Abstract
The RNA-dependent RNA polymerase of influenza virus consists of three subunits, PB1, PB2, and PA, and synthesizes three kinds of viral RNAs, vRNA, cRNA, and mRNA. PB1 is a catalytic subunit; PB2 recognizes the cap structure for generation of the primer for transcription; and PA is thought to be involved in viral RNA replication. However, the process of polymerase complex assembly and the exact nature of polymerase complexes involved in synthesis of the three different RNA species are not yet clear. ts53 virus is a temperature-sensitive (ts) mutant derived from A/WSN/33 (A. Sugiura, M. Ueda, K. Tobita, and C. Enomoto, Virology 65:363-373, 1975). We confirmed that the mRNA synthesis level of ts53 remains unaffected at the nonpermissive temperature, whereas vRNA synthesis is largely reduced. Sequencing of the gene encoding ts53 PA and recombinant virus rescue experiments revealed that an amino acid change from Leu to Pro at amino acid position 226 is causative of temperature sensitivity. By glycerol density gradient analyses of nuclear extracts prepared from wild-type virus-infected cells, we found that polymerase proteins sediment in three fractions: one (H fraction) consists of RNP complexes, another (M fraction) contains active polymerases but not viral RNA, and the other (L fraction) contains inactive forms of polymerases. Pulse-chase experiments showed that polymerases in the L fraction are converted to those in the M fraction. In ts53-infected cells, polymerases accumulated in the L fraction. These results strongly suggest that PA is involved in the assembly of functional viral RNA polymerase complexes from their inactive intermediates.
The genome of influenza type A viruses consists of a set of eight single-stranded RNA segments of negative polarity. The viral RNA genome (vRNA) is transcribed into mRNA and replicated through a cRNA intermediate to produce a large quantity of progeny vRNA. These RNAs are synthesized by the viral RNA-dependent RNA polymerase complex. The RNA polymerase complex consists of three subunits, polymerase basic protein 1 (PB1), polymerase basic protein 2 (PB2), and polymerase acidic protein (PA). PB1 functions as a polymerase catalytic subunit for the sequential addition of nucleotides to RNA transcripts (3, 4). It contains the conserved motifs characteristic of RNA-dependent RNA polymerases (32). It also contains sites for sequence-specific binding to conserved 5′- and 3′-terminal sequences of vRNA and cRNA molecules (7). The PB2 subunit binds to cap-1 structures of host pre-mRNAs and is involved in transcription initiation by the cap-stealing mechanism, in which cellular capped pre-mRNAs are used to generate primers for viral transcription (4, 45). It has been reported that the endonuclease domain for endonucleolytic cleavage of cellular pre-mRNA resides in PB1 but not in PB2 (17). However, much less is known about the function of PA.
A large collection of influenza virus temperature-sensitive (ts) mutants, derived from different strains, had been generated in several laboratories (18, 19, 37, 39-41). ts53 virus was generated from strain A/WSN/33 by 5-fluorouracil mutagenesis by Sugiura et al. in 1975 (41). A complementation test by mixed infection predicted that ts53 virus contains a mutation(s) in the gene encoding PA protein (34). The mRNA synthesis level of ts53 remains unaffected at the nonpermissive temperature, whereas vRNA synthesis is largely reduced (15). Thus, PA was predicted to be involved in viral genome replication. It has been reported that PA induces proteolysis of both viral and host proteins (35). Perals et al. showed that the replication activity of the polymerase is associated with the proteolysis activity of PA, because point mutations at amino acid positions 157 (PA T157A) and 162 (PA T162A) of PA, potential sites for phosphorylation by casein kinase II (CKII), resulted in reduction of both proteolytic induction and viral genome replication by model vRNP complexes formed in vivo from cloned cDNAs (31, 36). However, the role of the proteolysis-inducing activity of PA in viral transcription and replication is not completely understood. Recently, the defect in replication of the PA T157A mutant virus was suggested to be caused by an abnormality of PA nuclear transport (12).
