Abstract
This study was guided by the hypothesis that the aging central nervous system progressively loses its ability to process rapid acoustic changes that are important for speech recognition. Specifically, we hypothesized that age-related deficits in neural synchrony and neuronal oscillatory activity occur independently in older adults and disrupt auditory temporal processing. Neural synchrony is largely dependent on phase locking within the central auditory pathway, beginning at the auditory nerve. In contrast, the resonance characteristics of oscillatory activity are dependent on the integrity and structure of long range cortical connections. We tested our hypotheses by assessing age-related differences in electrophysiologic correlates of neural synchrony and peak oscillatory frequency in younger and older adults with normal hearing and determining their associations with a behavioral measure of gap detection. Phase-locking values (PLVs) were smaller (poorer neural synchrony) and peak alpha frequency (PAF) was lower for older than younger adults and decreased as gap-detection thresholds increased; variations in PLV and PAF uniquely predicted gap-detection thresholds. These effects were driven, in large part, by associations in older adults. These results reveal dissociable neural mechanisms associated with distinct underlying pathology that may differentially present in older adults and contribute to auditory processing declines.
1. Introduction
Clinical practice characterizes hearing loss as a loss of hearing sensitivity, as determined by elevated pure-tone thresholds. Concomitant with or independent of peripheral changes that affect detection abilities (hearing loss) in older adults, aging may also disrupt suprathreshold auditory processes, including temporal processing, which can contribute to their communication difficulties (e.g., Humes et al., 2013; Schoof and Rosen, 2014). We hypothesized that age-related deficits observed in behavioral measures of auditory temporal processing, such as gap detection, may arise from poorer sensory encoding, as demonstrated by reduced phase locking, or slowed neuronal oscillations, or both. The limited success of current rehabilitation methods for older adults with hearing loss and the difficulties experienced by older adults with clinically normal hearing underscore the importance of identifying specific neural mechanisms contributing to age-related declines in auditory processing.
Impaired auditory temporal processing and subsequent speech recognition deficits may be the result of age-related disruptions of neural synchrony (e.g. Anderson, et al., 2012,Tremblay, et al., 2004). In younger adults, poorer neural synchrony at the brainstem is predictive of poorer auditory processing and speech recognition (Bharadwaj, et al., 2015,Bones and Plack, 2015). Aging may exacerbate such deficits in neural synchrony as age-related morphologic and metabolic deficits in the auditory nerve, brainstem, and cortex, can result in atypical coding of temporal properties. Results of studies with animal models of presbyacusis have shown that aging can lead to a loss or inactivity of certain afferent auditory nerve fibers prior to elevated hearing thresholds (Lang, et al., 2010,Xing, et al., 2012). Aged animals also exhibit changes in excitatory and inhibitory neurotransmission (Caspary, et al., 2008), which can further disrupt neural synchrony. This dyssynchrony may lead to disruptions in coding of onset and offset cues (Frisina, 2001), which can lead to poorer neural representation of temporal gaps in ongoing acoustic signals.
Synchronized neural responses to incoming stimuli or salient acoustic changes, such as gaps in noise, occur as a relatively small change in excess of ongoing neural oscillatory activity at the cortex. The phase of this ongoing neural activity, particularly in the alpha frequency domain, has been shown to modulate stimulus sensitivity (detection, reaction time) and also affect perception (Cecere, et al., 2015,Henry, et al., 2016,Klimesch, et al., 1996,Samaha and Postle, 2015). These phasic effects have been reported across modalities and have led to the hypothesis that perception is not simply the representation of the stimuli in the neural response from the sensory system but occurs within discrete intervals whose durations are governed by or reflected in the frequency of alpha oscillations (Cecere, et al., 2015,Chakravarthi and Vanrullen, 2012,Gho and Varela, 1988). Changes in alpha phase across a cycle are thought to create a gating function that oscillates between periods of ‘best’ and ‘worst’ phases of target detection; this association may help describe why the same stimulus may be perceived during some trials but not others. Consistent with this hypothesis, variation in the frequency of alpha oscillations reflect visual and auditory-visual temporal resolution (Cecere, et al., 2015,Samaha and Postle, 2015), such that higher alpha frequencies are typically associated with more accurate discrimination. These observations are complemented by evidence that slower oscillatory activity results in larger windows of integration, reduces neural efficiency, and results in poorer neural processing (Cecere, et al., 2015). With increasing age, there is an increase of slow wave alpha activity, resulting in a shift to lower dominate alpha frequency (Klimesch, et al., 1996,Roubicek, 1977,Woodruff and Kramer, 1979). This age-related slowing of alpha is associated with slower reaction times to simple visual and auditory stimuli and behavioral measures of processing speed (e.g. Klimesch, 1999,Klimesch, et al., 1996,Woodruff and Kramer, 1979) . Slower neural oscillatory activity with increasing age is unlikely to occur only within the auditory system specifically, but instead may result from widespread global gray matter and white matter (WM) declines in cerebellar and prefrontal cortex (Eckert, et al., 2010). In this report, we present new analyses of neural synchrony and neural oscillatory activity in younger and older adults with the goal of identifying neural mechanisms that may account for age-related differences in gap detection. Previously, we examined age-related changes in cortical event-related potentials during attended and passive listening and reported that older adults exhibit poorer auditory temporal processing (gap detection) than younger adults and may be less able than younger adults to compensate for deficits in sensory processing by exerting increased attention (Harris et al., 2012). To examine the role of neural synchrony, the current report presents new analysis of our previously reported EEG data that estimates age-related changes in phase locking. In addition, we present new EEG data collected from the same participants at rest to estimate age-related slowing of peak neuronal oscillatory frequency. Measures of peak neuronal oscillatory frequency represent global domain-general age-related changes that arise from wide-spread cortical declines and are independent of stimulus characteristics and modality. Thus, specific neurophysiologic markers associated with synchrony and peak neural oscillatory activity are used to reduce functional interdependence and examine the extent to which deficits contribute to declining auditory temporal processing in older adults.
2. Methods
2.1 Subjects
Two groups of adults participated in this experiment: younger [n=25; mean age=24.19 (3.42) years; 17 females] and older [n=25; mean age=69.82 (7.11) years; 17 females]. All participants were right-handed monolingual native speakers of American English. Each participant completed the Mini Mental State Examination (Folstein, et al., 1983) with three or fewer errors, indicating little or no cognitive impairment (as reviewed in (Tombaugh and McIntyre, 1992). Behavioral measures of gap detection followed by EEG recordings were obtained during two, two-hour sessions scheduled on different days.
Ideally, hearing thresholds of younger and older adults would be well matched; however, it is rare to find older adults whose auditory thresholds are equivalent to those of younger adults, especially at higher frequencies (Morrell, et al., 1996). Nevertheless, all participants had pure-tone thresholds ≤ 25 dB HL at 250, 500, 1000, 2000, 3000 and 4000 Hz; differences in thresholds between right and left ears did not exceed 15 dB at each frequency. Participants provided written informed consent before participating in this MUSC Institutional Review Board approved study.
