Abstract
With the advent of targeted therapies, there has been a revolution in the treatment of cancer across multiple histologies. Immune checkpoint blockade has made it possible to take advantage of receptor-ligand interactions between immune and tumor cells in a wide spectrum of malignancies. Toxicity in healthy tissue, however, can limit our use of these agents. Immune checkpoint blockade has been approved in advanced melanoma, renal cell cancer, non-small cell lung cancer, relapsed refractory Hodgkin's lymphoma, and urothelial cancer. Though FDA-approved indications for use of some of these novel agents depend on current, protein-based PD1 and PD-L1 assays, detection methods come with several caveats. Additional predictive tools must be interrogated to discern responders from non-responders. Some of these include measurement of microsatellite instability, PD-L1 amplification, CD8 infiltrate density, and tumor mutational burden. This review serves to synthesize biomarker detection at the DNA, RNA, and protein level to more accurately forecast benefit from these novel agents.
Keywords: Checkpoint inhibitor, tumor microenvironment, neoantigen presentation, predictive biomarker, tumor mutational burden, immunooncology
Introduction
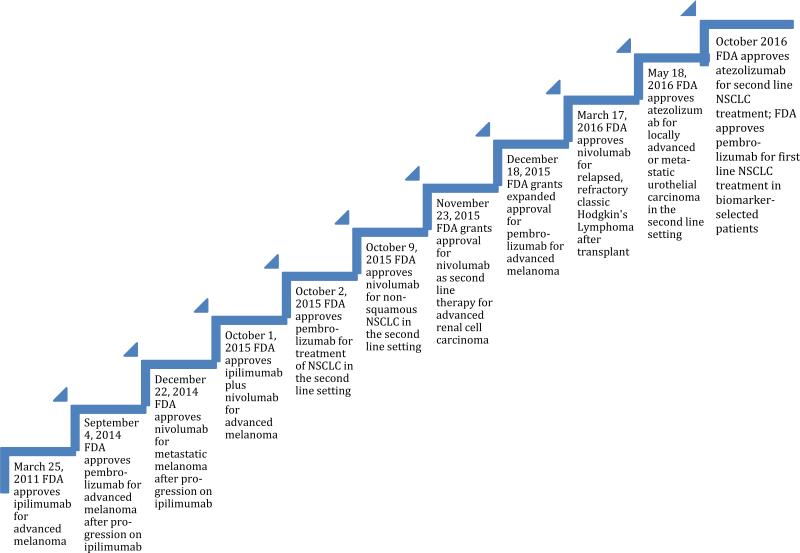
With the advent of targeted therapies, there has been a revolution in the treatment of cancers across histologies. In non-small cell lung cancer (NSCLC), for example, targetable driver mutations such as EGFR, ALK, or ROS1 have been discovered. [1] Nonetheless, there remains a significant need for additional therapies due to the development of acquired resistance, as well as the fact that many patients have multiple genomic alterations varying from individual to individual. [2] In the not so distant past, as researchers began to understand tumor biology, the importance of the immune microenvironment came to the forefront. Attempts at utilizing early immunotherapies, such as interleukin-2 (IL-2) for metastatic renal cell carcinoma and melanoma, however, were characterized by low rates of response. Nonetheless, durable remissions were seen in ~8-12% of patients. [3] Only years later, immune checkpoint blockade targeting T cell inhibitory signals entered the oncology vernacular. [4-6] The role of the cancer mutanome and immunogenic neoantigen generation became increasingly appreciated. Tumor histologies associated with a high mutational burden and neoantigen generation were found to be susceptible to the host's adaptive immunity. [7,8] With the ability to partially reverse cancer immunosuppression, checkpoint inhibitors, in particular anti-CTLA-4 and anti-PD-1/PD-L1, have now been approved across histologies. (Fig. 1)
Fig. 1.
Timeline of FDA approved indications for checkpoint inhibitors. Currently there are four FDA-approved checkpoint inhibitors: ipilimumab, nivolumab, pembrolizumab and most recently atezolizumab. They have been approved for a variety of indications over the last 5 years
[generated with Word - can be black and white]
Immune checkpoint blockade takes advantage of receptor-ligand interactions between T cells and tumor cells. This binding can occur early in the T cell activation cascade, e.g. in the tumor draining lymph nodes, or in the tumor microenvironment itself. One such interaction between CD28 and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) is up regulated after T cell activation so as to attenuate cytotoxic response. This is normally protective in that it prevents over-activation of the immune system to antigen stimulation. Additionally, in some cases, activated T cells up-regulate ligands that bind to receptors on antigen presenting cells (APCs) and can also send an inhibitory signal. One such interaction is between programmed death ligand-1 (PD-L1) on tumor cells and APCs that binds to programmed death-1 (PD-1) receptors on T cells. Blocking these inhibitory signals can unleash robust immune responses against tumor cells. [9]
Unfortunately, our ability to accurately predict response to these agents has been suboptimal. Moreover, toxicity related to action in healthy tissue has limited our use of these agents. For this reason, PD-L1 immunohistochemistry (IHC) was developed to help discern responders from non-responders across histologies. Despite IHC assays, however, 0% to 17% of patients that are considered to be PD-L1-“negative” will still respond, while 36% to 100% of PD-L1 “positive” patients will still respond to treatment. This dichotomization is important and suggests that additional factors are involved in predicting response. [5] A central dogma of cancer immunotherapy should involve detailing tumor and immune characteristics at multiple levels (e.g. the DNA, RNA, and protein levels) in order to predict response to immunotherapy.
Checkpoint Inhibitors: An Overview
CTLA-4 and PD-1, when expressed on activated T-cells, inhibit immune-mediated attack on cells. Moreover, with higher expression of inhibitory signals, there is recruitment of immunosuppressive cells, a process called “immunoediting”. [10] CTLA-4, PD-1, and PD-L1 are among a group of molecules known as inhibitory T cell checkpoints. [9]
Broadly speaking, CTLA-4 is a molecule expressed on T cells in central lymphoid organs, where it regulates T cell activation at its early stages. When the T cell receptor binds to a recognized antigen, the co-stimulatory molecule CD28 is activated and this augments T cell activity. CD28 and CTLA-4 both compete for binding of B7·1 and B7·2: CTLA-4 binding attenuates T cell activation and CD28 binding augments it. CTLA-4 is upregulated on activated T-cells as a homeostatic, auto-regulatory mechanism to outcompete CD28 with its enhanced binding affinity for B7·1 and B7·2. [9]
In contrast to CTLA-4, PD-1 is expressed on NK-, B-, and T-cells in the periphery, specifically in a tumor microenvironment. Similar to CTLA-4, the role of PD-1/PD-L1 binding is to attenuate the immune response. PD-1 is a receptor similarly upregulated on T cells during peripheral activation. Additionally, PD-1 is found on T regulatory cells and, when blocked, can reduce the suppressive activity of intratumoral T regulatory cells. [11]
PD-L1 IHC
Currently, predictive biomarker testing in the patient care setting exists in the form of PD-1/PD-L1 IHC detection on tumor specimens. However, there remains a need to harmonize IHC assays to determine clinically relevant cutoffs for the use of anti-PD-1/PD-L1 immunotherapies.
