Abstract
Neointimal hyperplasia, which results from the activation, proliferation and migration of vascular smooth muscle cells (SMCs), is a detrimental condition for vascular stents or vascular grafts that leads to stenosis. Preventing neointimal hyperplasia of vascular grafts is critically important for the success of arterial vascular grafts. We hypothesized that tropoelastin seeding onto the luminal surface of the graft would prevent neointimal hyperplasia through suppressing neointimal smooth muscle cell proliferation. In this study, we investigated the efficacy of tropoelastin seeding in preventing neointimal hyperplasia of bioresorbable arterial vascular grafts. Poly (glycolic acid) (PGA) fiber mesh coated with poly (l-lactic-co-ε-caprolactone) (PLCL) scaffolds reinforced by poly (l-lactic acid) (PLA) nano-fibers were prepared as bioresorbable arterial grafts. Tropoelastin was then seeded onto the luminal surface of the grafts. Tropoelastin significantly reduced the thickness of the intimal layer. This effect was mainly due to a substantial reduction the number of cells that stained positive for SMC (α-SMA) and PCNA in the vessel walls. Mature elastin and collagen type I and III were unchanged with tropoelastin treatment. This study demonstrates that tropoelastin seeding is beneficial in preventing SMC proliferation and neointimal hyperplasia in bioresorbable arterial vascular grafts.
Keywords: Bioresorbable vascular grafts, Small diameter arterial grafts, Tropoelastin, Intimal hyperplasia, Smooth muscle cells
Graphical abstract
Small resorbable vascular grafts can block due to the over-proliferation of smooth muscle cells in neointimal hyperplasia. We show here that this the proliferation of these cells is restricted in this type of graft.
This is achieved with a simple dip, non-covalent coating of tropoelastin. This is in principle amendable to other grafts and is therefore an attractive process.
This study is particularly significant because: (1) it shows that smooth muscle cell proliferation can be reduced while still accommodating the growth of endothelial cells, (2) small vascular grafts with an internal diameter of less than 1 mm are amenable to this process, and (3) this process works for resorbable grafts.
1. Introduction
Vascular grafts have been widely used in the treatment of vascular diseases, such as cardiovascular or peripheral vascular diseases. Autologous vessels are commonly recommended for this procedure, but in some cases, there are limitations in using autografts such as insufficient availability of viable tissue in patients with widespread atherosclerotic vascular disease. This lack of viable tissue necessitates the use of alternative synthetic grafts. However, current commercialized grafts composed of expanded-polytetrafluoroethylene or polyethylene terephthalate have not yet shown clinical effectiveness for small-diameter arteries (< 6 mm) due to their poor patency rate.1 To address this challenge, our group demonstrated the feasibility of novel bioresorbable small-diameter arterial grafts that restore vascular function in a mouse aortic implantation model.2, 3
Bioresorbable vascular grafts are biologically active grafts which are entirely reconstituted by host-derived cells over the course of an inflammation-mediated degradation process.4 The application of bioresorbable vascular grafts has several advantages to the patient, such as growth potential, favorable biocompatibility, and low risk of infection. One of the most detrimental events for a vascular graft is stenosis, which is often proceeded by intimal hyperplasia. Smooth muscle cells (SMCs), the predominant cells in the arterial wall, are essential for the structural and functional integrity of the neovessel. However, excessive proliferation of SMCs leads to neointimal hyperplasia followed by graft stenosis and occlusion. Some growth factors, such as transforming growth factor-beta (TGF-β) and platelet-derived growth factor (PDGF), are understood to promote SMC recruitment and proliferation leading to neointimal hyperplasia.5, 6
Elastin is an integral part of the vasculature as a major component of the internal elastic lamina and in arteries in alternating elastic lamellae.7 Elastin also regulates the phenotypic modulation, proliferation and migration of SMCs and is a crucial signaling molecule that directly controls SMC biology and stabilizes arterial structure.8 On this basis, in an undamaged artery SMCs are sandwiched between elastic lamellae. Elastin is predominantly comprised of the 60–70 kDa protein monomer tropoelastin, which is crosslinked and organized into elastin polymers that form elastic fibers around the arterial lumen which provide the compliance that arteries need to absorb and transmit hemodynamic forces.9, 10 We hypothesize that an abundant presence of tropoelastin will inhibit the migration and proliferation of SMCs into the bioresorbable vascular grafts. The purpose of the present study is to use a murine aortic implantation model to confirm whether a tropoelastin seeded bioresorbable arterial vascular graft inhibits SMC proliferation and thus prevents neointimal hyperplasia.