2.2 Audiometric thresholds
Pure-tone thresholds at conventional audiometric frequencies were measured with a Madsen OB922 clinical audiometer, calibrated to appropriate ANSI standards (ANSI, 2004) and equipped with TDH-39 headphones.
2.3 Gap detection: stimuli and task design
As described previously (Harris, et al., 2010, Harris, et al., 2012), auditory temporal processing was assessed behaviorally with gap detection thresholds. A single 500-ms broadband noise burst was presented in each trial. As reported here, gap detection thresholds were measured by adjusting gap duration adaptively with the gap location fixed at the midpoint of the total noise duration. Threshold was defined as the gap duration corresponding to the 50% point on the psychometric function.
2.4 EEG acquisition
EEGs were recorded with a 64-channel Neuroscan QuickcapTM based on the international 10-20 system and connected to a SynAmpsRT [sampling rate=1000 Hz, bandpass filtered 0.1 Hz to 200 Hz]. Bipolar electrodes were placed on the skin above and below the left eye for recording vertical electro-oculogram (VEOG) activity. SCAN version 4.4 acquisition software was used to record the EEG signal. Data were re-referenced to an average reference.
2.5 Neural Synchrony: Phase locking values (PLV), stimuli and processing
EEGs were recorded while subjects silently read and passively listened to silent gaps of 15 ms distributed every 2 to 2.2 s within a continuous broadband noise. As reported in our previous paper, this gap duration elicited an ERP in all subjects. To minimize the effect of elevated thresholds at higher frequencies in these otherwise normal-hearing adults, the noise was low-pass filtered at 5000 Hz. Stimuli were digitally generated using Tucker Davis Technologies (TDT) system 3 hardware (RP2). Stimuli were attenuated (TDT PA5), passed through a headphone buffer (TDT HB7), and presented monaurally to the right ear through a TDH-39 headphone at an overall level of 80 dB SPL. Stimuli were monitored by oscilloscope at the input to the earphone and acoustically calibrated with the earphone placed in a NBS-9A coupler using a Larson Davis Model 800B sound level meter equipped with a one inch pressure microphone (Model 2575).
In the single-unit literature, neural synchrony can refer to a neuron's ability to phase lock to a stimulus, recorded as spike discharge timing to the phase of the incoming signal, or as the neuron's response to the onset of a transient stimulus, recorded as a distribution over time to the onset. In this case, neural synchrony is measured as the relative number of responses at a particular phase or time-post onset relative to the stimulus. In the latter case, if a single neuron responds to each click at exactly 1 ms post stimulus onset, that neuron would have perfect synchrony; however, if the neuron responded at various time points with each presentation, that neuron would have poor neural synchrony. Although not necessarily a straightforward relationship, spiking activity, which is associated with the output of a neuron or a population of neurons, and field potentials that generate the EEG, which reflects the slow postsynaptic potential fluctuations and synchronized membrane potentials, are functionally related. Neural synchrony in the EEG may be assessed in the aggregate response of all neurons responding to the incoming stimuli. As a far-field response, PLV represents the trial-to-trial phase coherency of the EEG signal across a specific time window and frequency range (e.g., Zhu, et al., 2013). In this case, neural synchrony reflects both how a given neuron responds to a stimulus across multiple presentations and the timing across all of the units that are responding. PLVs were computed using time-frequency analysis performed using a continuous Morlet wavelet transform as implemented in EEGlab, using linear spaced frequencies from 2 to 30 Hz in 2-Hz steps (Delorme, et al., 2007). If the distribution of phase across trials is uniform and the trials are independent, then the relative phase will also have a uniform distribution and the PLV will be 0. In contrast, if phase across trials is strongly coupled, PLV will approach 1.0. Continuous recordings were epoched offline from -1000 to 2000 ms and time-locked to the onset of the gap. Epochs containing unique, non-stereotyped artifacts were rejected (<20% of trials per subject and condition). The runica algorithm in EEGlab was applied to the remaining concatenated trials to remove common EEG artifacts, such as eye blinks (Delorme et al., 2007). PLVs were calculated from a cluster of electrodes where the EEG showed maximal power and PLV (Fz, FCz, Cz, FC1, FC2). An average of a cluster of electrodes was chosen rather than a single electrode to improve the signal-to noise ratio and reduce the impact of variability in peak response across subjects (Harris, et al., 2012,Herrmann, et al., 2016,Herrmann, et al., 2013). Note that statistical results were qualitatively unaffected when using an electrode cluster rather an individual electrode (e.g., FCz). PLV was computed from a time-frequency window of 50 to 250 ms and focused on theta (4-8 Hz) frequencies, where PLV was significantly above baseline activity for younger and older subjects. This time frame is consistent with event-related potential results we reported previously that showed robust N1 and P2 responses to the 15-ms gap during passive listening (Harris et al., 2012). Group analyses were performed in EEGlab using family-wise error correction.
2.6 Neuronal Oscillatory Frequency: Peak alpha frequency (PAF)
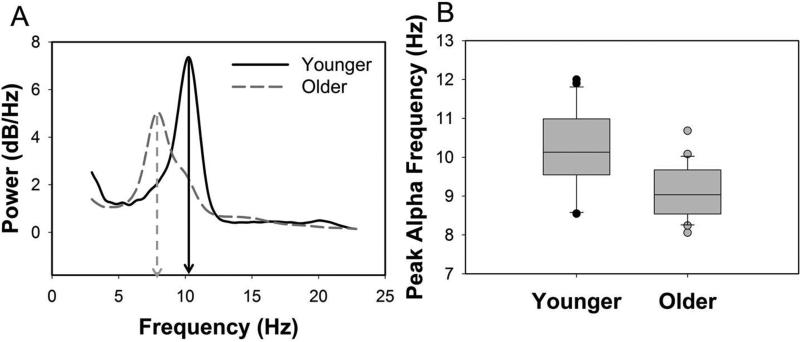
Alpha oscillations are hypothesized to contribute to top-down control and the integration of information by providing temporal frames for neural interactions via the timing of functional inhibition and facilitation. As such, higher resonance frequencies allow for more efficient alterations between ‘open’ and ‘closed’ states of information transfer and more efficient integration within and across brain regions. To assess PAF, EEGs were recorded during a 5-min interval in which participants rested quietly with their eyes closed, while passively listening to a broadband noise presented continuously (same noise as used for the EEG measure of gap detection but with no silent gaps). Data were visually inspected for artifacts and bandpass filtered between 0.3 Hz and 30 Hz. Power spectra of the EEG were computed using Welch's modified periodogram method in MATLAB (Welch, 1967); we extracted the peak in the expanded alpha frequency band (7–14 Hz), retaining only peak amplitudes that were at least 1.5 times higher than the average amplitude at surrounding frequencies (5–15 Hz), from PO3, PO4, P3, P4, O1, O2, and Oz electrodes (Brotzner, et al., 2014,Sokoliuk and VanRullen, 2013) (Figure 3A). Using this criterion, PAF was measurable in all but one younger and two older adults.