Functional forms of polymerase complexes involved in synthesis of the three different RNA species and the assembly regulation of the polymerase complexes and their subcomplexes, if any, are not completely understood. It has been reported that PB1 alone transcribes RNA templates in vitro (14). PB2-PB1 binary complexes showed essentially the same catalytic properties as PB2-PB1-PA complexes, whereas PB1-PA binary complexes catalyzed de novo initiation of RNA synthesis in the absence of primers in vitro (10). Furthermore, it was suggested by use of a minireplicon system with a model viral genome in vivo that PB1 alone catalyzes cRNA synthesis, and PB1-PA complexes appear to replicate vRNA and synthesize uncapped poly(A)+ transcripts (23, 24). These could be interpreted as meaning that PB1 and PB1 complexed with PB2 or PA have minimal functional properties. For efficient synthesis of all three viral RNAs, however, all three polymerase subunits are thought to be required (5, 9). For instance, a mutant with a change from His to Ala at the amino acid position 510 in the PA protein showed the normal level of replicational activity, whereas its transcriptional activity is negligible due to a defect in endonuclease activity (5). Moreover, PB2 mutant viruses showed delayed kinetics of cRNA and vRNA accumulation, although their primary transcription rates were indistinguishable from those of wild-type virus (9). Taken together, it is quite likely that all three polymerase subunits are involved in construction of structural and functional integrity as fully active polymerase complexes in transcription and replication, each subunit keeping its individual potential.
In the course of the analysis of the PA function using a temperature-sensitive mutant, ts53, we found that at the nonpermissive temperature polymerase proteins prepared from cells infected with ts53 accumulate in particular fractions of glycerol density gradient in which polymerases are inactive. Polymerases with the same properties as these in this fraction were also formed in cells infected with wild-type virus. Of interest was that inactive polymerases are recovered in polymerase-active fractions along with progression of infection. Thus, the results strongly suggest that PA is involved in the assembly process of functionally active polymerases from inactive intermediate ones.
MATERIALS AND METHODS
Biological materials.
Madin-Darby canine kidney (MDCK) cells were grown in minimal essential medium (MEM) (Nissui) containing 10% fetal bovine serum. Wild-type influenza virus strain A/WSN/33 (A/WSN/33) and the ts53 mutant (kindly provided by K. Tobita) were used after single-plaque isolation. MDCK cells were infected with the wild-type strain or ts53 at a multiplicity of infection (MOI) of 0.1 PFU/cell. After incubation at 34°C for 24 h, the culture fluid was harvested and centrifuged at 1,700 × g for 10 min. The virus suspension was stored at −80°C until use. vRNP was prepared from purified influenza A/PR/8/34 virus as previously described (46).
Rat polyclonal antibodies against PB1, PB2, and PA were generated by immunization of 5-week-old male rats (SD; Tokyo Jikken Doubutsu) with 100 μg of hexahistidine-tagged PB1 (His-PB1), His-PB2, and His-PA, respectively, in Freund's complete adjuvant (Sigma). For the expression of His-PB1, His-PB2, and His-PA, we cloned open reading frames corresponding to these genes into pET14b, a bacterial expression vector (Novagen). DNA fragments were generated by PCR amplification from pcDNA-PB1, pcDNA-PB2, or pcDNA-PA (kind gifts from Y. Kawaoka) with specific primers 5′-GATCCCGGGCATATGGATGTCAATCCGACCTTAC-3′ and 5′-GAACTCGAGAAGCTTATTTTTGCCGTCTGAGCTCT-3′ for PB1 cDNA, 5′-GAAAGAATAAAAGAACTAAGAAATCTAATGTCGCAGTC-3′ and 5′-CGCGCTCGAGCTAATTGATGGCCATCCGAATTC-3′ for PB2 cDNA, and 5′-GATCCCGGGCATATGGAAGATTTTGTGCGACAATG-3′ and 5′-TAGGATCCGCTAGCTCAATGCATGTGTAAGGAAGG-3′ for PA cDNA. PB1 fragments were digested with NdeI and XhoI and then were cloned into pET14b, which had been digested with NdeI and XhoI. PB2 fragments were digested with XhoI and were cloned into pET14b, which had been digested with XhoI. PA fragments were digested