Abstract
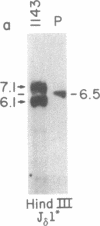
We cloned the t(10;14) recurrent translocation from CD3-negative T-cell acute lymphoblastic leukemia cells. The breakpoint at 14q11 involved an intermediate rearrangement of the delta T-cell receptor locus, suggesting that the translocation arose at the time of antigen receptor assemblage. Translocation introduced chromosome segment 10q24 as proven by hybridization of a breakpoint-derived probe to flow-sorted chromosomes and metaphase chromosomes. Two t(10;14) breakpoints were clustered within a 600-base-pair region of 10q24 but no heptamer-spacer-nonamer motifs resembling T-cell receptor/immunoglobulin rearrangement signals were noted at the breakpoint. A locus distinct from terminal deoxynucleotidyltransferase was found at 10q24. Evolutionarily conserved regions surrounding the 10q24 breakpoint were examined for transcriptional activity. A region telomeric to the 10q24 breakpoint, expected to translocate to the der(14) chromosome, recognized an abundant 2.9-kilobase RNA in a t(10;14) T-cell leukemia. This locus was not active in a variety of other normal and neoplastic T cells, arguing that it was deregulated by the introduction of the T-cell receptor. This locus is a candidate for a putative protooncogene, TCL3, involved in T-cell neoplasia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakhshi A., Jensen J. P., Goldman P., Wright J. J., McBride O. W., Epstein A. L., Korsmeyer S. J. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985 Jul;41(3):899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Bennett J. M., Catovsky D., Daniel M. T., Flandrin G., Galton D. A., Gralnick H. R., Sultan C. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976 Aug;33(4):451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Boehm T., Baer R., Lavenir I., Forster A., Waters J. J., Nacheva E., Rabbitts T. H. The mechanism of chromosomal translocation t(11;14) involving the T-cell receptor C delta locus on human chromosome 14q11 and a transcribed region of chromosome 11p15. EMBO J. 1988 Feb;7(2):385–394. doi: 10.1002/j.1460-2075.1988.tb02825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T., Buluwela L., Williams D., White L., Rabbitts T. H. A cluster of chromosome 11p13 translocations found via distinct D-D and D-D-J rearrangements of the human T cell receptor delta chain gene. EMBO J. 1988 Jul;7(7):2011–2017. doi: 10.1002/j.1460-2075.1988.tb03040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Smith S. D., Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986 Oct 10;47(1):19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Bregni M., Erikson J., Patterson D., Gallo R. C., Croce C. M. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dariavach P., Lefranc M. P. First genomic sequence of the human T-cell receptor delta 2 gene (TRDV2). Nucleic Acids Res. 1989 Jun 26;17(12):4880–4880. doi: 10.1093/nar/17.12.4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaven L. L., Van Dilla M. A., Bartholdi M. F., Carrano A. V., Cram L. S., Fuscoe J. C., Gray J. W., Hildebrand C. E., Moyzis R. K., Perlman J. Construction of human chromosome-specific DNA libraries from flow-sorted chromosomes. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):159–167. doi: 10.1101/sqb.1986.051.01.019. [DOI] [PubMed] [Google Scholar]
- Desiderio S. V., Yancopoulos G. D., Paskind M., Thomas E., Boss M. A., Landau N., Alt F. W., Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984 Oct 25;311(5988):752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- Dubé I. D., Raimondi S. C., Pi D., Kalousek D. K. A new translocation, t(10;14)(q24;q11), in T cell neoplasia. Blood. 1986 Apr;67(4):1181–1184. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Felix C. A., Wright J. J., Poplack D. G., Reaman G. H., Cole D., Goldman P., Korsmeyer S. J. T cell receptor alpha-, beta-, and gamma-genes in T cell and pre-B cell acute lymphoblastic leukemia. J Clin Invest. 1987 Aug;80(2):545–556. doi: 10.1172/JCI113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockett R. D., de Villartay J. P., Pollock K., Poplack D. G., Cohen D. I., Korsmeyer S. J. Human T-cell antigen receptor (TCR) delta-chain locus and elements responsible for its deletion are within the TCR alpha-chain locus. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9694–9698. doi: 10.1073/pnas.85.