The initial phase I nivolumab_trial revealed that patients who were PD-L1 positive (defined as greater than 5% of tumor cells express PD-L1 by IHC) had an ORR of 36%. This was compared to an ORR of 18%, 28%, and 27% in all-comers for NSCLC, melanoma, and renal cell carcinoma, respectively. [12] On the other hand, in the phase II (CheckMate 016) and phase III (CheckMate 017) studies comparing nivolumab to docetaxel in advanced squamous cell NSCLC, expression of PD-L1 on tumor cells was neither prognostic nor predictive. [13] The CheckMate 016 trial demonstrated a 3-month median OS advantage in the nivolumab arm and a duration of response that was prolonged (42% of patients alive at 1 year compared to 24% in the docetaxel arm). In contrast, in the phase III study comparing nivolumab with docetaxel in advanced non-squamous NSCLC (CheckMate 057), a similar ORR (17·6%) and OS (16% at 3 years) was observed, however, in the subgroup analysis patients with higher PD-L1 expression (>5% by IHC) had improved ORR and OS. [14] Although these studies utilized the same diagnostic antibody (28-8) and therapeutic antibody (nivolumab), there is discordance between response rates.
Also in the NSCLC setting, the KEYNOTE-010 trial compared pembrolizumab versus docetaxel for previously treated PD-L1 positive patients. The cutoff for inclusion was a PD-L1 expression with the 22C3 antibody of at least 1%. The trial then compared those “strongly positive” with PD-L1 expression by IHC of 50% or greater to all-comers. It was found that in those with at least 50% of tumor cells expressing PD-L1, progression free survival (PFS) was longer than in those who received docetaxel. Median OS followed suit (17·3 months versus 8·2 months) in those receiving pembrolizumab at 10mg/kg. [15] The disparate results in relation to the predictive capacity of PD-L1 IHC between the squamous and nonsquamous NSCLC cohorts further highlights the need for additional predictive biomarkers.
Atezolizumab was similarly studied in the advanced metastatic urothelial cancer setting. When assessing tumor specimens, PD-L1 expression on stromal immune cells (IC) was specifically distinguished from PD-L1 expression on tumor cells (TC) utilizing the SP142 antibody. Groups with the highest IC PD-L1 expression had better ORR. [16] Similarly, in the phase II multicenter POPLAR study comparing single agent atezolizumab versus docetaxel for pre-treated NSCLC, PD-L1 expression was measured on both immune cells as well as on tumor cells. OS was 12·6 months for atezolizumab versus 9·7 months for docetaxel (p=0·04) and increasing improvement in OS was directly related to increasing PD-L1 expression. [17] In conjunction with another randomized-controlled study, POPLAR lead to the approval of atezolizumab for NSCLC in the second line setting. This will be discussed later.
Another anti-PD-L1 agent being interrogated in the urothelial cancer setting is durvalumab. PD-L1 IHC utilizing a different antibody, SP263, found that both tumor and immune cells expressing greater than 25% PD-L1 was predictive of response. Further research into the disparate results in terms of tumor cell expression of PD-L1 by SP142 with atezolizumab versus SP263 with durvalumab is warranted. [18]
From the data presented, there is clearly discrepancy in how we measure PD-L1 expression. Issues include which cutoff to use for “positive” and “negative” and which detection antibody to use for IHC. Furthermore, given the uneven expression of PD-L1 in tumor cell populations, small biopsies may not give an accurate representation of true PD-L1 density. Finally, the question arises whether we should be using information from both tumor cell and infiltrating immune cell staining or focusing on only one technique to harmonize our recommendations for therapy. [5]
Beyond PD-L1 IHC
Generally speaking, the data suggest that PD-L1 IHC can be an effective positive predictive marker for response to anti-PD-1/PD-L1 therapy. However, as mentioned above, patients with PD-L1 negative tumors can still respond to immune checkpoint blockade, and a substantial fraction of PD-L1 positive patients do not respond. For this reason, there is a need to delve deeper into the drivers of PD targeted responses. As the central dogma of molecular biology dictates: “DNA codes for RNA, RNA codes for protein”, so too does this apply to cancer immunotherapy. There are drivers (and passengers) at the DNA level and RNA level that, if detected, can allow us to personalize our immunotherapeutic armament to the correct patient population.
DNA Level Changes
Mismatch Repair Mechanisms
Deficiencies in DNA mismatch repair (MMR) mechanisms result in microsatellite instability (MSI). The accumulation of cancer genomic errors results in microsatellite fragments that can be detected by current assays. MSI can result in the development of various cancers, such as colorectal cancer and endometrial cancer. [19] MSI-high (MSI-H) status has prognostic significance in colorectal cancer with relation to adjuvant chemotherapy in addition to predictive significance for anti-PD-1 directed therapy. MMR status can be determined via IHC or the more specific polymerase chain reaction (PCR) assay at specific microsatellite foci. [20]
Tumor Mutational Burden
Patients with higher tumor mutational burden (TMB) and neoantigen load have improved response rates to immune checkpoint blockade. [21] This is related to the concept that with increased TMB, the probability of a cognate T-cell clonally expanding against a specific tumor antigen will increase. [22]
To illustrate this, we can build on our knowledge of microsatellite unstable tumors discussed above. In one study, microsatellite marker MSH2, MSH6, MLH1 or PMS2 loss resulted in increased sensitivity to immune checkpoint blockade. Pembrolizumab was studied in three cohorts (total N=48): MMR-deficient colorectal cancers (which harbored a mean of 1782 somatic mutations in their tumors), MMR-stable colorectal cancers (which harbored a mean of 73 somatic mutations in their tumor), and MMR-deficient non-colorectal cancer tumors. In the MMR-deficient (MSI unstable or MSI-H) cohorts, median ORR was about 60% regardless of histologic type compared to 0% in the MMR stable colorectal cancer patients. Predictably, the MMR stable cohorts did not have increased PD-L1 expression. [8] MSI-high tumors always have high TMB, but MSI-high status alone is neither necessary nor sufficient to account for total mutational burden. [23]
At least one additional factor contributing to hypermutation is dysfunction of DNA polymerase. POLε (DNA polymerase epsilon) and POLδ (DNA polymerase delta) are required for reliable DNA replication. In POLε germline variant carriers there is a higher association with microsatellite instability. De novo POLε/POLδ mutations can also occur and predispose to high TMB and therefore the development of colorectal cancer. [24] It was found that, mutations in the POLε proofreading domain of DNA polymerase in endometrial tumors leads to high TMB, neoepitope generation, and PD-1 expression in tumor infiltrating cells. This in turn can be predictive of improved antitumor activity of checkpoint inhibitors due to increased intra-tumoral T cell activation. [25] For this reason, POLε/POLδ IHC or PCR can potentially be used to screen for germline or somatic mutations and may predict response to immune checkpoint blockade.
