Abstract
Recurrent acute pancreatic attacks is a rare clinical condition (2-5% of all acute pancreatis) in children and is mainly idiopathic in most cases. Sometimes it may be associated with congenital anomalies, metabolic diseases or hereditary conditions. Isovaleric acidemia (IVA) is a rare autosomal recessive amino acid metabolism disorder associated with isovaleryl coenzyme A dehydrogenase deficiency presenting the clinical findings such metabolic acidosis with increased anion gap, hyperammonemia, ketonemia, hypoglycemia, “the odor of sweaty feet,” abdominal pain, vomiting, feeding intolerance, shock and coma. Recurrent acute pancreatitis associated with IVA have been rarely reported. Herein; we report a child who admitted with recurrent acute pancreatic attacks and had the final diagnosis of IVA. Mutation analysis revealed a novel homozygous mutation of (p.E117K [c.349G>A]) in the IVA gene. Organic acidemias must kept in mind in the differential diagnosis of recurrent acute pancreatic attacks in children.
Keywords: Acute, Recurrent, Pancreatitis, Isovaleric acidemia, Child
INTRODUCTION
Acute pancreatitis (AP) is an inflammatory disease characterized by sudden onset abdominal pain together with elevation of pancreatic enzymes in serum and/or urine and radiographic changes in the pancreas [1]. Drugs, infections, metabolic disturbances, trauma and structural anomalies such as choledochal cyst are frequently involved in the etiology of childhood AP [2]. Diseases such as hemolytic uremic syndrome, malignancies and genetic mutations may also cause AP in childhood [3,4]. Activation of proenzymes (zymogens), an inactive form of digestive enzymes, outside the duodenum (acinar cells, the pancreatic duct, tissue) is involved in the pathophysiology [5].
Isovaleric acidemia (IVA) is a form of organic acidemia deriving from the inability to metabolize leucine, a branched-chain amino acid, developing in association with isovaleryl coenzyme A dehydrogenase deficiency. Laboratory findings include increased anion gap, metabolic acidosis, hyperammonemia, ketonemia, neutropenia and hypoglycemia, and patients may be admitted with clinical findings such as “the odor of sweaty feet,” abdominal pain, vomiting, feeding intolerance, shock and coma. Chronic intermittent or asymptomatic forms have been reported in addition to the acute form. Prolonged fasting, infections and a protein-rich diet may trigger acute attacks in chronic form [6].
Herein, we report a child with recurrent AP attacks and had the final diagnosis of IVA. We want to emphasize that; although it is rare, recurrent AP attacks may be a manifestation of organic acidemia.
CASE REPORT
An 8-year-old male patient admitted to our pediatric emergency clinic due to acute abdominal pain, nausea and non-bilious vomiting. The pain was severe, intermittent and localized in the umbilical region. There was no history of trauma and chronic drug use.
He had been investigated due to hypotonia in the neonatal period but no etiological factor had been determined. At the age of six years, he was performed cranial magnetic resonance imaging due to mild mental retardation and speech impairment and reported to be normal. Additionally; he had been hospitalized and treated for AP attack in our pediatric gastroenterology clinic two years previously. No causative factor was determined for the AP attack. Thereafter; he had admitted two times to another medical center due to recurrent vomiting and abdominal pain attacks. No aggravating factor was determined by the physicians for the abdominal pain attacks.
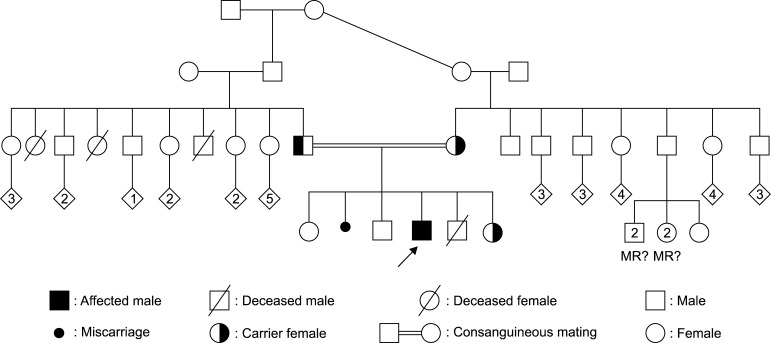
Third-degree parental consanguinity was present in family history. Our patient was the mother's third pregnancy (gravidity 6, parity 5, alive 4, and miscarriage 1). Two siblings had idiopathic epilepsy.