Figure 3.
Peak alpha frequency (PAF) was significantly lower for older than younger adults. A. Representative EEG power spectra recorded at rest and averaged across electrodes PO3, PO4, P3, P4, O1, O2, and Oz from one younger and one older adult. The PAF is indicated for each subject with a downward arrow (x-axis). B. Median values (line in center of box), 25th and 75th percentiles (box boundary), and 10th and 90th percentile (error bars), and outliers (circles) for PAF for younger and older adults.
2.7 Statistical analyses
Age group differences between younger and older adults were assessed using independent sample t tests with Bonferroni corrections. Using an individual differences approach, Pearson correlation coefficients determined the extent to which PLVs and PAF predicted gap detection thresholds. Associations were assessed across the entire sample and within groups of older and younger adults; subscripts indicate the sample size. In addition, multiple regression analyses assessed interactions and determined the extent to which PLV and PAF uniquely related to variance in gap detection thresholds after controlling for the other variables. Analyses were performed in R (version 3.0.3).
3. Results
3.1 Audiometric thresholds
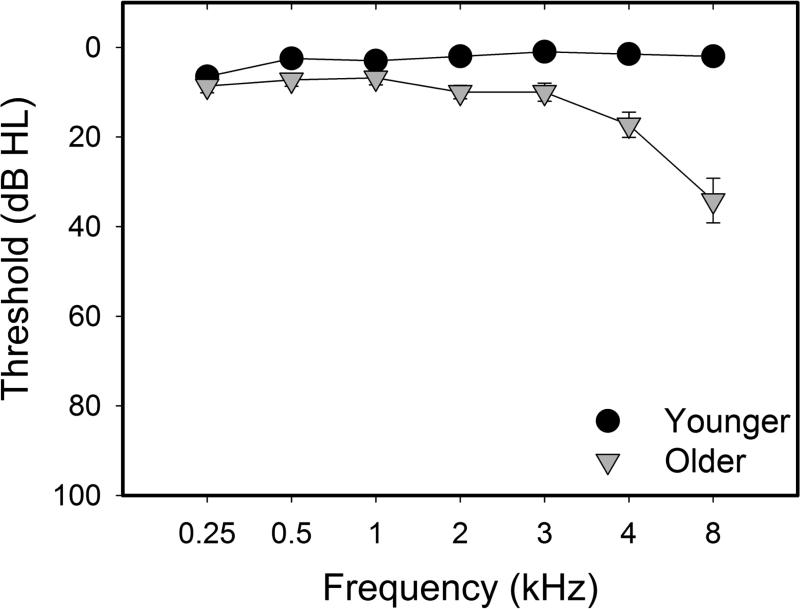
Mean pure-tone thresholds for the right ear (±1 S.E.M.) are shown in Figure 1. Thresholds for younger and older adults from 250-4000 Hz were within clinically normal limits (e.g., ≤ 25 dB HL). Nevertheless, thresholds for older subjects were significantly higher than those of younger subjects at 3000, 4000, and 8000 Hz. Despite these differences, pure-tone thresholds at all frequencies were not significantly associated with neural or behavioral measures (p>0.05).
Figure 1.
Mean pure-tone thresholds (dB HL) and standard errors (±1 S.E.M) for the test (right) ear of younger adults (black) and older adults (gray) plotted as a function of frequency (kHz).
3.2 Effects of age on gap detection
Consistent with age-related declines in auditory temporal processing, gap detection thresholds were higher for older than younger adults. For the younger adults gap detection thresholds averaged 4.33 ms. For the older adults, gap detection thresholds averaged 5.23 ms. Gap detection thresholds were significantly higher for older than younger adults [t(2,49)=-5.09, p<0.001].
3.3 Neural synchrony (PLV)
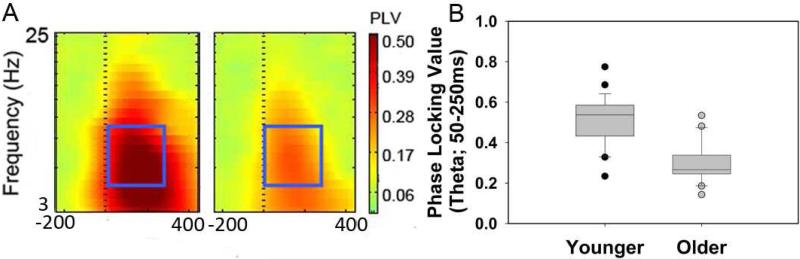
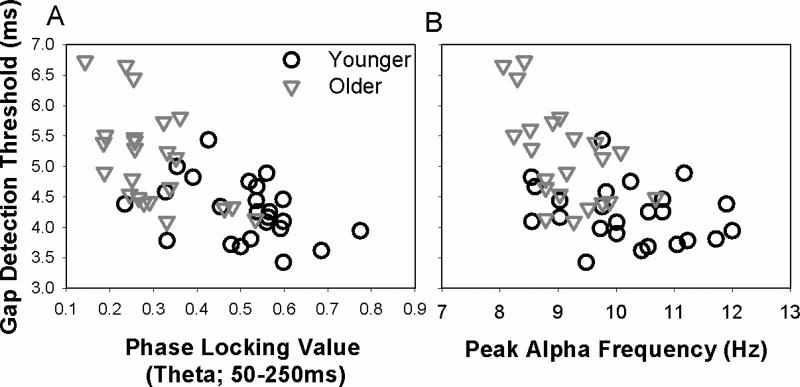
Consistent with age-related deficits in neural synchrony, PLVs were significantly lower for older than younger adults [t(2,48)=5.47, p<0.001] (Figure 2). To account for the age-related bimodal distribution present in the data, partial correlations controlling for age were examined. Higher gap detection thresholds were associated with lower PLVs [partial r48=-0.36, p=0.013]. This association between gap detection and PLV was significant in older adults [r24=-0.45, p=0.03] but not younger adults [r24=-0.24, p=0.253] (Figure 4A).
Figure 2.
Phase locking values (PLV) were significantly lower for older than younger adults. A. Grand-average PLV time–frequency transformations for younger and older adults across electrodes Fz, FCz, Cz, FC1, FC2. Significant effects of age in PLV (p<0.05, FDR-corrected) were observed from 0 ms (dotted line) to 400 ms in the frequency range from 3 Hz to 10 Hz. The blue boxes indicate the time/frequency range used to calculate an average PLV for each subject, which corresponded to the peak in PLVs and the time course of the evoked potential (50-250 ms, 4-8 Hz). B. Median values (line in center of box), 25th and 75th percentiles (box boundary), and 10th and 90th percentile (error bars), and outliers (circles) for PLV for younger and older adults.
Figure 4.