2. Materials and Methods
2.1. Bioresorbable arterial vascular graft preparation
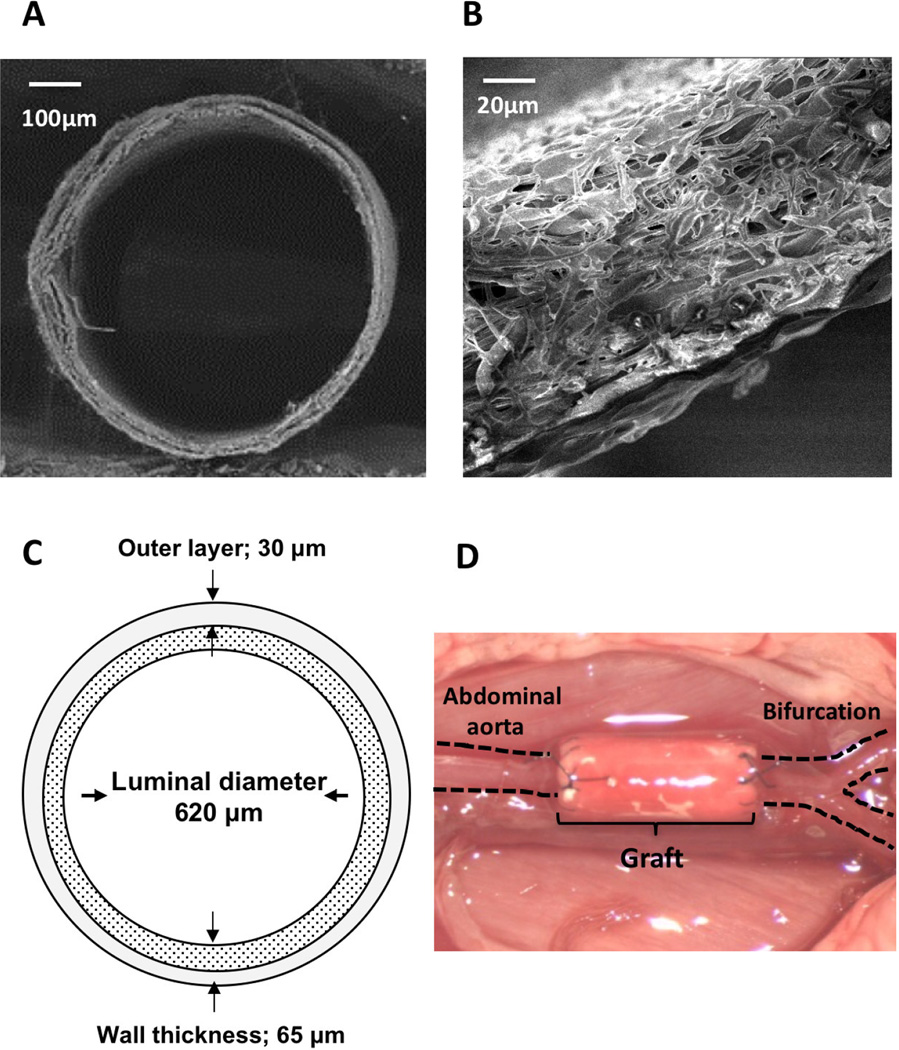
Bioresorbable arterial vascular grafts were constructed using a dual cylinder chamber molding system from a nonwoven poly (glycolic acid) (PGA) fiber mesh (Biomedical Structures, Warwick, RI) coated with a 50:50 copolymer sealant solution of poly(l-lactic-co-ε-caprolactone) (PLCL) (Gunze, Kyoto, Japan), as previously described.11 These scaffolds were reinforced by electrospinning poly (l-lactic acid) (PLA) nano-fiber (30µm thickness) onto the outer side of the PLCL scaffold to endure the high pressure arterial environment; the wall thickness of the grafts in all groups was around 65 µm, and the luminal diameter of each graft was around 620 µm (Fig. 1A, B, C). All grafts were provided by Gunze Ltd (Kyoto, Japan).
Fig. 1.
Mouse bioresorbable arterial graft images before and after implantation. Scanning electron migrographs of the mouse bioresorbable arterial graft at (A) low and (B) high magnification. (C) Schematic of the mouse bioresorbable arterial graft. (D) mouse abdominal aortic interposition graft.
2.2. Tropoelastin coating
Grafts were incubated under aseptic conditions with a sterile phosphate-buffered saline solution of 1 mg/ml tropoelastin for at 1 hr at room temperature, corresponding to the most common 60 kDa mature form of the secreted protein after removal of the signal peptide, which generates a monolayer coating of physisorbed tropoelastin molecules as described previously.12
2.3. Animal model and surgical implantation
All animals received humane care in compliance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Bioresorbable vascular grafts of 3mm in length were implanted in 8–10 week old female mice (n = 10 for each group) as infra-renal aortic interposition conduits using standard microsurgical techniques (Fig. 1D).13 Low pressure vascular clamps (Fine Science Tools, Foster City, CA) were used for cross clamping and 400 U/kg of heparin was injected intramuscularly 5 minutes before unclamping. Animals were followed for 8 weeks to evaluate neotissue formation. An aspirin-mixed diet (0.1% of diet) was fed to mice in each group 3 days before and 3 days after surgery to prevent acute thrombosis.
