Abstract
Functional gastrointestinal disorders (FGIDs) are common worldwide and cover a wide range of disorders attributable to the gastrointestinal tract that cannot be explained by structural or biochemical abnormalities. The diagnosis of these disorders relies on the symptom-based Rome criteria. In 2016 the Rome criteria were revised for infants/toddlers and for children and adolescents. In this review, we discuss the novel Rome IV criteria for infants and toddlers. The criteria for infant colic were drastically changed, whereas only minor changes were made for regurgitation, cyclic vomiting syndrome, functional diarrhea, infant dyschezia and functional constipation. In addition to this, the new Rome IV discusses underlying mechanisms of pain in infants and toddlers, including the neuro-development of nociceptive and pain pathways, the various factors that are involved in pain experience, and methods of pain assessment in infants and toddlers is essential for the clinician who encounters functional pain in this age group. Overall, the Rome IV criteria have become more distinctive for all disorders in order to improve the process of diagnosing pediatric FGIDs.
Keywords: Functional gastrointestinal disorders, Infant, Toddlers
INTRODUCTION
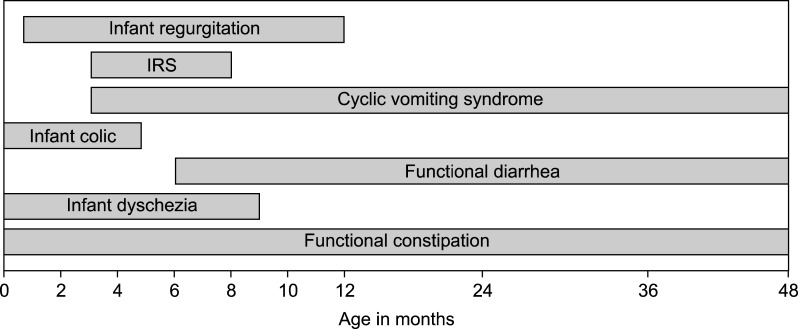
Functional gastrointestinal disorders (FGIDs) in infants and toddlers are common worldwide and cover a variety of disorders associated with chronic, recurrent symptoms attributable to the gastrointestinal tract, but not explained by structural or biochemical abnormalities [1]. The diagnosis of these disorders relies on symptom-based criteria, the so-called Rome criteria, which find their origin in 1994 [2]. A group of gastroenterology experts gathered in the city of Rome and created a classification system with diagnostic criteria for FGIDs in adults. These criteria were based on literature review and consensus process and considered as Rome I. The Rome working group updated the criteria in 1999 (the Rome II criteria); in this publication, specific standardized criteria for FGIDs in children were also provided by a group of pediatric gastroenterology experts [3]. The pediatric Rome II criteria were mainly based on knowledge of FGIDs in adults and a consensus process, because at that time literature on FGIDs in children was scarce. Since the publication of the Rome II criteria, the literature concerning these disorders in children has expanded. This led to revisions of the criteria and in 2006 the Rome III criteria were presented [4]. With the introduction of the Rome III criteria a distinction was made between FGIDs in younger children (neonate/toddler) and older children (child/adolescent) [5,6]. Over the past decade, knowledge regarding FGIDs in neonates and toddlers has further improved, which has resulted in the recently published Rome IV criteria [1]. Fig. 1 shows the various FGIDs in children under 4 years of age and the age at which they occur. The complete Rome IV criteria are provided in Appendix 1 [1].
Fig. 1. Rome IV diagnoses in infants and toddlers and the age at which they may occur.
IRS: infant rumination syndrome.
IMPACT
Reported prevalence rates of FGIDs in neonates and toddlers vary between 27.1% and 38.0%, with the most prevalent disorders being infant regurgitation and functional constipation (1-25.9% and 1-31%, respectively) [7]. Infants and toddlers with an FGID display a reduced quality of life and visit medical professionals more often compared to healthy controls [8]. Moreover, the impact on the families of affected children is considerable. Recurrent unexplained symptoms in young children can cause concerns for caretakers, especially because young children are unable to adequately describe emotions or pain. Whether these parental concerns result in health care utilization or not depends on the coping style of parents, their perception of their child's symptoms and previous experiences they have had. During a consultation, clinicians should realize that these parental factors are as important as the child's symptoms. Especially because the diagnosis of an FGID mainly relies on parental reports and interpretations of their child's symptoms. This makes the diagnostic process challenging and underlines the importance of strict criteria for FGIDs in this age group.
In this review article, we will discuss FGIDs that may occur in neonates and toddlers, focusing on the epidemiology, pathophysiology, diagnostic Rome IV criteria and treatment options. Only two population-based epidemiological studies assessing the prevalence of FGIDs in neonates and toddlers according to the Rome III criteria have been published in the English literature. van Tilburg et al. [8] conducted a community-based survey study amongst 1,447 mothers in the United States (US) assessing the prevalence of FGIDs through maternal reports based on the Questionnaire on Pediatric Gastrointestinal Symptoms-Rome III Version (QPGS-RIII). Chogle et al. [9], surveyed the parents of children aged 0-48 months presenting at primary care clinics for a well-child visit in Colombia, they used a Spanish translation of the QPGS-RIII.
INFANT REGURGITATION
Prevalence
Infant regurgitation is the most common FGID in infants (<12 months of age) with reported prevalence rates ranging from 8-26% in this age group [8,9]. At the peak age around 2-4 months, prevalence rates have been reported to be as high as 67-87% [10,11]. This wide range in prevalence rates might be explained by the different criteria that have been used to define regurgitation in the past [12].