PLV and PAF were negatively correlated with gap detection thresholds. A. Higher gap detection thresholds were associated with lower PLVs; this relationship was significant across the subject sample [r48=-0.59, p<0.001] and within older adults [r24=-0.45, p=0.03], but not within younger adults [r24=-0.24, p=0.253]. Correlations between PLV and gap detection for older adults remained significant after accounting for differences in gap detection thresholds attributed to PAF. B. Higher gap detection thresholds were associated with lower PAFs across the subject sample, after controlling for age-related effects across the groups [partial r48=-0.34, p=0.025] and within older adults [r23=-0.55, p=0.007], but not within younger adults [r24=-0.22, p=0.312]. Correlations between PAF and gap detection for older adults remained significant after accounting for differences in gap detection thresholds attributed to PLV.
3.4 Neuronal Oscillatory Frequency (PAF)
Representative power spectra recorded at rest are shown in Figure 3A for one younger and one older subject. Normalized individual and mean power spectra are shown for younger and older subjects in Supplementary Figure 1. PAFs were significantly lower in older than younger adults [t(2, 47)=4.13, p<0.001] (Figure 3B). To account for these age-related difference, partial correlations controlling for age, were used to examine associations between PAF and gap detection. Higher gap detection thresholds were associated with lower PAFs [partial r48=-0.34, p=0.025]. Again, this relationship was driven by stronger associations between PAF and gap detection in older adults [r23=-0.55, p=0.007] as compared to younger adults [r24=-0.22, p=0.312] (Figure 4B).
3.5 Unique contributions of PLV and PAF to age-related deficits in auditory temporal processing
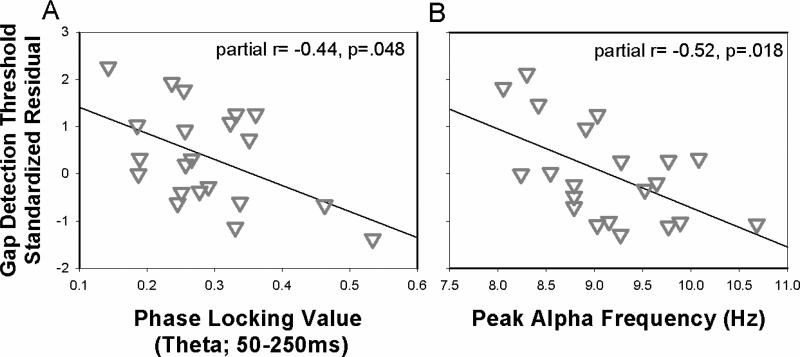
After separately characterizing relationships between neural markers of synchrony (PLV) and oscillatory activity (PAF) that could explain age-related differences in temporal processing, the next analysis included both measures to determine the extent to which these factors uniquely explained variance in temporal processing. As described earlier, PLV, PAF, and gap detection all showed robust correlations with age, driven by significant associations within older adults. Thus, we used a hierarchical regression analysis to first examine age effects on temporal processing, and then determine the extent to which PLV and PAF alternatively account for age-related differences. Regression results are provided in Table 1. The first model (Model 1) showed that age accounted for a significant portion of the variance in gap detection thresholds. However, when PLV and PAF were added to the model (Model 2), model fit was significantly improved (F=5.03, p=0.01), and PLV and PAF both were significant predictors of gap detection, with age as a non-significant factor. This suggests that PLV and PAF accounted for the age-related variance in gap detection. Excluding age from the model (Model 3) did not significantly affect model fit (F= 0.60, p=0.44). Separate regression models were employed to examine PLV and PAF effects for the younger adults (Model 4) and older adults (Model 5). For younger adults (Model 4), neither PLV nor PAF were related to gap detection thresholds. For older adults (Model 5), both PLV and PAF were significantly related to gap detection thresholds (Figures 5A and 5B). These results are consistent with the hypothesis that age-related differences in temporal processing can result from additive declines in neural synchrony and processing speed that may occur independently for older adults.
Table 1.
Age, PLV, and PAF associations with gap detection
| Model 1 | β | t score | p value |
|---|---|---|---|
| Age | 0.58 | 4.65 | <0.001 |
| Model 2 | β | t score | p value |
|---|---|---|---|
| Age | 0.15 | 0.78 | 0.441 |
| PLV | −0.37 | −2.14 | 0.038 |
| PAF | −0.31 | −2.34 | 0.025 |
| Model 3 | β | t score | p value |
|---|---|---|---|
| PLV | −0.46 | −3.74 | 0.001 |
| PAF | −0.35 | −2.83 | 0.007 |
| Younger Adults | β | t score | p value |
|---|---|---|---|
| PLV | −0.18 | −0.85 | 0.406 |
| PAF | −0.23 | −1.09 | 0.289 |
| Older Adults | β | t score | p value |
|---|---|---|---|
| PLV | −0.41 | −2.27 | 0.036 |
| PAF | −0.47 | −2.62 | 0.017 |
Multiple R squared = 0.34, p<.001
Multiple R squared=0.47 p=0.001
Multiple R squared=0.46, p=0.001
Multiple R squared=0.08, p=0.42
Multiple R squared=0.43 p=0.006
Figure 5.
Scatter plots showing the standardized residual gap detection thresholds plotted against PLV and PAF for older adults. A: Y-axis is the standardized residual gap detection threshold after controlling for PAF. B: Y-axis is the standardized residual gap detection threshold after controlling for PLV.
4. Discussion
Reduced auditory temporal processing has been shown to impact speech recognition in older adults (e.g. Fullgrabe, et al., 2014,Gordon-Salant, et al., 2011,Presacco, et al., 2016). The neural systems supporting temporal processing are complex and aging may differentially impact these systems. We hypothesized that age-related deficits in neural synchrony and slower neuronal oscillatory activity may occur independently with age and disrupt auditory temporal processing. Neural synchrony is largely dependent on phase locking within the central auditory pathway, beginning at the auditory nerve, whereas the resonance characteristics of oscillatory activity, and subsequent temporal resolution, is dependent on the integrity and structure of long range cortical connections. Consistent with our predictions, older adults exhibited poorer neural synchrony and slower peak oscillatory activity than younger adults, and each uniquely predicted auditory temporal processing. Complementary measures that have unique underlying mechanisms may provide a better understanding of an individual's auditory processing capacity.