2.4. Histology, immunohistochemistry, and immunofluorescence
Explanted grafts at 8 weeks after implantation were fixed in 4% para-formaldehyde, embedded in paraffin, sliced (5 µm thick sections), and stained with Hematoxylin and Eosin (H&E), Hart’s, and Picrosirius Red. Outer and luminal perimeters of the grafts were manually measured from H&E staining with Image J software (NIH, Bethesda, MD) to obtain luminal diameter and wall thickness measurements. Wall thickness of the graft was calculated by the following formula: (Wall thickness) = (Graft outer circumference)/2π - (Graft inner circumference)/2π. The area fraction, positively stained using Hart’s stain, was measured with Image J software to obtain area fraction of elastin deposition within the scaffold. Polarized microscopic images of Picrosirius Red staining were used to measure the area fraction of collagen type I and III deposition with Image J software. Identification of SMCs, proliferating-cell nuclear antigens (PCNA), and macrophages was done by immunohistochemical staining of paraffin-embedded explant sections with anti- α-smooth muscle actin (α-SMA) antibody (1:500, DAKO, Carpinteria, CA), anti- PCNA antibody (1:1000, Abcam, Cambridge, MA), and anti-MAC3 antibody (1:75, DAKO), respectively. Primary antibody binding was detected using biotinylated IgG (Vector, Burlingame, CA), and was followed by the binding of streptavidin-horseradish peroxidase and color development with 3,3-diaminobenzidine. SMCs identified by α-SMA expression, PCNA positive cells in the neointimal layer, and MAC3 positive macrophages were quantified by manual counting. One representative section from each explant was stained and imaged. Low magnification (5×) images were divided into nine sections (3×3). Four of these regions (upper middle, center, lower right, and lower left) were selected to obtain high magnification (40×) images. All positively stained nuclei were counted from high magnification images. Averages of these four regions provided the number of positive cells in each section. Immunofluorescent staining for CD31 as a marker of endothelial cells and for α-SMA was performed using rabbit anti-CD31 primary antibody (1:50, Abcam) and mouse anti-α-SMA primary antibody (1:500, DAKO), followed by Alexa Fluor 488 anti-rabbit IgG secondary antibody (1:300, Invitrogen, Carlsbad, CA), and Alexa Fluor 647 anti-mouse IgG secondary antibody (1:300, Invitrogen), respectively. Fluorescence images were obtained with an Olympus IX51 microscope (Olympus, Tokyo, Japan).
2.5. Statistical analysis
Numeric values are listed as mean ± standard deviation. The number of experiments is shown in each case. Data of continuous variables with normal distribution were evaluated by student’s t test. P values less than 0.05 indicated statistical significance.
3. Results
3.1. Animal Survival
Ten grafts for each group were implanted as infra-renal interposition aortic conduits and all animals were followed for 8 weeks. In the Unseeded group, 2 mice were sacrificed due to lower limb paralysis from acute thrombosis and 1 mouse died of undetermined causes. In the Tropoelastin seeded group, 4 mice were sacrificed due to lower limb paralysis from acute thrombosis. The grafts in the surviving mice were all patent. There was no aneurysmal formation or graft rupture in both groups. These findings were confirmed by autopsy within 24 hours after death or severe paralysis of lower limbs.