In addition to intrinsic hypermutation, there are several additional factors contributing to a patient's mutational landscape, including the molecular ‘smoking signature’. The molecular smoking signature is the conglomerate of deregulated genes in cigarette smokers. It has been shown that there are many differentially expressed gene transcripts in smokers versus nonsmokers leading to a differential mutational landscape between the two groups. [26] It has also been shown in NSCLC that this mutational landscape determines sensitivity to PD-1 blockade. In one study, whole-exome sequencing of NSCLC was performed in patients treated with pembrolizumab. In all-comers, those tumors with high total exomic mutational burden, especially nonsynonymous mutations, and those who expressed a molecular smoking signature demonstrated increased PFS. [27]
It appears that response to checkpoint blockade is not only predicted broadly by mutational burden and neoantigen expression, but more so by clonal neoantigens. Clonal neoantigens refer to those antigens that are conserved with high fidelity throughout the tumor cell. Swanton et al. demonstrated that, in 139 primary lung adenocarcinoma specimens, a higher percentage of tumor infiltrating cells directed at clonal neoantigens expressed higher PD-1. Therefore, these particular specimens were more sensitive to checkpoint blockade. [28] This demonstrates that mutational burden and neoantigen load at the DNA level, as well as the manner of TIL activation and expansion, are all relevant in predicting anti-tumor response.
PD-L1 Gene Amplification
Because of caveats with PD1/PD-L1 surface protein detection mentioned earlier, PD-L1 amplification at the genetic level might be a more useful predictive tool. In a study evaluating PD-L1 and PD-L2 gene copy number gains among resected NSCLC specimens, PD-L1 genomic augmentation more consistently and reliably correlated with true PD-L1 protein expression. This was demonstrated by an increased concordance of PD-L1 copy number gains versus PD-L1 protein expression in the primary tumor and associate lymph node metastases (86·8% concordance versus 64·4% concordance, respectively). Moreover, the study demonstrated a significant difference in overall survival between PD-L1 positive and negative patients (7·2 years versus 14·0 years, respectively). [29] For this reason, PD-L1 amplification assays may be useful predictive biomarkers that may one day replace, or be performed in addition to, PD-L1 IHC.
T-Cell Receptor Clonality
The diverse T cell repertoire of the human immune system is eventually tailored as antigenic peptides on MHC interact with the T cell receptor (TCR). Much of this clonal tailoring occurs at the complementarity-determining region 3 (CDR3) variable domain on the T cell receptor. Using nucleotide level sequencing of the CDR3 domain, a signature corresponding to TCR clonality may be detected. [30] A study of 46 patients with metastatic melanoma treated with pembrolizumab were evaluated with baseline and on-treatment quantitative IHC, immunofluorescence, and next generation sequencing of their T-cell antigen receptors. Those patients who met criteria for radiographic response were shown to have a 10-fold higher expansion of T-cell clonal populations compared to those who progressed. [31] T-cell receptor clonality testing intratumorally, particularly of CDR3 variable domains, can be performed with PCR protocols and may represent a potential on-treatment biomarker to predict response earlier in the therapeutic course. [32]
RNA Level Changes
Another method of identifying inter-patient heterogeneity as it relates to treatment response is by assessing RNA level changes. Transcriptional signatures could be the clue to interrogating T-cell rich tumor microenvironments.
One important discovery highlighting the power of identifying transcriptional and post-transcriptional elements as predictive biomarkers is the identification of microRNA expression. MicroRNAs (miRNAs) are small non-coding post-transcriptional regulators. One of the pathways involving these regulators is known as the signal transducers and activators of transcription (STAT) pathway. STAT1 is a transcriptional regulator that recruits effector cells during the immune response and STAT3 down-regulates this response to interferons and hence can facilitate tumor progression. The ratio of these post-transcriptional modulators was found to be key in predicting therapeutic effects of IFNa2b in melanoma. In one study, for example, those with a higher STAT1:STAT3 ratio prior to treatment had prolonged OS (P=0·032). [33]
Many circulating serum miRNAs have been proposed as predictive biomarkers for various malignancies. [34] One of these is miRNA-21. This miRNA not only upregulates STAT3, but is also a circulating molecule that can be detected noninvasively. This can make it a useful surrogate for STAT3 activation and, therefore, useful as a predictive biomarker for immunotherapy. [35]
Along with detection of surrogate regulators of transcription, direct detection of interferon and immune transcriptional signatures has shown promise in predicting response to checkpoint blockade. In a 2015 study utilizing discovery sets from the phase 1b KEYNOTE-001 study in which pembrolizumab was applied to several advanced solid malignancies, RNA was extracted and various gene signatures were analyzed. A 10-gene interferon gamma signature and a 28-gene “expanded immune” signature were shown to correlate significantly with ORR and PFS. Independent testing in head and neck, gastric, and melanoma tumors yielded similar results. [36]
Expanding on this, detection of CD8+ T cell infiltrates in the tumor microenvironment has been shown to have prognostic significance. Using RNA sequencing data from The Cancer Genome Atlas (TCGA), 3,485 tumor specimens over 11 different tumor types were analyzed for lymphocyte and macrophage gene expression. High expression of B-and T-cell signatures predicted improved overall survival. The CD8+ T cell hazard ratio reduction was particularly striking (for example, HR for breast cancer = 0·36, p = 0·01). [37] Corroborating this, in the realm of head and neck cancer, it has been shown that HPV-positive tumors have an increased number of CD8+ T-cell infiltrates as compared to HPV-negative tumors. In high CD8+, HPV+ tumors, there was an increase in pro-inflammatory chemokines and also a higher expression of PD1 mRNA compared to HPV-negative tumors. The presence of high intratumoral CD8+ T lymphocytes is purported to play an important role in immunotherapy response. [38]
Adding to this interesting biomarker detection approach, recent data suggest that tumor immune microenvironment characterization with novel anti-PD-L1 agents is important. In the phase II POPLAR study comparing atezolizumab versus docetaxel in previously treated NSCLC, exploratory analysis of pretreatment gene signatures was performed. Genes assessed had all been previously associated with activated T cells. A high biomarker group was defined as one with greater than or equal to the median level of gene expression in tumor samples. Those with high biomarker T effector-interferon-gamma gene signatures had improved OS with atezolizumab (HR 0·43 versus HR 1·1 in the low biomarker group). Additionally, these T-effector associated interferon-gamma gene signatures were directly associated with PD-L1 expression on tumor infiltrating cells. This suggests that “pre-existing immunity” within tumor cells was beneficial. [17]
Currently, Wallden et al. have developed additional predictive testing for anti-PD1 therapy. The anti-PD1 gene expression (“Gx”) probe developed by NanoString gives a predictor score (PS) based on composite RNA signatures from formalin fixed, paraffin embedded (FFPE) tissue. [39] These signatures are overexpressed in certain tumor tissues (e.g. melanoma) and could predict efficacy of immunotherapeutic agents such as pembrolizumab. Aside from melanoma, studies have also been done on specimens from anal, biliary tract, colorectal, esophageal, and ovarian cancer. Early phase validation studies are still needed. [40]
Additionally, in development are methods to perform large-scale analysis of RNA on complex tissues. IHC and flow cytometry can only assess for a limited number of phenotypic markers and often preparation of tissue results in non-evaluable cells. [41] As illustrated by Newman et al, one method to detect RNA transcripts in bulk is known as CIBERSORT (cell-type identification by estimating relative subsets of RNA transcripts). Developed at Stanford, this computational method can identify several gene expression signatures and extrapolate complex admixtures of cells within bulk tumor down to components of 0·5%. [42] CIBERSORT has been applied in triple negative and BRCA 1 and 2 mutated breast cancer tumor lines. Pre-treatment biopsies from the PrECOG 0105 trial (evaluating neoadjuvant carboplatin, gemcitabine, and iniparib in the stage I-IIIA setting) were evaluated by CIBERSORT flow cytometry. Tumors were given an “immune score” as defined by intratumoral TIL assessment. There was a correlation between higher immune scores by CIBERSORT and pathologic complete response in all tumor types (p<0·05). [43] This type of technology could be applied to evaluate bulk stromal and tumor cells in order to predict response to novel immunotherapies.