4.1 Neural mechanisms contributing to age-related changes in neural synchrony and associations with temporal processing
Age-related impairments in auditory processing are well documented in normal aging and have been attributed to degradation of the auditory signal through sensory processing declines (e.g., Harkrider, et al., 2005,Ruggles, et al., 2011,Tremblay, et al., 2006,Tremblay, et al., 2002). Age-related deficits in gap detection are seen as early as the brainstem (Poth, et al., 2001). However, deficits in cognition and selective attention are also present in the course of normal aging and are believed to contribute to auditory processing declines (Eckert, et al., 2008,Harris, et al., 2009,Harris, et al., 2012,Tun, et al., 2009,Wostmann, et al., 2015). To help disambiguate these two alternatives, Leung et al. (2015) and our previous work (Harris et al., 2012) compared ERPs during passive and active listening to identify the contribution of sensory or automatic levels of stimulus processing and the effects of attention, respectively. In younger adults, gap detection is related in part to attentional processing of the stimuli, such that the degree to which attention modulates the EEG response is predictive of behavior. Older adults exhibited degraded cortical responses to the gap, and significantly less attention modulation than younger adults, as such behavioral performance was more strongly associated with sensory processing during passive listening. We hypothesized that reduced abilities to benefit from auditory attention may leave older adults dependent on sensory systems that are subject to age-related declines. Our work and others suggested that these age-related differences in sensory processing may arise from declines in neural synchrony (e.g., Harkrider et al. 2005; Harris et al. 2008; Tremblay et al. 2004) . Consistent with this hypothesis, in the current study, older adults exhibited deficits in neural synchrony that were associated with poorer temporal processing. Phase-locking measures from the cortex have been shown to be responsive to differences in signal fidelity and thought to be driven by bottom-up processes (Bowers, et al., 2014). Evidence from animal models suggests that aging results in cochlear synaptopathy (Sergeyenko, et al., 2013) and myelin degeneration (Xing, et al., 2012), changes that may not affect detection thresholds, but may disrupt neural synchrony for higher level signals. These changes result in functional, physiologic, and morphologic declines throughout the auditory system, which may contribute to or amplify changes in sensory processing observed at the cortex (Caspary, et al., 2013,Chambers, et al., 2016). Therefore, our changes observed at the cortex may reflect central compensation for changes at the periphery or the accumulation of changes in synchrony throughout the auditory system (Chambers, et al., 2016). In line with the bottom-up hypothesis, similar deficits in neural synchrony have been reported in older adults at the level of the brainstem (Marmel, et al., 2013).
Neural synchrony is thought to be dependent in part, on white matter integrity, with increasing synchrony associated with greater myelination of axonal fibres, and refinement of GABAergic neurotransmission (as reviewed in (Uhlhaas, et al., 2009). Therefore, our results suggesting that better temporal processing is associated with greater neural synchrony is broadly consistent with neuroimaging findings suggesting that normal variation in WM measures of diffusion and anatomy of Heschl's Gyrus are predictive of the ability to process rapid acoustic change (Golestani, et al., 2007,Zatorre, et al., 2002). It is still uncertain the extent to which age-related changes in neural synchrony and cortical structure are compensatory responses to decreased input related to more peripheral degeneration and reflect homeostatic plasticity or are the result of independent age-related pathology at the cortex (Caspary, et al., 2013,Norena, 2011,Richardson, et al., 2011,Richardson, et al., 2013,Schmidt, et al., 2010). In the current study, neural synchrony as estimated from PLVs did not vary significantly with pure-tone thresholds in these generally normal-hearing younger and older adults. Nevertheless, declines in neural synchrony may still arise in response to more substantial changes in the periphery that result in elevated pure-tone thresholds, or due to loss, degeneration, or inactivity of auditory nerve fibers, which can occur while pure-tone thresholds remain within normal limits (Chambers, et al., 2016).
While the current study focused on aging, cochlear synaptopathy and subsequent deficits in neural synchrony have also been shown in response to noise-exposure in young animals. Consistent with this hypothesis, Mehraei et al., (2016) reported that individual differences in measures of neural synchrony from the brainstem were predictive of perceptual temporal sensitivity in a group of younger adults with normal hearing. In the current study, although not statistically significant, poorer gap detection thresholds were generally associated with poorer neural synchrony in younger adults. This lack of significance in younger adults may be due to a preservation of gap detection abilities, which resulted in a restricted range of gap detection thresholds in younger adults (3.42 ms to 5.44 ms) as compared to older adults (4.01 ms to 6.73 ms). In fact, in the Mehraei experiment, temporal sensitivity was assessed using an interaural timing difference (ITD) comparison in the presence of off frequency background maskers. This paradigm created a greater range of ITD thresholds than previously reported. When the stimulus was presented with only a low-pass noise, ITD thresholds improved and the range of variability decreased, rendering the relationship between the brainstem EEG measure and ITD task non-significant. Stronger associations between PLV and gap detection in older adults may also be expected due to an increase in pathology associated with aging that may have more profound impact on the survival of cochlear afferents (Makary, et al., 2011) , which may be further exaggerated by age-related changes in the brainstem and cortex that disrupt neural synchrony and increase gap detection thresholds (Chambers, et al., 2016).
4.2 Neural mechanisms contributing to age-related changes in oscillatory activity and associations with temporal processing
There is growing evidence that resonance characteristics of the cortex can change with age, effectively slowing oscillatory activity, specifically PAF, which can have profound effects on sensory processing (Fries, et al., 2008,Kruglikov and Schiff, 2003,Lakatos, et al., 2005,Woodruff and Kramer, 1979). Individual differences and age-related changes in PAF have been shown to differentiate individuals and groups of adults by performance on memory tasks, reading ability, and processing speed (Angelakis, et al., 2004a,Angelakis, et al., 2004b,Klimesch, 1999,Klimesch, et al., 1996). Whereas psychophysical target detection and speech recognition have been shown to be modulated by slow oscillatory brain phase, including alpha (Henry and Obleser, 2012,Strauß, et al., 2015), phase and activity within these bands and the ability to entrain activity to incoming stimuli may be dependent in part on PAF (Cecere, et al., 2015,Sokoliuk and VanRullen, 2013). Our results provide new evidence for a role of oscillatory activity in auditory temporal processing and suggest that slowed processing in older adults reduces auditory temporal acuity. While our findings are correlational, in visual cortex a causal link between PAF and auditory-visual integration has been demonstrated using transcranial stimulation (Cecere, et al., 2015). Younger adults showed the same general trend as older adults, where slower PAF was associated with poorer gap detection, yet this association was not significant. This finding was surprising as individual differences in PAF, even in younger adults, have been shown to correlate with performance on several visual perception tasks, processing speed, and working memory. Similar to our PLV results, the lack of a significant association between PAF and gap detection in the current study may be due, in part, to a restricted range of gap detection thresholds in younger adults. Alternatively, stronger associations between PAF and auditory processing in older adults may be related to underlying neural pathologies, which are more likely to be found in older adults than younger adults and contribute to both slowed oscilliatory activity and deficits in temporal processing with age.
Changes in oscillatory activity have the potential to provide biological explanations for declines in auditory temporal processing and recognition of speech, especially in challenging conditions such as with temporally altered speech. These findings add to a growing literature demonstrating the importance of oscillatory activity in sensory processing and how the disruption of these neural networks may contribute to age-related deficits more generally. Better understanding of the role of slowed neural oscillatory activity may support development of evidence-based diagnostic tools and hearing loss interventions. Additional evidence comes from studies showing that processing speed training in older adults generalizes to untrained tasks (Edwards, et al., 2002) and that older adults who regularly listen to speeded speech have better recognition of temporally modulated speech than age-matched peers (Gordon-Salant and Friedman, 2011). For experimental and clinical applications, the relevance of PAF to auditory processing and aging is significant, in that PAF reflects an EEG characteristic or trait that can be measured at rest, thereby not confounded by reduced signal audibility.