with NdeI and BamHI and were cloned into pET14b, which had been digested with NdeI and BamHI. The resulting plasmids were used for transformation of Escherichia coli BL21(DE3). Recombinant proteins recovered as insoluble fractions were solubilized with a guanidine buffer (20 mM Tris-HCl [pH 7.4], 6 M guanidine hydrochloride, 500 mM NaCl). Solubilized recombinant proteins were purified using Ni-nitrilotriacetic acid resin in the presence of 6 M guanidine hydrochloride according to the recommended protocol of the manufacturer (Novagen). The animals were boosted three times with 100 μg of each protein in Freund's incomplete adjuvant at 2-week intervals. In the case of generation of a rabbit polyclonal antibody against PA, a His-PA deletion mutant spanning the region of PA between amino acid positions 159 and 379 with an additional hexahistidine tag at its amino terminus was used for the antigen. For the expression of the His-PA deletion mutant, we followed a previous report (1). The DNA fragment spanning the region of PA between nucleotide sequence positions 499 and 1161 was amplified from pET14b-PA (described above) by PCR with specific primers 5′-GCGCCTCGAGGCCGACTACACTCTCGAT-3′ and 5′-GCGCCTCGAGCTACTATACCTTTTCTGGTGCCATGTT-3′. Generated DNA fragments were digested with XhoI and then were cloned into pET14b, which had been digested with XhoI. The recombinant protein was purified by using the procedure described above. A 2-month-old female rabbit (Japan White; Tokyo Jikken Doubutsu) was immunized with 250 μg of the His-PA deletion mutant protein and boosted three times with 150 μg of the same protein at 2-week intervals. Rabbit anti-PB1 and anti-PB2 antibodies were kind gifts from T. Toyoda. Every antibody was used for immunological methods by appropriate dilution so that the titer of each diluted antibody was exactly the same as the others in Western blotting.
cDNA cloning and sequencing of segment 3 of the ts53 mutant.
Viral RNA was prepared from infected cells by phenol extraction followed by DNase I treatment. Single-stranded cDNAs were synthesized by reverse transcriptase (SuperScriptII; Lifetech) with either Bm-PA-1 primer (5′-TATTCGTCTCAGGGAGCGAAAGCAGGTAC-3′) corresponding to the 5′ portion of the PA cRNA, PA-664 primer (5′-ATCACAGGAACAATGCGCAAGC-3′) corresponding to the PA coding region between nucleotide sequence positions 643 to 664, or PA-1356 primer (5′-CACATTGCAAGCATGAGAAGGAAT-3′) corresponding to the PA coding region between nucleotide sequence positions 1333 to 1356. To make double-stranded cDNAs, PCR was carried out using Taq DNA polymerase with a combination of Bm-PA-1 primer and PA-717 primer (5′-CGGTTCGAATCCATCCACATAG-3′), the latter of which is complementary to the PA coding region between nucleotide sequence positions 717 to 738, a combination of PA-664 primer and PA-1398 primer (5′-ATTGATGTACACCCCCTTCATTATG-3′) complementary to the PA coding region between nucleotide sequence positions 1398 to 1422, and a combination of PA-1356 primer and PA-2233 primer (5′-ATATCGTCTCGTATTAGTAGAAACAAGGTACTT-3′) complementary to the 3′ portion of the PA cRNA. All cDNAs were purified and directly sequenced with PA-366 primer (5′-TTACTCCAATTTCGATGAATGTATTC-3′) complementary to the PA coding region between nucleotide sequence positions 366 to 391, PA717 primer, PA-1356 primer, PA-1398 primer, and PA-2233 primer using a Dyenamic ET sequencing system (ABI). Comparison of amino acid sequences around the mutation site was performed using the Influenza Sequence Database (http://www.flu.lanl.gov/).
Generation of recombinant viruses.
To construct the vector from which RNA polymerase I transcribes the ts53 virus segment 3 RNA, we cloned cDNA of segment 3 of ts53 into a pHH21 vector (25). Recombinant viruses containing viral RNAs of A/WSN/33 and ts53 in different combinations were generated by the method of Neumann et al. (25). After incubation at 34°C for 48 h posttransfection, aliquots of cell culture supernatants were used for virus amplification in MDCK cells. At 48 h postinfection at 34°C, the culture fluid was collected. The virus suspension was stored at −80°C until use.
Indirect immunofluorescence assay.