3.2. Reduced wall thickness in tropoelastin-treated grafts
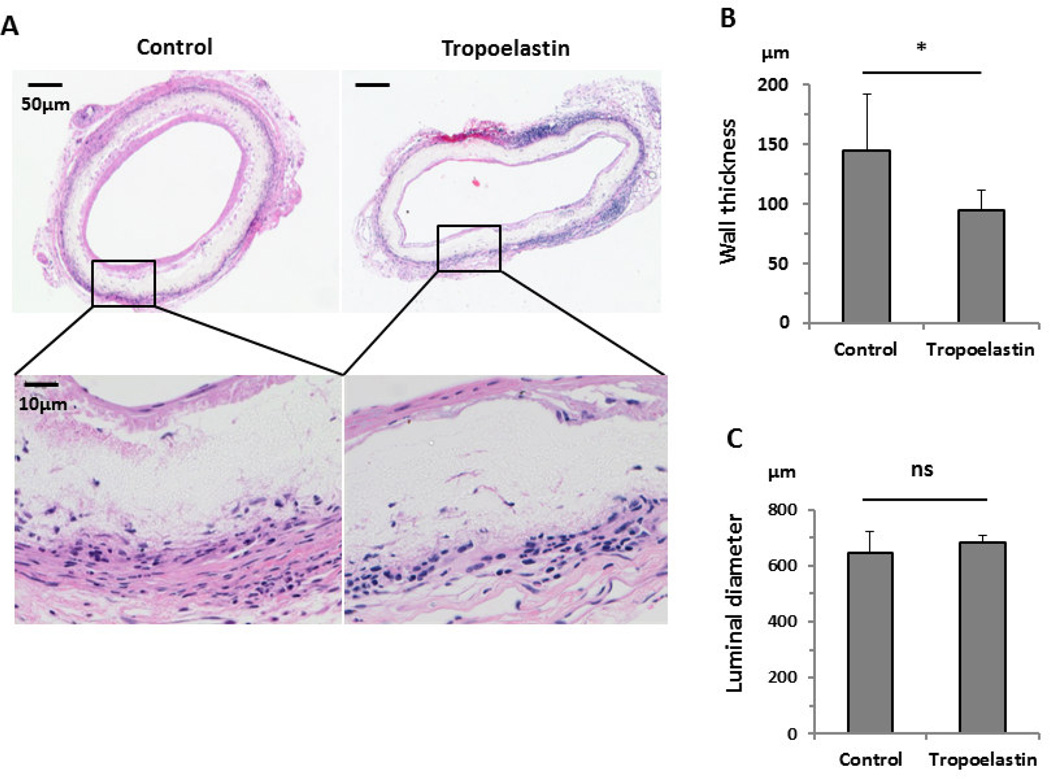
H&E staining showed slight cell infiltration within the scaffolds in both groups, and the cell infiltration main come from adventitia (Fig. 2A). The wall thickness of the grafts (excluding the connective tissue surrounding the graft) in the Unseeded group was significantly greater than in the Tropoelastin seeded group (Unseeded group: 145 ± 47 µm vs. Tropoelastin seeded group: 94 ± 17 µm, P=.047) (Fig. 2B). In contrast, the lumen diameter was unaffected (Unseeded group: 648 ± 75 µm vs. Tropoelastin seeded group: 682 ± 25 µm, P=.36) (Fig. 2C).
Fig. 2.
Histology and morphometric data of control and tropoelastin-seeded bioresorbable arterial vascular grafts. (A) H&E staining showed cell infiltration within the scaffolds, in both groups, 8 weeks after implantation. (B) Tropoelastin significantly decreased the wall thickness of the graft. (C) There was no significant difference between groups in the luminal diameter. *: P < 0.05, ns: no significant difference.
3.3. Comparable elastin and collagen deposition in both graft groups
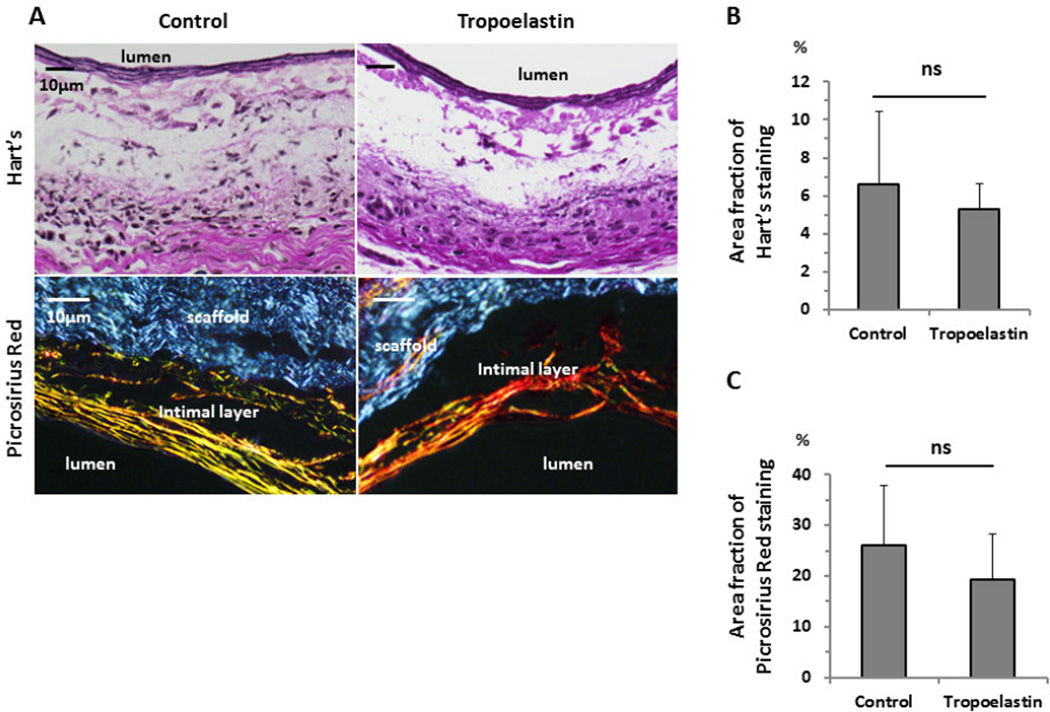
Hart’s staining was used to evaluate elastin deposition within the scaffold (Fig. 3A). There was no statistical difference between groups in the fraction of positively stained area (Unseeded group: 6.6 ± 3.9 % vs. Tropoelastin seeded group: 5.3 ± 1.3 %, P=.45) (Fig. 3B). Visualization of Picrosirius Red staining with polarized light microscopy was performed to evaluate the collagen fibers (type I and III) in each group for quantitative measurement of collagen deposition in the neointimal layer. There was no statistical difference between the groups in the deposition of collagen type I and III in the neointimal layer (Unseeded group: 26.0 ± 11.9 % vs. Tropoelastin seeded group: 19.2 ± 9.0 %, P=.31) (Fig. 3C).
