were defined as short-acting beta- agonist (SABA) and controller medications were defined as inhaled corticosteroids (ICS), leukotriene modifiers (LTM), or ICS-long acting beta agonists (ICS/LABA). Oral corticosteroid (OCS) prescription fills were categorized separately. Controller medication therapy was categorized as none, monotherapy (ICS or LTM only) or combination therapy (ICS + LABA or ICS + LTM). The asthma medication ratio (AMR) was calculated at baseline for the medications filled in the prior 12 months, i.e., the total number of controller medication canisters or equivalents as the numerator and the total number of controller and SABA canisters or equivalents as the denominator. (14) Caregiver report of SABA and controller medication use was recorded and compared to pharmacy records. Caregiver concern over side effects of asthma medication was ascertained using one item from the Pediatric Asthma Caregiver Quality of Life Questionnaire (PACQLQ) Scale (“How worried or concerned were you about your child’s asthma medications and side effects?”, scored ranging from “very, worried/concerned to not worried/concerned”(17) and categorized into 2 groups as “very, fairly, somewhat worried” versus “a little, hardly or not worried”. Internal consistency for the PACQLQ scale was high (Cronbach’s alpha: 0.86) and comparable to the alpha (0.90) noted on the PACQLQ in a sample of urban African American caregivers of children with asthma. (18) Sharing of medication in the home was based on caregiver report.
Asthma Morbidity and Healthcare Utilization
Using the REDCap© web based application the baseline survey interview, written at a fourth grade reading level, was administered by the RA. The questionnaire included health, sociodemographic items and the Pediatric Caregiver Quality of Life (PACQLQ) scale. Asthma severity and control measures were based on current National Asthma Education Prevention Program (NAEPP) guidelines (7) including the number of symptom days, days of short-acting beta-agonist (SABA) use reported as quick relief or prophylactic medication and activity limitation over the past 2 weeks and symptom nights over the past 4 weeks. Caregivers rated their child’s asthma control over the past 4 weeks as “controlled”, “not controlled” or “unsure”. Healthcare utilization measures included the number of asthma ED visits or hospitalizations and number of primary care provider (PCP) visits for routine asthma care over the past 3 months and receipt of any asthma specialty care over the past two years. Lifetime asthma ICU admission was based on electronic medical record (EMR) review. High agreement (99%) was noted between caregiver report (94.6%) and baseline medical record verification of Medicaid insurance (94.1%). Continuous Medicaid coverage over the prior12 months was not confirmed due to lack of consent for this information. Private health insurance included BCBS, Tricare and the Johns Hopkins Employee Health Plan.
Serologic allergen specific IgE test
Blood was obtained from the child during the index ED visit for specific IgE serologic testing to determine sensitization to ten common environmental allergens. Serum specific IgE testing was performed by a private commercial laboratory for analysis using the (ImmunoCap®) fluorescent enzyme immunoassay (FEIA) and determined specific IgE antibodies to mouse, cockroach, cat, dog, timothy grass, Alternaria and Aspergillus molds, oak tree, common ragweed and house dust mite. The degree of sensitization ranged from <0.35 to >100 kU/L of specific IgE to any of the allergens tested and results >0.35 kU/L were considered positive.
Cotinine Analysis for Second Hand Smoke (SHS) Exposure
Child saliva samples were collected during the index ED asthma visit using a 3-cm cotton swab (Salimetrics, State College, PA) that was placed under the child’s tongue for 1 minute to absorb 1 ml of saliva. The cotton roll was placed in a 2 ml vial and stored at −20° Centigrade prior to transport to the lab, then centrifuged and analyzed at the Johns Hopkins Institute for Clinical and Translational Research (ICTR) lab using enzyme Immunoassay (EIA) analysis. The cotinine analysis serves as a biomarker of nicotine exposure over the prior 24 hours. The lower limit of cotinine sensitivity was 0.05 ng/ml and average intra and inter-assay coefficients of variation were less than 5.8% and 7.9%, respectively. A cotinine cutoff level of 1.0 ng/ml was used to define positive SHS exposure based on prior reports of inner-city children with asthma. (19)
Statistical Analysis
Standard frequencies and means (SD) were used to describe sociodemographic and health characteristics of all children and caregivers. These characteristics were then examined in relation to controller medication usage pattern (none, monotherapy, combination therapy) using generalized ordinal regression, average number of controller medication fills using Poisson regression due to the high skew of the distribution and count nature of this outcome, and odds ratio for AMR ≥ 0.50 versus <0.50 using logistic regression analyses. Unadjusted analyses were conducted to examine bivariate relationships and then extended to determine the combination of factors predictive of each outcome. In the multivariate analyses, factors were stepped into the respective model in order of significance based on the unadjusted analyses and retained in the final model when p <0.05. Proportional odds ratios (P_OR) were produced to further describe factors significantly related to the odds of being on combination versus monotherapy or no therapy and, likewise, the odds of being on any controller therapy versus no therapy. Incidence rate ratios (IRR) were calculated to compare the ratio of expected number of controller medication fills between significant factor levels such as asthma specialty care (Yes versus No). Odds ratios were computed to highlight factors associated with increased odds AMR ≥ 0.50 versus <0.50. The Hosmer and Lemeshow test was used for goodness of fit of the final model. Analyses were conducted using SPSS Version 22 software. (20)
Results
Sociodemographic and Health Characteristics
Children were primarily male (64%), African American (93%) and Medicaid insured (94%) with a mean age of 6.4 (SD 2.7) years. (Table 1). Caregivers were primarily the child’s biological mother (92%), single (74%), received a high school education or more (80%), poor with a household income less than $30,000 (61%) and with a mean age of 31.3 (SD7.5) years. Asthma morbidity was high among these children with uncontrolled asthma with mean (SD) symptom days over the past 2 weeks was 5.9 (2.4) days, symptom nights over the past 4 weeks was 7.0 (2.6) nights and ED visits for asthma in past 3 months was 1.3 (1.1) visits (excluding the index ED visit for study enrollment) and 28% had an ICU asthma admission over their lifetime. Approximately half (51%) reported having a PCP visit within 3 months of the asthma ED visit, although specialty asthma care over the past 2 years was low at 20%. Indoor environmental exposures were high for mice (47%), cockroach (32%), and SHS (55%), and atopy or a positive sensitization to at least one of the allergens was high at 75%.
