Abstract
We examined the relationships between Alzheimer’s disease neuropathologic change (ADNC), Lewy body disease (LBD), and vascular brain injury (VBI) in two large autopsy samples. Because findings may differ between study populations, data came from U.S. Alzheimer’s Disease Centers contributing to the National Alzheimer’s Coordinating Center (NACC, n=2,742) and from the population-based Adult Changes in Thought study (ACT, n=499). Regardless of study population, over 50% of participants with ADNC had co-occurring LBD or VBI; the majority of whom had a clinical AD dementia diagnosis prior to death. Overlap of pathologies was similar between studies, especially after standardizing to the distribution of age and dementia status in the ACT population. LBD, but not VBI, was positively associated with ADNC in both studies. Interestingly, cortical LBD was more common in those with intermediate ADNC compared to low or high ADNC, especially in NACC (p<0.001). High prevalence of co-occurring neuropathologies among older adults with dementia has implications for accurate diagnosis of dementia etiologies and development of disease-modifying strategies.
Keywords: Alzheimer’s disease, Lewy body disease, vascular brain injury, mixed neuropathologies, older adults
1. INTRODUCTION
Vascular brain injury (VBI) and Lewy body disease (LBD) commonly co-occur with Alzheimer’s disease neuropathologic change (ADNC) in older adults (Wirths et al., 2000; Riekse et al., 2004; Rahimi and Kovacs, 2014). Pre-mortem cognitive impairment is associated with mixed neuropathologies at autopsy (Schneider et al., 2007; Montine et al., 2012; Kawas et al., 2015; White et al., 2016). Whether the co-occurrence of VBI and LBD in ADNC is due to synergistic interactions or to overlapping independent processes is unclear.
LBD is present in up to 60% of individuals with ADNC (Hamilton, 2000). ADNC are defined by amyloid plaques and tau neurofibrillary tangles (Hyman et al., 2012). LBD is typically a hallmark of Parkinson’s disease and Dementia with Lewy bodies and is characterized by Lewy bodies (inclusions of α-synuclein) (Spillantini et al., 1997). In brains of many people with ADNC, Lewy bodies are limited to the amygdala with little involvement of other regions (Hamilton, 2000). But cortical LBD is also associated with amyloid burden in most studies (Obi et al., 2008; Jellinger and Attems, 2008; Sonnen et al., 2010; Kotzbauer et al., 2012; Swirski et al., 2014) and with neurofibrillary tangles in some studies (Jellinger and Attems, 2008; Sonnen et al., 2010)but not others (Chung et al., 2015; Kotzbauer et al., 2012; Obi et al., 2008).
Between 30% and 70% of people with ADNC also have co-occurring vascular neuropathologies (Jellinger and Attems, 2005; Rahimi and Kovacs, 2014). A wide range of vascular lesions can be present; however, VBI with gross and microscopic infarcts is considered the most important vascular contributor to dementia (Gorelick et al., 2011). Prevalence of ADNC with co-occurring VBI is higher with older ages (James et al., 2012; Jellinger and Attems, 2010). In some studies, cortical infarcts and microinfarcts are common in those with ADNC (Jellinger, 2007; Okamoto et al., 2009). However, other studies have found no relationship between VBI and amyloid burden (Sonnen et al., 2011; Vemuri et al., 2015).
Inconsistencies in prior study findings could be due to small sample sizes as well as differences in study design, age distribution of study populations, sample selection, clinical assessments, neuropathologic assessment protocols, and classification criteria. Findings on associations between neuropathologies and dementia may differ between clinic-based convenience samples and community or population-based autopsy samples that include home study visit capacity (Crane et al., 2016). Mixed pathologies may be more common in community-based samples compared to clinic-based samples (Schneider et al., 2009). Data from large databases may more precisely characterize relationships between ADNC, LBD, and VBI in clinic and community-based settings. Estimates of brain comorbidity in clinic as well as community-based studies are relevant to developing clinical trials and disease modification strategies.
The objective of this study was to examine the co-occurrence of ADNC, LBD, and VBI at autopsy and test whether LBD or VBI occurred more frequently in those with ADNC than those without. A secondary objective was to compare characteristics of autopsied participants with ADNC only to those with co-occurring LBD or VBI, exploring potential predictors of mixed ADNC pathology. This study focused on the co-occurrence of ADNC, LBD, and VBI as these are the most common pathologies associated with dementia (Sonnen et al., 2007). We also examined the prevalence of other slightly less common pathologies, including hippocampal sclerosis (HS), which is associated with dementia but not strongly associated with ADNC in prior studies (Brenowitz et al., 2014; Nelson et al., 2016), as well as primary age-related tauopathy (PART), which is defined as AD-type neurofibrillary tangles but without co-occurring amyloid plaques (Crary et al., 2014). Data came from a large database of clinical research volunteers who were evaluated at U.S. National Institute on Aging (NIA)-funded U.S. Alzheimer’s Disease Centers (ADCs), as well from the Adult Changes in Thought study (ACT), a population-based study in Seattle, WA.
2. METHODS
2.1. Data sources and study populations
2.1.1. U.S. Alzheimer’s Disease Centers
The National Alzheimer’s Coordinating Center (NACC) maintains data from participants evaluated prospectively by one of 34 past and present NIA-funded ADCs (Beekly et al., 2007, 2004). Participants in the Uniform Data Set were evaluated annually at an ADC using a standardized protocol beginning September 2005; neuropathology data based on autopsy results was available for those who had died and consented to autopsy evaluation (Beekly et al., 2007; Morris et al., 2006). Individual ADCs recruit and enroll participants according to their own protocols. Some, but not all, ADCs require participants’ consent to autopsy prior to enrollment. Some ADCs include home study visit capacity and others do not. Participants enrolled with any level of cognition, ranging from normal to demented. Written informed consent was obtained from all participants and their study co-participants; institutional review board (IRB) approval was obtained from all individual ADCs. The University of Washington IRB approved this current study.
Between September 2005 and September 2015, 6,507 of 32,479 total participants died, and 3,835 had an autopsy; this analysis is based on subjects with neuropathology data. Because of low prevalence in population-based studies and potential for confounding, 1,063 autopsied participants with Down’s syndrome, prion disease, early-onset autosomal dominant genetic diseases, frontotemporal lobar degeneration, and other rare causes of dementia were excluded. Also excluded were 30 additional participants missing neuropathologic information on ADNC, LBD, or VBI. The analytic sample for the current study thus comprised 2,742 autopsied participants (See Supplementary Figure 1 for study flow chart).
We conducted additional sub-analyses on 246 participants in the Oregon Health & Science University (OHSU) and 97 in the University of Washington (UW) ADCs. Both ADCs upload data to NACC and serve as their own data repositories. These two ADCs collaborate in the Pacific Northwest Dementia and Aging Neuropathology Group (PANDA), which also includes the ACT study. Brain tissue collection, histochemical staining, and reporting follow standardized procedures through this agreement. Both ADCs recruit patients seen in clinic for diagnosis, treatment, or enrollment in clinical trials; however, autopsied participants seen at OHSU were recruited from a number of cohort studies focusing on healthy aging in older adults, which are described elsewhere (Howieson et al., 1993; Kaye et al., 2009, 2011; Petersen et al., 2015). Hereafter OHSU and UW ADCs are referred to collectively as the PANDA ADCs.
2.1.2. Adult Changes in Thought study
ACT [U01 AG006781] is a longitudinal community-based prospective cohort study of older adults. In contrast to NACC, participants in ACT were enrolled from a well-defined underlying population of community dwelling older adults receiving care in an integrated delivery system. ACT is described in detail elsewhere (Kukull et al., 2002; Larson et al., 2006; Crane et al., 2013). Briefly, a random sample of Group Health Cooperative members aged 65 and older in the Seattle area was drawn and individuals were invited to participate. Individuals with dementia at baseline were not enrolled. Participants were followed every 2 years until time of dementia diagnosis, death, or drop-out: 2,581 persons were enrolled in 1994–1996, 811 in 2000–2002, and continuous enrollment started in 2004; 5,074 participants had completed at least one visit between 1994 and May 2015 of whom 2,537 had died. Neuropathological assessments were conducted on 531 participants who had died and consented to autopsy and followed the PANDA protocol. The Group Health and University of Washington IRBs approved the ACT study. All participants provided written informed consent, and next of kin consented to autopsy. The University of Washington IRB approved the use of ACT data in the current study. The analytic sample for the current study consisted of 499 autopsied ACT participants (Supplementary Figure 1), having excluded 32 with missing pathologic information on ADNC, LBD, or VBI.
