Abstract
Human immunodeficiency virus type I (HIV-1) reverse transcriptase (RT) resistance mutations reduce the susceptibility of the virus to nucleoside analogues but may also impair viral DNA synthesis. To further characterize the effect of nucleoside analogue resistance mutations on the efficiency and kinetics of HIV-1 DNA synthesis and to evaluate the impact of the depletion of deoxynucleoside triphosphates (dNTP) on this process, DNA synthesis was evaluated by allowing DNA synthesis to proceed with natural HIV-1 templates and primers, either within permeabilized viral particles or in newly infected cells, and quantifying the products by real-time PCR. Three recombinant viruses derived from three pNL4-3 molecular clones expressing mutations associated with resistance to zidovudine: a clone expressing RT mutation M184V, a clone expressing mutations M41L plus T215Y (M41L+T215Y), and clinical isolate BV34 (carrying seven resistance mutations). Following infection of P4 cells, the BV34 mutant, but not viruses expressing the M184V mutation or M41L+T215Y, exhibited a defect in DNA synthesis. Importantly, however, for mutants carrying the M184V mutation or M41L+T215Y mutations, a defect could be detected by using target cells in which dATP pools had been reduced by pretreatment with hydroxyurea. Based on these observations, we developed a recombinant-virus assay to assess the effects of hydroxyurea pretreatment on infectivity of viruses carrying plasma-derived RT sequences from patients with nucleoside resistance. Using this assay, we found that many, but not all, viruses carrying RT resistance mutations display an increased sensitivity to hydroxyurea, suggesting that the impact of RT resistance mutations on viral replication may be more profound in cell populations characterized by smaller dNTP pools.
Nucleoside analogues are the most frequently prescribed antiretroviral agents for the treatment of human immunodeficiency virus type 1 (HIV-1)-infected patients. In most cases, these drugs are administered as part of combinations of antiretroviral drugs often referred to as highly active antiretroviral therapy (HAART), which succeed in suppressing detectable virus replication and reversing the evolution of HIV disease (41). In some patients, however, the suppressive effect of HAART is incomplete, permitting the selection of viral variants with resistance to antiretroviral agents, including nucleoside analogues (14, 16).
Although all nucleoside analogues inhibit HIV-1 replication by similar mechanisms, i.e., premature termination of viral DNA synthesis by the virally encoded reverse transcriptase (RT), HIV-1 resistance to these agents involves at least two radically different biochemical mechanisms. Some resistance mutations impair the incorporation of the analogue into the nascent chain of viral DNA. The M184V mutation involves a residue situated within the nucleoside triphosphate-binding site of RT, and the high-level resistance to lamivudine conferred by this mutation constitutes the prototypical example of resistance through impaired incorporation (19, 45, 49). This mechanism is also fostered by less-frequent RT mutations, such as K65R, and the Q151M multinucleoside resistance pathway (14, 44, 53). Other resistance mutations act through a primer rescue mechanism, by facilitating the ability of ATP or pyrophosphate to excise the analogue from the prematurely terminated strand of viral DNA (1, 9, 37). This mechanism is principally the consequence of mutations known as TAMs (thymidine analogue mutations), which include the M41L, D67N, K70R, L210W, T215Y/F, and K219Q/E substitutions, as well as the rare dual amino acid insertions at amino acid 69 (14, 16).
Although resistance mutations confer a decisive growth advantage to the mutated variants over their wild-type (WT) parental counterparts for replication in the presence of antiretroviral drugs, there is accumulating evidence that resistant HIV-1 strains are less fit than the WT for replication in the absence of drug and that, overall, the replicative capacities of many drug-resistant HIV variants are reduced. While this important phenomenon has been extensively studied for HIV-1 resistance to protease inhibitors (4, 12, 15, 34, 54), our understanding of the replicative impact of resistance to nucleoside analogues remains incomplete. The M184V mutation has received considerable attention. Reversion of M184V is observed in patients interrupting treatment with lamivudine (6, 10), indicating that the mutation impairs fitness. The M184V mutation has also been shown to decrease the processivity of the RT in vitro and to impair viral replicative capacity in tissue culture systems (3, 8, 32, 40, 47). Similarly, the L74V mutation, which reduces the incorporation of dideoxyadenosine triphosphate, the active metabolite of didanosine, has also been reported to impair the processivity of RT in most studies and to impair viral replicative capacity (18, 46-48).
The evaluation of the effect of mutations that promote primer rescue on viral replication capacity and enzyme function has given more-divergent results. The reversion of TAMs in patients not receiving treatment has been described (23), but some TAMs can persist for long periods in untreated patients. Studies evaluating the function of RTs expressing TAMs in vitro have identified defects in processivity for only some combinations of TAMs (11, 36, 46). Similarly, while several studies have found that viruses expressing TAMs have reduced replicative capacity compared to WT viruses in tissue culture systems (6, 24, 25, 27, 28, 30), the replicative capacities of viruses expressing other combinations of TAMs were not different from, or even better than, that of WT virus (11, 46).
It is noteworthy that the apparent effects of resistance mutations on viral replicative capacity can be strongly influenced by the conditions under which enzyme activity or viral replication is measured. In particular, Back and Berkhout (2) showed that the defect in RT activity resulting from the M184V mutation could be accentuated under conditions where deoxynucleoside triphosphates (dNTPs) are limiting, but the potential effect of reduced dNTP concentrations on the replicative capacity of viruses carrying other RT mutations has received scant attention (6, 11).
In this study, we have further analyzed the effect of reductions in dNTP concentrations on the replication of viruses carrying different RT resistance mutations. In initial studies, recombinant viruses, one carrying the M184V mutation, one carrying two TAMs (M41L plus T215Y [M41L+T215Y]), and a clinical isolate with multiple mutations, were evaluated. To reproduce the natural conditions of reverse transcription in the replicative environment of the virus with natural HIV-1 templates and primers, the efficiency and the kinetics of viral DNA synthesis within permeabilized viral particles and in newly infected cells were evaluated. These results were then extended to the evaluation of RTs carrying a variety of different combinations of mutations, including mutations that impair analogue incorporation and those that promote primer rescue, by developing a recombinant-virus assay permitting the evaluation of the effect of depletion of dATP in target cells by hydroxyurea pretreatment on viral replication.
MATERIALS AND METHODS
Construction of plasmids.
Several mutations known to decrease the susceptibility of HIV-1 to nucleoside analogues were introduced into the RT sequence of HIV-1 by site-directed mutagenesis as previously described (35). Mutagenesis was performed in the plasmid (pSK-RTS), which carries a fragment of the HIV-1 genome encompassing the entire pol open reading frame from HIV-1 cloned into pBluescript SKII (Stratagene) between the ClaI and EcoRI sites. The viral sequence in this plasmid was obtained from pNL4-3XCS, a variant of pNL4-3, which carries an XbaI site upstream of the protease coding region, a ClaI site at the junction between the protease and RT coding sequences, and a SnaBI site at the junction between the RT and RNase H coding sequences. For site-directed mutagenesis, the following plus-strand primers were used, together with their cognate minus-strand equivalents: M41L+, 5′-GTAGAAATTTGTACAGAGCTCGAAAAGGAAGGAAAAATTTC; M184V+, 5′-CATAGTCATCTATCAATACGTGGATGATTTGTATGTAGG; and T215Y+, 5′-GTTGAGGTGGGGATTTTACACTCCGGACAAAAAACATCAG. Following mutagenesis, a 1,308-bp fragment covering the coding sequence for the entire polymerase domain of the HIV-1 RT was excised from the mutated pSK-RTS plasmids by ClaI and SnaBI digestion and cloned in pNL4-3XCS, previously digested with the same enzymes.
For the construction of a recombinant virus carrying the HIV-1 RT sequences from a clinical isolate (BV34), a 1,325-bp segment was amplified by RT-PCR after extraction of RNA from plasma with primers RTC+ (5′-AGTCCTATCGATACTGTACCAGTA) and RTS− (5′-CCCATCTACGTAGAAAGTTTCTGC), which introduce unique ClaI and SnaBI sites near the ends of the amplification products. The amplified sequences were digested by ClaI and SnaBI, cloned into pSK-RTS, and cloned into pNL4-3XCS as described above. The RT coding sequences in pSK-RTS (after site-directed mutagenesis or cloning from a clinical isolate) used to generate the recombinant viruses were verified by sequencing.
Cell culture and preparation of viral stocks.
HeLa cells and P4 cells (HeLa CD4+ long terminal repeat-LacZ) were cultured in Dulbecco's modified Eagle's medium. MT4 cells were cultured in RPMI 1640 medium. All media were supplemented with 10% fetal calf serum, 50-μg/ml streptomycin, and 50-U/ml penicillin G. P4 cells were cultured in the presence of 500-μg/ml G418.