Table 1.
Baseline sociodemographic and health characteristics. N=222
| Subjective Data | |
|---|---|
|
| |
| Sociodemographic Characteristics | N (Valid %) or mean (SD) |
|
| |
| Child Age (y), Mean (SD) | 6.4 (2.7) |
|
| |
| Male Gender | 142 (64.0) |
|
| |
| Race African American | 207 (93.3) |
|
| |
| Health Insurance Type | |
| Medicaid Insured | 210 (94.6) |
| Private | 11 ( 5.0) |
| No insurance | 1 ( 0.4) |
|
| |
| Caregiver age (Y), Mean (SD) (range 18–62 years) | 31.3 (7.5) |
|
| |
| Caregiver’s Highest Education Level (N=219) | |
| < High School | 42 (19.2) |
| HS grad or GED | 88 (40.2) |
| Some College + | 89 (40.7) |
|
| |
| Caregiver marital status | |
| Single | 164 (73.9) |
| Married | 33 (14.9) |
| Divorced/Widow/other | 25 (11.2) |
|
| |
| Caregiver employed (Yes) | 122 (55.0) |
|
| |
| Household Income | |
| < $10,000 | 60 (27.0) |
| $10,000–$29,000 | 75 (33.8) |
| $30,000 + | 56 (25.2) |
| Refused/missing | 31 (14.0) |
|
| |
| Pest Exposure in home (Yes) | |
| Mice (n=219) | 104 (47.3) |
| Cockroach (n=218) | 68(31.5) |
|
| |
| Health Characteristics of Child | |
|
| |
| Daytime Symptoms Past 2 weeks, Mean (SD) | 5.9 (2.4) a |
|
| |
| Night time symptoms Past 4 weeks, Mean (SD) | 7.0 (2.6) a |
|
| |
| ED visits for asthma past 3 months, Mean (SD) | 1.3 (1.1) a |
|
| |
| PCP Visits past 3 months | |
| 0 visits | 112 (50.9) |
| 1–2 visits | 97 (44.1) |
| 3 or more visits | 11 ( 5.0) |
|
| |
| Specialty Care in past 2 years (YES) | 44 (19.8) |
|
| |
| Prior Allergy Testing | |
| YES | 62 (27.9) |
| NO | 154 (69.4) |
| Don’t know or missing | 6 ( 2.7) |
|
| |
| ICU Admission for asthma over lifetime (YES) | 62( 27.9) |
|
| |
| Nasal Allergy (YES) | 90 (40.5) |
|
| |
| Food Allergy (YES) | 60 (27.3) |
|
| |
| Eczema (YES) | 128 (57.7) |
|
| |
| Asthma well controlled (caregiver report) | 125 (56.4) |
|
| |
| Medication Attitudes and Beliefs | |
|
| |
| Family Share asthma medication with child (YES) | 40 (18.0) |
|
| |
| Worried about side effects of asthma medication (YES) | 79 (35.6) |
|
| |
| Objective Data | |
|
| |
| Pharmacy Fills Past 12 Months | |
|
| |
| HEDIS AMR, Mean (SD) (N=197 w/asthma Rx fills) | 0.45 (0.27) |
| < 0.50 | 94(47.7) |
| 0.51–0.69 | 75(38.1) |
| >= 0.70 | 28(14.2) |
|
| |
| Oral Corticosteroid fills, Mean (SD) | 1.5 (1.2) a |
|
| |
| Inhaled Corticosteroid (ICS) fills, Mean (SD) | 3.8 (1.9) a |
|
| |
| Short Acting Beta Agonist (SABA) fills, Mean (SD) | 3.1 (1.7) a |
|
| |
| Cotinine Results | |
|
| |
| Cotinine ≥ 1 (Positive SHS exposure) | 121 (54.5) |
| Mean (SD) Baseline cotinine | 2.9 (4.5) |
|
| |
| Allergen specific IgE tests | |
|
| |
| Atopic (1 or more positive serum specific IgE test) (YES) | 166 (74.8) |
| Mean (SD) positive serum specific IgE tests | 4.48 (3.4) |
Due to high skew of distribution and count nature of data, estimates produced from Poisson regression.