PAF is associated with WM density within distinct fiber tracts (Valdes-Hernandez, et al., 2010,Zaehle and Herrmann, 2011). In general, increased WM density is associated with higher resonance frequencies, suggesting that shorter propagation delays lead to faster information transfer (Zaehle and Herrmann, 2011). Specifically, a large-scale study examining PAF and WM architecture in a sample of 397 younger adults identified significant associations between PAF and WM in the posterior and superior corona radiata, consistent with the hypothesized role of the thalamus in alpha generation (Valdes-Hernandez, et al., 2010). Strong associations between PAF and corpus callosum were also observed, consistent with cortico-cortico roles of alpha (Valdes-Hernandez, et al., 2010). Our results are broadly consistent with these previous results suggesting that age-related changes in neural processing speed are the result of a unique pattern of gray matter and WM changes in cerebellar and prefrontal cortex (Eckert, et al., 2010) and pronounced deterioration of brain morphology associated with aging. These morphologic changes likely influence oscillatory neural activity across the brain contributing to changes in oscillatory dynamics observed in the EEG.
4.3 Identifying unique neuropathological factors contributing to deficits in temporal processing
In the current study, we observed significant age-related deficits in neural synchrony, neural oscillations, and gap detection. Our results support the hypothesis that, due to different underlying mechanisms, neural synchrony and peak neural oscillatory activity uniquely contribute to auditory temporal processing deficits in older adults. Moreover, significant associations between neural synchrony, PAF, and auditory temporal processing were driven by associations seen within the older adults, suggesting that these relationships are not indirectly related through age and instead may be associated with underlying causes of temporal processing deficits observed in older adults. Robust differences between age groups and stronger associations between neural and behavioral measures in older adults may be explained by several interrelated factors. Neural synchrony is thought to decline with age due to a loss or inactivity of auditory nerve fibers; these changes at the level of the auditory nerve may have profound impacts throughout the central auditory system (Chambers, et al., 2016), which can compound the lower level effects. Our current results of poorer PLV and temporal processing in older adults is consistent with this model of neural dyssynchrony. Stronger associations between PAF and auditory temporal processing in older adults may reflect the increased prevalence of neuropathologies with age, which can also affect cortical resonance and lead to slower PAF and poorer auditory processing.
5. Conclusions
The neuropathological factors underlying canonical age-related deficits in temporal acuity remain unknown. Addressing these issues and in light of our findings, we offer a model in which age-related deficits in neural synchrony and neuronal oscillatory activity uniquely affect temporal processing and occur independently of changes in hearing thresholds. This work builds on our previous results suggesting that reduced benefit from attention modulation makes older adults more dependent on the integrity of an auditory system in decline and highlights specific neural mechanisms that may contribute to these auditory deficits. Current hearing interventions for older adults with hearing loss focus primarily on the use of sound amplification to restore audibility of important speech information. However, while necessary and important, amplification alone cannot account for age-related dyssynchrony, slowed processing, and subsequent degraded auditory representations, which likely result in poorer speech recognition, particularly in challenging listening environments.
Supplementary Material
Highlights.
Neural synchrony at the level of the cortex declines in older adults and may result in poorer auditory temporal processing
Similar to the visual domain, individuals with higher alpha frequencies have finer auditory temporal resolution
Age-related deficits in neural synchrony and processing speed may occur independently and may have distinct consequences for temporal processing.
Acknowledgements
This work was supported (in part) by grants from the NIDCD (R01 DC014467 and P50 DC00422). The project also received support from the South Carolina Clinical and Translational Research (SCTR) Institute with an academic home at the Medical University of South Carolina, NIH/NCRR Grant number UL1RR029882. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR1 4516 from the National Center for Research Resources, NIH.
Abbreviations
- EEG
electrophysiology
- PLV
phase locking value
- PAF
peak alpha frequency
- WM
white matter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests
References
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N. Aging affects neural precision of speech encoding. J Neurosci. 2012;32(41):14156–64. doi: 10.1523/JNEUROSCI.2176-12.2012. doi:10.1523/JNEUROSCI.2176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelakis E, Lubar JF, Stathopoulou S. Electroencephalographic peak alpha frequency correlates of cognitive traits. Neurosci Lett. 2004a;371(1):60–3. doi: 10.1016/j.neulet.2004.08.041. doi:S0304-3940(04)01052-3 [pii]10.1016/j.neulet.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Angelakis E, Lubar JF, Stathopoulou S, Kounios J. Peak alpha frequency: an electroencephalographic measure of cognitive preparedness. Clin Neurophysiol. 2004b;115(4):887–97. doi: 10.1016/j.clinph.2003.11.034. doi:10.1016/j.clinph.2003.11.034S1388245703004632 [pii] [DOI] [PubMed] [Google Scholar]
- ANSI . Specification for Audiometrics. American National Standards Institute; New York: 2004. [Google Scholar]
- Bharadwaj HM, Masud S, Mehraei G, Verhulst S, Shinn-Cunningham BG. Individual differences reveal correlates of hidden hearing deficits. J Neurosci. 2015;35(5):2161–72. doi: 10.1523/JNEUROSCI.3915-14.2015. doi:10.1523/JNEUROSCI.3915-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bones O, Plack CJ. Subcortical representation of musical dyads: individual differences and neural generators. Hear Res. 2015;323:9–21. doi: 10.1016/j.heares.2015.01.009. doi:10.1016/j.heares.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Bowers AL, Saltuklaroglu T, Harkrider A, Wilson M, Toner MA. Dynamic modulation of shared sensory and motor cortical rhythms mediates speech and non-speech discrimination performance. Front Psychol. 2014;5:366. doi: 10.3389/fpsyg.2014.00366. doi:10.3389/fpsyg.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotzner CP, Klimesch W, Doppelmayr M, Zauner A, Kerschbaum HH. Resting state alpha frequency is associated with menstrual cycle phase, estradiol and use of oral contraceptives. Brain Res. 2014;1577:36–44. doi: 10.1016/j.brainres.2014.06.034. doi:10.1016/j.brainres.