To produce viral stocks for endogenous reverse transcription, HeLa cells (4 × 105 cells/well in six-well plates) were transfected with 1.5 μg of plasmid DNA by using PolyFect transfection reagent (QIAGEN, Valencia, Calif.) according to the manufacturer's instructions. After culture for 24 h, cells were washed extensively, and 3 ml of complete medium was added. After an additional 24 h of culture, medium was harvested and passed through a 0.45-μm-pore-size filter and aliquots were frozen. To produce viral stocks used for intracellular reverse transcription, virions (100 ng of p24) produced by HeLa cells were treated with DNase I and used to infect MT4 cells (5 × 106 cells in 10 ml of complete medium). The viability of the cultures was monitored daily. Culture medium obtained the day before the viability fell below 80% was filtered (0.45-μm-pore-size filter) and stored in aliquots. To verify that reversion of resistance mutations had not occurred during culture, viral RNA was purified, RT-PCR was performed, and the sequence of the RT was confirmed.
Endogenous reverse transcription.
Endogenous reverse transcription was performed by a modification of the technique of Borroto-Esoda and Boone (7). Viral stocks (30 μl) were mixed with an equal volume of buffer containing 100 mM Tris-HCl (pH 8.0), 2 mM CaCl2, 10 mM MgCl2, and 340 U of DNase I/ml and incubated at 37°C for 30 min. Forty microliters of a solution containing 7.5 mM EGTA, 0.25% (vol/vol) NP-40, and 1.25 mM (each) dNTP was added, and the mixture was incubated at 39°C for 120 min. DNA was purified with the QIAquick 8 PCR purification kit (QIAGEN). Parallel reactions in which dNTPs were omitted were performed.
Intracellular reverse transcription.
Viral stocks in complete medium were mixed with an equal volume of solution containing 100 mM Tris-HCl (pH 8.0), 0.2 mM CaCl2, 19.2 mM MgCl2, and 340 U of DNase I/ml and incubated at 37°C for 30 min. One hundred microliters of the solution was transferred onto P4 cells which had been plated at 105 cells/well in 0.2 ml of complete medium in 96-well culture plates 18 h before infection. P4 cells were infected by spinoculation at 860 × g for 120 min at 22°C (39). The medium was removed, the cells were rinsed once with phosphate-buffered saline, and 0.1 ml of complete medium was added. Cells were either processed immediately (time zero) or cultured at 37°C for various lengths of time. Medium was removed, cells were detached with proteinase K and lysed, and DNA was purified as described above.
Real-time PCR.
The quantities of DNA corresponding to sequences in U5, env, and gag were measured by real-time PCR with the primers and Taqman probes shown in Table 1. DNA was diluted 1:10 in 10 mM Tris-HCl (pH 8.0) containing 0.1 mM EDTA and 10 ng of salmon sperm DNA/ml. Reaction mixtures (final volume, 50 μl) contained 1× Taqman Universal PCR mixture (Applied Biosystems, Foster City, Calif.), 200 nM (each) primer, 100 nM Taqman probe, and 10 μl of diluted DNA. Amplification was performed with a 7000 sequence detection system (Applied Biosystems). Cycling conditions were as follows: 50°C for 2 min, 95°C for 10 min, and 40 cycles at 95°C for 15 s and 60°C for 1 min each. The same serial dilutions of linearized pNL4-3XCS plasmid were used as standards for the quantification of U5, env, and gag.
TABLE 1.
Primers and probes used for quantification of viral sequences by real-time PCR
| Gene and primer or probe | Sequencea |
|---|---|
| U5 | |
| Forward primer | 5′-CAATAAAGCTTGCCTTGAGTGCT |
| Reverse primer | 5′-TGACTAAAAGGGTCTGAGGGATCTC |
| Probe | 5′-(6-Fam)AGTGTGTGCCCGTCTGTTGTGTGACTC(Tamra)(phosphate) |
| env | |
| Forward primer | 5′-ACCATGCTCCTTGGGATATTGA |
| Reverse primer | 5′-AATAGAGTGGTGGTTGCTTCCTTC |
| Probe | 5′-(6-Fam)TGCTACAGAAAAATTGTGGGTCACAGTCTATTATGG(Tamra)(phosphate) |
| gag | |
| Forward primer | 5′-GCGAAAGTAAAGCCAGAGGAGAT |
| Reverse primer | 5′-TTTTGGCGTACTCACCAGTCG |
| Probe | 5′-(6-Fam)CTCGACGCAGGACTCGGCTTGCT(Tamra)(phosphate) |
Fam, carboxyfluorescein; Tamra, carboxytetramethylrhodamine.
Quantification of intracellular dNTP pools.
The methods used to quantify dNTP pools have been described (26). Briefly, P4 cells were cultivated in the presence or absence of hydroxyurea (250 μM) for 18 h, trypsinized, and washed twice with 0.9% NaCl (pH 7.5) at 4°C, and a cell pellet containing 2 × 107 cells was frozen at −70°C. Cell extracts were prepared as described previously (42) and analyzed by liquid chromatography coupled to tandem mass spectrometry without (dATP and dTTP) or with (dCTP and dGTP) prior periodate oxidation.
Determination of the effect of mutations in RT on replication in hydroxyurea-pretreated cells by a recombinant-virus assay.
To evaluate the effect of hydroxyurea pretreatment of target cells on the replication of viruses carrying resistance mutations in RT, a modification of the previously described recombinant-virus assay was used (29, 38, 43). The plasmid used in these studies (pSRT) was derived from pNL4-3 and contains deletions in the regions coding for RT (nucleotides 2618 to 3886) and envelope (nucleotides 6343 to 7611). A unique NruI restriction site is present at position 3890. RNA was isolated from plasma, and a region encoding the RT was amplified by nested RT-PCR. The oligonucleotides used in the first amplification were MJ3 (5′-AGTAGGACCTACACCTGTCA) and RT-EXT (5′-TTCCCAATGCATATTGTGAG). The oligonucleotides used in the nested amplification reaction were A35 (5′-TTGGTTGCACTTTAAATTTTCCCATTAGTCCT) and RT-IN (5′-CAATGCATATTGTGAGTCTG). To perform the test, subconfluent 293T cells in T25 flasks were transfected by the calcium phosphate precipitation method with 8 μg of NruI-linearized pSRT, 0.1 μg of the vesicular stomatitis virus G plasmid, and 0.5 to 1 μg of the HIV RT amplification product. The transfection precipitate was washed off the cells after 18 h, and medium was added. After a further 24 h of culture, supernatant was clarified by centrifugation (500 × g, 15 min) and transferred to P4 indicator cells (13) that had been preincubated for 4 h with serial dilutions of hydroxyurea. The level of expression of β-galactosidase in the P4 cell lysates was measured by a colorimetric assay based on the cleavage of chlorophenol red-β-d-galactopyranoside by β-galactosidase. To determine the 50% inhibitory concentration (IC50), optical density data were fitted to a sigmoid curve with variable slope.
Statistical methods.
Results are presented as means ± standard deviations (SD) unless otherwise indicated. Groups were compared by analysis of variance. Posttest comparisons (performed only if P was <0.05) were made with the Newman-Keuls multiple-comparison test. A P value of <0.05 was considered significant.
RESULTS
Quantification of endogenous reverse transcription in permeabilized virions.
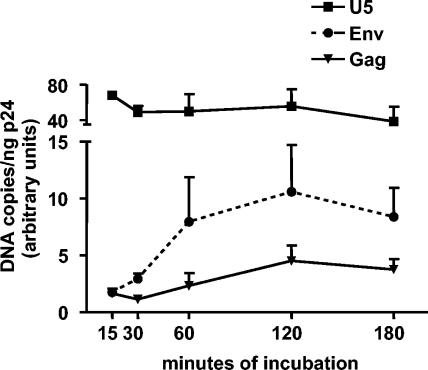
To quantify the efficiency of viral DNA synthesis by RT in the context of the natural HIV template and primers, endogenous RT reactions were conducted on permeabilized virion preparations. The kinetics of endogenous reverse transcription by WT virus is shown in Fig. 1. Synthesis of minus-strand strong-stop DNA (U5) occurred very quickly and was complete after 15 min, the shortest time examined. Reverse transcription into env and gag was detected later (30 and 60 min, respectively) but reached a plateau after 2 h. Synthesis of strong-stop DNA was considerably more efficient than extension of transcripts to env and gag, and after 2 h of incubation the percentages of U5 transcripts that had been extended to env and gag were 20.1% ± 5.0% and 7.7% ± 2.3%, respectively (n = 7). When virions were permeabilized and incubated in the absence of dNTPs for 2 h, only low levels of DNA were detected compared to that observed for virions incubated in the presence of 500 μM dNTPs. Thus, although small amounts of viral DNA have been detected in intact viral particles (33, 50), preformed DNA resulting from intraparticle reverse transcription or contamination with plasmid DNA represented only 0.6% ± 0.3% (U5), 1.8% ± 1.1% (env), and 2.5% ± 1.2% (gag) of total DNA detected after 2 h of incubation.