Pattern of Controller Medication Use
Agreement between caregiver report of prescribed controller medication and pharmacy records (none, monotherapy, combination) at two weeks prior to the baseline ED visit was moderately high at 78% (weighted Kappa=0.73 (95% CI 0.64–0.82). Pharmacy fill data over the past 12 months revealed non-guideline based medication use with low mean ICS fills at 3.8 (SD1.9) over 12 months (range 0–26). (Table 1) During the same time period, mean (SD) for OCS fills was 1.5 (1.2), range 0–10 fills and mean (SD) for SABA fills was 3.1 (1.7), range 0–25 fills indicating moderately high rescue medication use. Mean (SD) for AMR was 0.45 (0.27) and only 52% of children achieved AMR ≥ 0.50. Specific combinations of controller medications filled over the prior 12 months indicate non-guideline based care defined as no controller fills (24%) or monotherapy (44%) versus combination therapy (32%). (Table 2)
Table 2.
Pattern of Controller Medication use N=222
| Medication | N (%) Self-report |
N (%) RX data |
|---|---|---|
|
| ||
| No controller medication (24%) | 54 (24.3) | 12 (7.5) |
|
| ||
| Monotherapy (44%) | ||
|
| ||
| Flovent (fluticasone)Monotherapya | 86 (38.7) | 72 (45.0) |
|
| ||
| Singulair (montelukast) Monotherapyb | 1 (0.5) | 1 (0.6) |
|
| ||
| Advair (fluticasone/salmeterol)Monotherapy | 5 ( 2.3) | 4 (2.5) |
|
| ||
| Symbicort (budesonide/formoterol)Monotherapy | 2 (0.9) | 2 (1.3) |
|
| ||
| QVAR (beclovent) Monotherapy | 2 (0.9) | 2 (1.3) |
|
| ||
| Pulmicort (budesonide)Monotherpy | 1 (0.45) | 0 |
|
| ||
| Dulera (budesonide/formoterol) Monotherapy | 1 (0.45) | 0 |
|
| ||
| Combination Therapy (32%) | ||
|
| ||
| Combination with Singulair | 67 (30.2) | 66 (41.2) |
| Flovent + Singulair (n=48) or | ||
| Advair + Singulair (n=11)or | ||
| Pulmicort + Singulair ( n=2) or | ||
| Symbicort +Singulair (n=2) | ||
| Flovent + Advair + Singulair (n=2) | ||
| Symbicort + Advair + Singulair (n=1) | ||
| QVAR + Singulair (n=1) | ||
|
| ||
| Combination with Flovent (without Singulair) | 2 (0.90) | 1 (0.6) |
|
| ||
| Combination with Advair | 1 (0.45) | 0 |
|
| ||
| TOTAL | 222 | 160 |
Trade names are included.
Participant asthma severity was mild persistent.
Factors Associated with Level of Controller Medication Therapy
As shown in Table 3, increased odds of combination compared to monotherapy or no therapy was observed in children who received specialty care for asthma in past 2 years (P_OR=16.2, 95%CI 6.76, 39.04), in older children (P_OR=1.92, 95%CI 1.01, 3.63), those who had a prior ICU admission during their lifetime (P_OR=2.08, 95%CI 1.08, 4.02), and children positive to grass allergen (P_OR=2.3, 95%CI 1.24, 4.23). Odds of controller therapy (combination or monotherapy) compared to no controller therapy were higher in children whose caregiver reported minimal caregiver worry about asthma medication side effects (P_OR=2.4, 95%CI 1.32, 4.44). No additional statistically significant socioeconomic and health characteristics were noted in the adjusted analyses.
Table 3.
Percentage of patients on no controller therapy, monotherapy, or combination therapy based on pharmacy records, described by factors significantly related to outcome based on forward selection procedurea.
| None | Monotherapy | Combination Therapy | P_OR (95% CI), p-value a | |
|---|---|---|---|---|
|
| ||||
| Specialty care in past 2 yrs: | ||||
| Yes | 0.0% | 22.7% | 77.3% | 16.23 (6.76,39.04),p<.001 |
| No | 35.4% | 39.4% | 25.1% | Reference |
| Worried about side effects of asthma medications: | ||||
| Yes | 34.6% | 28.2% | 37.2% | 0.41 (0.22,0.76),p=.005 |
| No | 24.8% | 40.4% | 34.8% | Reference |
| Child age: | ||||
| 3–4 years | 35.7% | 40.0% | 24.3% | Reference |
| >=5 years | 24.5% | 35.1% | 40.4% | 1.92 (1.01, 3.63), p=.046 |
| ICU admission lifetime: | ||||
| Yes | 22.6% | 25.8% | 51.6% | 2.08 (1.08,4.02),p=.029 |
| No | 30.0% | 40.6% | 29.4% | Reference |
| Grass Allergy: | ||||
| Positive | 22.5% | 30.3% | 47.2% | 2.29 (1.24,4.23), p=.008 |
| Negative | 34.2% | 42.3% | 23.4% | Reference |
P_OR=Proportional odds ratios produced from generalized ordinal regression model, adjusted for factors listed in table.