Presentation
When stomach contents move back into the esophagus, mouth, and/or nose involuntarily, this is called gastroesophageal reflux (GER). The term regurgitation is used when these gastric contents can be visualized. This phenomenon is part of the normal development of an infant and uncomplicated regurgitation is therefore not a sign of pathology. Contrarily, gastroesophageal reflux disease (GERD) defines the situation where GER leads to complications or contributes to tissue damage or inflammation [13]. In 2009, the guideline published by the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and the European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) discriminated between infant regurgitation and GERD by adding “troublesome symptoms” as a diagnostic criterion for GERD [13]. Although infant regurgitation is a frequent reason for parental concern, it resolves spontaneously with age and it is not associated with negative long-term consequences.
Rome IV
The Rome Working Committee agreed to leave “troublesome” symptoms out of the new Rome IV criteria, since quantitative methods to define “troublesome” are lacking and infants are not able to communicate if they are bothered by certain symptoms. No revisions were made for infant regurgitation in the Rome IV compared to the Rome III [1,5].
Treatment
The most important part of treatment is reassurance. The relationship between the caregiver and the child can be improved by redeeming the caregiver's fears about their child's condition. Furthermore, sources of physical and emotional distress should be identified and a suitable plan to abate them needs to be made [1]. Medical interventions are not required in the management of regurgitation. Additional conservative treatments include thickened feedings, anti-regurgitation formulas and positioning after meals [14,15,16,17].
RUMINATION SYNDROME
Prevalence
Data regarding the prevalence of rumination syndrome in infants and toddlers are scarce and have only been published recently. van Tilburg et al. [8] showed that the prevalence of rumination syndrome was 2.4% in infants <1 year of age, and 1.9% in 1-3 year old children. Chogle et al. [9] found higher prevalence rates in these age groups (7.2% and 2.9%, respectively).
Presentation
In infant rumination syndrome, infants habitually and voluntarily regurgitate stomach contents into the oral cavity as an outing of self-stimulatory behavior, which is thought to arise in the context of longstanding social deprivation. Rumination syndrome may present clinically in patients from all ages, from infants to adults, and may occur in neurologically impaired children and adults [18].
Rome IV
Only two small changes have been made in the Rome IV criteria for rumination syndrome compared to the Rome III criteria. The duration of complaints was changed in 2 months instead of 3 months in order to be consistent with the criteria for rumination for the older age groups. Furthermore, the word nausea has been eliminated from the criteria due to the difficulty of assessing this symptom in infants.
Treatment
Malnutrition is one of the major concerns in infant rumination syndrome, since previously swallowed food is repeatedly and excessively lost. Therefore, management aims at eliminating the need for ruminative behavior in order to improve the infant's nutritional status [1].
CYCLIC VOMITING SYNDROME
Prevalence
Cyclic vomiting syndrome (CVS) occurs from infancy to middle-age adulthood, with a peak prevalence between 2 and 7 years [19]. Based on maternal reports, the prevalence of CVS in the US was 0.0% in infants <1 year, and 3.4% in toddlers [8]. The population-based survey study from Colombia found the prevalence of CVS to be 3.8% in infants <1 year and 6.1% in children from 1-4 years of age [9].
Presentation
CVS is presented as stereotypic, recurrent episodes of vomiting which last hours to days with symptom-free interval periods which last weeks to months [20]. In a series of 71 patients, the frequency of episodes varied from 1 to 70 per year, with an average of 12 per year [19]. Vomiting attacks may occur sporadically or at regular intervals, and they are characterized by onset at the same time of day, most often late at night or early in the morning.
Rome IV
For CVS, the most important decision regarding the Rome IV criteria involves the amount of recurrent episodes required to fulfil the diagnostic criteria. The committee reviewed the NASPGHAN Cyclic Vomiting Syndrome Consensus Statement [20] and International Headache Society [21] guideline for CVS, which recommend a minimum of 5 attacks of intense nausea and vomiting in any interval for the diagnosis of CVS in children. These statements criticized the minimum of 2 attacks in the Rome III criteria for lacking specificity. However, no studies have validated the NASPGHAN and International Headache guidelines. Furthermore, if a lack of specificity was suspected, one would expect significantly higher prevalence rates of studies using Rome III criteria. In contrast, 5 recent studies in infants, toddlers, children and adolescents using the Rome III criteria did not support this observation [22,23,24,25]. Based on these data and based on the impact of a CVS attack for the quality of life of the child and the family, the Rome IV working group decided that early diagnosis is important and left the minimum number of 2 episodes to diagnose CVS unchanged. Finally, the word nausea has been left out, as the working group is of the opinion that this symptom is difficult to assess in infants due to the inability to communicate the presence of nausea.
Treatment
Treatment focusses on two aspects of the CVS attacks. One aspect is the prevention of episodes in children who suffer from frequent, severe and prolonged attacks. Daily treatment with cyproheptadine, pizotifen, amitriptyline, propranolol or erythromycin can diminish the frequency of episodes or can even prevent them entirely [20]. The other aspect is to reduce the severity of the attacks. Oral acid-inhibiting drugs for the protection of esophageal mucosa and lorazepam for antiemetic, anxiolytic and sedative purposes can be considered.
INFANT COLIC
Prevalence
Prevalence rates for infant colic vary widely, mainly due to the use of different definitions in studies. A recent systematic review on outcome measures used in trials for infant colic, demonstrated that in 39 trials 20 different definitions for colic had been used [26]. According to a systematic review from 2001, prevalence rates for infant colic varied from 3-28%, based on 8 included prospective studies [27]. A more recent systematic review reported prevalence rates ranging from 2-73%, with a median of 17.7% [12]. Furthermore, two recent population-based survey studies in the US and Colombia reported prevalence rates of 5.9% and 10.4% respectively, based on the Rome III criteria [8,9]. One has to bear in mind that aside from the different definitions that are used, the prevalence of infant colic is affected by parental perception of the intensity and duration of crying episodes [28,29], methods used to collect data about crying, parents' well-being [30], and culturally dependent infant care practices [31].