FIG. 1.
Kinetics of synthesis of DNA by WT virus in endogenous transcription reactions. Viral stocks were treated with DNase I and incubated at 39°C in a solution containing 3 mM NP-40 (to permeabilize the virions) and 500 μM dNTPs. At the indicated times, DNA in the reaction mixtures was isolated and DNA corresponding to sequences in the U5, Env, and Gag regions was quantified by real-time PCR. Results are the means ± SEMs for three experiments. When virions were incubated for 2 h in the absence of dNTPs, the amount of DNA detected was <2% of that present in reactions containing dNTPs.
Effect of resistance mutations on endogenous retrotranscription.
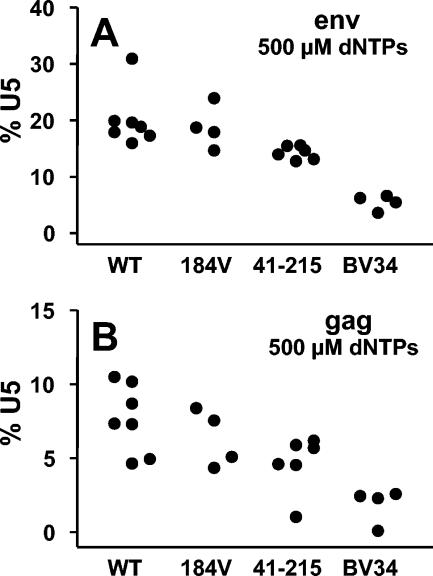
Using this system, we then compared the efficiencies of endogenous reverse transcription within permeabilized viral particles carrying RTs with different resistance mutations (Fig. 2). The M184V mutant has a substitution of a valine for a methionine at position 184 of HIV-1 RT, which induces high-level resistance to lamivudine. The M41L+T215Y mutant carries two classic TAMs, frequently associated with HIV-1 resistance to nucleoside analogues and conferring strong resistance to zidovudine. Mutant BV34, reconstructed from a clinical isolate exhibiting high-level resistance to several nucleoside analogues, carries seven mutations in RT: M41L, D67N, K70R, L74I, V75L, T215F, and K219Q.
FIG. 2.
Extension of transcripts to env and gag in endogenous transcription reactions. WT virions and those with the indicated resistance mutations in RT were evaluated in endogenous RT reactions. The final concentration of dNTPs in the reaction mixtures was 500 μM. Results are expressed as the number of env (A) or gag (B) sequences detected relative to the number of U5 transcripts. Each point represents the results of an independent experiment and is the mean of duplicate determinations. Statistical comparisons are presented in Results.
The amount of total U5 DNA synthesized per nanogram of p24 for WT virus was not significantly different from those for mutant viruses (data not shown; P = 0.36). This finding supports the conclusion that the viral stocks, normalized by measurement of p24, contained equivalent amounts of active reverse transcriptase. As shown in Fig. 2, the extension of transcripts to env and gag by viruses carrying the RT from clinical isolate BV34 was significantly reduced (P < 0.001 for both comparisons), and a small reduction in the extension of sequences to env and gag was observed for the RT with the M41L and T215Y mutations (P < 0.05 for both comparisons). In contrast, no significant difference in the extension of transcripts to env and gag between the M184V mutant RT and that of WT virus was observed.
Effect of reduced dNTP concentrations on endogenous reverse transcription.
In an effort to accentuate a defect in reverse transcription by resistant RT variants, endogenous reverse transcription was performed in the presence of suboptimal concentrations of dNTPs (i.e., <500 μM). When the dNTP concentration was reduced to 250 μM, the synthesis of U5 DNA per nanogram of p24 by WT virus fell to 31% ± 6% of that observed with 500 μM dNTPs. In addition, the proportion of U5 sequences extended to env and gag was also reduced (percentages of WT sequences extended to env: 500 μM dNTPs, 20.1% ± 5.0%; 250 μM dNTPs, 9.9% ± 1.7%; P < 0.01; percentages of WT sequences extended to gag: 500 μM dNTPs, 7.7% ± 2.3%, 250 μM dNTPs, 4.1% ± 1.1%; P < 0.01). As a result of this reduction in the synthesis of U5 DNA and the extension of transcripts, the background of the assay now represented 6.2% ± 3.4% (env) and 7.9% ± 2.8% (gag) of the total DNA detected in these samples (n = 5).
When endogenous transcription by viruses carrying resistance mutations was evaluated with 250 μM dNTPs, DNA synthesis was also found to be strongly reduced compared to that observed with 500 μM dNTPs. Because dNTP depletion reduced DNA synthesis without affecting the assay background, however, the background now represented 42.9% ± 9.8% (env; n = 7) and 51.8% ± 13.2% (gag; n = 7) of total DNA detected. In view of the small amplitude of the specific signals observed for the mutant viruses, the reliability of these measurements appeared uncertain, and this approach was not pursued.
Quantification of reverse transcription following infection of target cells.
To further characterize the effect of resistance mutations on reverse transcription by HIV-1, we developed techniques permitting the quantification of reverse transcription products synthesized after infection of HeLa-derived CD4+ P4 cells. Virions produced by transfected HeLa cells could not be used for these studies, because the amount of DNA synthesized following infection was less than that produced in endogenous RT reactions. As a result, residual plasmid DNA, even that following DNase treatment prior to infection, produced an unacceptable background. To reduce the background resulting from the residual plasmid DNA, we used viruses produced by infected cells in place of viruses produced directly by transfection. Thus, MT4 cells were infected with the virions produced by transfected HeLa cells, the medium was changed daily, and viruses produced over the 24-h interval preceding the time when the viability of the cultures fell below 80% were used. In these passaged viruses, no reversion of resistance mutations occurred during culture in MT4 cells, as confirmed by sequencing the RT region of RT-PCR-amplified virion RNA. These virions were treated with DNase and sedimented onto target P4 cells by spinoculation (39) at room temperature, and viral entry was initiated by culturing cells at 37°C. At various times after infection, DNA was isolated, and real-time PCR was used to quantify the transcripts as described above.
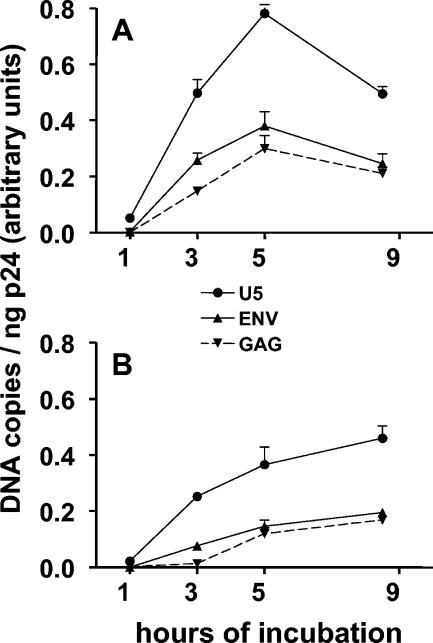
The kinetics of reverse transcription by WT virus is shown in Fig. 3A. Few transcripts were present 1 h after incubation of cells at 37°C, but, by 5 h, synthesis of U5, env, and gag had reached an apparent plateau. Unlike what was found for synthesis of DNA during endogenous reverse transcription, the extension of U5 transcripts to env and gag following infection was quite efficient, and 5 h after infection the products were present in amounts close to the expected 1:0.5:0.5 ratios (amounts of env and gag relative to U5, 0.59 ± 0.04 and 0.38 ± 0.02, respectively [n = 6]). Synthesis of U5, env, and gag increased linearly as a function of the amount of virus used for infection (data not shown).
FIG. 3.
Kinetics of synthesis of DNA by WT virus following infection of P4 cells. Viral stocks were prepared by infecting MT4 cells with virions produced by transfected HeLa cells and collecting culture medium on the day of peak virus production. The virions were treated with DNase I and used to infect P4 cells by spinoculation. The cells were then washed and incubated at 37°C. After various times of culture, DNA was extracted from the cells, and DNA corresponding to sequences in the U5, Env, and Gag regions was quantified by real-time PCR. (A) Kinetics of synthesis of viral DNA in unpretreated P4 cells. (B) Kinetics of synthesis of viral DNA in P4 cells that had been pretreated with 250 μM hydroxyurea for 18 h prior to infection. Results are the means ± SEMs for two experiments.
Effect of resistance mutations on reverse transcription in P4 cells.