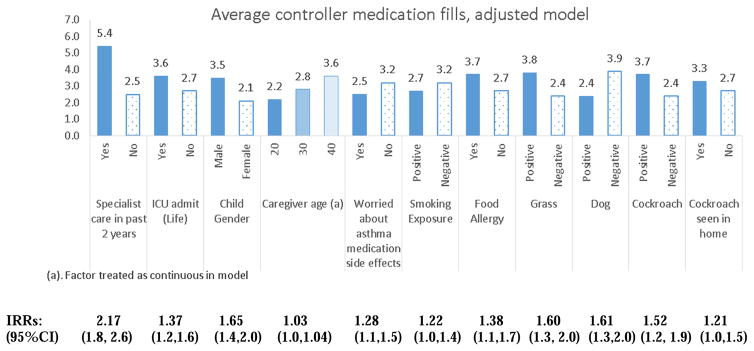
Comparison of average controller fills for significant factors are shown in Figure 2. Children who had received specialty care filled more than twice as many controller medication prescriptions in the past 12 months compared to those who had not received specialty care (IRR=2.17, 95%CI 1.8, 2.6, p<.001). Additional factors significantly associated with a higher average number of controller medication fills were increased caregiver age, a prior ICU admission, minimal caregiver worry about asthma medication side effects, lower SHS exposure, male gender, positive cockroach allergen sensitization and positive indoor exposure to cockroaches over the past 3 months. Positive sensitization to dog, grass and positive history of food allergy were not significantly associated with higher average number of controller fills.
Figure 2.
Average controller medication fills over 12 months described by contributory factors in respective adjusted models. Poisson regression was utilized to estimate average number of fills for respective outcome in final adjusted models. All Incidence Rate Ratios (IRR) p<.05.
Factors Associated with AMR ≥ 0.50 or Guideline-based Care
Unadjusted comparison of characteristics associated with AMR ≥ 0.5 versus < 0.5 are shown in Table 4. Factors significantly associated with AMR ≥ 0.50 included receipt of asthma specialty care in the past 2 years (OR 3.13, 95%CI 1.50, 6.54), lower mean cotinine level (OR: 0.91, 95%CI 0.84, 0.99), higher report of co-morbid condition of nasal allergy (OR: 1.92, 95%CI 1.07, 3.42) and a trend toward having a positive ragweed sensitization (OR: 1.85, 95%CI 0.99, 3.46) and higher report of co-morbid atopic dermatitis (OR 1.73, 95%CI 0.97, 3.08). Nasal allergy was significantly associated with receiving asthma specialty care (OR 1.75, 95%CI 1.03, 2.97). In the final multivariate model predicting odds AMR ≥ 0.50, children receiving specialty care for asthma were four times more likely (OR=4.87, 95%CI 2.06, 11.5), caregivers reporting “worried about side effects of asthma medications” were half as likely (OR=0.50, 95%CI 0.25, 1.00), children with a positive ragweed sensitization were almost four times more likely (OR=3.82, 95%CI 1.63, 8.96), and children with positive dust mite sensitization were less likely (OR=0.33, 95%CI 0.15, 0.76) to have an AMR ≥ 0.50. (Table 5) The adjusted analyses did not indicate additional contribution from any of the remaining sociodemographic characteristics nor health factors listed in Table 1. The final model predicting AMR ≥ 0.50 correctly identified 67.3% of children based on the AMR 0.50 threshold. Validity estimates showed 58.4% sensitivity, 76.9% specificity, 73.2% PPV and 63.0% NPV. Power to examine factors related to an AMR at the 0.70 threshold was limited due to the minimal number of children who met this criteria at baseline (n=28, 14.3%).
Table 4.
Sociodemographic and Health Characteristics Associated with AMR Level (≥0.50 vs < 0.50). N=197 (Unadjusted)
| <0.50 AMR N=94 N (47.7%) |
≥0.50 AMR N=103 N (52.3%) |
Statistic OR (95% CI), p-value |
|
|---|---|---|---|
|
| |||
| Sociodemographic Characteristics | |||
|
| |||
| Child Age | |||
| 3–4 years | 31(33.0) | 29 (28.4) | Reference |
| >= 5 years | 63 (67.0) | 73 (71.6) | OR: 1.24 (0.67,2.28), p=.490 |
|
| |||
| Child Gender | |||
| Female | 34 (36.2) | 31 (31.0) | Reference |
| Male | 60 (63.8) | 69 (69.0) | OR: 1.26 (0.69,2.29), p=.446 |
|
| |||