Protein Level Changes
As described earlier, the most widely utilized assays for predicting response to novel immunotherapeutics is measurement of protein expression by IHC. The FDA approval for pembrolizumab in NSCLC is for patients with PD-L1-positive tumors by IHC, and multiple upcoming approvals of novel anti-PD-1 and anti-PD-L1 agents will likely require biomarker expression. [5]
To review, in early studies, PD-L1 expression in at least 50% of NSCLC tumor cells correlated with improved efficacy of pembrolizumab. ORR in the total evaluable population with at least 50% PD-L1 expression was 45·2% compared to 16·5% and 10·7% in those with 1-49% and <1% expression, respectively. [17,18] Additionally, in the CheckMate 057 trial mentioned earlier, superior OS was quickly demonstrated with nivolumab over second line docetaxel and PD-L1 expression status positively correlated with OS. [14]
Due to conflicting data, FDA labels for pembrolizumab and nivolumab differ in terms of recommendations for PD-L1 testing in NSCLC. For Pembrolizumab, the recommendation is IHC testing with any PD-L1 IHC antibody. Studies support a PD-L1 “positive” cutoff of 50%, however, there is no specific cutoff for determining pembrolizumab use in NSCLC based on its FDA label. Nivolumab in the squamous cell NSCLC setting, on the other hand, does not have an FDA label for approved molecular testing. [44] For nivolumab in the non-squamous NSCLC setting, PD-L1 IHC with 28-8 antibody testing is optional (given subgroup analysis demonstrated that patients with PD-L1 overexpression derived preferential benefit). [45]
To illustrate variable PD-1/PD-L1 expression, a study of 321 patients with NSCLC was performed. PD-1 expression on TILs was found in 22% of samples and PD-L1 expression on tumors was found in 24% of samples. Improved OS was demonstrated in only certain subsets. These included: those with squamous cell histology, those who received adjuvant therapy, those who had large tumor size (T2-T4), or those who had positive nodes (N1-N3). [46]
A correlative study done in urothelial cancer also evaluated PD-L1 expression on TILs. The study found that PD-L1 expression was detected in approximately 20% of cases. Additionally, positive PD-L1 expression on TILs was associated with significantly longer survival in patients who developed metastatic disease (23 versus 12 months, P=0·02, N=89). [47] It is important to also note that PD-L1 expression is differentially predictive depending on tumor type and location (tumor PD-L1 expression versus immune cell PD-L1 expression). [48] In the aforementioned study in urothelial cancer, PD-L1 expression was only predictive for response to atezolizumab when assessed on TILs. This is in contrast to the majority of PD-L1 IHC testing which is detected on the tumor membrane itself. This adds to the earlier mentioned variables for PD-L1 IHC as a predictive biomarker, including differing cutoffs, heterogeneous methods of tissue preparation and processing, variability depending on biopsy site, and now expression on TILs versus tumor cells. [5]
It has been proposed that perhaps understanding the external and internal regulators of PD-L1 expression could assist in enhancing the sensitivity and specificity of PD1/PD-L1 detection methods. In more recent studies, for example, EGFR-mutant NSCLC tumors were found to display disproportionately elevated PD-L1 levels and EGFR-mutant mice showed improved response to anti-PD1 therapy (over EGFR-wild type counterparts). Despite this data in mice, a clinical trial combining nivolumab with erlotinib in EGFR-mutant patients demonstrated modest overall response in the advanced NSCLC setting (ORR 19% and PFS at 24 weeks was 51%). [49] Detection of a molecular smoking signature and JAK3 activation were also predictive in the same manner. [50] In one review outlining the complexity of epithelial PD-L1 expression in head and neck cancer, several intrinsic and extrinsic regulatory signaling mechanisms were described. A strong argument was made for improved understanding in this realm so as to optimize treatment approaches. [51]
PD-L2 is now also being investigated as an important ligand in T cell immune response. In one study retrospectively evaluating outcomes with atezolizumab (anti-PD-L1) in 4 cancer types (melanoma, NSCLC, urothelial cancer, and RCC), expression of PD-L2 correlated with response. Spearman correlations were stronger in melanoma (0·80), urothelial cancer (0·77), and NSCLC (0·74) than in RCC (0·43). Higher expression of PD-L2 was associated with improved OS with atezolizumab. When comparing biomarker high PD-L2 expression with biomarker low PD-L2 expression, the OS HR was 0·28 in the melanoma cohort. [52] Further studies regarding the role of PD-L2 are needed.
Aside from PD-1/PD-L1/PD-L2 protein expression, there are other important assays that could be useful in predicting response to checkpoint blockade. For example, antecedent viral infection of tumor cells could be predictive of immune checkpoint response. It was suggested this might be mediated through a broadening of neoantigen directed T-cell responses. [53] This was corroborated in a recent open-labeled phase III trial (CheckMate 141). The study randomized head and neck cancer patients who had progressed on platinum based chemotherapy to nivolumab versus investigator choice (IC). PD-L1 and HPV (p16 positive) status was assessed and correlated with response. Median OS in HPV positive patients was 9·1 months in the nivolumab arm versus 4·4 months with IC. In HPV negative patients median OS was 7·5 months in the nivolumab arm versus 5·8 months with IC (NCT02105636). [54]
Finally, at the cellular level, detection of other immune cell components may also be predictive of response to checkpoint blockade. In a study in the diffuse large B-cell population, it was shown that increased effector T-cell, NK-cell, and tumor macrophage infiltration correlated with a PD1 rich microenvironment and strong B-cell immune evasion mechanisms. [55] Detection of the cellular milieu within the tumor microenvironment may have important clinical significance apart from PD-1/PD-L1 expression.