2.2. Neuropathological features
Each ADC conducted neuropathologic assessments following consensus guidelines, but according to its own protocols, with the exception of the common assessment protocol used by PANDA ADCs. Results were uploaded to the NACC database using a standardized form. Additional information on number of microinfarcts as well as number, size, and approximate age of gross infarcts was abstracted from PANDA ADC neuropathology reports to supplement NACC data. In ACT, gross and microscopic lesions were collected using the same standardized assessment protocols used in the PANDA ADCs and as described previously (Sonnen et al., 2007). Measures were classified similarly across all data sources unless otherwise specified.
ADNC included Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) scores of neuritic plaque densities (none, sparse, moderate, frequent) (Mirra et al., 1991) and Braak stages for tau neurofibrillary pathology (none, I–II, III–IV, V–VI) (Braak et al., 2006). ADNC was categorized semi-quantitatively (low, intermediate, and high). Low ADNC was defined as no/sparse neuritic plaques & any Braak stage OR any neuritic plaques density & Braak stage 0-II. Intermediate ADNC was defined as moderate or frequent CERAD plaques & Braak stage III–IV; and high ADNC was defined as moderate or frequent plaques & Braak stage V–VI. Note that this assessment does not include Thal phasing (Thal et al., 2002) for amyloid plaques, so this operationalization overlaps with but does not correspond exactly to the levels of ADNC as defined by new NIA-Alzheimer’s Association criteria (Hyman et al., 2012). However, this has recently been shown not to contribute significantly to the correlation between cognitive function and neuropathologic assessment at autopsy (Serrano-Pozo et al., 2016). Primary age-related tauopathy (PART) was classified as present in participants with definite PART as defined by Braak Stage I–IV & no neuritic plaques (Crary et al., 2014).
Cerebrovascular pathology encompassed VBI and indicators of vessel disease. In all samples, VBI was defined as any gross infarcts or cortical microinfarcts. In NACC, gross infarcts (present, absent) were defined as large artery or lacunar infarcts identified macroscopically regardless of age. Cortical microinfarcts (present, absent) were defined as infarcts in the cortex that were only detected microscopically. In ACT, OHSU ADC, and UW ADCs old or chronic gross infarcts were defined as present or absent. Microinfarcts were assessed following methods developed in the Honolulu Asia Aging Study and defined as “a focal lesion attributed to ischemia, found only on microscopic examination, and judged to be temporally remote” (White et al., 2002). Microinfarcts were categorized as cortical (present, absent) or subcortical (present, absent). In all data sources, overall severity of cerebral amyloid angiopathy (identified with stains for amyloid) and atherosclerosis (identified grossly) were recorded as none, mild, moderate, or severe.
In all data sources, LBD was defined as presence of Lewy bodies in any brain region examined and categorized as present or absent. Presence of Lewy bodies was assessed according to established guidelines (McKeith et al., 2005). In NACC, LBD was classified as either none, brainstem predominant, limbic (transitional), cortical (diffuse), or region not specified/other. In ACT and PANDA ADCs, LBD was further classified as either none, brainstem predominant (Lewy bodies only found in brainstem: substantia nigra or locus ceruleus), limbic (Lewy bodies in brainstem and amygdala), cortical (Lewy bodies in cortex), or amygdala only (Lewy bodies in amygdala only).
Hippocampal sclerosis is considered a separate disease entity with potentially multiple etiologic origins (Nelson et al., 2013). In older NACC form versions (prior to 2014) hippocampal sclerosis was reported present as a primary or contributing neuropathologic diagnosis, while in the newest form version and in ACT presence of hippocampal sclerosis was recorded as unilateral, bilateral, or laterality unknown. Hippocampal sclerosis for this study was classified as present or absent.
2.3. Clinical characteristics
Demographic characteristics included age, sex, education, race/ethnicity, and cohort for ACT participants or ADC for NACC participants. For the purposes of this study, we focused on health histories as of the last study visit. In NACC, health history was obtained via co-participant or self-report, medical records, judgment of the examining clinician, or some combination. In ACT, histories of co-morbid medical conditions were obtained by questionnaire. In both studies, APOE ε4 allele status (at least one vs. none) was classified for consenting participants who underwent APOE genotyping. In NACC ADCs, either a single clinician or consensus group of clinicians made a diagnosis of normal cognition, impaired but not mild cognitive impairment (MCI), MCI, or demented after a review of all evaluation information available. Primary and contributing etiologic diagnoses are assigned for all participants with MCI or dementia, following established guidelines (Beekly et al., 2007). In ACT, dementia was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (American Psychiatric Association, 1995). A complete dementia work-up was only conducted on participants who had a Cognitive Abilities Screening Instrument (Teng et al., 1994) score 85 or below at their visit or who reported symptoms suggestive of dementia onset. Dementia due to AD (e.g. clinical AD dementia) was defined in both NACC and ACT as a primary clinical diagnosis of probable or possible AD according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria (McKhann et al., 1984).
2.4. Statistical analyses
We calculated the frequency and prevalence (calculated as the number of participants with the pathology / total autopsied participants) of ADNC (intermediate to high), LBD, VBI, and other major pathologies in NACC, PANDA ADCs, and ACT. A multivariable logistic regression model described differences between autopsied NACC and ACT participants, with study sample as the outcome and demographic, clinical, and pathologic characteristics included as predictors. We estimated the frequency of co-occurrence of intermediate to high ADNC, LBD, and VBI. The software eulerAPE (Micallef and Rodgers, 2014) was used to create area-proportional Venn/Euler diagrams that accurately illustrate the overlap of each pathology. Overlap of pathologies was examined in both samples overall as well as stratified by age at death (65–89 vs. 90+). Pearson χ2 tests or Fisher’s exact tests (if any categories included <10 participants) assessed whether estimates of co-occurrence differed by year of birth or death in NACC. In ACT, dates were not available per IRB approval; we assessed whether estimates of co-occurrence differed between those enrolled in the original cohort (1994–1996) or later.
Several sensitivity analyses were conducted to account for differences between participants who enrolled in NACC and ACT. Since age distribution and prevalence of dementia differed significantly between NACC and ACT populations, and are associated with pathologic burden (Nelson et al., 2010; Sonnen et al., 2007), estimated prevalence of single and mixed pathologies in each sample was standardized to the distribution of age at last visit and dementia status of all ACT participants, autopsied and non-autopsied. Standardized estimates were calculated as the weighted average of stratum-specific prevalence estimates for age (<80 years, 80–90, and 90+ years) and dementia status (non-demented, demented) weighted by the frequency of all enrolled ACT participants in each stratum (Zhou et al., 1999). To investigate whether differences in prevalence between samples was due to restriction in ACT enrollment of having to be non-demented and 65 years or older at baseline, we examined co-occurrence of pathologies in NACC participants most like ACT participants (e.g. who would have met criteria for ACT) and those least like ACT participants (e.g. those aged 65 and younger at dementia onset).
We next examined the prevalence of LBD and VBI along the continuum of ADNC (low, intermediate, and high). Pearson χ2 tests or Fisher’s exact tests (if any categories included <10 participants) assesed whether the prevalence of LBD subtype and VBI differed by level of ADNC. Because neuropathologic assessments may have differed between some ADCs and ACT, prevalence of pathologies was also examined separately among PANDA ADCs, which share neuropathologic protocols with ACT.
Finally, using descriptive statistics we examined clinical and demographic characteristics of those with ADNC and LBD (ADNC+LBD), ADNC and VBI (ADNC+VBI), or ADNC and LBD and VBI (ADNC+LBD+VBI) compared to those with ADNC only, defined as intermediate to high ADNC, no LBD, and no VBI. All tests were two-sided with α = 0.05. To account for multiple comparisons (n=18 tests), statistical significance using a Bonferroni correction was considered p<0.0028. Analyses were conducted using R (version 3.2.1, R Core Team, 2015).