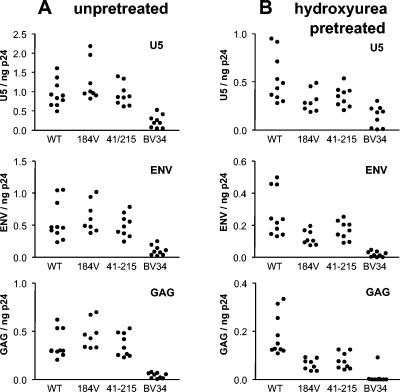
Using the system described above, we compared levels of reverse transcription in P4 cells exposed to viral particles containing mutant RTs. A 5-h incubation period was chosen for these studies because DNA synthesis by WT virus was near completion by that time, assuring a high signal/noise ratio, and because the 5-h point was near the time when DNA reached maximal values and therefore the system would be sensitive for the detection of delays in DNA synthesis. As shown in Fig. 4A, no reduction in DNA synthesis at 5 h was observed for either the M184V or the M41L+T215Y mutants (P > 0.05 for all comparisons with WT virus). By contrast, the synthesis of U5, env, and gag DNA by the highly mutated BV34 mutant was significantly reduced compared to that of WT virus (P < 0.001 for all comparisons).
FIG. 4.
Synthesis of viral DNA after infection of P4 cells with recombinant virions expressing WT RT or RT containing resistance mutations. Unpretreated P4 cells (A) and P4 cells cultured for 18 h in the presence of 250 μM hydroxyurea (B) were infected by spinoculation with WT virions or virions with the indicated resistance mutations in RT. The cells were then incubated at 37°C for 5 h, after which DNA was extracted. Viral DNA corresponding to sequences in the U5, Env, and Gag regions was quantified by real-time PCR. Each point represents the results of an independent experiment and is the mean of duplicate determinations. Statistical comparisons are presented in Results.
Effect of pretreatment of P4 cells with hydroxyurea on reverse transcription by WT virus.
Hydroxyurea, a free-radical scavenger that inhibits ribonucleotide reductase, has been shown to produce specific depletion of dATP, because no salvage pathway is available for the synthesis of this nucleotide. Consistent with results obtained for other cell types (5, 21), pretreatment of P4 cells with 250 μM hydroxyurea resulted in a specific twofold reduction in the amount of dATP, as measured by mass spectrometry (hydroxyurea-pretreated cells: dATP, 9.1 ± 0.6 pmol/106 cells; dTTP, 42.6 ± 2.9 pmol/106 cells; dCTP, 11.8 ± 2.2 pmol/106 cells; dGTP, 11.3 ± 0.6 pmol/106 cells; unpretreated cells: dATP, 18.7 ± 1.8 pmol/106 cells; dTTP, 45.1 ± 2.5 pmol/106 cells; dCTP, 12.6 ± 0.3 pmol/106 cells; dGTP, 10.6 pmol/106 cells; n = 2). To evaluate the effect of dATP depletion on reverse transcription, P4 cells were pretreated with hydroxyurea for 18 h before infection. The comparison of the kinetics of reverse transcription by WT virus in cells pretreated with 250 μM hydroxyurea with that observed in unpretreated cells is shown in Fig. 3 and demonstrated a delay in DNA synthesis in hydroxyurea-pretreated cells. This was particularly striking for the accumulation of gag sequences, especially at the 3-h time point. It is also noteworthy that U5, env, and gag sequences continued to accumulate between 5 and 9 h after infection of hydroxyurea-pretreated cells. Considering all experiments in which DNA synthesis was measured at 5 h, the amount of DNA synthesized by WT virus following infection of cells pretreated with 250 μM hydroxyurea was decreased by 51.6% ± 4.2% (U5), 46.2% ± 3.3% (env), and 48.1% ± 4.2% (gag), respectively, compared to that observed following infection of unpretreated cells with the same amount of virions (Fig. 4).
Effect of resistance mutations on reverse transcription in P4 cells pretreated with hydroxyurea.
To evaluate whether dATP depletion would accentuate a defect in reverse transcription by RT carrying resistance mutations, P4 cells pretreated with 250 μM hydroxyurea for 18 h prior to infection were infected with the recombinant viruses expressing RTs carrying resistance mutations. Although DNA synthesis by the mutants carrying the M184V and M41L+T215Y mutations was similar to that of WT virus following infection of unpretreated cells, DNA synthesis by these mutant viruses was reduced compared to that of WT virus following infection of hydroxyurea-pretreated cells. For both the M184V and M41L+T215Y mutants, the synthesis of U5 (P < 0.05 for both comparisons), env (P < 0.01 and 0.05, respectively), and gag (P < 0.001 for both comparisons) was reduced compared to that for WT virus. Furthermore, the extent of this reduction was significantly greater for gag (67% ± 10% and 64% ± 4% reductions, respectively, compared to WT) than for U5 (42% ± 18% and 39% ± 10% reductions, respectively; P < 0.05 and 0.01 for comparison of the decreases in gag and U5 synthesis). Consistent with the idea that reverse transcription by the viruses carrying the M184V and M41L+215Y resistance mutations progressed more slowly in hydroxyurea-pretreated cells, the proportion of env sequences extended to gag at 5 h was significantly reduced compared to that observed for WT viruses (Table 2). When incubation was extended to 9 h following infection of hydroxyurea-pretreated cells, however, the proportion of env sequences extended to gag for the M184V and M41L+215Y mutants approached values observed in unpretreated cells infected with these mutant viruses and that observed for WT virus replicating in hydroxyurea-pretreated cells. Although DNA synthesis at 5 h was reduced in hydroxyurea-pretreated cells, the detection of newly synthesized DNA generally remained robust. For WT viruses and the M184V and M41L+T215Y mutants, the assay background represented <6% (U5), <6% (env), and <7% (gag) of the total signal in all cases.
TABLE 2.
Percentage of env Sequences extended to gaga
| Resistance mutation(s) | % env sequences extended to gag
|
|||
|---|---|---|---|---|
| 5 h after infection
|
9 h after infection
|
|||
| Unpretreated | Hydroxyurea pretreated | Unpretreated | Hydroxyurea pretreated | |
| None (pNL4-3) | 71 ± 18 | 71 ± 15 | 86 ± 1 | 86 ± 4 |
| M41L + T215Y | 74 ± 11 | 46 ± 10** | 76 ± 7 | 84 ± 9 |
| M184V | 77 ± 9 | 54 ± 14* | 81 ± 15 | 81 ± 11 |
| BV34 mutationsb | 35 ± 7** | 3 ± 4** | 86 ± 2 | 66 ± 50 |
Unpretreated P4 cells or cells cultured in the presence of 250 μM hydroxyurea for 18 h were infected with virions expressing the indicated resistance mutations. The numbers of transcripts extended to env and gag were quantified by real-time PCR. Results are expressed as follows: (number of gag sequences)/(number of env sequences) × 100. Results are the means ± SD for 8 to 10 experiments (5 h) or 2 experiments (9 h). *, P < 0.05 compared to WT virus; **, P < 0.01 compared to WT virus.
Seven resistance mutations carried by clinical isolate BV34 (see Results).
As observed for infections of unpretreated cells, synthesis of U5, env, and gag by the BV34 mutant at 5 h was significantly reduced (P < 0.001 for all comparisons), and indeed synthesis of gag at 5 h was barely detectable (Fig. 4B). Because DNA synthesis by the BV34 mutant was more strongly inhibited in hydroxyurea-pretreated cells, assay background represented 8% ± 3% (U5), 14% ± 4% (env), and 66% ± 22% (gag) of the total signal.
Evaluation of the effect of hydroxyurea on the infectivity of virions carrying nucleoside analogue resistance mutations using a recombinant-virus infectivity assay.
In the studies evaluating viral DNA synthesis, the pretreatment of target cells with hydroxyurea had a greater impact on the three mutant viruses evaluated than on WT virus. To extend these observations, we sought to evaluate whether this impairment in DNA synthesis is associated with detectable reductions in viral infectivity and to develop a test that would facilitate the assessment of the effect of hydroxyurea pretreatment on the infectivity of viruses carrying other resistance genotypes. To do so, P4 cells, which express β-galactosidase under the control of a tar promoter, were pretreated with different concentrations of hydroxyurea and the cells were then infected with viruses obtained following the cotransfection of HeLa cells with a PCR product containing the RT gene to be evaluated and a linearized pNL4-3 plasmid in which the RT coding sequence had been deleted, a technique that generates a stock of recombinant viruses. Forty-eight hours after infection of P4 cells, viral infectivity was assessed by colorimetric detection of β-galactosidase activity.