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe M., Huebner K., Erikson J., Peterson R. C., Bollum F. J., Chang L. M., Croce C. M. Chromosome localization of the gene for human terminal deoxynucleotidyltransferase to region 10q23-q25. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5836–5840. doi: 10.1073/pnas.82.17.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J., Finan J., Letofsky J., Besa E. C., Nowell P. C., Croce C. M. Alpha-chain locus of the T-cell antigen receptor is involved in the t(10;14) chromosome translocation of T-cell acute lymphocytic leukemia. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4543–4546. doi: 10.1073/pnas.84.13.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J., Finger L. R., Letofsky J., Finan J., Nowell P. C., Croce C. M. Clustering of breakpoints on chromosome 10 in acute T-cell leukemias with the t(10;14) chromosome translocation. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4161–4165. doi: 10.1073/pnas.86.11.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G. The role of gene dosage and genetic transpositions in carcinogenesis. Nature. 1981 Nov 26;294(5839):313–318. doi: 10.1038/294313a0. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J., Arnold A., Bakhshi A., Ravetch J. V., Siebenlist U., Hieter P. A., Sharrow S. O., LeBien T. W., Kersey J. H., Poplack D. G. Immunoglobulin gene rearrangement and cell surface antigen expression in acute lymphocytic leukemias of T cell and B cell precursor origins. J Clin Invest. 1983 Feb;71(2):301–313. doi: 10.1172/JCI110770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- McGuire E. A., Hockett R. D., Pollock K. M., Bartholdi M. F., O'Brien S. J., Korsmeyer S. J. The t(11;14)(p15;q11) in a T-cell acute lymphoblastic leukemia cell line activates multiple transcripts, including Ttg-1, a gene encoding a potential zinc finger protein. Mol Cell Biol. 1989 May;9(5):2124–2132. doi: 10.1128/mcb.9.5.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellentin J. D., Smith S. D., Cleary M. L. lyl-1, a novel gene altered by chromosomal translocation in T cell leukemia, codes for a protein with a helix-loop-helix DNA binding motif. Cell. 1989 Jul 14;58(1):77–83. doi: 10.1016/0092-8674(89)90404-2. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Neve R. L., Colletti-Feener C., Bertelson C. J., Kurnit D. M., Kunkel L. M. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986 Oct 16;323(6089):646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- Morton C. C., Kirsch I. R., Taub R., Orkin S. H., Brown J. A. Localization of the beta-globin gene by chromosomal in situ hybridization. Am J Hum Genet. 1984 May;36(3):576–585. [PMC free article] [PubMed] [Google Scholar]
- Nunez G., Seto M., Seremetis S., Ferrero D., Grignani F., Korsmeyer S. J., Dalla-Favera R. Growth- and tumor-promoting effects of deregulated BCL2 in human B-lymphoblastoid cells. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4589–4593. doi: 10.1073/pnas.86.12.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi S. C., Behm F. G., Roberson P. K., Pui C. H., Rivera G. K., Murphy S. B., Williams D. L. Cytogenetics of childhood T-cell leukemia. Blood. 1988 Nov;72(5):1560–1566. [PubMed] [Google Scholar]
- Reynolds T. C., Smith S. D., Sklar J. Analysis of DNA surrounding the breakpoints of chromosomal translocations involving the beta T cell receptor gene in human lymphoblastic neoplasms. Cell. 1987 Jul 3;50(1):107–117. doi: 10.1016/0092-8674(87)90667-2. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Identification of the constant chromosome regions involved in human hematologic malignant disease. Science. 1982 May 14;216(4547):749–751. doi: 10.1126/science.7079737. [DOI] [PubMed] [Google Scholar]
- Seto M., Jaeger U., Hockett R. D., Graninger W., Bennett S., Goldman P., Korsmeyer S. J. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. 1988 Jan;7(1):123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R., Kirsch I., Morton C., Lenoir G., Swan D., Tronick S., Aaronson S., Leder P. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillinghast J. P., Russell J. H., Fields L. E., Loh D. Y. Protein kinase C regulation of terminal deoxynucleotidyl transferase. J Immunol. 1989 Oct 1;143(7):2378–2383. [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]