The Current Clinical Status of Checkpoint Inhibitors
Approved for use in metastatic melanoma, ipilimumab was the first FDA-approved checkpoint inhibitor. [56,57] Anti-CTLA-4 blockade has been evaluated in phase II/III clinical trials in NSCLC (in combination with chemotherapy), metastatic urothelial cancer, head and neck squamous cell carcinoma, and hormone-refractory prostate cancer. [58]
Tremelimumab, another anti-CTLA-4 antibody, has also been studied in advanced melanoma and a variety of other solid tumor types. A phase III study comparing overall survival (OS) with standard single agent chemotherapy in metastatic melanoma patients failed to demonstrate improvement in median OS as a first line treatment. [59]
The FDA approved nivolumab, an anti-PD-1 monoclonal antibody, in December 2014. With blockade of PD-1, inhibition of host immune T cell activation is abrogated. [60] Anti-PD-1 therapies in melanoma, NSCLC, renal cell carcinoma, head and neck squamous cell carcinoma, bladder cancer, and Hodgkin's lymphoma have yielded impressive results.
Pembrolizumab, another PD-1 inhibitor, was approved in September 2014 for metastatic melanoma, and in October 2015 for second line treatment of non-small cell lung cancer with tumors expressing PD-L1. [61,62] The KEYNOTE-001 trial demonstrated that in the advanced NSCLC setting, the overall response rate (ORR) to pembrolizumab in a biomarker-unselected population was 19·4%. ORRs in patients with PD-L1 tumor proportion scores >50% (at least 50% of cells stained positive for PD-L1) were an unprecedented 45·2%. [62] On October 24, 2016 the US FDA approved pembrolizumab for use as first-line treatment of NSCLC. This is the first FDA approval of checkpoint inhibitor therapy in the first-line NSCLC setting. The indication is for use in those patients without EGFR and ALK aberrations and at least 50% PD-L1 expression by an FDA approved assay. [63] The approval was based on an international, open labeled, randomized phase III trial (KEYNOTE-024) comparing pembrolizumab with investigator's choice (IC) of first line chemotherapy for NSCLC. In the pembrolizumab group, ORR was 44.8% compared to 27.8% in the IC group. Median PFS was 10.3 months compared to 6.0 months in the pembrolizumab group versus IC group, respectively. Median OS had not been reached, however at 6 months, 80.2% compared to 72.4% of patients were alive in the pembrolizumab group versus IC group, respectively. [64]
On October 18, 2016, the US FDA approved atezolizumab, another PD-L1 inhibitor for use in the second line treatment of NSLCL. The indication for use is progression on platinum-containing chemotherapy. [65] The approval is based on two, international randomized clinical trials (OAK and POPLAR). OAK demonstrated a 13.8 month median OS with atezolizumab versus 9.6 month median OS in the comparative docetaxel arm (HR 0.74). [66] Similarly, in the POPLAR trial, there was a median OS benefit of 2.9 months with atezolizumab compared to docetaxel. [17] Prior to this, in May 2016, atezolizumab was approved for the treatment of metastatic urothelial cancer. A multicenter phase II trial of atezolizumab in advanced and metastatic urothelial cancer patients who progressed with platinum based chemotherapy demonstrated a 26% ORR in patients with positive PD-L1 expression on immune cells within the tumor microenvironment. Ongoing responses were demonstrated in 84% of patients at median follow up of 11·7 months. Median OS had not yet been reached. [16] In an early phase trial of both squamous and non-squamous cell lung cancer, overall response rates were 23%. [17] It is currently in clinical trials across multiple other cancer types including melanoma, breast cancer and renal cell carcinoma. [16,17]
Another anti-PD-L1 monoclonal antibody, avelumab, has also shown clinical benefit in a subset of patients. This agent has the unique ability to mediate tumor cell death by checkpoint inhibition, but also direct antibody-dependent cell-mediated cytotoxicity (ADCC) and NK cell activation. [67] From recent data presented at the American Society of Clinical Oncology conference in 2016, avelumab was demonstrated to have significant anti-tumor activity in metastatic Merkel cell carcinoma. The open-label phase II study showed a 31·8% ORR (with 9·1% complete response rate). Median OS was 11·3 months. (NCT02155647)
In terms of toxicity, both anti-CTLA-4 and anti-PD1/PD-L1 agents have been associated with severe immune related adverse events (irAEs). In a retrospective review of 14 phase I to III ipilimumab trials involving nearly 1500 patients, 64·2% of patients experienced some degree of irAE. The most frequent toxicities were gastrointestinal, dermatologic, and endocrine. The rate of grade 3-4 toxicity was between 20% and 30%. [68] Anti-PD-1 agents are generally better tolerated with a 6% incidence of severe irAEs that include colitis, dermatitis, transaminitis, thyroid dysregulation, and a 1-2% pneumonitis rate. [12]
Numerous single agent and combination clinical trials with immunotherapy are currently ongoing in a variety of tumor types including hepatocellular cancer (HCC), lung cancer, melanoma, lymphoma, sarcoma, small cell lung cancer, multiple myeloma, myelodysplastic syndrome, colorectal cancer, bladder cancer, renal cell cancer, acute and chronic myelogenous leukemia, gastric cancer, pancreatic cancer, breast cancer, and head and neck cancer. [6,12,69]
Conclusion
Because checkpoint inhibitor therapies are being validated in an increasingly histo-agnostic fashion, there is a need for more discerning biomarkers to predict benefit. As we have seen, PD-L1 IHC expression is an important predictive biomarker, and currently the only biomarker approved for clinical use. Overall, across studies, 36% to 100% of patients that are PD-L1 IHC positive on tumor cells will respond to therapies targeting the PD1/PD-L1 axis. However, between 0% to 17% of PD-L1 negative patients also respond. [5] For this reason, additional biomarkers are required to fully interrogate the tumor immune microenvironment.
As assessment of HER2 status in breast and gastric cancer is standard of care, it is likely PD-L1 IHC will become a reflex biomarker given the clinical science. Analogously, HER2 detection by IHC is suboptimal in the intermediate ranges, and reflex testing utilizing FISH is required in some situations. Similarly, reflex testing at the DNA and RNA level may be required after initial PD-L1 protein-level testing. (Table 1)
Table 1.