| Caregiver Education Level | P=.692 | ||
| < High School Grad | 19 (20.4) | 16 (15.8) | Reference |
| HS grad or GED | 37 (39.8) | 41 (40.6) | OR: 1.32 (0.59, 2.93), p=.501 |
| Some College + | 37 (39.8) | 44 (43.6) | OR: 1.41 (0.64, 3.13), p=.395 |
|
| |||
| Season at enrollment | P=.614 | ||
| Fall | 32 (34.0) | 36 (35.0) | Reference |
| Winter | 21 (22.3) | 30 (29.1) | OR: 1.27 (0.61, 2.64), p=.523 |
| Spring | 22 (23.4) | 18 (17.5) | OR: 0.73 (0.33, 1.59), p=.426 |
| Summer | 19 (20.2) | 19 (18.4) | OR: 0.89 (0.40, 1.97), p=.771 |
|
| |||
| Health Characteristics | |||
|
| |||
| Number PCP visits past 3 months, Mean (SD) | 0.38 (1.0) | 0.46 (1.0) | OR (per one PCP visit increase) OR: 1.14 (0.78, 1.65), p=.505 |
|
| |||
| Cotinine at Baseline, Mean (SD) | 3.62 (5.8) | 2.08 (3.0) | OR (per one unit increase) OR: 0.91 (0.84, 0.99), p=.041 |
|
| |||
| Specialty Care in past 2 years | |||
| Yes | 12 (12.9) | 32 (31.7) | OR: 3.13 (1.50, 6.54), p=.002 |
| No | 81 (87.1) | 69 (68.3) | Reference |
|
| |||
| Nasal Allergy | |||
| Yes | 31 (33.0) | 49 (48.5) | OR: 1.92 (1.07, 3.42), p=.028 |
| No | 63 (67.0) | 52 (51.5) | Reference |
|
| |||
| Food Allergy | |||
| Yes | 21 (22.3) | 33 (32.7) | OR: 1.69 (0.89, 3.20), p=.109 |
| No | 73 (77.7) | 68 (67.3) | Reference |
|
| |||
| Eczema (Dry, itchy skin) | |||
| Yes | 49 (52.1) | 66 (65.3) | OR: 1.73 (0.97, 3.08), p=.062 |
| No | 45 (47.9) | 35 (34.7) | Reference |
|
| |||
| Child Share asthma medication with other family members | |||
| Yes | 15 (16.0) | 18 (18.0) | OR: 1.16 (0.55, 2.45), p=.705 |
| No | 79 (84.0) | 82 (82.0) | Reference |
|
| |||
| Asthma Well Controlled (caregiver rating) | |||
| Yes | 49 (55.7) | 56 (57.1) | OR: 1.06 (0.59–1.90), p=.841 |
| No | 39 (44.3) | 42 (42.9) | Reference |
|
| |||
| Worried about side effects of asthma medication | |||
| Yes | 35 (37.6) | 34 (33.7) | OR: 0.84 (0.47, 1.51), p=.564 |
| No | 58 (62.4) | 67 (66.3) | Reference |
|
| |||
| Cockroaches in home | |||
| Yes | 29 (30.9) | 31 (31.0) | OR: 1.01 (0.55, 1.85), p=.982 |
| No | 65 (69.1) | 69 (69.0) | Reference |
|
| |||
| Mice in home | |||
| Yes | 48(51.1) | 47 (47.5) | OR: 0.87 (0.49,1.52), p=.618 |
| No | 46 (48.9) | 52 (52.5) | Reference |
|
| |||
| Mouse Positive | 41 (49.4) | 46 (52.3) | OR: 1.12 (0.62, 2.05), p=.707 |
|
| |||
| Cockroach Positive | 34 (40.5) | 39 (42.9) | OR: 1.10 (0.60, 2.01), p=.750 |
|
| |||
| Cat Positive | 51 (59.3) | 51 (56.0) | OR: 0.88 (0.48, 1.59), p=.661 |
|
| |||
| Dog Positive | 54 (63.5) | 51 (56.0) | OR: 0.73 (0.40, 1.34), p=.312 |
|
| |||
| Ragweed Positive | 25 (29.8) | 40 (44.0) | OR: 1.85 (0.99, 3.46), p=.053 |
|
| |||
| Dust mite Positive | 40 (46.5) | 34 (37.4) | OR: 0.69 (0.38, 1.25), p=.218 |
Timothy Grass, Aspergillus, Alternaria, Oak tree sensitization levels were non-significant between groups.
Table 5.
Adjusted model based on forward selection of factors in order of significance of relationship to odds AMR ≥ 0.50
| Odds AMR ≥ 0.50 a, b |
|---|
| Specialty care in past 2 years (Y vs N): OR=4.87 (2.06, 11.5), p<.001 |
| Worried about side effects of asthma medications (yes vs no): OR=0.50 (0.25, 1.00) P=.051 |
| Ragweed allergen (+ vs −): OR=3.82 (1.63, 8.96), p=.002 |
| Dust mite allergen (+ vs −): OR= 0.33 (0.15, 0.76), p=.009 |
Logistic regression analyses was utilized to estimate odds ratios and to test significance of factor in adjusted model (forward selection (WALD)).
Hosmer and Lemeshow test: p=.82 indicating good fit of model.