3. RESULTS
3.1. Participant characteristics
NACC and ACT participants without dementia were relatively similar in demographic (Table 1) and pathologic characteristics (Table 2). NACC participants with dementia were on average almost 10 years younger at death and were more likely to have at least one APOE ε4 allele compared to ACT participants (Table 1). NACC participants with dementia had a higher prevalence of high ADNC and LBD but a lower prevalence of VBI than did ACT autopsied participants with dementia (Table 2), including when pathologies among those with clinical AD or non-AD dementia were examined separately (Supplementary Table 1). Other pathologies, such as severe cerebral amyloid angiopathy, severe atherosclerosis, HS, and PART were less common (<20%) in both samples (Table 2). Definite PART pathology was more frequent in participants without dementia compared to those with dementia; mean Braak Stage of those with PART was 2.2 (SD: 1.0) in NACC and 2.1 (SD:0.9) in ACT. In a multivariable regression model, younger age at death, college education, dementia at last visit, ADNC, LBD, severe cerebral amyloid angiopathy, severe atherosclerosis, and PART were significantly more common in NACC autopsied participants compared to ACT autopsied participants (Table 3).
Table 1.
Demographic and clinical characteristics of participants by clinical dementia status at last visit before death
| Characteristics* | NACC | ACT | ||||
|---|---|---|---|---|---|---|
| Non-demented | Demented | Total | Non-demented | Demented | Total | |
| Total autopsies, N | 585 | 2,157 | 2,742 | 275 | 224 | 499 |
| Age at death, mean (SD) | 87.3 (8.8) | 80.3 (10.3) | 81.8 (10.4) | 86.3 (7.0) | 89.5 (5.7) | 87.7 (6.7) |
| Female | 327 (55.9) | 941 (43.6) | 1,268 (46.2) | 145 (52.7) | 120 (53.6) | 265 (53.1) |
| Non-white | 24 (4.1) | 1,487 (6.9) | 171 (6.3) | 11 (4.0) | 9 (4.0) | 20 (4.0) |
| College graduate | 334 (57.1) | 1,180 (54.7) | 1,514 (55.2) | 120 (43.6) | 78 (34.8) | 198 (39.7) |
| History of stroke | 74 (12.7) | 263 (12.3) | 337 (12.4) | 42 (16.2) | 32 (17) | 74 (16.6) |
| APOE ε4 allele | 113 (21.5) | 1,015 (55.8) | 1,128 (48.1) | 59 (23.1) | 68 (34.9) | 127 (28.2) |
| Clinical AD dementia | NA | 1,776 (82.3) | 1,776 (42.9) | NA | 176 (78.6) | 178 (35.7) |
ACT, Adult Changes in Thought study; AD, Alzheimer’s disease; NA, not applicable; NACC, National Alzheimer’s Coordinating Center
N,% unless otherwise specified. Relative frequencies calculated based on complete data. Number of participants missing data in NACC: race=15 (<1%), education=25 (<1%), APOE genotype=395 (14.4%), stroke=22 (<1%), and age of onset=27 (1.3%). Number of participants missing data in ACT: APOE genotype=49 (9.8%), stroke=52 (10.4%).
Table 2.
Prevalence of individual pathologies in participants by clinical dementia status at last visit before death
| Characteristics* | NACC | ACT | ||||
|---|---|---|---|---|---|---|
| Non-demented | Demented | Total | Non-demented | Demented | Total | |
| Total autopsies, N | 585 | 2,157 | 2,742 | 275 | 224 | 499 |
| Alzheimer’s disease | ||||||
| neuropathologic change (ADNC)† | 29.6 | 82.7 | 71.3 | 26.2 | 63.4 | 42.9 |
| Intermediate ADNC | 16.8 | 13.9 | 14.5 | 15.6 | 13.3 | 14.6 |
| High ADNC | 12.8 | 68.8 | 56.9 | 10.5 | 50.0 | 28.3 |
| Lewy body disease (LBD) | 16.9 | 40.8 | 35.7 | 13.8 | 21.4 | 17.2 |
| Cortical LBD | 3.8 | 18.2 | 15.1 | 4.0 | 8.9 | 6.2 |
| Vascular brain injury‡ | 40.7 | 30.7 | 32.9 | 40.7 | 68.3 | 53.1 |
| Cortical microinfarcts | 20.2 | 16.2 | 17.1 | 29.8 | 45.5 | 36.9 |
| Gross infarcts | 31.0 | 22.5 | 24.3 | 23.9 | 46.0 | 33.9 |
| Other Pathologies | ||||||
| Severe atherosclerosis | 14.5 | 13.4 | 13.6 | 5.6 | 9.6 | 7.4 |
| Severe CAA | 5.6 | 15.1 | 13.1 | 1.8 | 2.2 | 2.0 |
| Hippocampal sclerosis | 2.6 | 9.5 | 8.0 | 5.6 | 12.5 | 8.7 |
| Primary age-related tauopathy | 32.3 | 5.6 | 11.3 | 25.8 | 10.7 | 19.0 |
ACT, Adult Changes in Thought study; CAA, cerebral amyloid angiopathy; NACC, National Alzheimer’s Coordinating Center
Percent autopsied unless otherwise noted, calculated based on complete data. Number of participants missing data in NACC: gross infarcts=3 (<1%), atherosclerosis=23 (<1%), CAA=62 (2.3%), hippocampal sclerosis=450 (16.4%). Number of participants missing data in ACT: gross infarcts=3 (<1%), atherosclerosis=14 (2.8%), CAA=2 (<1%), hippocampal sclerosis=14 (2.8%).
ADNC defined as moderate/frequent CERAD neuritic plaques & Braak stages of neurofibrillary degeneration III–IV (intermediate ADNC) or Braak stages V–VI (high ADNC).
VBI defined as any gross infarcts or cortical microinfarcts.
Table 3.
Multivariable regression model of NACC participants compared to ACT participants
| Characteristics | OR for NACC vs ACT* |
95%CI | p |
|---|---|---|---|
| Demographic and Clinical | |||
| Older age at death (yrs) | 0.66 | (0.60, 0.82) | <0.001 |
| Female | 1.40 | (1.08, 1.80) | 0.01 |
| Non-white | 1.03 | (0.57, 1.88) | 0.92 |
| College graduate | 1.77 | (1.38, 2.27) | <0.001 |
| History of stroke | 0.92 | (0.65, 1.30) | 0.64 |
| APOE ε4 allele | 1.31 | (0.99, 1.74) | 0.06 |
| Demented at last visit | 2.13 | (1.35, 3.36) | 0.001 |
| Clinical AD dementia | 1.68 | (1.07, 2.63) | 0.02 |
| Pathologic | |||
| Intermediate ADNC | 1.50 | (1.03, 2.20) | 0.03 |
| High ADNC | 1.55 | (1.09, 2.19) | 0.01 |
| Cortical LBD | 2.17 | (1.30, 3.63) | 0.003 |
| Cortical microinfarcts | 0.35 | (0.27, 0.46) | <0.001 |
| Gross infarcts | 1.01 | (0.76, 1.35) | 0.93 |
| Severe atherosclerosis | 6.07 | (2.64, 13.97) | <0.001 |
| Severe CAA | 3.17 | (1.98, 5.08) | <0.001 |
| Hippocampal sclerosis | 0.69 | (0.45, 1.07) | 0.10 |
| Primary age-related tauopathy | 1.72 | (1.21, 2.45) | 0.002 |
ACT, Adult Changes in Thought study; ADNC, Alzheimer’s disease neuropathologic change; CAA, cerebral amyloid angiopathy; LBD, Lewy body disease; NACC, National Alzheimer’s Coordinating Center
Based on a logistic regression model with ACT as the reference group; 498 NACC participants and 126 ACT participants excluded due to missing data; OR>1 if characteristic is more common in NACC compared to ACT
NACC participants were evaluated at 31 ADCs; 3 to 252 participants were seen per ADC. Because there may be some heterogeneity across ADCs, we also separately examined prevalence of pathologies among demented in PANDA (OHSU and UW) ADCs, which share neuropathologic protocols with ACT. Compared to ACT, LBD was more common across all ADCs, including PANDA ADCs (Supplementary Table 2). Prevalence of ADNC and VBI was more similar between OHSU and ACT compared to UW and other ADCs; OHSU participants were more like ACT participants in demographics (Supplementary Table 3).