DNA, RNA, and protein level detection methods for predicting response to anti-PD1/PD-L1 therapy. Assays for response to checkpoint inhibitors include MSI detection, tumor mutational burden analysis, PD-1 copy amplification and IHC
| Level of Detection | |||
|---|---|---|---|
| DNA | RNA | Protein | |
| Predictive Method | Detection of Microsatellite Instability (MSI)19,20 | Detection of immune infiltrates37,38 | Direct PD-L1 immunohistochemical (IHC) assays5,13-18,45-48,62 |
| Example | PCR detection of altered microsatellite foci in colorectal cancer19 | mRNA sequencing of CD8+ T cells in the tumor microenvironment37,38 | PD-L1 expression in NSLCL tumor samples13-15,69 |
| Predictive Method | Detection of tumor mutational burden and neoantigen load7,22-27,29,41 | Identification of interferon gamma and expanded immune signatures36 | |
| Example | Somatic and whole exome genetic sequencing to detect bulk alterations in melanoma and other histotypes27,41 | 10-gene and 28-gene RNA signatures in various solid malignancies36 | |
| Predictive Method | PD-L1 amplification29 | ||
| Example | Copy expansion assays to increase sensitivity of PD-L1 positivity across histotypes29 | ||
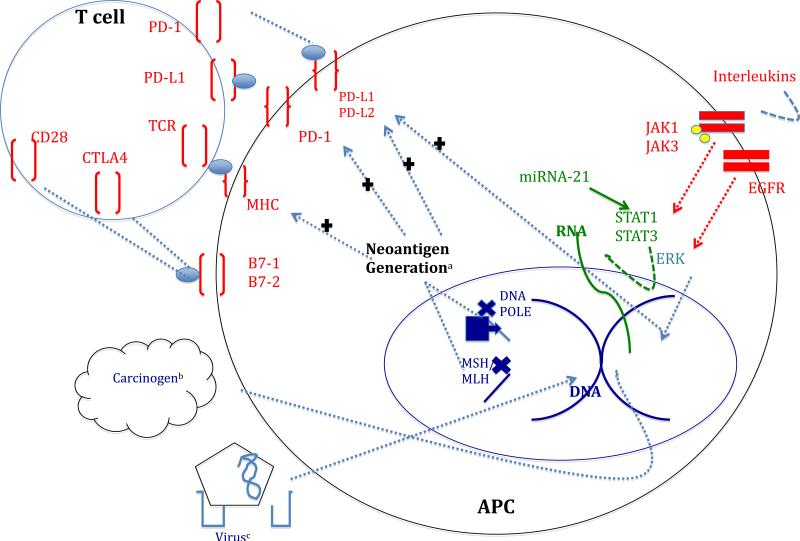
Changes at the DNA level resulting in microsatellite instability and mismatch repair defects can result in the development of higher mutational burden and a welcoming DNA fingerprint for use of novel immunotherapies. From the standpoint of anti-tumor effector cells, T-cells with evaluable TCR repertoires in an environment regulated by detectable miRNAs or type 1 interferon-gamma signatures might be used to categorize favorable transcriptional signatures. Finally, improved detection of PD1/PD-L1 proteins and precursors, as well as detection of infiltrating cell types will be crucial in predicting terminal effector interactions. (Fig. 2)
Fig. 2.
Intrinsic and extrinsic interactions influencing neoantigen generation and PD-1/PD-L1 expression. Red: protein level interactions. Purple: DNA level interactions. Green: RNA level interactions. a: neoantigen generation is influenced by DNA and RNA level changes and in turn influences the expression of PD surface proteins as well as antigen presentation to T cells on major histocompatibility complex (MHC). b: environmental influences alter DNA c: virally mediated DNA changes lead to expression of neoantigens (e.g. HPV exposure with p16 expression)
[generated with PowerPoint, edited with Word- should be color]
As the central dogma of molecular biology has ushered in the era of genome-guided precision medicine, the central dogma of immunotherapy remains an active area of research in the hopes of unlocking similar promise. Genomic alterations differ from patient to patient, making individualized therapy necessary. [70-72] Indeed, the fields of genomics and immunotherapy intersect, with the immune system recognizing genomic alterations within the tumor. To date, the major predictive markers for checkpoint inhibitor response include PD-L1 expression, high tumor mutational burden, microsatellite instability, CD8 infiltrates, and PD-L1 amplification. Evaluation of patient specific neoantigens and integration of the above markers into patient care will be necessary in order to fulfill the promise of immunotherapy.
Acknowledgements
Yulian Khagi, MD, Razelle Kurzrock, MD and Sandip Patel, MD completed the compilation of references, writing, editing and proofreading of this article. There were no additional parties involved in the completion of this work. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of Interest Statement
Dr. Khagi has no conflicts of interest. Dr. Patel receives research funding from MedImmune, Genentech, Pfizer, Amgen, Xcovery, Lilly, Bristol-Myers Squibb. Dr. Kurzrock receives research funding from Genentech, Merck Serono, Pfizer, Sequenom, Foundation Medicine, and Guardant.
Financial Disclosure: Dr. Khagi has no financial disclosures to report. Dr. Patel receives speaking fees from: Boehringer Ingelheim, Merck. Dr. Kurzrock receives consultant and advisory board fees from Actuate Therapeutics, and Xbiotech. She has an ownership interest in Novena, Inc. and Curematch, Inc. Dr. Kurzrock is funded in part by the Joan and Irwin Jacobs philanthropic fund.
REFERENCES
- 1.Johnson BE, Kris MG, Berry LD, Kwiatkowski DJ, Lafrate AJ, Varella-Garcia M, Wistuba II. A multicenter effort to identify driver mutations and employ targeted therapy in patients with lung adenocarcinomas: The Lung Cancer Mutation Consortium (LCMC). Journal of Clinical Oncology 2013 ASCO Annual Meeting Abstracts. 13(15) 052013. [Google Scholar]
- 2.Patel SP, Schwaederle M, Daniels GA, Fanta PT, Schwab RB, Shimabukuro KA, Kurzrock R. Molecular inimitability amongst tumors: implications for precision cancer medicine in the age of personalized oncology. Oncotarget. 2015;6(32):32602–32609. doi: 10.18632/oncotarget.5289. doi:10.18632/oncotarget.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Rosenberg SA. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 1999;17(7):2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 4.La-Beck NM, Jean GW, Huynh C, Alzghari SK, Lowe DB. Immune Checkpoint Inhibitors: New Insights and Current Place in Cancer Therapy. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2015;35(10):963–976. doi: 10.1002/phar.1643. doi:10.1002/phar.1643. [DOI] [PubMed] [Google Scholar]
- 5.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Molecular Cancer Therapeutics. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983. doi:10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 6.Patel SP, Osada T, Osada K, Hurwitz H, Lyerly HK, Morse MA. Modulation of Immune System Inhibitory Checkpoints in Colorectal Cancer. Current Colorectal Cancer Reports. 2013;9(4):391–397. doi:10.1007/s11888-013-0184-3. [Google Scholar]
- 7.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, Stratton MR. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. doi:10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Diaz LA. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. New England Journal of Medicine. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. doi:10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. doi:10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunology. 2002;3(11):991–998. doi: 10.1038/ni1102-991. doi:10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 11.Larsen SK. Cellular immune responses towards regulatory cells. Danish Medical Journal. 2016;63(1):B5188. [PubMed] [Google Scholar]
- 12.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England Journal of Medicine. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. doi:10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Spigel DR. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. doi:10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Brahmer JR. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. doi:10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (London, England) 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. doi:10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dreicer R. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (London, England) 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. doi:10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Rittmeyer A. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. The Lancet. 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. doi:10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 18.Massard C, Gordon MS, Sharma S, Rafil S, Wainberg ZA, Luke JJ, Curiel TJ. Safety and efficacy of durvalumab (MEDI4736), a PD-L1 antibody, in urothelial bladder cancer abstact #4502.. Presented at the ASCO 2016; Chicago, Il. 2016. [Google Scholar]