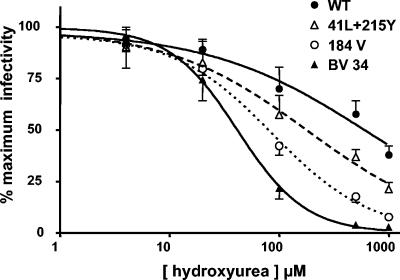
Figure 5 shows the hydroxyurea dose-response curves obtained when the PCR product containing the RT gene was derived from the four viruses for which DNA synthesis had been studied. IC50 values are shown in Table 3. The replication of WT virus was moderately impaired at the higher doses of hydroxyurea; 50% inhibition was achieved at the dose of 669 ± 141 μM hydroxyurea (mean ± standard error of the mean [SEM]; n = 5). As was observed in the studies evaluating DNA synthesis, the BV34 mutant showed the greatest sensitivity to replication in hydroxyurea-pretreated cells (IC50, 42 ± 8 μM hydroxyurea; P < 0.001 compared to WT). Similarly, levels of replication of both the M41L+T215Y mutant and the M184V mutant were more sensitive to replication in hydroxyurea-pretreated cells than that of the WT virus (IC50, 180 ± 59 and 84 ± 7 μM hydroxyurea, respectively; P < 0.01 for both comparisons). Although the replication of the M184V mutant appeared to show somewhat increased sensitivity to replication in hydroxyurea-pretreated cells compared to that of the M41L+T215Y mutant, the difference was not significant. It is noteworthy that in the studies evaluating DNA synthesis in cells pretreated with 250 μM hydroxyurea (Fig. 4), the impairment observed for the M184V mutant also appeared to be somewhat greater than that observed for the M41L+T215Y mutant, but the differences in this system were also not significant. Taken together, these findings suggest that the reduction in DNA synthesis observed at 5 h for the mutant viruses evaluated here could also be detected by measurement of the sensitivity of these viruses to inhibition by hydroxyurea of viral replication in the single-cycle replication assay.
FIG. 5.
Evaluation of the effect of hydroxyurea on the infectivity of virions carrying nucleoside analogue resistance mutations by a recombinant-virus infectivity assay. P4 cells, which express β-galactosidase under the control of a tar promoter, were pretreated with hydroxyurea at the indicated concentrations for 4 h, and the cells were then infected with viruses obtained following the transfection of HeLa cells with plasmids prepared by recombination between a PCR product containing the RT gene to be evaluated and a linearized pNL4-3 plasmid in which the RT sequence had been deleted. Forty-eight hours after infection of P4 cells, viral infectivity was assessed by colorimetric detection of β-galactosidase activity. In these studies, the same viruses used in the evaluation of DNA synthesis were studied: WT (pNL4-3), the M41L+T215Y and M184V mutants, and clinical isolate BV34. Results, expressed as percentages of the infectivity observed in unpretreated cells, are the means ± SD for four to six independent experiments.
TABLE 3.
Effect of resistance mutation in the RT on the inhibition of viral replication in P4 cells pretreated with hydroxyurea as measured by a single-cycle viral replication assaya
| Mutant | TAMs | Other NRTIb mutation(s) | NNRTI mutation(s) | Polymorphisms | Mean IC50 (μM) (SEM) |
|---|---|---|---|---|---|
| WT | |||||
| 1 (pNL4-3) | 102Q, 122K, 162C | 669 (141) | |||
| 2 | 83K, 122K, 123E, 135T, 162A, 177E, 211K, 214F | 777 (197)− | |||
| 3 | 39N, 64R, 123S, 135V, 162C, 173A, 174K, 177D/E, 200A, 207A, 211S, 214F | 483 (153)− | |||
| 4 | 178L, 169E/D | 364 (136)− | |||
| M184V present | |||||
| 5 (M184V) | 184V | 102Q, 122K, 162C | 84 (4)* | ||
| 6 | 184V | 83K, 122E, 123D/E, 200T/A, 214F | 211 (52)* | ||
| 7 | 184V | 49R, 122E, 177E, 202V | 146 (43)** | ||
| 8 | 41L, 215Y | 184V | 101Q | 121C, 135T, 162C, 178L, 211K, 214F | 49 (11)** |
| 20 | 67N, 215Y | 101K/N/Q/H, 181C | 72I, 74I, 75I, 86G, 89K, 91P, 93K, 95A, 136Q, 137H, 139M, 142V, 162Y, 177E, 207E, 214F | 562 (129)− | |
| 21 | 67N, 70R | 98S | 44K, 47N, 48T, 54K, 102K, 122Q, 123N, 166R, 177E, 211K, 214F | 382 (103)− | |
| 3 TAMs | |||||
| 22 | 67D, 70R, 219Q | 98G | 122K, 162N/D | 120 (38)* | |
| 23 | 41L, 210W, 215Y | 43S, 121H, 135T, 143R/K, 177E, 211K, 214F | 424 (112)− | ||
| 24 | 41L, 67N, 215Y | 42E, 86E, 110D/N, 122K, 135V, 147N/K, 162C, 178L, 204A, 207E, 214F | 826 (211)− | ||
| 25 | 41L, 67N, 215Y | 122E, 135V, 200I, 211K, 214F | 404 (88)− | ||
| 26 | 41L, 210W, 215Y | 135T, 178L, 211K, 214F | 195 (53)* | ||
| 5 TAMs | |||||
| 27 | 41M/L, 67N, 70R, 215F, 219Q | 101E | 122K, 123E, 135I/V, 162Y, 211K, 214F | 147 (18)** | |
| 28 | 41L, 67N, 70R, | 103N, 181C | 69E, 122K, 135T, 150L/Q/M, 162Y, | 70 (3)** | |
| 9 | 41L, 210W, 215Y | 184V | 39K, 43K, 122K, 214F | 101 (22)** | |
| 10 | 62V, 151M, 184V | 68G, 83K, 122K, 135K, 143S/G, 162A, 203E/D, 207Q/E, 211K, 214L/F | 89 (10)** | ||
| 11 | 41L, 67N, 210W, 215Y | 184V | 122K, 123E, 177E, 202V, 211K, 214F, 245E | 103 (26)** | |
| Other RT mutations present | |||||
| 12 (BV34) | 41L, 67N, 70R, 215F, 219Q | 74I, 75L | NAc | 42 (4)** | |
| 13 | 151M | 181C | 102Q, 122K, 162C | 107 (13)** | |
| 14 | 215Y, 219K/Q | 69S + VA, 62A, 74V | 98G, 181C, 190A | 83K, 200A, 203D | 46 (6)** |
| 15 | 41L, 70R, 219E | 65R, 75I, 151M, 178M | 103N, 108I, 190A | 68G, 112S, 121D/M, 142V, 177E, 211A, 214F | 61 (4)** |
| 16 | 41L, 67E, 210W, 215Y | 69S + SV, 62V, 74V, 118V/I | 98S, 101K/Q, 103N, 108V/I, 181C, 190A | 39A, 135T, 178I/L, 189V/I, 196E, 203D, 211K, 214F, 228R | 47 (4)** |
| 2 TAMs | |||||
| 17 (41 + 215) | 41L, 215Y | 102Q, 122K, 162C | 251 (45)* | ||
| 18 | 41L, 215Y | 103N, 181C | 102Q, 122E, 135T, 142V, 162C, 200A, 228R | 161 (36)* | |
| 19 | 210L/W, 215Y, 215F, 219Q | 49R, 83K, 178I/M, 196G/E, 211Q, 177G, 214F, 218E, 228H, 247R | 123 (8)* |
Viral infectivity in hydroxyurea-pretreated cells was evaluated as described in the Fig. 5 legend with RTs amplified from 25 clinical isolates and the recombinant virions used in the evaluation of DNA synthesis. IC50s were calculated by fitting the optical density data to a sigmoid curve. For each viral population studied, both the RT genotype and the IC50 for the inhibition of viral replication in hydroxyurea-pretreated cells were determined. The results are the means of four to six independent experiments. Statistical analysis was performed using analysis of variance (P < 0.0001), followed by the Newman-Keuls multiple-comparison test. −, not significantly different from the WT pNL4-3 virus; *, P < 0.01 compared to the WT pNL4-3 virus; **, P < 0.001 compared to the WT pNL4-3 virus.
NRTI, nucleoside RT inhibitor.
NA, not applicable.
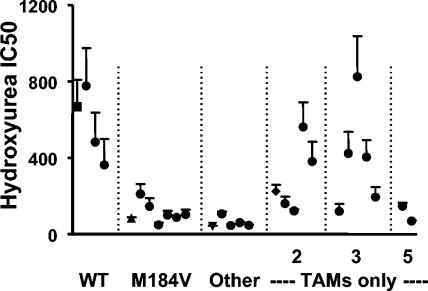
RTs from 25 clinical isolates containing a spectrum of mutations associated with resistance to reverse transcriptase inhibitors were also evaluated using this assay. The IC50s for inhibition by hydroxyurea are shown in Fig. 6. A complete listing of RT genotypes of these virions, including both resistance mutations and other polymorphisms, is included in Table 3.