Fig. 3.
Elastin and collagen type I and III deposition within control and tropoelastin seeded bioresorbable arterial vascular grafts. (A) Representative images of Hart’s (elastin) and Picrosirius Red staining. Visualization of Picrosirius Red staining with polarized light microscopy showed collagen fibers (type I and III) in the neointimal layer in each group. The groups showed the same extent of positively stained areas for (B) Hart’s and (C) Picrosirius Red staining. ns: no significant difference.
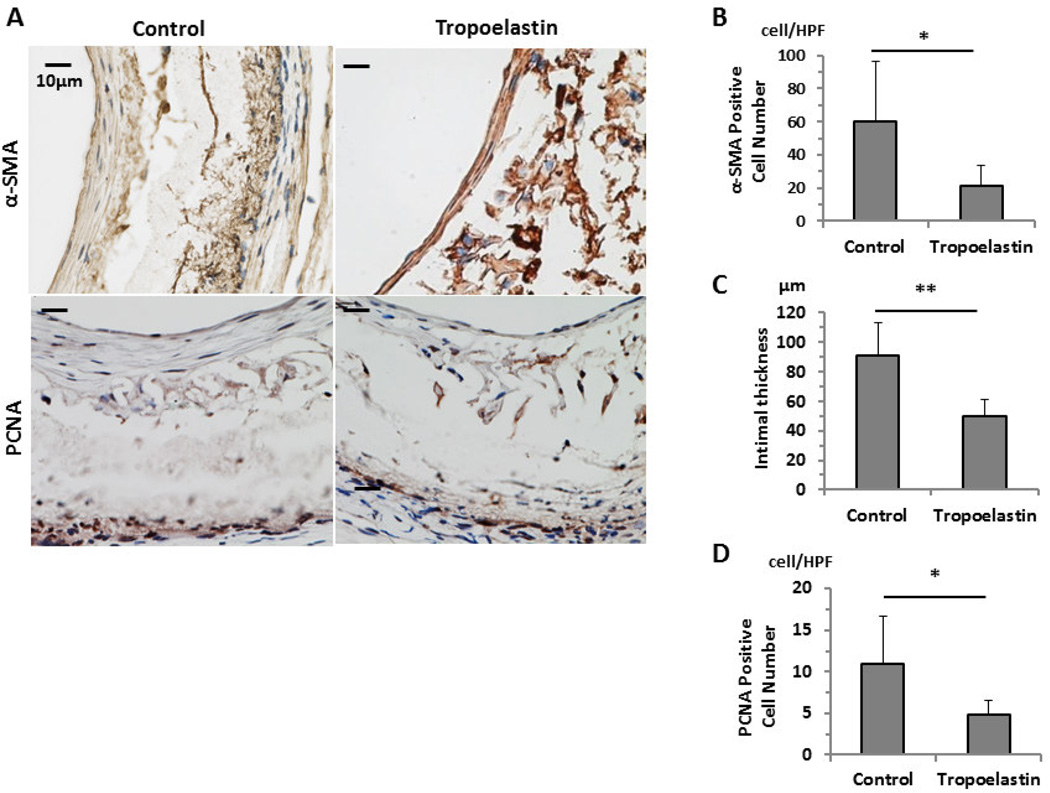
3.4. Decreased smooth muscle cell proliferation in tropoelastin-treated grafts
Tropoelastin treatment reduced the number of smooth muscle cells (Fig. 4A) as evidenced by reduced immunohistochemical staining for α-SMA (Unseeded group: 60 ± 36/HPF vs. Tropoelastin seeded group: 21 ± 13/HPF, P=.048, HPF; high power field) (Fig. 4B). The thickness of the intimal layer was significantly reduced in the Tropoelastin seeded group (Unseeded group: 91 ± 22 µm vs. Tropoelastin seeded group: 50 ± 11 µm, P=.004) (Fig. 4C). Immunohistochemical staining for PCNA was performed to evaluate smooth muscle cell proliferation in the neointimal layer. Tropoelastin-treated vessels displayed decreased PCNA staining, pointing to suppressed neointimal smooth muscle proliferation (Unseeded group: 11.0 ± 5.6/HPF vs. Tropoelastin seeded group: 4.8 ± 1.7/HPF, P=.043) (Fig. 4D).