Presentation
Infant colic can be considered as a behavioral phenomenon in infants aged 1 to 4 months and involves long periods of inconsolable crying and hard-to-calm behavior. The crying occurs for no apparent cause and this is one of the main reasons it is distressing and worrisome for parents [32]. The term “colic” refers to unexplained and acute abdominal pain. It is often assumed by clinicians that crying in infant colic has a gastrointestinal cause and that is why children with infant colic are commonly referred to pediatric gastroenterologists [33,34]. However, whether infant colic truly has a gastrointestinal origin is debatable [35]. The pathophysiology of infant colic is incompletely understood, but it has been postulated that it is caused by various factors such as a disturbance of pathways in the central nervous system [36,37,38,39], psychosocial causes (e.g., inadequate infant-family relationship or parental anxiety) [40,41,42], or gastrointestinal-related causes as cow's milk allergy, GER or motility disorders of the gastrointestinal tract [43]. Over the past decades, interest in the role of the microbiome in the pathophysiology of infant colic has rapidly increased. It has been suggested that altered microbiota affect gut motility and gas production, leading to colicky behavior [44]. Some studies have shown that the intestinal microbiota of infants with colic shows a lower microbiota diversity compared to healthy controls [45,46]. However, in most cases excessive crying remains unexplained and probably represents the upper end of the normal developmental “crying curve" of healthy infants [47,48,49]. In general, the crying of healthy babies increases after birth, reaches a peak around 5-6 weeks of gestational age and then declines at 3 months [29,49]. In less than 5% of children who present with inconsolable crying, an underlying organic disease is found [50]. Conclusively, it still uncertain whether infant colic reflects a normal developmental phenomenon, a central nervous system disbalance, an environmental disturbance or gastrointestinal discomfort. Nonetheless, it is a self-limiting disease that, in the absence of other alarming symptoms, should be treated with education and reassurance [51].
Rome IV
A major revision has been made for the criteria of infant colic in Rome IV; the modified Wessel's criteria used in Rome III were abandoned. The modified Wessel's criteria required the crying to occur for more than 3 hours per day, on at least 3 days per week of the last week described by Wessel et al. [52] (hence these criteria were referred to as Wessel's rule of threes). The committee decided to exclude Wessel's rule of threes from the Rome IV criteria, as it was stated that a minimum of 3 hours per day was too arbitrary since no evidence exists that a child who cries 2 hours and 50 minutes per day differs from one who cries 3 hours per day [53]. Furthermore, in some cultures infants cry more than in others and this makes the criteria culturally dependent [31]. In addition, filling out behavior diaries for 7 days is considered to be intensive and time-consuming, hence it is a struggle for parents to complete them [1]. Another reason for abandoning Wessel's rule was the focus on crying amount instead of the prolonged, unsoothable character of the crying episodes, which has been demonstrated to distress caregivers [54]. Moreover, the term ‘paroxysmal’ is left out, since evidence is lacking to support that infant colic differs in sound and starts more abrupt compared to normal crying bouts [55,56]. Finally, the Rome IV committee decided to add two criteria that have to be fulfilled for the diagnosis of infant colic in clinical research. In a telephone or face-to-face consult with a researcher or clinician, parents have to report that their infant has cried or fussed for 3 or more hours per day, during 3 or more days in the preceding week. In addition, parents have to keep a 24-hour behavior diary to confirm that the total amount of crying and fussing is more than 3 hours per 24 hours.
Treatment
One of the most important goals of treatment for infant colic is to help caregivers cope with their infant's symptoms and to provide support for the infant-family relationship [57]. Following the crying, caregivers may develop frustrations and insecurities concerning their nurturing skills [58,59]. It is essential for clinicians to recognize this and to offer continuous support, in order to influence the way parents view their ability to care for the child [60].
Moreover, recent evidence for the treatment of infant colic with Lactobacillus reuteri DSM 17938 is promising. Several randomized controlled trials demonstrated a favorable effect of this probiotic strain in infants with colic compared to healthy controls [61,62]. However, another recent randomized double-blind controlled trial with a larger amount of participants did not show this advantage of L. reuteri [63]. In addition, a systematic review reported that evidence for probiotic use for the management of infant colic, in particular in formula-fed infants, was lacking [64].
FUNCTIONAL DIARRHEA
Prevalence
Population-based survey studies utilizing the Rome III criteria have reported the prevalence of functional diarrhea in infants to be 2.4% in the US and 1.9% in Colombia [8,9]. For toddlers, the prevalence has been shown to be 6.4% in the US, whereas in children 1-4 years of age the prevalence was 0.5% in Colombia [8,9].
Presentation
Despite having frequent unformed stools, children with functional diarrhea do not suffer from failure to thrive as long as the caloric intake is adequate. The child appears unperturbed by the frequent, loose stools and the symptoms resolve spontaneously by the time the child reaches the school age. Functional diarrhea is therefore also called toddler's diarrhea. Dietary factors such as overfeeding, excessive fruit juice consumption, excessive carbohydrate (fructose) ingestion with low fat intake, and excessive sorbitol intake have been suggested to play a role in the pathophysiology of functional diarrhea.