Cells on the coverslips were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min and permeabilized with 0.1% NP-40 in PBS, and the coverslips were soaked in 1% nonfat dry milk in PBS. Coverslips were incubated at room temperature for 1 h with a primary antibody. After being washed twice with PBS, coverslips were incubated at room temperature for 1 h with a secondary antibody, fluorescein isothiocyanate (FITC)-conjugated goat anti-species-specific immunoglobulin. After being washed, coverslips were incubated at room temperature for 5 min with 3 μM 4′,6′-diamidino-2-phenylindole (DAPI). Coverslips were finally mounted on glass plates, and cells were observed under a fluorescence microscope (Carl Zeiss).
RNA analysis.
MDCK cells were infected with WSN/33 or ts53 at an MOI of 10 PFU/cell. Total RNA was isolated at indicated time points postinfection by using phenol extraction followed by DNase I treatment. Purified RNA was then subjected to primer extension assays with oligodeoxyribonucleotide primers, which were labeled at their 5′ end with [γ-32P]ATP and T4 polynucleotide kinase (Toyobo). The mixture of RNA and the primer was boiled, rapidly cooled on ice, and then transferred to 42°C. Primer extension assays were performed by the addition of 50 U of reverse transcriptase (SuperScriptII; Lifetech) at 42°C for 1 h. Segment 3 vRNA was reverse transcribed with PA-593 primer (5′-GTACTGTGTTCTTGAGATAGG-3′) corresponding to the PA cRNA between nucleotide sequence positions 1641 to 1661. Segment 7 vRNA was reverse transcribed with Seg7-150 primer (5′-TACGGTTTGAAAAGAGGGC-3′) corresponding to the segment 7 cRNA between nucleotide sequence positions 880 to 898. Segment 3 mRNA and cRNA were reverse transcribed with PA-366 primer. Segment 7 mRNA and cRNA were also reverse transcribed with Seg7-200 primer (5′-TCCCCTTAGTCAGAGGTGAC-3′) complementary to the M1 coding region between nucleotide sequence positions 179 to 198. The expected length of each product was 593 nucleotides for segment 3 vRNA, 132 nucleotides for segment 7 vRNA, 366 nucleotides for segment 3 cRNA, and 198 nucleotides for segment 7 cRNA. In the case of mRNAs, products were 10 to 13 nucleotides longer than that from cRNA due to the primer oligonucleotide derived from capped RNA. Products were separated through 5% polyacrylamide gels containing 7 M urea in Tris-borate-EDTA (TBE) buffer and were detected by autoradiography. For internal quality control, 10 ng of luciferase mRNA (Promega) was added to each sample when RNA isolation was started. For reverse transcription, the specific primer (5′-CCGGAATGATTTGATTGCC-3′) complementary to a part of the luciferase coding region was used.
Glycerol density gradient centrifugation.
MDCK cells infected with ts53 at 34 or 39.5°C were permeabilized with 100 μg of digitonin/ml in a digitonin buffer [50 mM HEPES-NaOH (pH 7.4), 220 mM potassium acetate (KOAc), 300 mM sodium acetate (NaOAc), 100 mM Mg(OAc)2, 25 mM EGTA] for 5 min and washed twice with the digitonin buffer. The permeabilized cells were lysed with immunoprecipitation (IP) buffer (20 mM HEPES-NaOH [pH 7.9], 100 mM NaCl, 30 mM KCl, 1 mM EDTA and 0.1% Nonidet P-40) containing 0.1 U of RNase inhibitor (Toyobo)/μl and then disrupted using a 27-gauge needle. Soluble nuclear proteins recovered from these lysates by centrifugation at 12,000 × g were loaded onto a 30 to 60% (wt/vol) glycerol density gradient in a solution containing 20 mM HEPES-NaOH (pH 7.9), 100 mM NaCl, 30 mM KCl, 1 mM EDTA, and 0.1% Nonidet P-40 and were subjected to centrifugation at 4°C in an SW28 rotor at 45,000 rpm for 5 h. Fractionation was carried out from the top of the gradient, and the fractions were stored at −80°C until use. In the case of metabolic labeling experiments, infected cells were pulse labeled at 34°C in the presence of 10 μCi of [35S]methionine and [35S]cysteine mixture/ml for 15 min at 4.5 h postinfection. Cells were further incubated for 15 min or 1 h in MEM containing excess amounts of nonlabeled methionine and cysteine. Metabolically labeled cell lysates were then subjected to the glycerol density gradient centrifugation assay as described above. Proteins in each fraction were separated in sodium dodecyl sulfate-7.5% polyacrylamide gel electrophoresis (SDS—7.5% PAGE) and visualized by autoradiography. For quantitative determination of proteins, the autoradiogram was scanned with the National Institutes of Health (NIH) image analyzing system.