- 19.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2005;23(3):609–618. doi: 10.1200/JCO.2005.01.086. doi:10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 20.Salipante SJ, Scroggins SM, Hampel HL, Turner EH, Pritchard CC. Microsatellite instability detection by next generation sequencing. Clinical Chemistry. 2014;60(9):1192–1199. doi: 10.1373/clinchem.2014.223677. doi:10.1373/clinchem.2014.223677. [DOI] [PubMed] [Google Scholar]
- 21.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, Pai SI. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Research. 2013;73(6):1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. doi:10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science (New York, N.Y.) 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. doi:10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 23.Lal N, Beggs AD, Willcox BE, Middleton GW. An immunogenomic stratification of colorectal cancer: Implications for development of targeted immunotherapy. Oncoimmunology. 2015;4(3):e976052. doi: 10.4161/2162402X.2014.976052. doi:10.4161/2162402X.2014.976052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palles C, Cazier J-B, Howarth KM, Domingo E, Jones AM, Broderick P, Tomlinson I. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nature Genetics. 2012;45(2):136–144. doi: 10.1038/ng.2503. doi:10.1038/ng.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Gool IC, Eggink FA, Freeman-Mills L, Stelloo E, Marchi E, de Bruyn M, Church DN. POLE Proofreading Mutations Elicit an Antitumor Immune Response in Endometrial Cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2015;21(14):3347–3355. doi: 10.1158/1078-0432.CCR-15-0057. doi:10.1158/1078-0432.CCR-15-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosse Y, Postma DS, Sin DD, Lamontagne M, Couture C, Gaudreault N, Laviolette M. Molecular Signature of Smoking in Human Lung Tissues. Cancer Research. 2012;72(15):3753–3763. doi: 10.1158/0008-5472.CAN-12-1160. doi:10.1158/0008-5472.CAN-12-1160. [DOI] [PubMed] [Google Scholar]
- 27.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (New York, N.Y.) 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. doi:10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGranahan N, Furness AJS, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Swanton C. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science (New York, N.Y.) 2016;351(6280):1463–1469. doi: 10.1126/science.aaf1490. doi:10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue Y, Yoshimura K, Mori K, Kurabe N, Kahyo T, Mori H, Sugimura H. Clinical significance of PD-L1 and PD-L2 copy number gains in non-small-cell lung cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.8528. doi:10.18632/oncotarget.8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruggiero E, Nicolay JP, Fronza R, Arens A, Paruzynski A, Nowrouzi A, von Kalle C. High-resolution analysis of the human T-cell receptor repertoire. Nature Communications. 2015;6:8081. doi: 10.1038/ncomms9081. doi:10.1038/ncomms9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. doi:10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groenen PJTA, Langerak AW, van Dongen JJM, van Krieken JHJM. Pitfalls in TCR gene clonality testing: teaching cases. Journal of Hematopathology. 2008;1(2):97–109. doi: 10.1007/s12308-008-0013-9. doi:10.1007/s12308-008-0013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, Edington HD, Rao UNM, Jukic DM, Land SR, Ferrone S, Kirkwood JM. Modulation of signal transducers and activators of transcription 1 and 3 signaling in melanoma by high-dose IFNalpha2b. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2007;13(5):1523–1531. doi: 10.1158/1078-0432.CCR-06-1387. doi:10.1158/1078-0432.CCR-06-1387. [DOI] [PubMed] [Google Scholar]
- 34.Brase JC, Wuttig D, Kuner R, Sültmann H. Serum microRNAs as non invasive biomarkers for cancer. Molecular Cancer. 2010;9(1):306. doi: 10.1186/1476-4598-9-306. doi:10.1186/1476-4598-9-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Han J, Cui Y, Fan K, Zhou X. Circulating microRNA-21 as noninvasive predictive biomarker for response in cancer immunotherapy. Medical Hypotheses. 2013;81(1):41–43. doi: 10.1016/j.mehy.2013.03.001. doi:10.1016/j.mehy.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Albright A, McClanahan T. Relationship between immune gene signatures and clinical response to PD-1 blockade with pembrolizumab (MK-3475) in patients with advanced solid tumors. Journal for ImmunoTherapy of Cancer. 2015;3(Suppl 2):P80. doi:10.1186/2051-1426-3-S2-P80. [Google Scholar]
- 37.Iglesia MD, Parker JS, Hoadley KA, Serody JS, Perou CM, Vincent BG. Genomic Analysis of Immune Cell Infiltrates Across 11 Tumor Types. Journal of the National Cancer Institute. 2016;108(11):djw144. doi: 10.1093/jnci/djw144. doi:10.1093/jnci/djw144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Partlová S, Bouček J, Kloudová K, Lukešová E, Zábrodský M, Grega M, Fialová A. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology. 2015;4(1):e965570. doi: 10.4161/21624011.2014.965570. doi:10.4161/21624011.2014.965570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallden B, Pekker I, Popa S, Dowidar N, Sullivan A, Hood T, Danaher P. Development and analytical performance of a molecular diagnostic for anti-PD1 response on the nCounterDx analysis system Abstract #3034.. Presented at the ASCO 2016; Chicago, Il. 2016. [Google Scholar]
- 40.Piha-Paul S, Bennouna J, Albright A, Nebozhyn M, McClanahan T, Ayers M, Ott P. T-cell inflamed phenotype gene expression signatures to predict clinical benefit from pembrolizumab across multiple tumor types Abstract #1536.. Presented at the ASCO 2016; Chicago, Il. 2016. [Google Scholar]
- 41.Shen-Orr SS, Gaujoux R. Computational deconvolution: extracting cell type-specific information from heterogeneous samples. Current Opinion in Immunology. 2013;25(5):571–578. doi: 10.1016/j.coi.2013.09.015. doi:10.1016/j.coi.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nature Methods. 2015;12(5):453–457. doi: 10.1038/nmeth.3337. doi:10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinayak S, Newman A, Adams S, Afghahi A, Jensen KC, Badve SS, Telli ML. Abstract P5-04-03: Deconvoluting immune cell populations using “in silico flow cytometry” with CIBERSORT: Association with neoadjuvant therapy response and genomic instability in TNBC. Cancer Research. 2015;75(9 Supplement):P5–04–03–P5–04–03. doi:10.1158/1538-7445.SABCS14-P5-04-03. [Google Scholar]
- 44.FDA Approves Personalized Lung Cancer Immunotherapy with New Kind of CDx. 102015, Retrieved from https://www.genomeweb.com/molecular-diagnostics/fda-approves-personalized-lung-cancer-immunotherapy-new-kind-cdx.