3.2. Co-occurrence of LBD and VBI in ADNC
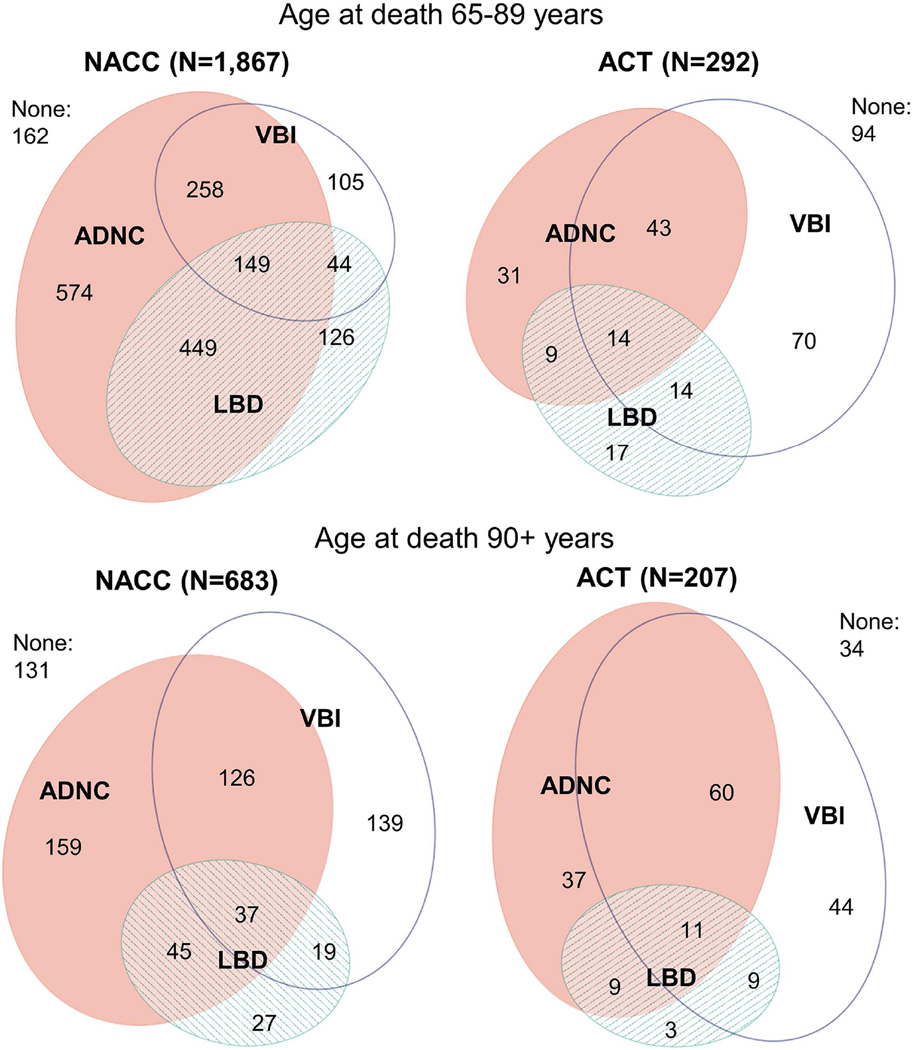
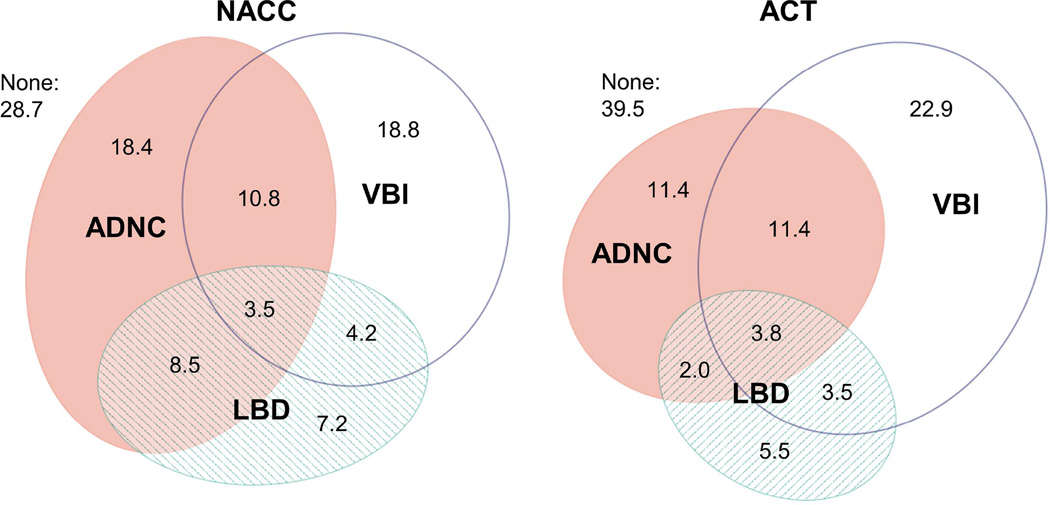
A majority of participants with ADNC had co-occurring LBD or VBI (59% of NACC participants and 68% of ACT participants). Co-occurrence of ADNC and LBD was slightly more common in NACC than ACT (38% vs. 20% of participants with ADNC), while the cooccurrence of ADNC and VBI was less common in NACC than ACT (30% vs. 60% of participants with ADNC). Overlap of pathologies was similar between NACC and ACT in age stratified samples (65–89 vs. 90+) (Figure 1) and after standardization to the age and dementia prevalence of all enrolled ACT participants (Figure 2). After adjustment for multiple comparisons, estimates of co-occurrence did not differ significantly by year of death (p=0.3) or year of birth (p=0.05) in NACC. In ACT, estimates of co-occurrence did not differ between those enrolled in the original cohort (1994–1996) or later (p=0.4). Co-occurrence of HS in those with ADNC was slightly under 15% for both NACC and ACT. About 10% of participants with LBD or VBI also had co-occurring HS, in both samples. Co-occurrence of PART was slightly higher in those with VBI (14% in NACC and 17% in ACT) than those with LBD (8% in NACC and 14% in ACT).
Figure 1. Co-occurrence of Alzheimer’s disease neuropathologic change (ADNC), Lewy body disease (LBD), and vascular brain injury (VBI) stratified by age.
ACT, Adult Changes in Thought study; NACC, National Alzheimer’s Coordinating Center. ADNC = moderate/frequent neuritic plaques & Braak stage III–VI; LBD = Lewy bodies in any brain region examined; VBI = gross infarcts and cortical microinfarcts. Note: 191 NACC participants with age of death less than 65 years were excluded.
Figure 2. Prevalence (percent) of Alzheimer’s disease neuropathologic change (ADNC), Lewy body disease (LBD), and vascular brain injury (VBI) standardized to ACT overall study population.
Prevalence estimates (percent) standardized to the distribution of age (<80yrs, 80–90 years, and 90+) and dementia status (demented, non-demented) at last visit among all ACT participants (autopsied and non-autopsied). Confidence intervals for estimates shown in Table 4. ACT, Adult Changes in Thought study; NACC, National Alzheimer’s Coordinating Center. ADNC = moderate/frequent neuritic plaques & Braak stage III–VI; LBD = Lewy bodies in any brain region examined; VBI = gross infarcts and cortical microinfarcts.
Estimated prevalence of VBI and low neuropathology in NACC was higher after standardization to the age and dementia prevalence of ACT participants (Supplementary Table 4). There were 885 participants from NACC who would have met basic ACT entry criteria of being non-demented and 65 years or older at baseline (mean age at baseline= 83.4 (SD 7.8); 53% female). Co-occurrence of ADNC, LBD, and VBI was similar between those 885 “ACT-like” NACC participants and ACT participants: approximately 40% of participants had ADNC of whom 26% had co-occurring LBD and 38% had co-occurring VBI in NACC while in ACT 20% had co-occurring LBD and 60% had co-occurring VBI. In contrast, among 576 NACC participants who were younger than 65 at dementia onset and who would have not been eligible for ACT, 85.6% had ADNC, 45.6% of whom had co-occurring LBD and just 16.8% had co-occurring VBI.