Rome IV
In Rome IV, the defecation frequency required for the diagnosis of functional diarrhea has changed from 3 to 4 stools per day, based on findings from the study by van Tilburg et al. [8] which showed that a frequency of 3 stools per day is common in young children. Moreover, the passing of stools during sleep has been abandoned because of its low specificity; 25% of mothers reported that their child passes stools in their sleep [8]. These changes will likely influence the results of future epidemiological studies.
Treatment
Parents need to be educated and reassured; this condition is not harmful and is indeed self-limiting. It is recommended to evaluate fruit juice and fructose intake and to provide the parents with adequate dietary advice to normalize the child's diet. No medical interventions are needed.
INFANT DYSCHEZIA
Prevalence
According to the Rome III criteria, the prevalence of infant dyschezia in infants under 6 months of age has been reported to be 2.4% in the US and 3.2% in Colombia. A study in the Netherlands found that at the ages of 1 and 3 months, 3.9% and 0.9% infants, respectively, fulfilled the Rome III criteria for infant dyschezia. In the same study, 0.9% of 9-month old infants would have fulfilled the diagnostic criteria for infant dyschezia if the maximum age limit for this diagnosis had not been 6 months according to the Rome III criteria.
Presentation
Infant dyschezia is characterized by straining, screaming, crying, and turning red or purple in the face while making an effort to defecate in a child that has daily soft stools. These symptoms usually persist for 10-20 minutes, which can be distressing to parents. The underlying mechanism behind infant dyschezia is considered to be related to the failure to coordinate increased intra-abdominal pressure with relaxation of the pelvic floor muscles. Infant dyschezia is easily mistaken for constipation and it is important to distinguish between these two disorders.
Rome IV
Based on the study by Kramer et al. [65], the age limit for this diagnosis has been modified and is now 9 months in Rome IV. Furthermore, straining and crying which are characteristic symptoms for infant dyschezia no longer have to be associated with successful defecation only, but may also be associated with unsuccessful passage of stools.
Treatment
Since this behavior resolves spontaneously, effective reassurance is of key importance in the management of infant dyschezia and medical interventions are not necessary. Caregivers are advised to avoid rectal stimulation, since this may be disturbing for the child or may condition the child to wait for stimulation to defecate. There is no need for laxatives.
FUNCTIONAL CONSTIPATION
Prevalence
The reported prevalence of functional constipation in infants and toddlers varies between studies and ranges from 5% to 27% [8,9]. The prevalence in toddlers is frequently reported to be higher than in infants [8,9], which is in line with recent findings from a retrospective chart review study which described that the median age of onset of functional constipation in children was 2.3 years [66].
Presentation
The presentation of functional constipation may vary in young children. Symptoms may include hard, painful bowel movements and toddlers sometimes exhibit fecal incontinence. Withholding behavior is considered to play an important role in the pathophysiology of functional constipation in young children; unpleasant experiences with defecation (most notably painful defecation) may cause the child to voluntarily retain their stools to avoid this unpleasant experience. This can lead to a vicious cycle, where voluntary stool retention leads to increased water absorption from the feces and thereby harder stools and inherently more difficult and painful defecation.
Rome IV
In Rome IV, a differentiation has now been made between children who are toilet trained and children who are not. This is especially relevant for the criterion of fecal incontinence and for the description of large stools. Furthermore, the criteria for infants and toddlers and those for children and adolescents have been adjusted to match one another in Rome IV.
Treatment
Non-pharmacological treatment for functional constipation consists of education, demystification, regular dietary advice (sufficient fiber and fluid intake) and in older children toilet training, a reward system and a stool diary [67]. It is important to reduce fear and, if possible, to make the child and parents understand the underlying pathophysiological mechanisms and the need to learn how to recognize these in daily life. If a fecal mass is suspected to be present, disimpaction should be attempted, followed by maintenance treatment with laxatives. For both the disimpaction phase and the maintenance phase, polyethylene glycol is the primary preferred medication [68].
PAIN
Pain is an important component of many FGIDs in neonates and toddlers. A better understanding of the neurodevelopment of nociception, the various factors that are involved in pain experience, and methods of pain assessment in infants and toddlers is essential for the clinician who encounters functional pain in this age group. The traditional acute pain model, which describes pain as a signal following anatomic or biochemical pathology, is not suitable for the management of functional pain, in which the pain does not function as a warning of a demonstrable anatomic or biochemical substrate but where there is a dysfunction in the mechanisms involved in pain perception. Moreover, this model does not consider the influence of other elements on the interpretation and response to nociceptive information, such as psychosocial factors, environmental factors, genetic predisposition and impaired pain regulatory systems [69].
Development
The development of nociceptive systems starts as early as the prenatal period, with the development of cutaneous innervation in infants with a gestational age of 8 weeks. Between 10 and 15 weeks of gestational age, afferent synapses to the spinal cord develop and the spinal cord is laminated. Thalamocortical pathways begin to function around 30 weeks of gestational age and at this moment the infant is capable to perceive pain [70,71,72]. In early life, the pain threshold is low and this threshold increases with age. In combination with a decreased inhibitory control for modulating pain experiences, infants may experience painful stimuli more intense than older children [72,73]. On the long-term, painful experiences in newborn infants may result in prolonged alterations in pain processing mechanisms, which may result in visceral hyperalgesia at a later age [71,74,75]. This is considered to be an important pathophysiological mechanism underlying functional abdominal pain.