Discussion
Our data confirm that most inner city children with persistent asthma do not receive guideline based preventive asthma care (21, 22) despite NAEPP guidelines implemented over twenty-five years ago. The results of our study indicate that children with frequent asthma ED visits have poor use of controller medication based on pharmacy record data. Moreover, our data are consistent with previous research highlighting diverse factors associated with non-guideline based preventive asthma care including lack of provider knowledge of child allergen sensitizations and home exposure status, (6) lack of asthma specialty care (23, 24) and parental medication concerns about the side effects of anti-inflammatory medications.(25, 26)
Only one out of five enrolled children received asthma specialty care in the previous two years, yet receipt of specialty care was significantly associated with increased controller medication fills and a higher level of therapy (e.g., combination therapy versus monotherapy). Others have reported similar findings that suggest guideline-based care is less consistently delivered in primary care offices. (22, 27, 28) Major benefits of asthma specialty care include increased (1) knowledge of updated treatment options, (2) likelihood to prescribe inhaled and systemic corticosteroids, (11) (3) testing to identify sensitization to potential allergen triggers, (4) use of written Asthma Action Plans for self-management, (21, 24) (5) routine evaluation of inhaler use and (6) receipt of pulmonary function testing, (22) all aligned with guideline- based care. (7) Unadjusted results indicate that caregiver report of allergic rhinitis was significantly associated with an AMR ≥ 0.50 and with receiving asthma specialty care. Perhaps nasal allergy symptoms prompt increased use of controller medication or allergic comorbidities prompt receipt of specialty care that may increase controller medication use. Anecdotally, we noted high caregiver satisfaction when receiving results of testing of allergen sensitization obtained during the ED visit, in that most children (69%) had no prior allergy testing or knew their atopy status.
Exposure to indoor allergens and pollutants is highly associated with increased asthma morbidity and unscheduled healthcare utilization (6, 29, 30) and are potential indicators of high risk asthma. (31) Our finding that 75% of enrolled children were atopic is congruent with prior allergen sensitization rates in children with asthma, (32) but lower than the 94% allergy sensitization rate noted in inner city children with moderate to severe asthma and tested for similar allergens (Inner City Asthma Study, ICAS). (29) Perhaps the higher rate of sensitization in the ICAS group is due to an older age group (Mean age 7.7 years) with increased time for sensitization to occur. Although we found high indoor mouse exposure in this group of children, likely due to the suboptimal housing stock, (6) positive mouse sensitization was not associated with increased controller medication use. Regarding outdoor allergens, positive ragweed sensitization was the only outdoor allergen consistently associated with increased controller medication use. Perhaps ragweed allergy with associated nasal symptoms prompts a caregiver to administer allergy, SABA and/or controller medication during the ragweed season to control symptoms. Our data supports Beck and colleagues(6) who recommend reevaluation of inpatient, and as we suggest, acute outpatient asthma care, to incorporate the identification of allergen sensitizations and indoor exposures prior to discharge from the hospital or ED visit to enhance clinical decision making.(6) Based on sensitization and exposure status, step up medication therapy may be initiated along with co-treatment with antihistamines, (6) and targeted, intensive environmental control education and home remediation referral to link hospital care to the home. Pre and post one year comparison of the implementation of the Inner-City Asthma Intervention (ICAI), based on the National Cooperative Inner-City Asthma Study (NCICAS) model of a social worker and physician team focusing on psychosocial issues, medication use and environmental control education, indicated an increase in prescribed controller medication and allergy testing, (33, 34) but no test of AMR by intervention status. Future research testing the effectiveness of a specialty care + social work + environmental home assessment/remediation in enhancing AMR is indicated.
Patient beliefs about treatment benefits and risks are a strong determinant of health management behaviors including medication adherence. (26, 35) Over one-third of caregivers expressed “worry about side effects of asthma medication” in our research, with those endorsing concern more likely to have a child with lower controller medication use. Caregivers may under appreciate the benefit of controller medications and focus on suspected side effects and subsequently under dose or discontinue the controller medication. (35) It is possible that the comparable mean ICS and SABA fills over 12 months (3.8 and 3.1 fills, respectively) reflects caregivers administering controller medications on an intermittent basis and instead relying on SABA to control symptoms on a more routine basis. In children with persistent asthma, NAEPP guidelines recommend lowering the inhaled corticosteroid dose when fewer symptoms are experienced rather than discontinue the medication. However, at the time of the ED visit, most enrolled children reported symptom frequency compatible with uncontrolled asthma suggesting that caregivers may be treating the child’s asthma as episodic rather than chronic disease. (36) Alternatively, they may be initiating anti-inflammatory medication too late to control acute symptoms perpetuating poor asthma control.
One strength of the study is recruitment of high ED utilizers with frequent asthma exacerbations. This increased the validity of recent symptom reports and asthma medication use. However, there were several limitations to this study. First, the asthma diagnosis was based on physician evaluation as reported by the caregiver. Yet, all enrolled children were diagnosed with an acute asthma exacerbation at the time of the enrollment ED visit and had to have 2 or more prior asthma ED visits over the past 12 months to be enrolled in the study. This increased the likelihood of a valid asthma diagnosis. Second, pharmacy fill data may have been incomplete or inaccurate although pharmacy records were requested from every pharmacy used over the past year as reported by the caregiver. Prescription records have been shown to be a reliable source of drug exposure. (37) Although we obtained pharmacy records regarding the number of prescriptions dispensed over 12 months, we are unable to determine actual medication use without confirmation of use of samples, formulary change resulting in an inability to obtain or reluctance of caregiver to administer new medication, or misuse by other family members. We were unable to discern the reason for the low controller medication fills as either lack of insurance coverage, provider under-prescribing, parent misunderstanding of medication use, family sharing of child’s medications. Additionally, our recruitment methods may be biased toward inclusion of more motivated families based on a high decline rate or recruitment bias with missed recruitment of some eligible children. Caregiver report of sharing of medications and controller medication use may have resulted in a social desirability bias. However, with a 78% agreement between caregiver report of prescribed controller medication and pharmacy records, caregivers most likely reported valid medication use. Furthermore, the possibility of caregiver underreporting of specialty and primary care and single items on the survey may lead to invalid results. Last, our study was also limited to children with high asthma ED visits from one institution thereby limiting generalizability.