At physical examination, his appearance was lethargic. His body weight was 22 kg (10-25 percentile) and height was 132 cm (50-75 percentile). At laboratory analysis; complete blood count, electrolyte, liver-kidney function tests, lipid levels, lactate were within the normal levels. The initial ammonia level was 69 µmol/L (normal range, 16-53 µmol/L). The mild hyperammonemia improved spontaneously on second day. The blood gas analysis was normal (pH, 7.41; PCO2, 38 mmHg; PO2, 93 mmHg; HCO3, 21 mmol/L; SPO2, 98; BE, 1.2 mmol/L). Serum amylase and amylase clearance were 1,442 U/L (normal range, 25-90 U/L) and 6% (normal interval, <5%), respectively. Abdominal ultrasonography revealed increased pancreatic tail echogenecity and size with perisplenic and pelvic free fluid. The patient was hospitalized for the second time with a diagnosis of AP on the basis of clinical, laboratory and radiological findings. Oral nutrition was discontinued and the patient was started on somatostatin, intravenous fluid and antibiotics. The clinical and laboratory findings of the patients improved on the sixth day.
Magnetic resonance cholangiography performed due to recurrent AP attacks and revealed minimal dilation of 3 mm at the widest point in the main pancreatic duct (normal interval, 2-3 mm). There was no pancreatic stone, congenital malformation or other anomaly.
Metabolic tests including tandem mass spectrometry and urinary organic acids performed due to mental retardation, family history, consanguinity and recurrent attacks (abdominal pain and vomiting). There were an increase in isovaleric acid and its derivatives in urinary organic acids (isovaleryl-glycine >20×normal upper limit), an increase in C5 isovaleryl-carnitine (3.38 µM; normal upper limit, 0.47) and a decrease in C0 free carnitine (5.43 µM; normal lower limit, 7.5) and C3 propionyl-carnitine (0.48 µM; normal lower limit, 0.55) at tandem mass spectrometry which analyzed in serum data. A diagnosis of IVA was made based on metabolic test results. Molecular genetic analysis revealed a homozygous mutation of (p.E117K [c.349G>A]). The patient was started on protein and leucine-restricted diet (1.5 g/kg), glycine (200 mg/day) and carnitine (100 mg/kg). We have been monitoring the patient for 8 months and no AP attacks have occurred following therapy.
Molecular genetic analysis of parents and a healthy sibling were studied because of parental consanguinity history and revealed a heterozygous mutation of (p.E117K [c.349G>A]). The other siblings with epilepsy could not studied (Fig. 1).
Fig. 1. Pedigree of the family. MR: mental retardation.
DISCUSSION
IVA is a rare autosomal recessive amino acid metabolism disorder associated with isovaleryl coenzyme A dehydrogenase deficiency. The disease occurs in acute and chronic forms. The acute form appears with findings of lethargy, vomiting, dehydration, convulsion and coma in the first week of life. The chronic intermittent form appears at subsequent periods of childhood following conditions of stress, such as viral or bacterial infections, prolonged fasting or high protein intake [6]. In our patient, clinical presentation and advanced age were compatible with the chronic intermittent form. The diagnosis of IVA in our case was made based on clinical, laboratory (metabolic tests) findings and molecular genetic analysis. Molecular genetic analysis revealed a homozygous p.E117K (c.349G>A) mutation in the IVA gene. The most common mutations associated with IVA have been identified as c.1222G>A and c.890C>T mutations in Turkey in a previous study [7]. p.E117K (c.349G>A) mutation in IVD gene have not been defined before. This mutation was evaluated high probability the cause of IVA according to PolyPhen-2, SIFT and Mutation Taster data [8].
The treatment of AP consists of; the fluid and electrolyte supplementation, enzyme inhibition therapy, pain control with analgesics and antibiotic to prevent infections. Infectious complications are still regarded as the primary cause of mortality in severe pancreatitis [9]. Somatostatin analogues are used to inhibition of exocrine pancreatic secretion and prevent complications [4].
Recurrent AP is frequently idiopathic, but may also develop in association with trauma, drugs, infections, gall stones or congenital biliary system anomalies. Additionally; hereditary forms of recurrent AP attacks associated with mutation in the trypsinogen gene (PRSS1) have been well defined in children previously [10]. Structural anomalies were excluded at magnetic resonance cholangiography in our case, and there was no history of drug use, trauma or family history of pancreatic attacks.