Pain assessment
Several pain scoring instruments for adults and older children exist, which typically rely on self-report of pain on a numeric rating scale [76]. For younger children, modified rating scales with pain faces instead of numbers are used [77]. However, infants and most toddlers are not capable of scaling their pain and link an internal sensation to an associated image of a face on these scales. Despite the help caregivers provide by translating perceptions of their child's discomfort, it is a challenge for clinicians to assess pain directly from the child. Certain techniques, such as the evaluation of physiological factors (heart rate, blood pressure, oxygen saturation) and behaviors associated with pain (facial expressions, motoric behavior, crying), have been developed to support the clinician [1]. However, a study on behavioral pain assessment with infrared spectroscopy in pre-term babies who underwent heel stick procedures demonstrated that one-third of the babies showed evidence of a cortical hemodynamic response associated with pain while lacking an externally observable behavioral response. Consequently, the authors suggested that the available techniques for clinical assessment of pain in children might be inadequate [78]. The assessment of chronic pain compared to acute pain is even more difficult, since many of the expected behaviors related to (acute) pain might not be present in patients chronic pain. Unfortunately, no instruments are available at the moment for the assessment of pain by infants and toddlers.
CONCLUSION
In Rome IV, the diagnostic criteria for FGIDs in infants and toddlers have been refined. These revisions are expected to improve clinical care in infants and toddlers with FGIDs. Adequate diagnoses will enable choosing the appropriate treatment, which is expected to result in an improvement in the clinical outcomes of these patients. Furthermore, these internationally accepted criteria should be adhered to in future medical research, to establish homogeneity in study design, which enables comparison of study results.
ACKNOWLEDGEMENTS
M.A. Benninga was part of the pediatric working committee of the Rome Foundation which developed the Rome IV criteria for infants and toddlers discussed in this review. M.A. Benninga is a scientific consultant for Shire, Sucampo, Astrazeneca, Norgine, Zeria, Coloplast, Danone, Friesland Campina, Sensus, Novalac. The authors report no other relevant potential conflicts of interest.
Appendix 1
Childhood Functional Gastrointestinal Disorders: Neonate/Toddler
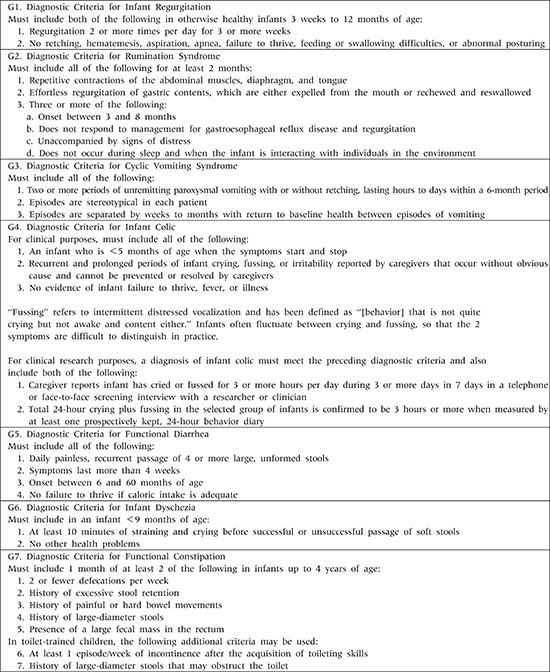
Adapted from Benninga et al. Gastroenterology 2016;150:1443-55.e2 [1].
References
- 1.Benninga MA, Faure C, Hyman PE, St James Roberts I, Schechter NL, Nurko S. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. 2016;150:1443–1455.e2. doi: 10.1053/j.gastro.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016;150:1262–1279.e2. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 3.Rasquin-Weber A, Hyman PE, Cucchiara S, Fleisher DR, Hyams JS, Milla PJ, et al. Childhood functional gastrointestinal disorders. Gut. 1999;45(Suppl 2):II60–II68. doi: 10.1136/gut.45.2008.ii60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Hyman PE, Milla PJ, Benninga MA, Davidson GP, Fleisher DF, Taminiau J. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. 2006;130:1519–1526. doi: 10.1053/j.gastro.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 6.Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira-Maia AP, Matijasevich A, Wang YP. Epidemiology of functional gastrointestinal disorders in infants and toddlers: a systematic review. World J Gastroenterol. 2016;22:6547–6558. doi: 10.3748/wjg.v22.i28.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Tilburg MA, Hyman PE, Walker L, Rouster A, Palsson OS, Kim SM, et al. Prevalence of functional gastrointestinal disorders in infants and toddlers. J Pediatr. 2015;166:684–689. doi: 10.1016/j.jpeds.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 9.Chogle A, Velasco-Benitez CA, Koppen IJ, Moreno JE, Ramírez Hernández CR, Saps M. A population-based study on the epidemiology of functional gastrointestinal disorders in young children. J Pediatr. 2016;179:139–143.e1. doi: 10.1016/j.jpeds.2016.08.095. [DOI] [PubMed] [Google Scholar]
- 10.Osatakul S, Sriplung H, Puetpaiboon A, Junjana CO, Chamnongpakdi S. Prevalence and natural course of gastroesophageal reflux symptoms: a 1-year cohort study in Thai infants. J Pediatr Gastroenterol Nutr. 2002;34:63–67. doi: 10.1097/00005176-200201000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Nelson SP, Chen EH, Syniar GM, Christoffel KK Pediatric Practice Research Group. Prevalence of symptoms of gastroesophageal reflux during infancy. A pediatric practice-based survey. Arch Pediatr Adolesc Med. 1997;151:569–572. doi: 10.1001/archpedi.1997.02170430035007. [DOI] [PubMed] [Google Scholar]