Despite these limitations, our findings have several important implications. Many innercity children with uncontrolled persistent asthma are lacking guideline-based preventive asthma management. Identification of allergen sensitizations and indoor exposures during an inpatient or acute outpatient visit may enhance clinical decision making including step up medication therapy and provision of targeted, intensive environmental control education and home remediation referrals. Effective interventions are needed to ensure that caregivers are informed and understand the purpose, benefit and side effects of all asthma medications to improve the child’s self-management and asthma control.
Acknowledgments
Funding source: National Institute of Nursing Research, National Institutes of Health (NIH), [grant number R01 NR013486].
Funding Declaration
This work was supported by the National Institute of Nursing Research, National Institutes of Health (NIH), [grant number R01 NR013486]. This publication was made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR) which is funded in part by Grant Number UL1 TR 000424-06 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
We thank Mary Gates and Amanda Manning who contributed to the data collection and thank the many caregivers and their children who participated in the study.
Abbreviations
- ED
Emergency Department
- ICS
Inhaled corticosteroid
- SABA
Short acting beta-agonist
- OCS
Oral corticosteroid
- AMR
Asthma Medication Ratio
Footnotes
Clinical trial registration is with ClinicalTrials.gov: NCT01981564
Conflict of Interest Statement
Drs. Butz, Kub, Bellin, Ogborn, Mudd, Bollinger, Tsoukleris and Ms. Morphew and Ms. Lewis-Land declared no conflicts of interest with respect to the research, authorship and/or publication of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murdoch JR, Lloyd CM. Chronic Inflammation and Asthma. Mutation Research. 2010;690:24–39. doi: 10.1016/j.mrfmmm.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use and mortality: United States: 2005–2009. Natl Health Stat Rep. 2011;12:1–14. [PubMed] [Google Scholar]
- 3.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(suppl 3):S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 4.Akinbami LJ, Simon AE, Rossen LM. Changing trends in asthma prevalence among children. Pediatrics. 2016;137(1):1–7. doi: 10.1542/peds.2015-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloom B, Jones LI, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2012. National Center for Health Statistics. Vital Health Stat. 2013;10(258) [PubMed] [Google Scholar]
- 6.Beck AF, Huang B, Kercsmar CM, et al. Allergen Sensitization Profiles in a Population-Based Cohort of Children Hospitalized for Asthma. Ann Am Thorac Soc. 2015;12(3):376–384. doi: 10.1513/AnnalsATS.201408-376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services. National Asthma Education Prevention Program. Expert Panel Report 3 (EPR3): Guidelines for the Diagnosis and Management of Asthma. NIH Publication 07-4051. Washington DC: US Department of Health and Human Services; 2007. [Google Scholar]
- 8.Celano M, Geller RJ, Phillips KM, Ziman R. Treatment adherence among low-income children with asthma. J Pediatric Psychol. 1998;23(6):345–349. doi: 10.1093/jpepsy/23.6.345. [DOI] [PubMed] [Google Scholar]
- 9.Bender B, Wamboldt FS, O’Connor SL, et al. Measurement of children’s asthma medication adherence by self-report, mother report, canister weight and Doser CT. Ann Allergy Asthma Immunol. 2000;85(5):416–421. doi: 10.1016/s1081-1206(10)62557-4. [DOI] [PubMed] [Google Scholar]
- 10.Butz A, Ogborn J, Mudd S, et al. Factors associated with high short-acting β2-agonist use in urban children with asthma. Ann Allergy Asthma Immunol. 2015;114(5):385–392. doi: 10.1016/j.anai.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson S, Tolstykh I, Selby JV, Mendoza G, Iribarren C, Eisner MD. The impact of allergy and pulmonary specialist care on emergency asthma utilization in a large managed care organization. Health Services Research. 2005;40:1443–1465. doi: 10.1111/j.1475-6773.2005.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flores G, Snowden-Bridon C, Torres S, et al. Urban minority children with asthma: Substantial morbidity, compromised quality and access to specialists and the importance of poverty and specialty care. J Asthma. 2009;46(4):392–398. doi: 10.1080/02770900802712971. [DOI] [PubMed] [Google Scholar]
- 13.Flood C, Sheehan K, Crandall M. Predictors of Emergency Department Utilization among Children in Vulnerable families. Pediatric Emergency Care. 2016 doi: 10.1097/PEC.0000000000000658. epub online at www.pec-online.com. [DOI] [PubMed]
- 14.Schatz M, Zeiger RS, Vollmer WM, et al. The controller-to-total asthma medication ratio is associated with patient-centered as well as utilization outcomes. Chest. 2006;130(1):43–50. doi: 10.1378/chest.130.1.43. [DOI] [PubMed] [Google Scholar]