AP has also been reported as a rare complication of organic acidemias. The pathogenesis of pancreatitis in organic acidemias is still unclear. Factors such as mitochondrial dysfunction due to ATP deficiency, accumulation of toxic metabolites in the pancreatic cell membrane, carnitine deficiency, decreased levels of antioxidant agents such as vitamins C and E, glutathione and selenium and increased free oxygen radicals have been implicated [11].
The patients can be diagnosed IVA after recurrent AP attacks or AP attacks can occur during the follow-up of IVA [5,11]. In a study; 9 of the 108 patients (8.3%) with organic acidemia had pancreatitis (7 acute, 2 chronic). Three of the 9 patients with pancreatitis had IVA, and the diagnosis of IVA was made following AP attacks in these patients, as in our case [11]. Our patient was diagnosed with IVA following advanced tests after recurrent AP attacks. Although, our patient did not have metabolic acidosis and severe hyperammonemia, the presence of mental retardation and family history suggest a preliminary diagnosis of metabolic disease in our patient.
In conclusion; metabolic diseases must be kept in mind in the differential diagnosis of recurrent AP attacks in children even in the absence of metabolic disturbances.
References
- 1.Kandula L, Lowe ME. Etiology and outcome of acute pancreatitis in infants and toddlers. J Pediatr. 2008;152:106–110. 110.e1. doi: 10.1016/j.jpeds.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 2.Nydegger A, Couper RT, Oliver MR. Childhood pancreatitis. J Gastroenterol Hepatol. 2006;21:499–509. doi: 10.1111/j.1440-1746.2006.04246.x. [DOI] [PubMed] [Google Scholar]
- 3.Masamune A, Mizutamari H, Kume K, Asakura T, Satoh K, Shimosegawa T. Hereditary pancreatitis as the premalignant disease: a Japanese case of pancreatic cancer involving the SPINK1 gene mutation N34S. Pancreas. 2004;28:305–310. doi: 10.1097/00006676-200404000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki M, Sai JK, Shimizu T. Acute pancreatitis in children and adolescents. World J Gastrointest Pathophysiol. 2014;5:416–426. doi: 10.4291/wjgp.v5.i4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pohl JF, Uc A. Paediatric pancreatitis. Curr Opin Gastroenterol. 2015;31:380–386. doi: 10.1097/MOG.0000000000000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantadakis E, Chrysafis I, Tsouvala E, Evangeliou A, Chatzimichael A. Acute pancreatitis with rapid clinical improvement in a child with isovaleric acidemia. Case Rep Pediatr. 2013;2013:721871. doi: 10.1155/2013/721871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozgul RK, Karaca M, Kilic M, Kucuk O, Yucel-Yilmaz D, Unal O, et al. Phenotypic and genotypic spectrum of Turkish patients with isovaleric acidemia. Eur J Med Genet. 2014;57:596–601. doi: 10.1016/j.ejmg.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 8.The Institute of Medical Genetics in Cardiff. The human gene mutation database [Internet] Cardiff (UK): The Institute of Medical Genetics in Cardiff; [cited 2016 Feb]. Available from: http://www.hgmd.cf.ac.uk/ac/index.php. [Google Scholar]
- 9.Manes G, Uomo I, Menchise A, Rabitti PG, Ferrara EC, Uomo G. Timing of antibiotic prophylaxis in acute pancreatitis: a controlled randomized study with meropenem. Am J Gastroenterol. 2006;101:1348–1353. doi: 10.1111/j.1572-0241.2006.00567.x. [DOI] [PubMed] [Google Scholar]
- 10.Minen F, De Cunto A, Martelossi S, Ventura A. Acute and recurrent pancreatitis in children: exploring etiological factors. Scand J Gastroenterol. 2012;47:1501–1504. doi: 10.3109/00365521.2012.729084. [DOI] [PubMed] [Google Scholar]
- 11.Kahler SG, Sherwood WG, Woolf D, Lawless ST, Zaritsky A, Bonham J, et al. Pancreatitis in patients with organic acidemias. J Pediatr. 1994;124:239–243. doi: 10.1016/s0022-3476(94)70311-6. [DOI] [PubMed] [Google Scholar]