In vitro RNA synthesis.
In vitro RNA synthesis was performed as previously reported (20, 21). Briefly, in vitro RNA synthesis was carried out at 30°C for 60 min in a final volume of 25 μl containing 50 mM HEPES-NaOH (pH 7.9), 3 mM MgCl2, 50 mM KCl, 1.5 mM dithiothreitol, 500 μM each ATP, CTP, and UTP, 25 μM GTP, 5 μCi of [α-32P]GTP (3,000 Ci/mmol), 4 U of RNase inhibitor, 25 μg of actinomycin D/ml, 250 μM ApG dinucleotide, 10 ng of a 53-nucleotide-long model RNA template (53-mer; 5′-AGUAGAAACAAGGGUGUUUUUUCAUAUCAUUUAAACUUCACCCUGCUUUUGCU-3′) of negative polarity, and glycerol density gradient centrifugation fractions as an enzyme source. RNA products were purified, subjected to 10% PAGE in the presence of 8 M urea, and visualized by autoradiography. vRNP complexes obtained from purified virions by glycerol density gradient (21, 38, 46) was used in in vitro RNA synthesis as a positive control.
RESULTS
The change of Leu 226 to Pro 226 in PA causes temperature sensitivity of ts53 virus.
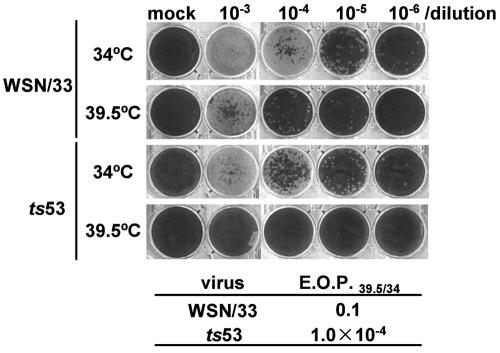
To study the function of the PA subunit of the influenza virus RNA polymerase in transcription and replication of the viral RNA genome, we decided to start with analysis of the properties of ts53 virus. It was previously suggested by complementation assays that the ts53 virus contains a mutation(s) in the PA gene and gives a defect in the viral genome replication process (15, 34). We first confirmed the temperature sensitivity of ts53 by plaque assays (Fig. 1). Plaque assays were carried out at the permissive temperature (34°C) and the nonpermissive temperature (39.5°C) for wild-type WSN/33 and ts53 viruses. The efficiency of plaque formation at 39.5°C is indicated as the ratio relative to that at 34°C (EOP39.5/34). The EOP39.5/34 for ts53 was 1/1,000 less than that for WSN/33 (Fig. 1).
FIG. 1.
Temperature sensitivity of ts53 virus in plaque formation. Plaque assays were carried out at 34°C (permissive temperature) and 39.5°C (nonpermissive temperature) with diluted (103 to 106 times) wild-type WSN/33 virus and ts53 virus. The efficiency of plaque formation of WSN/33 virus at 39.5°C relative to that at 34°C (EOP39.5/34) was 0.1, whereas the EOP39.5/34 of ts53 virus was 1.0 × 10−4.