- 12.Vandenplas Y, Abkari A, Bellaiche M, Benninga M, Chouraqui JP, Çokura F, et al. Prevalence and health outcomes of functional gastrointestinal symptoms in infants from birth to 12 months of age. J Pediatr Gastroenterol Nutr. 2015;61:531–537. doi: 10.1097/MPG.0000000000000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) J Pediatr Gastroenterol Nutr. 2009;49:498–547. doi: 10.1097/MPG.0b013e3181b7f563. [DOI] [PubMed] [Google Scholar]
- 14.Horvath A, Dziechciarz P, Szajewska H. The effect of thickened-feed interventions on gastroesophageal reflux in infants: systematic review and meta-analysis of randomized, controlled trials. Pediatrics. 2008;122:e1268–e1277. doi: 10.1542/peds.2008-1900. [DOI] [PubMed] [Google Scholar]
- 15.Vandenplas Y, Leluyer B, Cazaubiel M, Housez B, Bocquet A. Double-blind comparative trial with 2 antiregurgitation formulae. J Pediatr Gastroenterol Nutr. 2013;57:389–393. doi: 10.1097/MPG.0b013e318299993e. [DOI] [PubMed] [Google Scholar]
- 16.van Wijk MP, Benninga MA, Davidson GP, Haslam R, Omari TI. Small volumes of feed can trigger transient lower esophageal sphincter relaxation and gastroesophageal reflux in the right lateral position in infants. J Pediatr. 2010;156:744–748.e1. doi: 10.1016/j.jpeds.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Task Force on Sudden Infant Death Syndrome. Moon RY. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:e1341–e1367. doi: 10.1542/peds.2011-2285. [DOI] [PubMed] [Google Scholar]
- 18.Fleisher DR. Infant rumination syndrome: report of a case and review of the literature. Am J Dis Child. 1979;133:266–269. [PubMed] [Google Scholar]
- 19.Fleisher DR, Matar M. The cyclic vomiting syndrome: a report of 71 cases and literature review. J Pediatr Gastroenterol Nutr. 1993;17:361–369. [PubMed] [Google Scholar]
- 20.Li BU, Lefevre F, Chelimsky GG, Boles RG, Nelson SP, Lewis DW, et al. North american society for pediatric gastroenterology, hepatology, and nutrition consensus statement on the diagnosis and management of cyclic vomiting syndrome. J Pediatr Gastroenterol Nutr. 2008;47:379–393. doi: 10.1097/MPG.0b013e318173ed39. [DOI] [PubMed] [Google Scholar]
- 21.Headache Classification Committee of the International Headache Society (IHS) The international classification of headache disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 22.Saps M, Adams P, Bonilla S, Chogle A, Nichols-Vinueza D. Parental report of abdominal pain and abdominal pain-related functional gastrointestinal disorders from a community survey. J Pediatr Gastroenterol Nutr. 2012;55:707–710. doi: 10.1097/MPG.0b013e3182662401. [DOI] [PubMed] [Google Scholar]
- 23.Saps M, Nichols-Vinueza DX, Rosen JM, Velasco-Benítez CA. Prevalence of functional gastrointestinal disorders in Colombian school children. J Pediatr. 2014;164:542–545.e1. doi: 10.1016/j.jpeds.2013.10.088. [DOI] [PubMed] [Google Scholar]
- 24.Devanarayana NM, Adhikari C, Pannala W, Rajindrajith S. Prevalence of functional gastrointestinal diseases in a cohort of Sri Lankan adolescents: comparison between Rome II and Rome III criteria. J Trop Pediatr. 2011;57:34–39. doi: 10.1093/tropej/fmq039. [DOI] [PubMed] [Google Scholar]
- 25.Lewis ML, Palsson OS, Whitehead WE, van Tilburg MA. Prevalence of functional gastrointestinal disorders in children and adolescents. J Pediatr. 2016;177:39–43.e3. doi: 10.1016/j.jpeds.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Steutel NF, Benninga MA, Langendam MW, de Kruijff I, Tabbers MM. Reporting outcome measures in trials of infant colic. J Pediatr Gastroenterol Nutr. 2014;59:341–346. doi: 10.1097/MPG.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 27.Lucassen PL, Assendelft WJ, van Eijk JT, Gubbels JW, Douwes AC, van Geldrop WJ. Systematic review of the occurrence of infantile colic in the community. Arch Dis Child. 2001;84:398–403. doi: 10.1136/adc.84.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James-Roberts IS, Conroy S, Wilsher K. Clinical, developmental and social aspects of infant crying and colic. Infant Child Dev. 1995;4:177–189. [Google Scholar]
- 29.St James-Roberts I, Halil T. Infant crying patterns in the first year: normal community and clinical findings. J Child Psychol Psychiatry. 1991;32:951–968. doi: 10.1111/j.1469-7610.1991.tb01922.x. [DOI] [PubMed] [Google Scholar]
- 30.Rautava P, Helenius H, Lehtonen L. Psychosocial predisposing factors for infantile colic. BMJ. 1993;307:600–604. doi: 10.1136/bmj.307.6904.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolke D. Behavioural treatment of prolonged infant crying: evaluation, methods, and a proposal. In: Barr RG, St James-Roberts I, Keefe MR, editors. New evidence on unexplained early infant crying: its origins, nature and management. Skillman, NJ: Johnson & Johnson Pediatric Institute; 2001. pp. 187–208. [Google Scholar]
- 32.Barr RG, St. James-Roberts I, Keefe MR. New evidence on unexplained early infant crying: its origins, nature and management. Skillman, NJ: Johnson & Johnson Pediatric Institute; 2001. [Google Scholar]