FIG. 6.
Inhibition of the infectivity of virions carrying nucleoside analogue resistance mutations in hydroxyurea-pretreated cells measured by a recombinant-virus infectivity assay. Viral infectivity in hydroxyurea-pretreated cells was evaluated as described in the Fig. 5 legend with RTs amplified from 25 clinical isolates and the recombinant virions used in the evaluation of DNA synthesis. IC50s were calculated by fitting the optical density data to a sigmoid curve. The results are presented as the means ± SEMs for three to six independent experiments. The precise genotypes of the virions studied are detailed in Table 3. The group labeled M184V had this mutation either alone or in combination with other resistance mutations. The group labeled “other” had a variety of mutations, including both TAMs and other resistance mutations (e.g., L74I/V, V75T, Q151M, and 69 insertions). The virions used in the evaluation of DNA synthesis are indicated by the following symbols: WT (pNL4-3), square; M184V, triangle; M41L+T215Y, diamond; clinical isolate BV34, inverted triangle.
The impairment of viral replication in hydroxyurea-pretreated cells was significantly enhanced for all recombinant viruses expressing the M184V mutation, regardless of the presence of other resistance mutations. A substantial impairment in viral replication was also observed for the five recombinant viruses carrying a combination of TAMs with other nucleoside mutations (e.g., 151M, K65R, L74V, and 69 insertions), an impairment that approached that observed for the BV34 clinical isolate, the mutant investigated in the studies evaluating viral DNA synthesis (Fig. 5).
The effect of TAMs on viral replication in hydroxyurea-pretreated cells was more variable. RTs containing M41L+T215Y showed greater sensitivity to replication in hydroxyurea-treated cells than viruses with WT RT, as did the virus expressing 210W+215Y, but not viruses with 67N+215Y or 67N+70R. Similarly, some viruses with three TAMs demonstrated greater sensitivity to replication in hydroxyurea-pretreated cells, while others did not. The two viruses with M41L+D67N+T215Y did not demonstrate the reduced sensitivity seen for viruses expressing only M41L+T215Y. Both viruses with five TAMs were found to have substantially increased sensitivity to replication in hydroxyurea-pretreated cells. RTs expressing only nonnucleoside RT inhibitor (NNRTI) mutations in isolation were not evaluated in this assay. However, several of the viruses with TAMs that did not demonstrate increased sensitivity to replication in hydroxyurea-pretreated cells also expressed NNRTI mutations, suggesting that NNRTI mutations were not necessarily deleterious for viral replication under these conditions.
DISCUSSION
The impairment in viral replicative capacity resulting from the acquisition of mutations conferring resistance to antiretroviral drugs may have implications for viral pathogenicity, viral transmission, and the treatment of HIV-1 infection. Despite the potential importance of the extent and mechanisms of resistance-associated loss of replicative capacity during evolution of RT resistance to nucleoside analogues, our understanding of them is incomplete. Our results extend previous observations in several ways. First, our studies indicate that RT resistance mutations previously shown to impair viral fitness in tissue culture systems and/or to impair RT activity in vitro produce a measurable defect in DNA synthesis following infection of target cells with virus expressing these mutations. Importantly, however, for mutants carrying the M184V (2) and M41L+T215Y mutations, this defect could be detected only with target cells in which dATP pools had been reduced by pretreatment with hydroxyurea. The impairment of RT activity in these mutant viruses could also be detected by their increased sensitivity to inhibition of viral infectivity in hydroxyurea-pretreated cells, as measured by a single-cycle recombinant-virus assay. Using this assay, we found that many, but not all, viruses carrying RT resistance mutations display similar increased sensitivities to hydroxyurea, suggesting that the impact of RT resistance mutations on viral replication may be more profound in cell populations characterized by smaller dNTP pools.
At least two distinct mechanisms could account for the impairment in DNA synthesis observed in hydroxyurea-pretreated cells. Our finding of a clear delay in the extension of nascent DNA transcripts to gag is compatible with a general defect in the processivity of RT, a defect that could be accentuated by resistance mutations, as has been observed in vitro for the M184V mutant (3, 32). It is also known, however, that the initiation of DNA synthesis is an important rate-limiting step in reverse transcription, and resistance mutations, including M184V, can also impair the initiation of DNA synthesis (18, 31, 52). In this regard, the synthesis of U5 DNA by WT virus was reduced in hydroxyurea-pretreated cells compared to that observed in unpretreated cells, and U5 synthesis by the mutant viruses was reduced to an even greater extent in hydroxyurea-pretreated cells. These findings are compatible with the possibility that the depletion of dNTPs impaired the initiation of reverse transcription and that this effect was accentuated by resistance mutations. It should be stressed, however, that factors other than an impairment in initiation could have contributed to the reductions in U5 synthesis observed. First, hydroxyurea pretreatment blocked cell replication and, although untreated and hydroxyurea-treated cells were plated at the same density, the number of cells present in hydroxyurea-treated cultures after 18 h was reduced by approximately 50% (data not shown), which may have reduced the number of virions that attached to these cells during spinoculation and reduced the number of infection events occurring in these cultures. Such an effect could explain the lower synthesis of U5 by WT viruses in hydroxyurea-pretreated cells than in unpretreated cells but would not account for the observation that WT virus synthesized more U5 in hydroxyurea-pretreated cells than mutant viruses. In addition, the synthesis of the last two of the four strands of U5 generated by RT must be preceded by the completion of the synthesis of gag, and we found that gag synthesis by the mutant viruses was decreased to a greater extent than U5 synthesis. Thus, the synthesis of the last two copies of U5 by the M184V and M41L+T215Y mutants, which must be preceded by the extension of sequences to gag, may not have advanced by 5 h after infection to the extent that it had advanced in cells infected with WT virus. Because we did not specifically evaluate the kinetics of U5 synthesis at early time points, our data do not permit conclusions concerning the relative importance of delays in the initiation of DNA synthesis and the extension of nascent transcripts to the overall impairment. We did find, however, that the synthesis of U5 by the mutant viruses was reduced to a smaller extent than the synthesis of gag, suggesting that a delay in DNA synthesis, reflecting a delay in initiation and/or elongation, not an irreversible block in initiation, was the predominant effect.
Our observation that the M184V mutation leads to an impairment of DNA synthesis in hydroxyurea-pretreated cells is entirely compatible with prior observations that the defect in the processivity of RT containing this mutation measured in vitro is accentuated by reductions in dNTP concentrations and the observation that viral replication is impaired in primary cells (smaller dNTP pools), but not in some transformed T-cell lines (large dNTP pools) (2, 3). Using the recombinant-virus assay, we found that the replication of all viruses carrying an RT with the M184V mutation in hydroxyurea-pretreated cells was considerably reduced compared to that of WT virus, suggesting that compensatory mutations to correct this defect may not be readily available. Other mutations in RT known to inhibit the incorporation of nucleoside triphosphate analogs have also been studied and have been found to impair to a variable extent RT processivity and viral replicative capacity. This is the case for mutation L74V, selected by didanosine when used as a single nucleoside analogue, and K65R, associated with resistance to zalcitabine and tenofovir (17, 18, 44, 46-48, 53). Consistent with the possibility that the defects in RT activity produced by these mutations are accentuated by low dNTP concentrations, we found that the replication of viruses carrying an RT with these mutations, as measured by the single-cycle assay, was more sensitive to inhibition in hydroxyurea-pretreated cells than was that of WT virus.
Our results provide further insights into the impact of TAMs on viral replication. No defect in DNA synthesis for viruses carrying the M41L+T215Y mutations could be detected either in endogenous RT reactions or following infection of unpretreated P4 cells. In this regard, no clear defect for this mutant was observed when the DNA-dependent DNA polymerase activity of the mutant RT was evaluated in vitro (36), although an approximately 15% reduction in fitness was observed in a cell culture system (24). In our studies, however, DNA synthesis by viruses carrying the M41L+T215Y mutations was clearly delayed following infection of hydroxyurea-pretreated cells. Thus, as was observed for the M184V mutation, defects in RT activity produced by these TAMs appear to be accentuated by depletion of dNTP pools. The results obtained by the recombinant-virus assay suggest that both the number of TAMs and the specific TAMs present can influence their impact on RT activity. The replication of both viruses whose RTs contained combinations of five TAMs was considerably more sensitive to inhibition in hydroxyurea-pretreated cells than was that of WT virus. In contrast, although some viruses whose RTs contained two or three TAMs showed sensitivity to replication in hydroxyurea-pretreated cells, others did not. It is noteworthy that four of the five RTs without increased sensitivity to replication in hydroxyurea-pretreated cells expressed the D67N or K70R mutation or both and that, although both viruses expressing the M41L+T215Y mutations showed increased sensitivity to replication in hydroxyurea-pretreated cells, the two viruses with the M41L+D67N+T215Y combination did not. In this regard, prior studies have shown that the K70R mutation had only a small impact on viral replication in vitro and that replication of a virus expressing the D67N+K70R+T215Y+K219Q combination of TAMs was actually superior to that of WT virus under conditions thought to be associated with small dNTP pools (25). Together with our findings, these studies indicate that, although TAMs frequently impair RT activity, at least when dNTP levels are suboptimal, some combinations appear to have little effect and may actually improve replication under these conditions. The recombinant-virus assay described here may prove to be a useful method to define the impact of various combinations of TAMs and other RT mutations on viral replication under conditions of suboptimal nucleotide pools.