Fig. 4.
Immunohistochemistry and quantification data of control and tropoelastin seeded bioresorbable arterial vascular grafts. (A) Representative immunohistochemical images of smooth muscle cells (α-SMA) and PCNA. (B) Tropoelastin significantly reduced the number of cells that stained positive for α-SMA. (C) Tropoelastin significantly reduced the thickness of the intimal layer. (D) Tropoelastin significantly reduced the number of cells that stained positive for PCNA in the SMC layer. HPF: high power field. *: P < 0.05, **: P < .01.
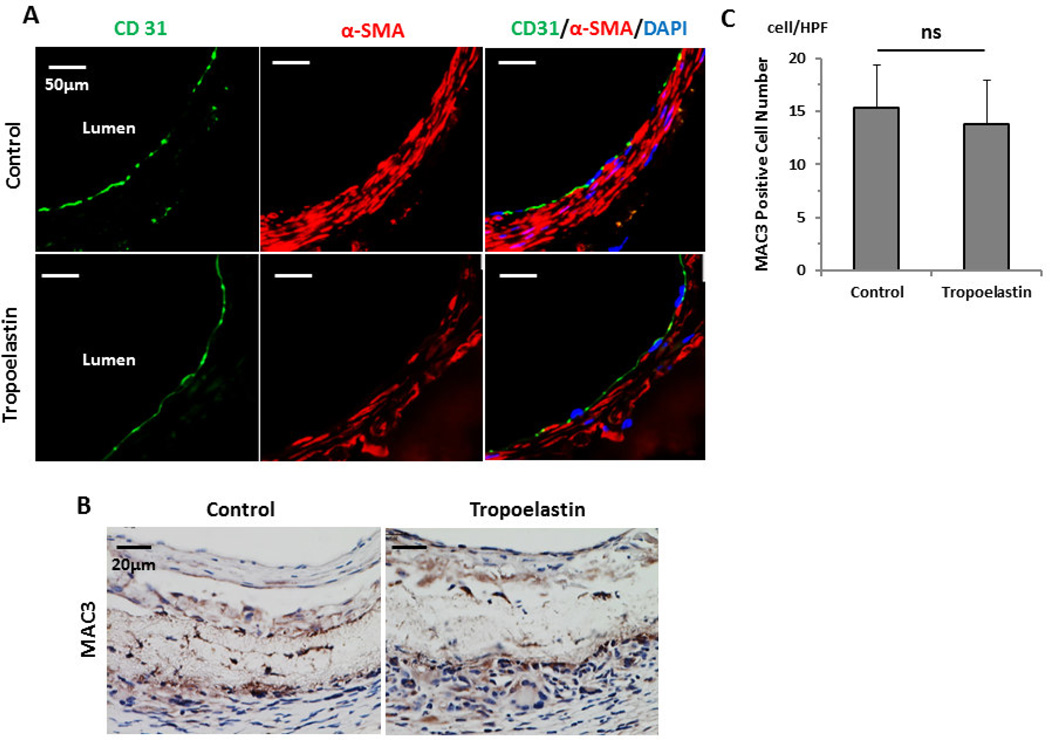
3.5. Tropoelastin did not affect endothelialization or inflammation
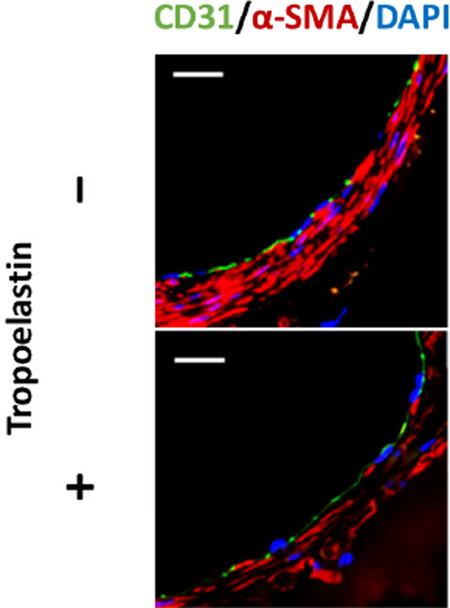
CD31 staining identified endothelial cell coverage on the luminal side of the scaffolds, which was internal to the SMC layer in both groups equally (Fig. 5A). There was no significant difference between groups in the number of cells staining positive for MAC3 (Unseeded group: 15.3 ± 4.1/HPF vs. Tropoelastin seeded group: 13.8 ± 4.2/HPF, P=.58) (Fig. 5B, C)
Fig. 5.