- 33.Treem WR. Infant colic. A pediatric gastroenterologist's perspective. Pediatr Clin North Am. 1994;41:1121–1138. doi: 10.1016/s0031-3955(16)38848-4. [DOI] [PubMed] [Google Scholar]
- 34.Treem WR. New evidence on unexplained early infant crying: its origins, nature and management. Skillman, NJ: Johnson & Johnson Pediatric Institute; 2001. Assessing crying complaints: the interaction with gastroesophageal reflux and cow's milk protein intolerance; pp. 165–176. [Google Scholar]
- 35.Shamir R. Infant colic and functional gastrointestinal disorders: is there more than a “gut feeling”? J Pediatr Gastroenterol Nutr. 2013;57:S1–S2. [Google Scholar]
- 36.Barr RG, Young SN, Wright JH, Gravel R, Alkawaf R. Differential calming responses to sucrose taste in crying infants with and without colic. Pediatrics. 1999;103:e68. doi: 10.1542/peds.103.5.e68. [DOI] [PubMed] [Google Scholar]
- 37.Lester BM, Boukydis CZ, Garcia-Coll CT, Hole WT. Colic for developmentalists. Infant Ment Health J. 1990;11:321–333. [Google Scholar]
- 38.DeSantis A, Coster W, Bigsby R, Lester B. Colic and fussing in infancy, and sensory processing at 3 to 8 years of age. Infant Ment Health J. 2004;25:522–539. [Google Scholar]
- 39.Milidou I, Søndergaard C, Jensen MS, Olsen J, Henriksen TB. Gestational age, small for gestational age, and infantile colic. Paediatr Perinat Epidemiol. 2014;28:138–145. doi: 10.1111/ppe.12095. [DOI] [PubMed] [Google Scholar]
- 40.Douglas P, Hill P. Managing infants who cry excessively in the first few months of life. BMJ. 2011;343:d7772. doi: 10.1136/bmj.d7772. [DOI] [PubMed] [Google Scholar]
- 41.Talge NM, Neal C, Glover V Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health. Antenatal maternal stress and longterm effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Berg MP, van der Ende J, Crijnen AA, Jaddoe VW, Moll HA, Mackenbach JP, et al. Paternal depressive symptoms during pregnancy are related to excessive infant crying. Pediatrics. 2009;124:e96–e103. doi: 10.1542/peds.2008-3100. [DOI] [PubMed] [Google Scholar]
- 43.Indrio F, Riezzo G, Raimondi F, Di Mauro A, Francavilla R. Gut motility alterations in neonates and young infants. J Pediatr Gastroenterol Nutr. 2013;57:S9–S11. [Google Scholar]
- 44.Gupta SK. Is colic a gastrointestinal disorder? Curr Opin Pediatr. 2002;14:588–592. doi: 10.1097/00008480-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Rhoads JM, Fatheree NY, Norori J, Liu Y, Lucke JF, Tyson JE, et al. Altered fecal microflora and increased fecal calprotectin in infants with colic. J Pediatr. 2009;155:823–828.e1. doi: 10.1016/j.jpeds.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 46.de Weerth C, Fuentes S, Puylaert P, de Vos WM. Intestinal microbiota of infants with colic: development and specific signatures. Pediatrics. 2013;131:e550–e558. doi: 10.1542/peds.2012-1449. [DOI] [PubMed] [Google Scholar]
- 47.Nooitgedagt JE, Zwart P, Brand PL. Causes, treatment and clinical outcome in infants admitted because of excessive crying to the paediatric department of the Isala clinics, Zwolle, the Netherlands, 1997/'03. Ned Tijdschr Geneeskd. 2005;149:472–477. [PubMed] [Google Scholar]
- 48.Lucassen PL, Assendelft WJ, Gubbels JW, van Eijk JT, van Geldrop WJ, Neven AK. Effectiveness of treatments for infantile colic: systematic review. BMJ. 1998;316:1563–1569. doi: 10.1136/bmj.316.7144.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barr RG. The normal crying curve: what do we really know? Dev Med Child Neurol. 1990;32:356–362. doi: 10.1111/j.1469-8749.1990.tb16949.x. [DOI] [PubMed] [Google Scholar]
- 50.Freedman SB, Al-Harthy N, Thull-Freedman J. The crying infant: diagnostic testing and frequency of serious underlying disease. Pediatrics. 2009;123:841–848. doi: 10.1542/peds.2008-0113. [DOI] [PubMed] [Google Scholar]
- 51.Shamir R, St James-Roberts I, Di Lorenzo C, Burns AJ, Thapar N, Indrio F, et al. Infant crying, colic, and gastrointestinal discomfort in early childhood: a review of the evidence and most plausible mechanisms. J Pediatr Gastroenterol Nutr. 2013;57(Suppl 1):S1–S45. doi: 10.1097/MPG.0b013e3182a154ff. [DOI] [PubMed] [Google Scholar]
- 52.Wessel MA, Cobb JC, Jackson EB, Harris GS, Jr, Detwiler AC. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics. 1954;14:421–435. [PubMed] [Google Scholar]
- 53.Barr RG. Excessive crying. In: Sameroff AJ, Lewis M, Miller SM, editors. Handbook of developmental psychopathology. 2nd ed. New York: Kluwer Academic/Plenum Publishers; 2000. pp. 327–350. [Google Scholar]
- 54.Fujiwara T, Barr RG, Brant R, Barr M. Infant distress at five weeks of age and caregiver frustration. J Pediatr. 2011;159:425–430.e1-2. doi: 10.1016/j.jpeds.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 55.Gustafson G, Wood R, Green J. Can we hear the causes of infants' crying? In: Barr RG, Hopkins B, Green JA, editors. Crying as a sign, a symptom, and a signal. London: Mac Keith Press; 2000. pp. 8–22. [Google Scholar]