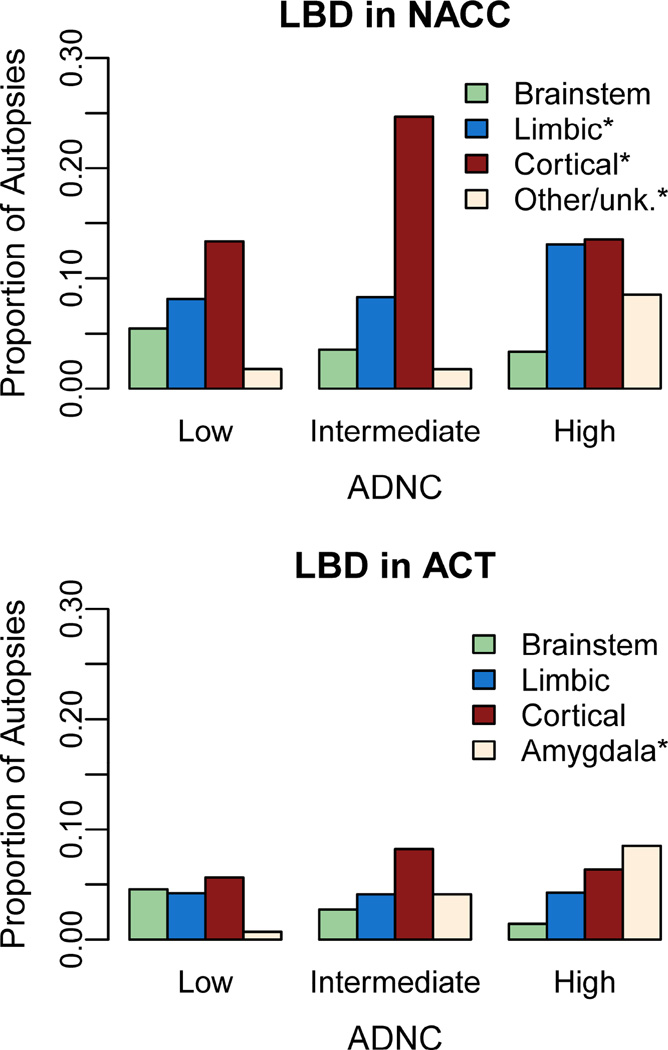
Prevalence of LBD by regional subtype is shown across levels of ADNC in Figure 3. The proportion of participants with limbic LBD [NACC] or amygdala only LBD [ACT] was greater in those with high ADNC compared to low and intermediate ADNC (both p<0.001). Interestingly in NACC, cortical LBD was more common in those with intermediate ADNC compared to those with low or high ADNC (p<0.001).
Figure 3. Prevalence of Lewy body disease (LBD) subtypes in participants with low, intermediate, and high Alzheimer’s disease neuropathologic change (ADNC).
ACT, Adult Changes in Thought study; NACC, National Alzheimer’s Coordinating Center. Low ADNC= no/sparse CERAD neuritic plaques & any Braak stage OR any neuritic plaques & Braak stage 0-II; intermediate ADNC= moderate/frequent neuritic plaques & Braak stage III–IV; high ADNC = moderate/frequent neuritic plaques & Braak stages V–VI. *p<0.001 for difference in prevalence of LBD subtype by level of ADNC based on χ2 (NACC) or Fisher’s exact test (ACT).
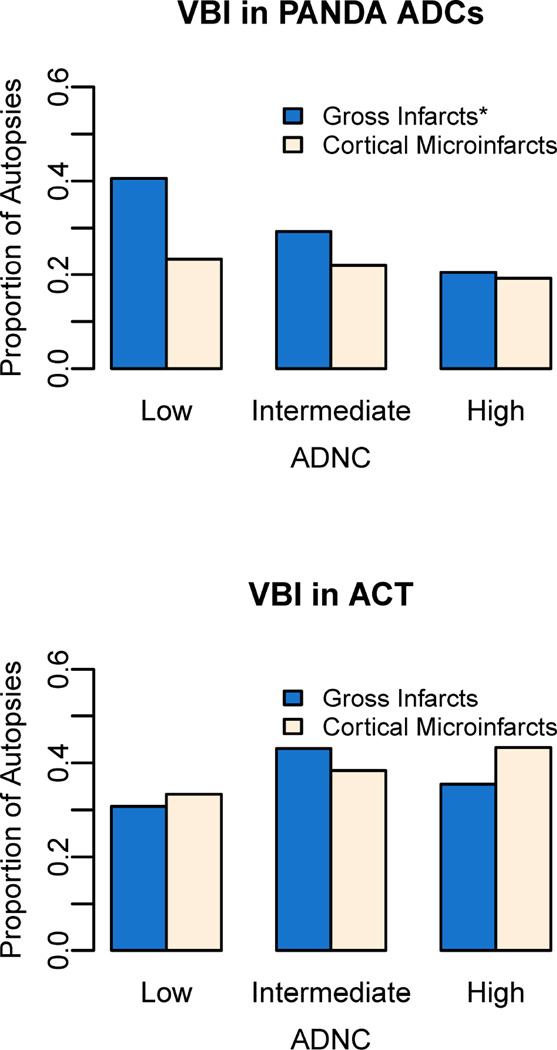
VBI were less common in higher levels of ADNC, in NACC, (p<0.001) but VBI did not differ significantly by level of ADNC in ACT after correction for multiple comparisons (p=0.03). We also compared specific VBI sub-types between participants from PANDA ADCs and ACT (Figure 4) since they all used the same neuropathological assessment protocol. Gross infarcts were less common in intermediate or high ADNC, in a graded fashion, in PANDA ADCs (p<0.001) but not in ACT (p=0.13); prevalence of cortical microinfarcts did not differ by level of ADNC in PANDA ADCs (p=0.22) or ACT (p=0.13).
Figure 4. Prevalence of vascular brain injury (VBI) in participants with low, intermediate, and high Alzheimer’s disease neuropathologic change (ADNC).
ACT, Adult Changes in Thought study; PANDA ADCs, Pacific Northwest Dementia and Aging Neuropathology Group Alzheimer’s Disease Centers (Oregon Health & Science University and University of Washington). Low ADNC = no/sparse CERAD neuritic plaques & any Braak stage OR any neuritic plaques & Braak stage 0-II; Intermediate ADNC = moderate/frequent neuritic plaques & Braak stage III–IV; High ADNC = moderate/frequent neuritic plaques & Braak stages V–VI. *p<0.05 for difference in prevalence of VBI by level of ADNC based on fisher’s exact test.
3.3. Clinical characteristics of ADNC only vs. mixed ADNC
Clinical characteristics of autopsied participants with ADNC, with and without co-occurring LBD or VBI are shown for NACC (Table 4) and ACT (Table 5). Male sex and the APOE ε4 allele were more common in participants with ADNC and co-occurring LBD compared to participants with ADNC only. Male sex, and history of stroke were more likely in participants with ADNC and co-occurring VBI compared to participants with ADNC only. The majority of participants with mixed ADNC had dementia and a clinical AD diagnosis; although sensitivity of clinical AD diagnosis was slightly lower in those with mixed ADNC than ADNC only.
Table 4.
Demographic and clinical characteristics of NACC participants with Alzheimer’s disease neuropathologic change (ADNC) with and without co-occurring Lewy body disease (LBD) or vascular brain injury (VBI)*
| Characteristics† | ADNC only | ADNC+LBD | ADNC+VBI | ADNC+LBD +VBI |
|---|---|---|---|---|
| Total autopsies, N | 810 | 559 | 394 | 193 |
| Age at death, mean (SD) | 80.2 (10.6) | 77.4 (9.5) | 84.4 (8.4) | 82.9 (10.9) |
| Female | 403 (49.8) | 228 (40.8) | 184 (46.7) | 76 (39.4) |
| Non-white | 37 (4.6) | 35 (6.3) | 35 (9) | 24 (12.5) |
| College graduate | 466 (57.5) | 314 (56.2) | 203 (51.5) | 103 (53.4) |
| History of stroke | 54 (6.8) | 30 (5.4) | 102 (26.3) | 28 (14.6) |
| APOE ε4 allele | 378 (54.9) | 307 (64.9) | 185 (54.9) | 105 (62.5) |
| Demented | 726 (89.6) | 538 (96.2) | 337 (85.5) | 182 (94.3) |
| Clinical AD dementia | 658 (90.6) | 421 (78.3) | 306 (90.8) | 159 (87.4) |
NACC, National Alzheimer’s Coordinating Center
ADNC = moderate/frequent neuritic plaques & Braak stage III-VI; LBD = Lewy bodies in any brain region examined; VBI = gross infarcts and cortical microinfarcts.
N,% unless otherwise specified. Relative frequencies presented for complete data. Number of participants missing data: race=11 (<1%), education=19 (1.0%), stroke=20 (1.0%), and APOE genotype=289 (14.8%).