The tumor cells commonly used for the measurement of drug susceptibility have markedly higher intracellular pools of dNTPs than most primary human cells, including phytohemagglutinin-stimulated peripheral blood mononuclear cells (5, 20). As was already shown for M184V mutants (3), our results suggest that viruses with a wide variety of resistance mutations in RT could be significantly impaired in their capacity for replication in primary cells, in spite of apparently normal replication kinetics in tumor cell-derived CD4+ cell types in tissue culture. It is noteworthy that viruses with distinct resistance genotypes have been identified in vivo in different cellular compartments (22, 51). Although differences in the penetration of antiretroviral agents into these compartments is likely to be an important factor, differences in the dNTP pools in different cell types could also influence the fitness cost of resistance mutations and favor the persistence of less-mutated or even WT virus at some sites. Further studies, involving larger panels of viruses with diverse RT resistance mutation profiles, should focus on the impact of resistance on HIV DNA synthesis and replication in primary cells, with an emphasis on cell types with the lowest DNA turnover and smallest dNTP pools.
Acknowledgments
We gratefully acknowledge the help of Virginie Trouplin and Esther Race in performing these studies.
This work was supported in part by grant R03050HS from the Agence Nationale de Recherches sur le SIDA.
REFERENCES
- 1.Arion, D., N. Kaushik, S. McCormick, G. Borkow, and M. A. Parniak. 1998. Phenotypic mechanism of HIV-1 resistance to 3′-azido-3′-deoxythymidine (AZT): increased polymerization processivity and enhanced sensitivity to pyrophosphate of the mutant viral reverse transcriptase. Biochemistry 37:15908-15917. [DOI] [PubMed] [Google Scholar]
- 2.Back, N. K., and B. Berkhout. 1997. Limiting deoxynucleoside triphosphate concentrations emphasize the processivity defect of lamivudine-resistant variants of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 41:2484-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Back, N. K., M. Nijhuis, W. Keulen, C. A. Boucher, B. O. Oude Essink, A. B. van Kuilenburg, A. H. van Gennip, and B. Berkhout. 1996. Reduced replication of 3TC-resistant HIV-1 variants in primary cells due to a processivity defect of the reverse transcriptase enzyme. EMBO J. 15:4040-4049. [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour, J. D., T. Wrin, R. M. Grant, J. N. Martin, M. R. Segal, C. J. Petropoulos, and S. G. Deeks. 2002. Evolution of phenotypic drug susceptibility and viral replication capacity during long-term virologic failure of protease inhibitor therapy in human immunodeficiency virus-infected adults. J. Virol. 76:11104-11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi, V., E. Pontis, and P. Reichard. 1986. Changes of deoxyribonucleoside triphosphate pools induced by hydroxyurea and their relation to DNA synthesis. J. Biol. Chem. 261:16037-16042. [PubMed] [Google Scholar]
- 6.Bleiber, G., M. Munoz, A. Ciuffi, P. Meylan, and A. Telenti. 2001. Individual contributions of mutant protease and reverse transcriptase to viral infectivity, replication, and protein maturation of antiretroviral drug-resistant human immunodeficiency virus type 1. J. Virol. 75:3291-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borroto-Esoda, K., and L. R. Boone. 1994. Development of a human immunodeficiency virus-1 in vitro DNA synthesis system to study reverse transcriptase inhibitors. Antiviral Res. 23:235-249. [DOI] [PubMed] [Google Scholar]
- 8.Boyer, P. L., and S. H. Hughes. 1995. Analysis of mutations at position 184 in reverse transcriptase of human immunodeficiency virus type 1. Antimicrob Agents Chemother. 39:1624-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyer, P. L., S. G. Sarafianos, E. Arnold, and S. H. Hughes. 2001. Selective excision of AZTMP by drug-resistant human immunodeficiency virus reverse transcriptase. J. Virol. 75:4832-4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner, B. G., J. P. Routy, M. Petrella, D. Moisi, M. Oliveira, M. Detorio, B. Spira, V. Essabag, B. Conway, R. Lalonde, R. P. Sekaly, and M. A. Wainberg. 2002. Persistence and fitness of multidrug-resistant human immunodeficiency virus type 1 acquired in primary infection. J. Virol. 76:1753-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caliendo, A. M., A. Savara, D. An, K. DeVore, J. C. Kaplan, and R. T. D'Aquila. 1996. Effects of zidovudine-selected human immunodeficiency virus type 1 reverse transcriptase amino acid substitutions on processive DNA synthesis and viral replication. J. Virol. 70:2146-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrillo, A., K. D. Stewart, H. L. Sham, D. W. Norbeck, W. E. Kohlbrenner, J. M. Leonard, D. J. Kempf, and A. Molla. 1998. In vitro selection and characterization of human immunodeficiency virus type 1 variants with increased resistance to ABT-378, a novel protease inhibitor. J. Virol. 72:7532-7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charneau, P., G. Mirambeau, P. Roux, S. Paulous, H. Buc, and F. Clavel. 1994. HIV-1 reverse transcription. A termination step at the center of the genome. J. Mol. Biol. 241:651-662. [DOI] [PubMed] [Google Scholar]
- 14.Clavel, F., and A. J. Hance. 2004. HIV drug resistance. N. Engl. J. Med. 350:1023-1035. [DOI] [PubMed] [Google Scholar]
- 15.Croteau, G., L. Doyon, D. Thibeault, G. McKercher, L. Pilote, and D. Lamarre. 1997. Impaired fitness of human immunodeficiency virus type 1 variants with high-level resistance to protease inhibitors. J. Virol. 71:1089-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deeks, S. G. 2003. Treatment of antiretroviral-drug-resistant HIV-1 infection. Lancet 362:2002-2011. [DOI] [PubMed] [Google Scholar]
- 17.Deval, J., K. L. White, M. D. Miller, N. T. Parkin, J. Courcambeck, P. Halfon, B. Selmi, J. Boretto, and B. Canard. 2004. Mechanistic basis for reduced viral and enzymatic fitness of HIV-1 reverse transcriptase containing both K65R and M184V mutations. J. Biol. Chem. 279:509-516. [DOI] [PubMed] [Google Scholar]
- 18.Diallo, K., B. Marchand, X. Wei, L. Cellai, M. Gotte, and M. A. Wainberg. 2003. Diminished RNA primer usage associated with the L74V and M184V mutations in the reverse transcriptase of human immunodeficiency virus type 1 provides a possible mechanism for diminished viral replication capacity. J. Virol. 77:8621-8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao, H. Q., P. L. Boyer, S. G. Sarafianos, E. Arnold, and S. H. Hughes. 2000. The role of steric hindrance in 3TC resistance of human immunodeficiency virus type-1 reverse transcriptase. J. Mol. Biol. 300:403-418. [DOI] [PubMed] [Google Scholar]
- 20.Gao, W. Y., A. Cara, R. C. Gallo, and F. Lori. 1993. Low levels of deoxynucleotides in peripheral blood lymphocytes: a strategy to inhibit human immunodeficiency virus type 1 replication. Proc. Natl. Acad. Sci. USA 90:8925-8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao, W. Y., D. G. Johns, S. Chokekuchai, and H. Mitsuya. 1995. Disparate actions of hydroxyurea in potentiation of purine and pyrimidine 2′,3′-dideoxynucleoside activities against replication of human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 92:8333-8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosn, J., J. P. Viard, C. Katlama, M. de Almeida, R. Tubiana, F. Letourneur, L. Aaron, C. Goujard, D. Salmon, M. Leruez-Ville, C. Rouzioux, and M. L. Chaix. 2004. Evidence of genotypic resistance diversity of archived and circulating viral strains in blood and semen of pre-treated HIV-infected men. AIDS 18:447-457. [DOI] [PubMed] [Google Scholar]