Endothelialization and macrophage infiltration of control and tropoelastin seeded bioresorbable arterial vascular grafts. (A) Representative immunofluorescent images of endothelial cells (CD31) and smooth muscle cells (α-SMA). A layer of endothelial cell coverage on the luminal side of the scaffolds is surrounded by a SMC layer in both groups equally. (B) Representative immunohistochemical images for MAC3. (C) MAC3 levels for the two groups were indistinguishable. HPF: high power field. ns: no significant difference.
4. Discussion
In the present study, we demonstrate that a tropoelastin seeded bioresorbable arterial vascular graft inhibits SMC proliferation and prevents neointimal hyperplasia. Although SMCs are an essential component of neotissue development in bioresorbable vascular grafts, neointimal hyperplasia, which results from the activation, proliferation and migration of SMCs, is a detrimental condition leading to graft stenosis. However, vascular SMCs are able to exist in a quiescent contractile state or a proliferative non-contractile state.14 The elastin matrix is a potent autocrine factor that regulates arterial morphogenesis by instructing vascular SMCs to localize around the elastic fibers and remain in a quiescent, contractile state.8 This cell-matrix interaction is mediated via a heterotrimeric G-protein signaling pathway that activates downstream rho GTPases where it stabilizes and maintains the structure of the mature artery. When this morphogenic signal is absent during arterial development, the unregulated migration and proliferation of vascular SMCs results in occlusion of the arterial lumen.15 This beneficial effect of tropoelastin is unlikely to be accounted for solely by interactions between SMCs and tropoelastin; contributions embrace those due to cross-talk between cell populations, particularly endothelial cell upregulation of nitric oxide (Hiob et al. in press) and concomitant SMC proliferative control.
There was no significant difference between groups in elastin deposition 8 weeks after implantation. This implies that the regulatory function for tropoelastin was in the early phase of neotissue formation where it served to reduce SMC proliferation. After a good elastic fiber layer is formed, it could regulate SMC proliferation and prevent neointimal hyperplasia. In a range of studies, we have quantified tropoelastin persistence when presented as a monolayer in vitro and in vivo and confirm tropoelastin’s persistence9.
Numerous in vitro studies have demonstrated the ability of matrix proteins such as collagen, fibronectin and laminin to affect SMC activity, including phenotypic modulation, migration and proliferation.16, 17 These data might suggest that there is overlap with regard to the function of different vascular matrix proteins. Elastin, however, is unique among matrix elements in that the disruption of this gene leads to vascular SMC proliferation.8 Disruption of other genes encoding vascular matrix proteins, including fibulin, fibrillin and collagen, is associated with arterial tortuosity, dissection or aneurysm formation in vivo.18–20 However, those proteins are not associated with proliferative or occlusive vascular pathology. In addition, there was no significant difference in collagen deposition in this study. Thus, elastin, when compared with other matrix proteins, is effective in regulating SMC regeneration. It was not possible to reliably quantify or assess the stability of this monolayer coating of molecular tropoelastin due to the small amounts of protein on these surfaces, which is exacerbated during prolonged incubation in vitro and in vivo where there is a plethora of other molecules.
Previously, emphasis was placed on the major role of inflammatory cells in regulating vascular SMCs through the secretion of cytokines and growth factors.6, 21 In this study, however, there was no significant difference in macrophage numbers in the scaffold between the groups. In situ degradation of elastin by macrophages and their proteases might act to release vascular SMCs from their mature contractile state to migrate, proliferate and form neointimal hyperplasia.
There are several limitations in this study. One limitation is from a clinical standpoint. Small caliber human grafts would be 2–5 mm in diameter and it is not clear if the tropoelastin seeding would make a significant difference in the neointimal thickness in larger grafts. Another limitation is the luminal roughness of the graft. Luminal roughness is one of the most important factors in the thrombogenicity of a graft. We observed more acute thrombosis in the Tropoelastin treated group, which can be accounted for by occasional tropoelastin aggregation in these cases contributing to a rougher luminal surface on the scaffold. Also we note that the benefit of tropoelastin was not to change the inherent dimensions of the conduit, but to mainly deliver to a substantial reduction in risk of occlusion by decreasing the number of SMCs.
In conclusion, the present study demonstrates that tropoelastin treatment of bioresorbable arterial grafts controls the proliferation of vascular SMCs and reduces neointimal hyperplasia in vivo. These data provide a proof of concept that seeding tropoelastin could be a useful method to prevent intimal hyperplasia for bioresorbable arterial vascular grafts and lay the foundation for large animal studies.