Table 5.
Demographic and clinical characteristics of ACT participants with Alzheimer’s disease neuropathologic change (ADNC) with and without co-occurring Lewy body disease (LBD) or vascular brain injury (VBI)*
| Characteristics† | ADNC only | ADNC+LBD | ADNC+VBI |
ADNC+LBD +VBI |
|---|---|---|---|---|
| Total autopsies, N | 68 | 18 | 103 | 25 |
| Age at death, mean (SD) | 89.1 (6.7) | 88.9 (5.8) | 90.6 (5.9) | 87.8 (6.3) |
| Female | 43 (63.2) | 7 (38.9) | 60 (58.3) | 13 (52.0) |
| Non-white | 5 (7.4) | 1 (5.6) | 7 (6.8) | 1 (4.0) |
| College graduate | 26 (38.2) | 9 (50.0) | 31 (30.1) | 11 (44.0) |
| History of stroke | 2 (3.4) | 1 (6.7) | 25 (26.9) | 1 (4.5) |
| APOE ε4 allele | 20 (33.3) | 6 (37.5) | 33 (34.4) | 12 (60.0) |
| Demented | 35 (51.5) | 14 (77.8) | 76 (73.8) | 17 (68.0) |
| Clinical AD dementia | 33 (94.3) | 10 (71.4) | 61 (80.2) | 14 (82.3) |
ACT, Adult Changes in Thought study
ADNC = moderate/frequent neuritic plaques & Braak stage III-VI; LBD = Lewy bodies in any brain region examined; VBI = gross infarcts and cortical microinfarcts.
N,% unless otherwise specified. Relative frequencies presented for complete data. Number of participants missing data: stroke=25 (11.7%), and APOE genotype=22 (10.3%).
4. DISCUSSION
We conducted a thorough examination of mixed neuropathologies in clinic and community-based samples, with particular focus on the relationships between ADNC and LBD or VBI. Compared to the ACT study, ADC participants were on average younger and a higher proportion had dementia and co-occurring LBD and ADNC at autopsy. Despite these differences, mixed neuropathologies were common in both populations, especially the co-occurrence of LBD or VBI with ADNC. The overall overlap of neuropathologies was also similar between the two cohorts, especially after standardization to the distribution of age and dementia status in the ACT population. Although slightly less common, 10–15% of participants also had co-occurring HS, and about 15% of participants with VBI had co-occurring PART. Prevalence of limbic LBD was higher among those with high ADNC in NACC, and similarly amygdala LBD was more common among those with higher ADNC in ACT. Interestingly, cortical LBD was associated with intermediate ADNC, most evidently in NACC. Evidence for a positive association between VBI and ADNC in either NACC or ACT was lacking. Characteristics of autopsied participants with mixed ADNC neuropathologies were also remarkably similar between studies: the majority had dementia and a clinical AD-diagnosis.
Although prior research has found mixed neuropathologies to be common, estimates of the prevalence of mixed neuropathologies are varied (Rahimi and Kovacs, 2014). In both study samples considered here, the majority of brains had mixed pathologies, contrary to a prior study in which mixed pathologies were more common in a community-based sample (Schneider et al., 2009). This difference from the earlier report may be due to combining data from multiple ADCs that have heterogeneous study populations. Although ADNC+LBD was more common in NACC than in ACT, this may be partly because ACT enrollment criteria would exclude those with earlier dementia onset. Prevalence of mixed pathologies was quite similar between ACT and NACC in our sensitivity analyses among those non-demented and age 65+ at baseline, among the oldest-old, and after standardization, further suggesting that differences in age and dementia prevalence may drive much of the differences in prevalence. After we standardized to the overall ACT population distribution of age and dementia prevalence, ADNC+VBI was the most common mixed pathology in both NACC and ACT. Additionally, OHSU findings were more similar to ACT in contrasts to UW and other ADCs; likely because many OHSU participants were also part of cohort studies that have strict enrollment criteria and focus on healthy aging (Howieson et al., 2003).
Our study adds to the evidence of positive association between ADNC and LBD (Obi et al., 2008; Jellinger and Attems, 2008; Sonnen et al., 2010; Kotzbauer et al., 2012; Swirski et al., 2014). Limbic LBD, in NACC, and amygdala LBD only, in ACT, were more common in those with high ADNC compared to low or intermediate ADNC. In NACC, LBD in the amygdala may have been classified as limbic or as other/unknown, which may account for the observed associations with ADNC in those regions. Interestingly, cortical LBD was associated with intermediate but not high levels of ADNC, especially in NACC. This finding has not been previously reported, but it is consistent with prior evidence that cortical LBD is associated with higher amyloid burden, but not higher Braak stage (Obi et al., 2008), and that demented individuals with mixed neuropathologies typically have lower levels of ADNC compared to individuals with ADNC only (Nagy et al., 1997; Postupna et al., 2015). Discrepancies in prior findings regarding LBD and neurofibrillary tangles, in which some studies found positive associations (Iseki et al., 2003; Jellinger and Attems, 2008; Sonnen et al., 2010) while others did not (Chung et al., 2015; Kotzbauer et al., 2012; Obi et al., 2008; Schneider et al., 2012) may be accounted for by the non-monotonic association we observed between cortical LBD and level of ADNC. Interactions between amyloid and α-synuclein may lead to an alternative pathologic and clinical presentation than ADNC only, in which neurofibrillary tangles are more predominant (Jellinger and Attems, 2008; Swirski et al., 2014; Wirths et al., 2000).
Overall, there was not strong evidence for a positive association between VBI and ADNC, consistent with other studies (Sonnen et al., 2011), including a recent study using biomarker data (Vemuri et al., 2015). Presence of VBI was inversely associated with ADNC in NACC overall as well as in the PANDA ADCs. In ACT, although VBI was somewhat related to level of ADNC, such an association was not consistent when looking at individual VBI sub-types, unlike other studies in which cortical VBI were associated with ADNC (Jellinger, 2007; Okamoto et al., 2009). Since the PANDA ADCs share neuropathologic assessment with ACT, differences in VBI assessment are unlikely to explain these findings. This result may be because individuals with ADNC in NACC may be more likely to die prior to development of VBI. In NACC, prevalence of ADNC is lower in those who died after age 80 while prevalence of VBI is higher among those with older ages at death (Brenowitz et al., 2014).
Most participants with ADNC mixed with LBD or VBI had dementia prior to death (70–96%). Higher proportions of those with ADNC and co-occurring LBD were male and had an APOE ε4 allele compared to ADNC only, consistent with other studies (Chung et al., 2015; Tsuang et al., 2005). A higher proportion of participants with ADNC and co-occurring VBI were male and had a history of stroke compared to ADNC only, characteristics associated with VBI in general (Gorelick et al., 2011; Jellinger, 2013). As in prior studies (Lim et al., 1999), clinical diagnosis of AD dementia was fairly sensitive (generally 70–90% of those with ADNC), even in those with additional pathologies. However, sensitivity of clinical AD was slightly lower for those with mixed ADNC than participants with ADNC only. Additional research is needed to determine the clinical relevance of these findings.
Our findings should be considered in light of the limitations of this study. We focused on combinations of the most common pathologies to simplify our analyses. Future research that can further examine combinations of relatively less common pathologies, including HS and PART would be beneficial, especially amongst the oldest-old (Nelson et al., 2016). Newly identified pathologic features, such as TAR DNA-binding protein 43 (TDP-43) (Neumann et al., 2006), were not available for most participants. NACC and ACT studies include predominantly Caucasian and well-educated older adults, which may limit generalizability. Dementia status may have been misclassified in some prior to death; in particular, clinic-only assessment may have underestimated the prevalence of dementia in NACC compared to ACT, which conducts home-based assessments as well (Crane et al., 2016). Prevalence of stroke history in ACT may have been underestimated as participants did not undergo ACT visits after dementia diagnosis. There are likely other important differences between NACC and ACT participants that we were unable to quantify; for instance selection criteria for ADCs or information on depression and psychiatric disorders, which were not comparable between NACC and ACT. Autopsied participants are a select sample and may differ in characteristics (e.g. dementia status and demographics) from the overall NACC and ACT study populations (Haneuse et al., 2009); prevalence estimates standardized to the overall ACT population based on age and dementia status suggest that a higher prevalence of low neuropathology would be expected in the overall ACT sample. The smaller ACT sample size resulted in analyses that were relatively underpowered in comparison to NACC; because this could impact the significance tests conducted, we also described qualitative differences both between and within study populations.