- 15.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 16.Butz AM, Tsoukleris M, Donithan M, et al. Patterns of inhaled anti-inflammatory medication use in young underserved children with asthma. Pediatrics. 2006;118(6):2504–2513. doi: 10.1542/peds.2006-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffin LE, Townsend M. Measuring quality of life in children with asthma. Qual Life Res. 1996;5(1):35–46. doi: 10.1007/BF00435967. [DOI] [PubMed] [Google Scholar]
- 18.Everhart RS, Miadich AS, Leibach GG, Borschuk AP, Koinis-Mitchell D. Acculturation and quality of life in urban, African American caregivers of children with asthma. J Asthma. 2016;53(9):983–988. doi: 10.3109/02770903.2016.1167904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarville M, Sohn M, Oh E, Weiss K, Gupta R. Environmental tobacco smoke and asthma exacerbations and severity: the difference between measured and reported exposure. Archives of Disease in Childhood. 2013;98(7):510–514. doi: 10.1136/archdischild-2012-303109. [DOI] [PubMed] [Google Scholar]
- 20.IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp; [Google Scholar]
- 21.Yee BA, Fagnano M, Halterman JS. Preventive asthma care delivery in the primary care office: missed opportunities for children with persistent asthma symptoms. Academic Pediatrics. 2013;13(2):98–104. doi: 10.1016/j.acap.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wisnivesky JP, Lorenzo J, Lyn-Cook R, et al. Barriers to adherence to asthma management guidelines among inner-city primary care providers. Ann Allergy Asthma Immunol. 2008;101(3):264–270. doi: 10.1016/S1081-1206(10)60491-7. [DOI] [PubMed] [Google Scholar]
- 23.Sheares BJ, Mellins RB, Dimango E, et al. Do patients of subspecialist physicians benefit from written asthma action plans? Am J Resp Crit Care Med. 2015;191(12):1374–1383. doi: 10.1164/rccm.201407-1338OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheares BJ, Du Y, Vasquez TL, Mellins RB, Evans D. Use of written treatment plans for asthma by specialist physicians. Pediatr Pulmonol. 2007;42:348–356. doi: 10.1002/ppul.20586. [DOI] [PubMed] [Google Scholar]
- 25.Klok T, Lubbers S, Kaptein AA, Brand PL. Every parent tells a story: why non-adherence may persist in children receiving guideline-based comprehensive asthma care. J Asthma. 2014;51(1):106–112. doi: 10.3109/02770903.2013.841191. [DOI] [PubMed] [Google Scholar]
- 26.Yilmaz O, Eroglu N, Ozalp D, Yuksel H. Beliefs about medications in asthmatic children presenting to emergency department and their parents. J Asthma. 2012;49:282–287. doi: 10.3109/02770903.2011.654021. [DOI] [PubMed] [Google Scholar]
- 27.Diette GB, Skinner EA, Nguyen TT, Markson L, Clark BD, Wu AW. Comparison of quality of care by specialists and generalists physicians as usual source of asthma care for children. Pediatrics. 2001;108:432–437. doi: 10.1542/peds.108.2.432. [DOI] [PubMed] [Google Scholar]
- 28.Lee GB, Lee TT. Training pediatricians to adhere to asthma guidelines. Pediatric Allergy Immunol Pulmonol. 2013;26(3):110–114. doi: 10.1089/ped.2013.0265. [DOI] [PubMed] [Google Scholar]
- 29.Gruchalla RS, Pongracic J, Plaut M, et al. Inner city asthma study: Relationships among sensitivity, allergen exposure and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Rosenstreich D, Eggleston P, Kattan M, Baker D, Slavin R, Gergen P. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Eng J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Visness CM, Calatroni A, Gergen PJ, Mitchell HE, Sampson HA. Effect of environmental allergen sensitization on asthma morbidity in inner-city asthmatic children. Clin Exp Allergy. 2009;39:1381–1389. doi: 10.1111/j.1365-2222.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320(5):271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 33.Portnoy JM, Jennings D. Utilization patterns in an asthma intervention. Ann Allergy Asthma Immunol. 2006;97(Suppl 1):S25–S30. doi: 10.1016/s1081-1206(10)60782-x. [DOI] [PubMed] [Google Scholar]
- 34.Rosen CM, Rodriquez L. The Inner-city Asthma Intervention asthma counselor program: a collaborative model between physician and social worker to help empower families. Ann Allergy Asthma Immunol. 2006;97(Suppl 1):S16–S19. doi: 10.1016/s1081-1206(10)60780-6. [DOI] [PubMed] [Google Scholar]
- 35.Menckeberg TT, Bpouvy ML, Bracke M, et al. Beliefs about medicines predict refill adherence to inhaled corticosteroids. J Psychosom Res. 2008;64:47–54. doi: 10.1016/j.jpsychores.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 36.Halm EA, Mora P, Leventhal H. No symptoms, no asthma: the acute episodic disease belief is associated with poor self-management among inner-city adults with persistent asthma. Chest. 2006;129:573–580. doi: 10.1378/chest.129.3.573. [DOI] [PubMed] [Google Scholar]
- 37.McKenzie DA, Semradek J, McFarland BH, Mullooly JP, McCamant LE. The validity of Medicaid pharmacy claims for estimating drug use among elderly nursing home residents: the Oregon experience. J Clin Epidemiology. 2000;53:1248–1257. doi: 10.1016/s0895-4356(00)00259-6. [DOI] [PubMed] [Google Scholar]