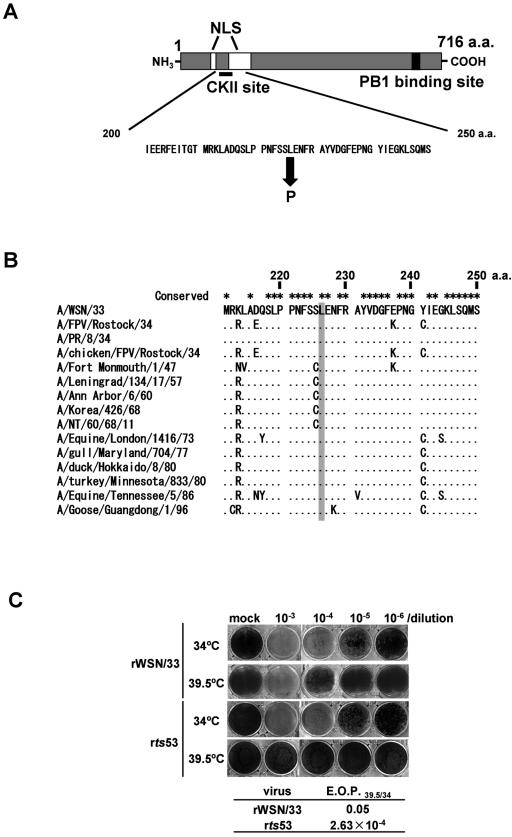
We next determined a mutation site(s) by cloning and sequencing the cDNA of the segment coding for PA (segment 3) of the ts53 mutant. RNA was prepared from ts53 virus-infected cells, and single-stranded cDNAs were synthesized by reverse transcriptase with several kinds of primers (see Materials and Methods). The single-stranded cDNA was subjected to amplification by PCR. Double-stranded cDNA corresponding to segment 3, alternatively designated the PA gene, were directly sequenced. Compared to the wild-type nucleotide sequence, we found one nucleotide change from T to C at the nucleotide position of 701 (where the 5′ terminal nucleotide of cRNA is referred to as nucleotide position 1) (data not shown). This nucleotide change leads to an amino acid change from Leu to Pro at amino acid position 226 (Fig. 2A). This amino acid position is located near nuclear localization signals (NLS) and putative phosphorylation sites, possibly phosphorylated by CKII (27, 28, 31). Additionally, we performed RACE-PCR (rapid amplification of cDNA ends-PCR) to determine nucleotide sequences within untranslated regions. We did not find any other mutations in segment 3 of ts53 (data not shown). Comparison of amino acid sequences around the mutation site among different influenza A virus strains revealed that Leu at amino acid 226 (Leu226) is highly conserved (Fig. 2B), suggesting that Leu226 is important for the function and/or structure of PA.
FIG. 2.
Mutation point in the PA gene of ts53 virus. (A) A single point mutation is located near nuclear localization signals (NLS) and putative phosphorylation sites. This mutation causes an amino acid change from Leu to Pro at amino acid position 226. The PB1 binding site is also shown (28). (B) The conservation of Leu at amino acid position 226 among several influenza A viruses. The mutated amino acid in the PA of ts53 is indicated by a gray box. This amino acid is highly conserved among several influenza A viruses. (C) Responsibility of the mutation at amino acid position 226 for the temperature sensitivity of ts53 virus. A recombinant virus was generated by reverse genetics that contains the ts53 PA gene and seven other genes derived from wild-type virus. Plaque assays were carried out at 34 or 39.5°C. The EOP39.5/34 of recombinant WSN/33 virus was 0.05, whereas the EOP39.5/34 of recombinant ts53 virus was 2.63 × 10−4. a.a., amino acid.
To test whether the single change from L226 to P226 in PA is causative of the temperature-sensitive defect in ts53, a recombinant virus was generated by reverse genetics that contains the ts53 PA gene and seven other genes of the wild-type virus. The recombinant virus was subjected to plaque assays at 34 and 39.5°C. The EOP39.5/34 of the recombinant virus was 2.63 × 10−4 (Fig. 2C), whereas that of ts53 virus was 1.0 × 10−4 (Fig. 1). Thus, the recombinant virus containing the ts53 PA gene was found to be phenotypically similar to the original ts53 virus. Therefore, it is concluded that the amino acid change from L226 to P226 is responsible for the temperature sensitivity of ts53.
Intracellular localization of ts53 PA.