- 56.James-Roberts IS, Conroy S, Wilsher K. Bases for maternal perceptions of infant crying and colic behaviour. Arch Dis Child. 1996;75:375–384. doi: 10.1136/adc.75.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyer EC, Coll CT, Lester BM, Boukydis CF, McDonough SM, Oh W. Family-based intervention improves maternal psychological well-being and feeding interaction of preterm infants. Pediatrics. 1994;93:241–246. [PubMed] [Google Scholar]
- 58.Vik T, Grote V, Escribano J, Socha J, Verduci E, Fritsch M, et al. Infantile colic, prolonged crying and maternal postnatal depression. Acta Paediatr. 2009;98:1344–1348. doi: 10.1111/j.1651-2227.2009.01317.x. [DOI] [PubMed] [Google Scholar]
- 59.Kurth E, Kennedy HP, Spichiger E, Hösli I, Stutz EZ. Crying babies, tired mothers: what do we know? A systematic review. Midwifery. 2011;27:187–194. doi: 10.1016/j.midw.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 60.Murray L, Cooper P. The impact of irritable infant behavior on maternal mental state: a longitudinal study and a treatment trial. In: Barr RG, St James-Roberts I, Keefe MR, editors. New evidence on unexplained early infant crying: its origins, nature and management. Skillman, NJ: Johnson & Johnson Pediatric Institute; 2001. pp. 149–164. [Google Scholar]
- 61.Savino F, Cordisco L, Tarasco V, Palumeri E, Calabrese R, Oggero R, et al. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics. 2010;126:e526–e533. doi: 10.1542/peds.2010-0433. [DOI] [PubMed] [Google Scholar]
- 62.Szajewska H, Gyrczuk E, Horvath A. Lactobacillus reuteri DSM 17938 for the management of infantile colic in breastfed infants: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2013;162:257–262. doi: 10.1016/j.jpeds.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 63.Sung V, Hiscock H, Tang ML, Mensah FK, Nation ML, Satzke C, et al. Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial. BMJ. 2014;348:g2107. doi: 10.1136/bmj.g2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sung V, Collett S, de Gooyer T, Hiscock H, Tang M, Wake M. Probiotics to prevent or treat excessive infant crying: systematic review and meta-analysis. JAMA Pediatr. 2013;167:1150–1157. doi: 10.1001/jamapediatrics.2013.2572. [DOI] [PubMed] [Google Scholar]
- 65.Kramer EA, den Hertog-Kuijl JH, van den Broek LM, van Leengoed E, Bulk AM, Kneepkens CM, et al. Defecation patterns in infants: a prospective cohort study. Arch Dis Child. 2015;100:533–536. doi: 10.1136/archdischild-2014-307448. [DOI] [PubMed] [Google Scholar]
- 66.Malowitz S, Green M, Karpinski A, Rosenberg A, Hyman PE. Age of onset of functional constipation. J Pediatr Gastroenterol Nutr. 2016;62:600–602. doi: 10.1097/MPG.0000000000001011. [DOI] [PubMed] [Google Scholar]
- 67.Koppen IJ, Lammers LA, Benninga MA, Tabbers MM. Management of functional constipation in children: therapy in practice. Paediatr Drugs. 2015;17:349–360. doi: 10.1007/s40272-015-0142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tabbers MM, DiLorenzo C, Berger MY, Faure C, Langendam MW, Nurko S, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58:258–274. doi: 10.1097/MPG.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 69.von Baeyer CL, Champion GD. Commentary: multiple pains as functional pain syndromes. J Pediatr Psychol. 2011;36:433–437. doi: 10.1093/jpepsy/jsq123. [DOI] [PubMed] [Google Scholar]
- 70.Lee SJ, Ralston HJ, Drey EA, Partridge JC, Rosen MA. Fetal pain: a systematic multidisciplinary review of the evidence. JAMA. 2005;294:947–954. doi: 10.1001/jama.294.8.947. [DOI] [PubMed] [Google Scholar]
- 71.Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 72.Lowery CL, Hardman MP, Manning N, Hall RW, Anand KJ, Clancy B. Neurodevelopmental changes of fetal pain. Semin Perinatol. 2007;31:275–282. doi: 10.1053/j.semperi.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 73.Fitzgerald M, Walker SM. Infant pain management: a developmental neurobiological approach. Nat Clin Pract Neurol. 2009;5:35–50. doi: 10.1038/ncpneuro0984. [DOI] [PubMed] [Google Scholar]
- 74.Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103:765–774. doi: 10.1111/j.1572-0241.2007.01722.x. quiz 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gebhart GF. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. Am J Physiol Gastrointest Liver Physiol. 2000;278:G834–G838. doi: 10.1152/ajpgi.2000.278.6.G834. [DOI] [PubMed] [Google Scholar]
- 76.Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41:1073–1093. doi: 10.1016/j.jpainsymman.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 77.Tomlinson D, von Baeyer CL, Stinson JN, Sung L. A systematic review of faces scales for the self-report of pain intensity in children. Pediatrics. 2010;126:e1168–e1198. doi: 10.1542/peds.2010-1609. [DOI] [PubMed] [Google Scholar]
- 78.Slater R, Cantarella A, Gallella S, Worley A, Boyd S, Meek J, et al. Cortical pain responses in human infants. J Neurosci. 2006;26:3662–3666. doi: 10.1523/JNEUROSCI.0348-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]