Despite the limitations, this study has important strengths. This study used data from two large autopsy samples with extensive clinical and pathologic information, which allowed us to examine the co-occurrence of ADNC, LBD, and VBI from multiple perspectives. We conducted thorough qualitative and quantitative comparisons between autopsied clinical research volunteers included in NACC and autopsied participants in ACT, a population-based study. Weighting techniques allowed us to compare the prevalence of ADNC, LBD, and VBI standardized to the ACT population (of autopsied and non-autopsied participants) by age and dementia status. To our knowledge no prior studies have used standardization or weighting techniques to attempt to account for demographic differences between autopsy samples. This study also included sub-analyses among ACT, and the PANDA ADCs that shared identical neuropathologic assessment protocols. This approach suggests that differences between NACC and ACT were related to the populations studied rather than potential heterogeneity in neuropathological assessment protocols.
Assessing the similarities, we find further evidence that ADNC are common with LBD or VBI, especially in demented patients. Our findings point to an association between ADNC and LBD; whether these factors are synergistic or related to shared pathogenic processes remains to be determined. Over half of participants with ADNC in our study had co-occurring LBD or VBI and the majority of those with ADNC mixed with LBD or VBI were demented, regardless of study population. Thus, it is likely that patients with dementia – including those diagnosed with clinical AD – have multiple pathologies. Our findings suggest accurate clinical diagnosis of patients with multiple pathologies may be challenging. Effective prevention and treatment of clinical AD may need to target multiple disease processes.
Supplementary Material
Highlights.
We examined overlap of three common neuropathologies in two large autopsy studies
The majority with Alzheimer’s disease changes (ADNC) had co-occurring pathologies
Cortical Lewy body disease was associated with intermediate levels of ADNC
Overlap of pathologies was similar between studies after standardization
Acknowledgments
We are deeply grateful to all of the study participants, clinicians, and other workers at the ADCs, NACC, and ACT that made this research possible. We also thank NACC, ACT, UW, and OHSU staff for help obtaining data. Preliminary versions of this work were included in a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy from the Department of Epidemiology, University of Washington (WDB).
The ACT study is supported by NIH (U01-AG006781, MPI Eric Larson, MD MPH, and Paul Crane, MD MPH). The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIAfunded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI Marie-Francoise Chesselet, MD, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P50 AG005136 (PI Thomas Montine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), and P50 AG047270 (PI Stephen Strittmatter, MD, PhD). PANDA ADCs are supported by NIH P50 AG005136 (UW ADC) and NIH grants P30 AG008017, R01 AG024059, M01 RR000334, UL1 RR024140 as well as Intel Corporation, and Department of Veterans Affairs (OHSU ADC).
Abbreviations
- ACT
Adult Changes in Thought
- AD
Alzheimer’s disease
- ADC
Alzheimer’s Disease Center
- ADNC
Alzheimer’s disease neuropathologic change
- CAA
cerebral amyloid angiopathy
- CI
confidence interval
- LBD
Lewy body disease
- SD
standard deviation
- PANDA
Pacific Northwest Dementia and Aging Neuropathology Group
- PART
Primary age-related tauopathy
- NACC
National Alzheimer’s Coordinating Center
- OHSU
Oregon Health & Science University
- UW
University of Washington
- VBI
vascular brain injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
REFERENCES
- American Psychiatric Association. Diagnostic criteria from DSM-IV TM; 3rd print. Washington, D.C.: American Psychiatric Association; 1995. [Google Scholar]
- Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA. The National Alzheimer’s Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis. Assoc. Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- Beekly DL, Ramos EM, van Belle G, Deitrich W, Clark AD, Jacka ME, Kukull WA. The National Alzheimer’s Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis. Assoc. Disord. 2004;18:270–277. [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz WD, Monsell SE, Schmitt FA, Kukull WA, Nelson PT. Hippocampal sclerosis of aging is a key Alzheimer’s disease mimic: clinical-pathologic correlations and comparisons with both alzheimer’s disease and non-tauopathic frontotemporal lobar degeneration. J. Alzheimers Dis. 2014;39:691–702. doi: 10.3233/JAD-131880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung EJ, Babulal GM, Monsell SE, Cairns NJ, Roe CM, Morris JC. Clinical Features of Alzheimer Disease With and Without Lewy Bodies. JAMA Neurol. 2015;72:789–796. doi: 10.1001/jamaneurol.2015.0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane PK, Gibbons LE, McCurry SM, McCormick W, Bowen JD, Sonnen J, Keene CD, Grabowski T, Montine TJ, Larson EB. Importance of home study visit capacity in dementia studies. Alzheimers Dement. 2016;12:419–426. doi: 10.1016/j.jalz.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, Haneuse S, Craft S, Montine TJ, Kahn SE, McCormick W, McCurry SM, Bowen JD, Larson EB. Glucose levels and risk of dementia. N. Engl. J. Med. 2013;369:540–548. doi: 10.1056/NEJMoa1215740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Gearing M, Grinberg LT, Hof PR, Hyman BT, Jellinger K, Jicha GA, Kovacs GG, Knopman DS, Kofler J, Kukull WA, Mackenzie IR, Masliah E, McKee A, Montine TJ, Murray ME, Neltner JH, Santa-Maria I, Seeley WW, Serrano-Pozo A, Shelanski ML, Stein T, Takao M, Thal DR, Toledo JB, Troncoso JC, Vonsattel JP, White CL, Wisniewski T, Woltjer RL, Yamada M, Nelson PT. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128:755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular Contributions to Cognitive Impairment and Dementia A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RL. Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10:378–384. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneuse S, Schildcrout J, Crane P, Sonnen J, Breitner J, Larson E. Adjustment for selection bias in observational studies with application to the analysis of autopsy data. Neuroepidemiology. 2009;32:229–239. doi: 10.1159/000197389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howieson DB, Camicioli R, Quinn J, Silbert LC, Care B, Moore MM, Dame A, Sexton G, Kaye JA. Natural history of cognitive decline in the old old. Neurology. 2003;60:1489–1494. doi: 10.1212/01.wnl.0000063317.44167.5c. [DOI] [PubMed] [Google Scholar]
- Howieson DB, Holm LA, Kaye JA, Oken BS, Howieson J. Neurologic function in the optimally healthy oldest old Neuropsychological evaluation. Neurology. 1993;43:1882–1882. doi: 10.1212/wnl.43.10.1882. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseki E, Togo T, Suzuki K, Katsuse O, Marui W, de Silva R, Lees A, Yamamoto T, Kosaka K. Dementia with Lewy bodies from the perspective of tauopathy. Acta Neuropathol. 2003;105:265–270. doi: 10.1007/s00401-002-0644-3. [DOI] [PubMed] [Google Scholar]