- 23.Goudsmit, J., A. de Ronde, E. de Rooij, and R. de Boer. 1997. Broad spectrum of in vivo fitness of human immunodeficiency virus type 1 subpopulations differing at reverse transcriptase codons 41 and 215. J. Virol. 71:4479-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrigan, P. R., S. Bloor, and B. A. Larder. 1998. Relative replicative fitness of zidovudine-resistant human immunodeficiency virus type 1 isolates in vitro. J. Virol. 72:3773-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrigan, P. R., I. Kinghorn, S. Bloor, S. D. Kemp, I. Najera, A. Kohli, and B. A. Larder. 1996. Significance of amino acid variation at human immunodeficiency virus type 1 reverse transcriptase residue 210 for zidovudine susceptibility. J. Virol. 70:5930-5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hennere, G., F. Becher, A. Pruvost, C. Goujard, J. Grassi, and H. Benech. 2003. Liquid chromatography-tandem mass spectrometry assays for intracellular deoxyribonucleotide triphosphate competitors of nucleoside antiretrovirals. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 789:273-281. [DOI] [PubMed] [Google Scholar]
- 27.Imamichi, T., S. C. Berg, H. Imamichi, J. C. Lopez, J. A. Metcalf, J. Falloon, and H. C. Lane. 2000. Relative replication fitness of a high-level 3′-azido-3′-deoxythymidine-resistant variant of human immunodeficiency virus type 1 possessing an amino acid deletion at codon 67 and a novel substitution (Thr→Gly) at codon 69. J. Virol. 74:10958-10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jellinger, R. M., R. W. Shafer, and T. C. Merigan. 1997. A novel approach to assessing the drug susceptibility and replication of human immunodeficiency virus type 1 isolates. J. Infect. Dis. 175:561-566. [DOI] [PubMed] [Google Scholar]
- 29.Kellam, P., and B. A. Larder. 1994. Recombinant virus assay: a rapid, phenotypic assay for assessment of drug susceptibility of human immunodeficiency virus type 1 isolates. Antimicrob. Agents Chemother. 38:23-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosalaraksa, P., M. F. Kavlick, V. Maroun, R. Le, and H. Mitsuya. 1999. Comparative fitness of multi-dideoxynucleoside-resistant human immunodeficiency virus type 1 (HIV-1) in an in vitro competitive HIV-1 replication assay. J. Virol. 73:5356-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanchy, J. M., C. Ehresmann, S. F. Le Grice, B. Ehresmann, and R. Marquet. 1996. Binding and kinetic properties of HIV-1 reverse transcriptase markedly differ during initiation and elongation of reverse transcription. EMBO J. 15:7178-7187. [PMC free article] [PubMed] [Google Scholar]
- 32.Larder, B. A., S. D. Kemp, and P. R. Harrigan. 1995. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science 269:696-699. [DOI] [PubMed] [Google Scholar]
- 33.Lori, F., F. di Marzo Veronese, A. L. de Vico, P. Lusso, M. S. Reitz, Jr., and R. C. Gallo. 1992. Viral DNA carried by human immunodeficiency virus type 1 virions. J. Virol. 66:5067-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mammano, F., C. Petit, and F. Clavel. 1998. Resistance-associated loss of viral fitness in human immunodeficiency virus type 1: phenotypic analysis of protease and Gag coevolution in protease inhibitor-treated patients. J. Virol. 72:7632-7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mammano, F., V. Trouplin, V. Zennou, and F. Clavel. 2000. Retracing the evolutionary pathways of human immunodeficiency virus type 1 resistance to protease inhibitors: virus fitness in the absence and in the presence of drug. J. Virol. 74:8524-8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer, P. R., J. Lennerstrand, S. E. Matsuura, B. A. Larder, and W. A. Scott. 2003. Effects of dipeptide insertions between codons 69 and 70 of human immunodeficiency virus type 1 reverse transcriptase on primer unblocking, deoxynucleoside triphosphate inhibition, and DNA chain elongation. J. Virol. 77:3871-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer, P. R., S. E. Matsuura, A. M. Mian, A. G. So, and W. A. Scott. 1999. A mechanism of AZT resistance: an increase in nucleotide-dependent primer unblocking by mutant HIV-1 reverse transcriptase. Mol. Cell 4:35-43. [DOI] [PubMed] [Google Scholar]
- 38.Meynard, J. L., M. Vray, L. Morand-Joubert, E. Race, D. Descamps, G. Peytavin, S. Matheron, C. Lamotte, S. Guiramand, D. Costagliola, F. Brun-Vezinet, F. Clavel, and P. M. Girard. 2002. Phenotypic or genotypic resistance testing for choosing antiretroviral therapy after treatment failure: a randomized trial. AIDS 16:727-736. [DOI] [PubMed] [Google Scholar]
- 39.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oude Essink, B. B., N. K. Back, and B. Berkhout. 1997. Increased polymerase fidelity of the 3TC-resistant variants of HIV-1 reverse transcriptase. Nucleic Acids Res. 25:3212-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palella, F. J., Jr., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, and S. D. Holmberg. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 42.Prabu-Jeyabalan, M., E. A. Nalivaika, N. M. King, and C. A. Schiffer. 2003. Viability of a drug-resistant human immunodeficiency virus type 1 protease variant: structural insights for better antiviral therapy. J. Virol. 77:1306-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Race, E., E. Dam, V. Obry, S. Paulous, and F. Clavel. 1999. Analysis of HIV cross-resistance to protease inhibitors using a rapid single-cycle recombinant virus assay for patients failing on combination therapies. AIDS 13:2061-2068. [DOI] [PubMed] [Google Scholar]
- 44.Ray, A. S., A. Basavapathruni, and K. S. Anderson. 2002. Mechanistic studies to understand the progressive development of resistance in human immunodeficiency virus type 1 reverse transcriptase to abacavir. J. Biol. Chem. 277:40479-40490. [DOI] [PubMed] [Google Scholar]
- 45.Sarafianos, S. G., K. Das, A. D. Clark, Jr., J. Ding, P. L. Boyer, S. H. Hughes, and E. Arnold. 1999. Lamivudine (3TC) resistance in HIV-1 reverse transcriptase involves steric hindrance with beta-branched amino acids. Proc. Natl. Acad. Sci. USA 96:10027-10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma, P. L., and C. S. Crumpacker. 1997. Attenuated replication of human immunodeficiency virus type 1 with a didanosine-selected reverse transcriptase mutation. J. Virol. 71:8846-8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma, P. L., and C. S. Crumpacker. 1999. Decreased processivity of human immunodeficiency virus type 1 reverse transcriptase (RT) containing didanosine-selected mutation Leu74Val: a comparative analysis of RT variants Leu74Val and lamivudine-selected Met184Val. J. Virol. 73:8448-8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.St Clair, M. H., J. L. Martin, G. Tudor-Williams, M. C. Bach, C. L. Vavro, D. M. King, P. Kellam, S. D. Kemp, and B. A. Larder. 1991. Resistance to ddI and sensitivity to AZT induced by a mutation in HIV-1 reverse transcriptase. Science 253:1557-1559. [DOI] [PubMed] [Google Scholar]
- 49.Tisdale, M., S. D. Kemp, N. R. Parry, and B. A. Larder. 1993. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc. Natl. Acad. Sci. USA 90:5653-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trono, D. 1992. Partial reverse transcripts in virions from human immunodeficiency and murine leukemia viruses. J. Virol. 66:4893-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Rompay, K. K., T. B. Matthews, J. Higgins, D. R. Canfield, R. P. Tarara, M. A. Wainberg, R. F. Schinazi, N. C. Pedersen, and T. W. North. 2002. Virulence and reduced fitness of simian immunodeficiency virus with the M184V mutation in reverse transcriptase. J. Virol. 76:6083-6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei, X., C. Liang, M. Gotte, and M. A. Wainberg. 2003. Negative effect of the M184V mutation in HIV-1 reverse transcriptase on initiation of viral DNA synthesis. Virology 311:202-212. [DOI] [PubMed] [Google Scholar]
- 53.White, K. L., N. A. Margot, T. Wrin, C. J. Petropoulos, M. D. Miller, and L. K. Naeger. 2002. Molecular mechanisms of resistance to human immunodeficiency virus type 1 with reverse transcriptase mutations K65R and K65R+M184V and their effects on enzyme function and viral replication capacity. Antimicrob. Agents Chemother. 46:3437-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, Y. M., H. Imamichi, T. Imamichi, H. C. Lane, J. Falloon, M. B. Vasudevachari, and N. P. Salzman. 1997. Drug resistance during indinavir therapy is caused by mutations in the protease gene and in its Gag substrate cleavage sites. J. Virol. 71:6662-6670. [DOI] [PMC free article] [PubMed] [Google Scholar]