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Hughes LF, Ling LL. Age-related GABAA receptor changes in rat auditory cortex. Neurobiol Aging. 2013;34(5):1486–96. doi: 10.1016/j.neurobiolaging.2012.11.009. doi:10.1016/j.neurobiolaging.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211(Pt 11):1781–91. doi: 10.1242/jeb.013581. doi:211/11/1781 [pii]10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecere R, Rees G, Romei V. Individual differences in alpha frequency drive crossmodal illusory perception. Curr Biol. 2015;25(2):231–5. doi: 10.1016/j.cub.2014.11.034. doi:10.1016/j.cub.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthi R, Vanrullen R. Conscious updating is a rhythmic process. Proc Natl Acad Sci U S A. 2012;109(26):10599–604. doi: 10.1073/pnas.1121622109. doi:10.1073/pnas.1121622109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB. Central Gain Restores Auditory Processing following Near-Complete Cochlear Denervation. Neuron. 2016;89(4):867–79. doi: 10.1016/j.neuron.2015.12.041. doi:10.1016/j.neuron.2015.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage. 2007;34(4):1443–9. doi: 10.1016/j.neuroimage.2006.11.004. doi:S1053-8119(06)01109-8 [pii]10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Keren NI, Roberts DR, Calhoun VD, Harris KC. Age-related changes in processing speed: unique contributions of cerebellar and prefrontal cortex. Front Hum Neurosci. 2010;4:10. doi: 10.3389/neuro.09.010.2010. doi:10.3389/neuro.09.010.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR. Age-related effects on word recognition: reliance on cognitive control systems with structural declines in speech-responsive cortex. J Assoc Res Otolaryngol. 2008;9(2):252–9. doi: 10.1007/s10162-008-0113-3. doi:10.1007/s10162-008-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J, Wadley V, Myers R, Roenker DL, Cissell G, Ball K. Transfer of a speed of processing intervention to near and far cognitive functions. Gerontology. 2002;48(5):329–40. doi: 10.1159/000065259. doi:65259. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40(7):812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- Fries P, Womelsdorf T, Oostenveld R, Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J Neurosci. 2008;28(18):4823–35. doi: 10.1523/JNEUROSCI.4499-07.2008. doi:28/18/4823 [pii]10.1523/JNEUROSCI.4499-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisina RD. Subcortical neural coding mechanisms for auditory temporal processing. Hearing Research. 2001;158(1–2):1–27. doi: 10.1016/s0378-5955(01)00296-9. doi: http://dx.doi.org/10.1016/S0378-5955(01)00296-9. [DOI] [PubMed] [Google Scholar]
- Fullgrabe C, Moore BC, Stone MA. Age-group differences in speech identification despite matched audiometrically normal hearing: contributions from auditory temporal processing and cognition. Front Aging Neurosci. 2014;6:347. doi: 10.3389/fnagi.2014.00347. doi:10.3389/fnagi.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gho M, Varela FJ. A quantitative assessment of the dependency of the visual temporal frame upon the cortical rhythm. Journal de physiologie. 1988;83(2):95–101. [PubMed] [Google Scholar]
- Golestani N, Molko N, Dehaene S, LeBihan D, Pallier C. Brain structure predicts the learning of foreign speech sounds. Cereb Cortex. 2007;17(3):575–82. doi: 10.1093/cercor/bhk001. doi:bhk001 [pii]10.1093/cercor/bhk001. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ, Yeni-Komshian GH. Auditory Temporal Processing and Aging: Implications for Speech Understanding of Older People. Audiology Research. 2011;1(1):e4. doi: 10.4081/audiores.2011.e4. doi:10.4081/audiores.2011.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Salant S, Friedman SA. Recognition of rapid speech by blind and sighted older adults. J Speech Lang Hear Res. 2011;54(2):622–31. doi: 10.1044/1092-4388(2010/10-0052). doi:1092-4388_2010_10-0052 [pii]10.1044/1092-4388(2010/10-0052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkrider AW, Plyler PN, Hedrick MS. Effects of age and spectral shaping on perception and neural representation of stop consonant stimuli. Clin Neurophysiol. 2005;116(9):2153–64. doi: 10.1016/j.clinph.2005.05.016. doi:S1388-2457(05)00224-5 [pii] 10.1016/j.clinph.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Harris KC, Dubno JR, Keren NI, Ahlstrom JB, Eckert MA. Speech recognition in younger and older adults: a dependency on low-level auditory cortex. J Neurosci. 2009;29(19):6078–87. doi: 10.1523/JNEUROSCI.0412-09.2009. doi:29/19/6078 [pii] 10.1523/JNEUROSCI.0412-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KC, Eckert MA, Ahlstrom JB, Dubno JR. Age-related differences in gap detection: effects of task difficulty and cognitive ability. Hear Res. 2010;264(1-2):21–9. doi: 10.1016/j.heares.2009.09.017. doi:S0378-5955(09)00241-X [pii]10.1016/j.heares.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KC, Wilson S, Eckert MA, Dubno JR. Human evoked cortical activity to silent gaps in noise: effects of age, attention, and cortical processing speed. Ear Hear. 2012;33(3):330–9. doi: 10.1097/AUD.0b013e31823fb585. doi:10.1097/AUD.0b013e31823fb585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry MJ, Herrmann B, Obleser J. Neural Microstates Govern Perception of Auditory Input without Rhythmic Structure. J Neurosci. 2016;36(3):860–71. doi: 10.1523/JNEUROSCI.2191-15.2016. doi:10.1523/JNEUROSCI.2191-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry MJ, Obleser J. Frequency modulation entrains slow neural oscillations and optimizes human listening behavior. Proceedings of the National Academy of Sciences. 2012;109(49):20095–100. doi: 10.1073/pnas.1213390109. doi:10.1073/pnas.1213390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann B, Henry MJ, Johnsrude IS, Obleser J. Altered temporal dynamics of neural adaptation in the aging human auditory cortex. Neurobiol Aging. 2016;45:10–22. doi: 10.1016/j.neurobiolaging.2016.05.006. doi:10.1016/j.neurobiolaging.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Herrmann B, Henry MJ, Obleser J. Frequency-specific adaptation in human auditory cortex depends on the spectral variance in the acoustic stimulation. J Neurophysiol. 2013;109(8):2086–96. doi: 10.1152/jn.00907.2012. doi:10.1152/jn.00907.2012. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29(2-3):169–95. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schimke H, Pachinger T. Alpha frequency, reaction time, and the speed of processing information. J Clin Neurophysiol. 1996;13(6):511–8. doi: 10.1097/00004691-199611000-00006. [DOI] [PubMed] [Google Scholar]