- 45.Borczuk AC, Allen TC. PD-L1 and Lung Cancer: The Era of Precision-ish Medicine? Archives of Pathology & Laboratory Medicine. 2016;140(4):351–354. doi: 10.5858/arpa.2015-0509-SA. doi:10.5858/arpa.2015-0509-SA. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt LH, Kümmel A, Görlich D, Mohr M, Bröckling S, Mikesch JH, Hartmann W. PD-1 and PD-L1 Expression in NSCLC Indicate a Favorable Prognosis in Defined Subgroups. PLOS ONE. 2015;10(8):e0136023. doi: 10.1371/journal.pone.0136023. doi:10.1371/journal.pone.0136023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellmunt J, Mullane SA, Werner L, Fay AP, Callea M, Leow JJ, Signoretti S. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2015;26(4):812–817. doi: 10.1093/annonc/mdv009. doi:10.1093/annonc/mdv009. [DOI] [PubMed] [Google Scholar]
- 48.Wu C, Zhu Y, Jiang J, Zhao J, Zhang X-G, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochemica. 2006;108(1):19–24. doi: 10.1016/j.acthis.2006.01.003. doi:10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Rizvi NA, Chow L, Borghaei H, Shen Y, Harbison C, Alaparthy S, Chen AC. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC Abstract #8022.. Presented at the ASCO 2014; Chicago, Il. 2014. [Google Scholar]
- 50.Ghebeh H, Mohammed S, Al-Omair A, Qattant A, Lehe C, Al-Qudaihi G, Dermime S. The B7-H1 (PD-L1) T Lymphocyte-Inhibitory Molecule Is Expressed in Breast Cancer Patients with Infiltrating Ductal Carcinoma: Correlation with Important High-Risk Prognostic Factors. Neoplasia. 2006;8(3):190–198. doi: 10.1593/neo.05733. doi:10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ritprajak P, Azuma M. Intrinsic and extrinsic control of expression of the immunoregulatory molecule PD-L1 in epithelial cells and squamous cell carcinoma. Oral Oncology. 2015;51(3):221–228. doi: 10.1016/j.oraloncology.2014.11.014. doi:10.1016/j.oraloncology.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 52.Schmid P, Hegde P, Zou W, Kowanetz M, Mariathasan S, Molinero L, Gadgeel S. Association of PD-L2 expression in human tumors with atezolizumab activity Abstract #11506.. Presented at the ASCO 2016; Chicago, Il. 2016. [Google Scholar]
- 53.Woller N, Gürlevik E, Fleischmann-Mundt B, Schumacher A, Knocke S, Kloos AM, Kühnel F. Viral Infection of Tumors Overcomes Resistance to PD-1-immunotherapy by Broadening Neoantigenome-directed T-cell Responses. Molecular Therapy: The Journal of the American Society of Gene Therapy. 2015;23(10):1630–1640. doi: 10.1038/mt.2015.115. doi:10.1038/mt.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bristol-Meyers Squibb. (n.d.). An Open Label, Randomized Phase 3 Clinical Trial of Nivolumab vs Therapy of Investigator's Choice in Recurrent or Metastatic Platinum-refractory Squamous Cell Carcinoma of the Head and Neck (SCCHN). (NCT02105636).
- 55.Keane C, Vari F, Hertzberg M, Cao K-AL, Green MR, Han E, Gandhi MK. Ratios of T-cell immune effectors and checkpoint molecules as prognostic biomarkers in diffuse large B-cell lymphoma: a population-based study. The Lancet Haematology. 2015;2(10):e445–e455. doi: 10.1016/S2352-3026(15)00150-7. doi:10.1016/S2352-3026(15)00150-7. [DOI] [PubMed] [Google Scholar]
- 56.Jefferson E. FDA Approves New Treatment for a Type of Late-Stage Skin Cancer. US Food and Drug Administration; 04252011, Retrieved from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm1193237.htm. [Google Scholar]
- 57.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annual Review of Immunology. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. doi:10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 58.Bracarda S, Altavilla A, Hamzaj A, Sisani M, Marrocolo F, Del Buono S, Danielli R. Immunologic checkpoints blockade in renal cell, prostate, and urothelial malignancies. Seminars in Oncology. 2015;42(3):495–505. doi: 10.1053/j.seminoncol.2015.02.004. doi:10.1053/j.seminoncol.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Ribas A, Kefford R, Marshall MA, Punt CJA, Haanen JB, Marmol M, Hauschild A. Phase III Randomized Clinical Trial Comparing Tremelimumab With Standard-of-Care Chemotherapy in Patients With Advanced Melanoma. Journal of Clinical Oncology. 2013;31(5):616–622. doi: 10.1200/JCO.2012.44.6112. doi:10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer immunology, immunotherapy: CII. 2005;54(4):307–314. doi: 10.1007/s00262-004-0593-x. doi:10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.US Food and Drug Administration FDA Approves Keytruda for Advanced Non-Small Cell Lung Cancer. 10/02/2015.
- 62.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Gandhi L. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. doi:10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 63.US Food and Drug Administration Pembrolizumab (KEYTRUDA) Checkpoint Inhibitor. 2016 Retrieved from http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm526430.htm.
- 64.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Brahmer JR. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2016 doi: 10.1056/NEJMoa1606774. doi:10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 65.US Food and Drug Administration Atezolizumab (TECENTRIQ) 2016 Retrieved from http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm525780.htm.
- 66.Hoffman-La Roche A Randomized Phase 3 Study of Atezolizumab (an Engineered Anti-PDL1 Antibody) Compared to Docetaxel in Patients with Locally Advanced or Metastatic Non-Small Cell Lung Cancer Who Have Failed Platinum Therapy-“OAK.”. 2016 Retrieved from https://www.clinicaltrials.gov/ct2/show/NCT02008227?term=OAK&rank=2.
- 67.Boyerinas B, Jochems C, Fantini M, Heery CR, Gulley JL, Tsang KY, Schlom J. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti-PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunology Research. 2015;3(10):1148–1157. doi: 10.1158/2326-6066.CIR-15-0059. doi:10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Rosenberg SA. Prognostic Factors Related to Clinical Response in Patients with Metastatic Melanoma Treated by CTL-Associated Antigen-4 Blockade. Clinical Cancer Research. 2007;13(22):6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. doi:10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel SP. Immune Checkpoint Blockade for Lung Cancer: State of the Art. Transl Cancer Res. 2015;4(4):415–422. [Google Scholar]
- 70.Wheler J, Lee JJ, Kurzrock R. Unique molecular landscapes in cancer: implications for individualized, curated drug combinations. Cancer Research. 2014;74(24):7181–7184. doi: 10.1158/0008-5472.CAN-14-2329. doi:10.1158/0008-5472.CAN-14-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wheler JJ, Parker BA, Lee JJ, Atkins JT, Janku F, Tsimberidou AM, Kurzrock R. Unique molecular signatures as a hallmark of patients with metastatic breast cancer: implications for current treatment paradigms. Oncotarget. 2014;5(9):2349–2354. doi: 10.18632/oncotarget.1946. doi:10.18632/oncotarget.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurzrock R, Giles FJ. Precision oncology for patients with advanced cancer: the challenges of malignant snowflakes. Cell Cycle (Georgetown, Tex.) 2015;14(14):2219–2221. doi: 10.1080/15384101.2015.1041695. doi:10.1080/15384101.2015.1041695. [DOI] [PMC free article] [PubMed] [Google Scholar]