Acknowledgments
This study was supported in part by grants from the National Institutes of Health (R01-HL098228 to Dr. Breuer) and from Gunze Ltd. (Kyoto, Japan) (Drs. Breuer and Shinoka). Dr. Sugiura was the recipient of a Funding Award from Kanae Foundation for the Promotion of Medical Science (Tokyo, Japan) and from Astellas Foundation for Research on Metabolic Disorders (Tokyo, Japan) in 2013. Dr. Tara was recipient of a Banyu Fellowship from Banyu Life Science Foundation International (Tokyo, Japan) in 2012. We thank Dr. Suzanne Mithieux for treating the constructs with tropoelastin. Dr. Weiss acknowledges grant support from the Australian Research Council and the National Health & Medical Research Council. Part of this research was funded by NIH EB014283 (A.S.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statements
Dr. Weiss is the Scientific Founder of Elastagen Pty Ltd. The authors declare no conflict of interest.
References
- 1.Kannan RY, Salacinski HJ, Butler PE, Hamilton G, Seifalian AM. Current status of prosthetic bypass grafts: a review. Journal of biomedical materials research Part B, Applied biomaterials. 2005;74(1):570–581. doi: 10.1002/jbm.b.30247. [DOI] [PubMed] [Google Scholar]
- 2.Tara S, Kurobe H, Maxfield MW, Rocco KA, Yi T, Naito Y, Breuer CK, Shinoka T. Evaluation of remodeling process in small-diameter cell-free tissue-engineered arterial graft. J Vasc Surg. 2014 doi: 10.1016/j.jvs.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Tara S, Kurobe H, Rocco KA, Maxfield MW, Best CA, Yi T, Naito Y, Breuer CK, Shinoka T. Well-organized neointima of large-pore poly(l-lactic acid) vascular graft coated with poly(l-lactic-co-epsilon-caprolactone) prevents calcific deposition compared to small-pore electrospun poly(l-lactic acid) graft in a mouse aortic implantation model. Atherosclerosis. 2014;237(2):684–691. doi: 10.1016/j.atherosclerosis.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, Yi T, Mirensky TL, Nalbandian A, Udelsman B, Hibino N, Shinoka T, Saltzman WM, Snyder E, Kyriakides TR, Pober JS, Breuer CK. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(10):4669–4674. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goumans MJ, Liu Z, ten Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19(1):116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 6.Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89(3):957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130(2):411–423. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 9.Wise SG, Yeo GC, Hiob MA, Rnjak-Kovacina J, Kaplan DL, Ng MK, Weiss AS. Tropoelastin: a versatile, bioactive assembly module. Acta Biomater. 2014;10(4):1532–1541. doi: 10.1016/j.actbio.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res. 1967;20(1):99–111. doi: 10.1161/01.res.20.1.99. [DOI] [PubMed] [Google Scholar]
- 11.Roh JD, Nelson GN, Brennan MP, Mirensky TL, Yi T, Hazlett TF, Tellides G, Sinusas AJ, Pober JS, Saltzman WM, Kyriakides TR, Breuer CK. Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model. Biomaterials. 2008;29(10):1454–1463. doi: 10.1016/j.biomaterials.2007.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holst J, Watson S, Lord MS, Eamegdool SS, Bax DV, Nivison-Smith LB, Kondyurin A, Ma L, Oberhauser AF, Weiss AS, Rasko JE. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat Biotechnol. 2010;28(10):1123–1128. doi: 10.1038/nbt.1687. [DOI] [PubMed] [Google Scholar]
- 13.Mirensky TL, Nelson GN, Brennan MP, Roh JD, Hibino N, Yi T, Shinoka T, Breuer CK. Tissue-engineered arterial grafts: long-term results after implantation in a small animal model. J Pediatr Surg. 2009;44(6):1127–1132. doi: 10.1016/j.jpedsurg.2009.02.035. discussion 32–3. [DOI] [PubMed] [Google Scholar]
- 14.Owens GK. Molecular control of vascular smooth muscle cell differentiation. Acta Physiol Scand. 1998;164(4):623–635. doi: 10.1111/j.1365-201x.1998.tb10706.x. [DOI] [PubMed] [Google Scholar]
- 15.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393(6682):276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 16.Raines EW. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int J Exp Pathol. 2000;81(3):173–182. doi: 10.1046/j.1365-2613.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedin U, Roy J, Tran PK, Lundmark K, Rahman A. Control of smooth muscle cell proliferation--the role of the basement membrane. Thromb Haemost. 1999;82(Suppl 1):23–26. [PubMed] [Google Scholar]
- 18.Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415(6868):168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 19.Arteaga-Solis E, Gayraud B, Ramirez F. Elastic and collagenous networks in vascular diseases. Cell Struct Funct. 2000;25(2):69–72. doi: 10.1247/csf.25.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dietz HC, Mecham RP. Mouse models of genetic diseases resulting from mutations in elastic fiber proteins. Matrix Biol. 2000;19(6):481–488. doi: 10.1016/s0945-053x(00)00101-3. [DOI] [PubMed] [Google Scholar]
- 21.Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]