- Kruglikov SY, Schiff SJ. Interplay of electroencephalogram phase and auditory-evoked neural activity. J Neurosci. 2003;23(31):10122–7. doi: 10.1523/JNEUROSCI.23-31-10122.2003. doi:23/31/10122 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94(3):1904–11. doi: 10.1152/jn.00263.2005. doi:00263.2005 [pii]10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- Lang H, Jyothi V, Smythe NM, Dubno JR, Schulte BA, Schmiedt RA. Chronic reduction of endocochlear potential reduces auditory nerve activity: further confirmation of an animal model of metabolic presbyacusis. J Assoc Res Otolaryngol. 2010;11(3):419–34. doi: 10.1007/s10162-010-0214-7. doi:10.1007/s10162-010-0214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary CA, Shin J, Kujawa SG, Liberman MC, Merchant SN. Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol. 2011;12(6):711–7. doi: 10.1007/s10162-011-0283-2. doi:10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmel F, Linley D, Carlyon RP, Gockel HE, Hopkins K, Plack CJ. Subcortical Neural Synchrony and Absolute Thresholds Predict Frequency Discrimination Independently. JARO: Journal of the Association for Research in Otolaryngology. 2013;14(5):757–66. doi: 10.1007/s10162-013-0402-3. doi:10.1007/s10162-013-0402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell CH, Gordon-Salant S, Pearson JD, Brant LJ, Fozard JL. Age- and gender-specific reference ranges for hearing level and longitudinal changes in hearing level. J Acoust Soc Am. 1996;100(4 Pt 1):1949–67. doi: 10.1121/1.417906. [DOI] [PubMed] [Google Scholar]
- Norena AJ. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci Biobehav Rev. 2011;35(5):1089–109. doi: 10.1016/j.neubiorev.2010.11.003. doi:10.1016/j.neubiorev.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Poth EA, Boettcher FA, Mills JH, Dubno JR. Auditory brainstem responses in younger and older adults for broadband noises separated by a silent gap. Hear Res. 2001;161(1-2):81–6. doi: 10.1016/s0378-5955(01)00352-5. [DOI] [PubMed] [Google Scholar]
- Presacco A, Simon JZ, Anderson S. Evidence of degraded representation of speech in noise, in the aging midbrain and cortex. J Neurophysiol. 2016 doi: 10.1152/jn.00372.2016. jn 00372 2016. doi:10.1152/jn.00372.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BD, Ling LL, Uteshev VV, Caspary DM. Extrasynaptic GABA(A) receptors and tonic inhibition in rat auditory thalamus. PLoS One. 2011;6(1):e16508. doi: 10.1371/journal.pone.0016508. doi:10.1371/journal.pone.0016508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BD, Ling LL, Uteshev VV, Caspary DM. Reduced GABA(A) receptor-mediated tonic inhibition in aged rat auditory thalamus. J Neurosci. 2013;33(3):1218–27a. doi: 10.1523/JNEUROSCI.3277-12.2013. doi:10.1523/JNEUROSCI.3277-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubicek J. The electroencephalogram in the middle-aged and the elderly. J Am Geriatr Soc. 1977;25(4):145–52. doi: 10.1111/j.1532-5415.1977.tb00282.x. [DOI] [PubMed] [Google Scholar]
- Ruggles D, Bharadwaj H, Shinn-Cunningham BG. Normal hearing is not enough to guarantee robust encoding of suprathreshold features important in everyday communication. Proc Natl Acad Sci U S A. 2011;108(37):15516–21. doi: 10.1073/pnas.1108912108. doi:10.1073/pnas.1108912108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha J, Postle BR. The Speed of Alpha-Band Oscillations Predicts the Temporal Resolution of Visual Perception. Curr Biol. 2015;25(22):2985–90. doi: 10.1016/j.cub.2015.10.007. doi:10.1016/j.cub.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Redecker C, Bruehl C, Witte OW. Age-related decline of functional inhibition in rat cortex. Neurobiol Aging. 2010;31(3):504–11. doi: 10.1016/j.neurobiolaging.2008.04.006. doi:10.1016/j.neurobiolaging.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 2013;33(34):13686–94. doi: 10.1523/JNEUROSCI.1783-13.2013. doi:10.1523/jneurosci.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoliuk R, VanRullen R. The flickering wheel illusion: when alpha rhythms make a static wheel flicker. J Neurosci. 2013;33(33):13498–504. doi: 10.1523/JNEUROSCI.5647-12.2013. doi:10.1523/JNEUROSCI.5647-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauß A, Henry MJ, Scharinger M, Obleser J. Alpha Phase Determines Successful Lexical Decision in Noise. The Journal of Neuroscience. 2015;35(7):3256–62. doi: 10.1523/JNEUROSCI.3357-14.2015. doi:10.1523/jneurosci.3357-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Billings C, Rohila N. Speech evoked cortical potentials: effects of age and stimulus presentation rate. J Am Acad Audiol. 2004;15(3):226–37. doi: 10.3766/jaaa.15.3.5. quiz 64. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Kalstein L, Billings CJ, Souza PE. The neural representation of consonant-vowel transitions in adults who wear hearing AIDS. Trends Amplif. 2006;10(3):155–62. doi: 10.1177/1084713806292655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P. Aging alters the neural representation of speech cues. Neuroreport. 2002;13(15):1865–70. doi: 10.1097/00001756-200210280-00007. [DOI] [PubMed] [Google Scholar]
- Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol Aging. 2009;24(3):761–6. doi: 10.1037/a0014802. doi:2009-13203-027 [pii]10.1037/a0014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, Nikolic D, Singer W. Neural synchrony in cortical networks: history, concept and current status. Frontiers in integrative neuroscience. 2009;3:17. doi: 10.3389/neuro.07.017.2009. doi:10.3389/neuro.07.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes-Hernandez PA, Ojeda-Gonzalez A, Martinez-Montes E, Lage-Castellanos A, Virues-Alba T, Valdes-Urrutia L, Valdes-Sosa PA. White matter architecture rather than cortical surface area correlates with the EEG alpha rhythm. Neuroimage. 2010;49(3):2328–39. doi: 10.1016/j.neuroimage.2009.10.030. doi:10.1016/j.neuroimage.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Welch PD. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroacoustics. 1967;15:70–3. [Google Scholar]
- Woodruff DS, Kramer DA. EEG alpha slowing, refractory period, and reaction time in aging. Exp Aging Res. 1979;5(4):279–92. doi: 10.1080/03610737908257205. doi:10.1080/03610737908257205. [DOI] [PubMed] [Google Scholar]
- Wostmann M, Herrmann B, Wilsch A, Obleser J. Neural alpha dynamics in younger and older listeners reflect acoustic challenges and predictive benefits. J Neurosci. 2015;35(4):1458–67. doi: 10.1523/JNEUROSCI.3250-14.2015. doi:10.1523/JNEUROSCI.3250-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Samuvel DJ, Stevens SM, Dubno JR, Schulte BA, Lang H. Age-related changes of myelin basic protein in mouse and human auditory nerve. PLoS One. 2012;7(4):e34500. doi: 10.1371/journal.pone.0034500. doi:10.1371/journal.pone.0034500PONE-D-11-17944 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaehle T, Herrmann CS. Neural synchrony and white matter variations in the human brain--relation between evoked gamma frequency and corpus callosum morphology. Int J Psychophysiol. 2011;79(1):49–54. doi: 10.1016/j.ijpsycho.2010.06.029. doi:10.1016/j.ijpsycho.2010.06.029. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: music and speech. Trends Cogn Sci. 2002;6(1):37–46. doi: 10.1016/s1364-6613(00)01816-7. [DOI] [PubMed] [Google Scholar]
- Zhu L, Bharadwaj H, Xia J, Shinn-Cunningham B. A comparison of spectral magnitude and phase-locking value analyses of the frequency-following response to complex tones. J Acoust Soc Am. 2013;134(1):384–95. doi: 10.1121/1.4807498. doi:10.1121/1.4807498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.