- James BD, Bennett DA, Boyle PA, Leurgans S, Schneider JA. Dementia From Alzheimer Disease and Mixed Pathologies in the Oldest Old. Jama. 2012;307:1798–1800. doi: 10.1001/jama.2012.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front. Aging Neurosci. 2013;5:17. doi: 10.3389/fnagi.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA. The enigma of mixed dementia. Alzheimers Dement. 2007;3:40–53. doi: 10.1016/j.jalz.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 2010;119:421–433. doi: 10.1007/s00401-010-0654-5. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol. 2008;115:427–436. doi: 10.1007/s00401-008-0347-5. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Prevalence and pathogenic role of cerebrovascular lesions in Alzheimer disease. J. Neurol. Sci. 2005;229-230:37–41. doi: 10.1016/j.jns.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology. 2015;85:535–542. doi: 10.1212/WNL.0000000000001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J, Michael Y, Calvert J, Leahy M, Crawford D, Kramer P. Exceptional brain aging in a rural population-based cohort. J. Rural Health. 2009;25:320–325. doi: 10.1111/j.1748-0361.2009.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye JA, Maxwell SA, Mattek N, Hayes TL, Dodge H, Pavel M, Jimison HB, Wild K, Boise L, Zitzelberger TA. Intelligent Systems For Assessing Aging Changes: home-based, unobtrusive, and continuous assessment of aging. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2011;(66 Suppl 1) doi: 10.1093/geronb/gbq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzbauer PT, Cairns NJ, Campbell MC, Willis AW, Racette BA, Tabbal SD, Perlmutter JS. Pathologic accumulation of α-synuclein and Aβ in Parkinson disease patients with dementia. Arch. Neurol. 2012;69:1326–1331. doi: 10.1001/archneurol.2012.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, van Belle G, Jolley L, Larson EB. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch. Neurol. 2002;59:1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann. Intern. Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- Lim A, Tsuang D, Kukull W, Nochlin D, Leverenz J, McCormick W, Bowen J, Teri L, Thompson J, Peskind ER, Raskind M, Larson EB. Clinico-neuropathological correlation of Alzheimer’s disease in a community-based case series. J. Am. Geriatr. Soc. 1999;47:564–569. doi: 10.1111/j.1532-5415.1999.tb02571.x. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VMY, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Micallef L, Rodgers P. eulerAPE: Drawing Area-Proportional 3-Venn Diagrams Using Ellipses. PLoS ONE. 2014;9:e101717. doi: 10.1371/journal.pone.0101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Sonnen JA, Montine KS, Crane PK, Larson EB. Adult Changes in Thought Study: Dementia is an Individually Varying Convergent Syndrome with Prevalent Clinically Silent Diseases that may be Modified by Some Commonly Used Therapeutics. Curr. Alzheimer Res. 2012;9:718. doi: 10.2174/156720512801322555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, DeCarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): Clinical and Cognitive Variables and Descriptive Data From Alzheimer Disease Centers. Alzheimer Dis. Assoc. Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Esiri MM, Jobst KA, Morris JH, King EM, McDonald B, Joachim C, Litchfield S, Barnetson L, Smith AD. The effects of additional pathology on the cognitive deficit in Alzheimer disease. J. Neuropathol. Exp. Neurol. 1997;56:165–170. doi: 10.1097/00005072-199702000-00007. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Davis DG, Poduska JW, Patel E, Mendiondo MS, Markesbery WR. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20:66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Smith CD, Abner EL, Wilfred BJ, Wang W-X, Neltner JH, Baker M, Fardo DW, Kryscio RJ, Scheff SW, Jicha GA, Jellinger KA, Van Eldik LJ, Schmitt FA. Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol. 2013;126:161–177. doi: 10.1007/s00401-013-1154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Trojanowski JQ, Abner EL, Al-Janabi OM, Jicha GA, Schmitt FA, Smith CD, Fardo DW, Wang W-X, Kryscio RJ, Neltner JH, Kukull WA, Cykowski MD, Eldik LJV, Ighodaro ET. “New Old Pathologies”: AD, PART, and Cerebral Age-Related TDP-43 With Sclerosis (CARTS) J. Neuropathol. Exp. Neurol. 2016;75:482–498. doi: 10.1093/jnen/nlw033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM-Y. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Obi K, Akiyama H, Kondo H, Shimomura Y, Hasegawa M, Iwatsubo T, Mizuno Y, Mochizuki H. Relationship of phosphorylated a-synuclein and tau accumulation to Aβ deposition in the cerebral cortex of dementia with Lewy bodies. Exp. Neurol. 2008;210:409–420. doi: 10.1016/j.expneurol.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Ihara M, Fujita Y, Ito H, Takahashi R, Tomimoto H. Cortical microinfarcts in Alzheimer’s disease and subcortical vascular dementia. Neuroreport. 2009;20:990–996. doi: 10.1097/WNR.0b013e32832d2e6a. [DOI] [PubMed] [Google Scholar]
- Petersen J, Austin D, Mattek N, Kaye J. Time Out-of-Home and Cognitive, Physical, and Emotional Wellbeing of Older Adults: A Longitudinal Mixed Effects Model. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0139643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postupna N, Keene CD, Crane PK, Gonzalez-Cuyar LF, Sonnen JA, Hewitt J, Rice S, Howard K, Montine KS, Larson EB, Montine TJ. Cerebral cortical Aβ42 and PHF-τ in 325 consecutive brain autopsies stratified by diagnosis, location, and APOE. J. Neuropathol. Exp. Neurol. 2015;74:100–109. doi: 10.1097/NEN.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- Rahimi J, Kovacs GG. Prevalence of mixed pathologies in the aging brain. Alzheimers Res. Ther. 2014;6:82. doi: 10.1186/s13195-014-0082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riekse RG, Leverenz JB, McCormick W, Bowen JD, Teri L, Nochlin D, Simpson K, Eugenio C, Larson EB, Tsuang D. Effect of vascular lesions on cognition in Alzheimer’s disease: a community-based study. J. Am. Geriatr. Soc. 2004;52:1442–1448. doi: 10.1111/j.1532-5415.2004.52405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The Neuropathology of Older Persons with and Without Dementia from Community versus Clinic Cohorts. J. Alzheimers Dis. 2009;18:691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain J. Neurol. 2012;135:3005–3014. doi: 10.1093/brain/aws234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A, Qian J, Muzikansky A, Monsell SE, Montine TJ, Frosch MP, Betensky RA, Hyman BT. Thal Amyloid Stages Do Not Significantly Impact the Correlation Between Neuropathological Change and Cognition in the Alzheimer Disease Continuum. J. Neuropathol. Exp. Neurol. 2016;75:516–526. doi: 10.1093/jnen/nlw026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Craft S, Leverenz JB, Montine TJ. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann. Neurol. 2007;62:406–413. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- Sonnen JA, Postupna N, Larson EB, Crane PK, Rose SE, Montine KS, Leverenz JB, Montine TJ. Pathologic correlates of dementia in individuals with Lewy body disease. Brain Pathol. 2010;20:654–659. doi: 10.1111/j.1750-3639.2009.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnen JA, Santa Cruz K, Hemmy LS, Woltjer R, Leverenz JB, Montine KS, Jack CR, Kaye J, Lim K, Larson EB, White L, Montine TJ. Ecology of the aging human brain. Arch. Neurol. 2011;68:1049–1056. doi: 10.1001/archneurol.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini M, Schmidt M, Lee V, Trojanowski J, Jakes R, Goedert G. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Swirski M, Miners JS, de Silva R, Lashley T, Ling H, Holton J, Revesz T, Love S. Evaluating the relationship between amyloid-β and α-synuclein phosphorylated at Ser129 in dementia with Lewy bodies and Parkinson’s disease. Alzheimers Res. Ther. 2014;6 doi: 10.1186/s13195-014-0077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, Sugimoto K, Yamaguchi T, Sasaki H, Chiu D, White LR. The Cognitive Abilities Screening Instrument (CASI): A Practical Test for Cross-Cultural Epidemiological Studies of Dementia. Int. Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Tsuang DW, Wilson RK, Lopez OL, Luedecking-Zimmer EK, Leverenz JB, DeKosky ST, Kamboh MI, Hamilton R. Genetic association between the APOE*4 allele and Lewy bodies in Alzheimer disease. Neurology. 2005;64:509–513. doi: 10.1212/01.WNL.0000150892.81839.D1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Preboske GM, Kantarci K, Raman MR, Machulda MM, Mielke MM, Lowe VJ, Senjem ML, Gunter JL, Rocca WA, Roberts RO, Petersen RC, Jack CR. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain. 2015;138:761–771. doi: 10.1093/brain/awu393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, Masaki K, Launer L, Markesbery WR. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann. N. Y. Acad. Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- White LR, Edland SD, Hemmy LS, Montine KS, Zarow C, Sonnen JA, Uyehara-Lock JH, Gelber RP, Ross GW, Petrovitch H, Masaki KH, Lim KO, Launer LJ, Montine TJ. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging Studies. Neurology. 2016;86:1000–1008. doi: 10.1212/WNL.0000000000002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O, Weickert S, Majtenyi K, Havas L, Kahle PJ, Okochi M, Haass C, Multhaup G, Beyreuther K, Bayer TA. Lewy body variant of Alzheimer’s disease: alpha-synuclein in dystrophic neurites of A beta plaques. Neuroreport. 2000;11:3737–3741. doi: 10.1097/00001756-200011270-00029. [DOI] [PubMed] [Google Scholar]
- Zhou XH, Castelluccio P, Hui SL, Rodenberg CA. Comparing two prevalence rates in a two-phase design study. Stat. Med. 1999;18:1171–1182. doi: 10.1002/(sici)1097-0258(19990530)18:10<1171::aid-sim113>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.