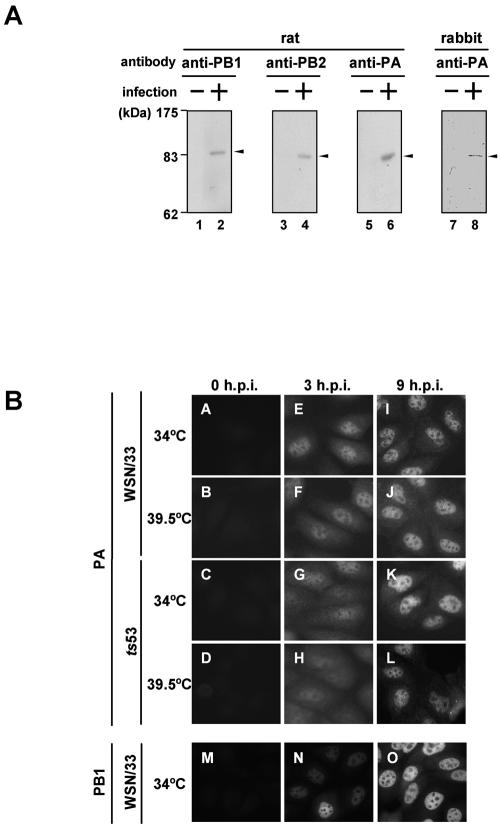
To identify each subunit of the influenza virus polymerase, we generated antibodies by immunization of rats and a rabbit with purified PB1, PB2, and PA expressed in bacteria as recombinant proteins with an N-terminal hexahistidine tag. In the case of a rabbit anti-PA antibody, a His-PA deletion mutant protein spanning amino acid positions 159 to 379 of PA with an additional hexahistidine tag at its amino terminus was used as the antigen. Anti-PB1, anti-PB2, and anti-PA antibodies recognized PB1, PB2, and PA in influenza virus-infected cells, respectively (Fig. 3A). Using these antibodies, we examined the intracellular localization of ts53 PA (Fig. 3B), because the amino acid position of 226 is located near the NLS (Fig. 2A). MDCK cells were infected with WSN/33 or ts53 virus at a multiplicity of infection of 10. After incubation at either 34 or 39.5°C for 3 (Fig. 3E, F, G, and H) or 9 (Fig. 3I, J, K, and L) h, PA was visualized by the indirect immunofluorescence method using the anti-PA rat antibody. Previous reports showed that in early phases of infection (for instance, at 3 h postinfection), PB1 and PB2 are present mainly in the nucleus, whereas PA is present in both the cytoplasm and the nucleus (1). In contrast, in the late phases of infection (for instance, at 9 h postinfection), all three polymerase proteins are localized in the nucleus. These localization patterns were confirmed for a wild-type virus at 34°C (Fig. 3E and I for PA and 3N and O for PB1) and 39.5°C (Fig. 3F and J for PA and data not shown). At 3 h postinfection, PB1 was distinctly localized in the nucleus (Fig. 3N), while PA was localized in both the cytoplasm and the nucleus. In ts53-infected cells at 39.5°C, PA was localized in both the cytoplasm and the nucleus at 3 h postinfection (Fig. 3H) and in the nucleus at 9 h postinfection (Fig. 3L), as it was at 34°C (Fig. 3G and K). The nuclear accumulation level of PA in ts53 virus-infected cells at 39.5°C (Fig. 3L) seemed somehow lower than that in ts53 virus-infected cells at 34°C (Fig. 3K) or WSN/33-infected cells at 34 and 39.5°C (Fig. 3I and J), in agreement with the fact that the viral protein synthesis decreased slightly in ts53 virus-infected cells at 39.5°C (data not shown). Recently, Fodor and Smith reported that PA is localized in both the cytoplasm and the nucleus in cells expressing only the PA subunit (8). In addition, it was shown that PB1 stimulates the nuclear accumulation of PA, and PB1 also requires the expression of PA for efficient nuclear accumulation. It has been suggested that efficient nuclear accumulation of PB1 is dependent on the N-terminal one-third of PA (8). Thus, it is quite possible that PA is transported to the nucleus by itself and/or as PA-PB1 complexes. We found that ts53 PA, when expressed alone at 34 or 39.5°C, is localized in both the nucleus and the cytoplasm (data not shown), and PB1 accumulates in the nucleus efficiently even in ts53 virus-infected cells at 39.5°C (data not shown). These results indicate that the nuclear localization of ts53 PA is normal even at the nonpermissive temperature.
FIG. 3.
Intracellular localization of ts53 PA. (A) Generation of rat polyclonal antibodies against PB1, PB2, and PA and a rabbit polyclonal antibody against PA. Details of the protocol for preparation of the antibodies are described in Materials and Methods. Lysates prepared from WSN/33 virus-infected MDCK cells (lanes 2, 4, 6, and 8) or mock-infected MDCK cells (lanes 1, 3, 5, and 7) were subjected to Western blotting using each antibody. Proteins corresponding to antigens are indicated by arrowheads. (B) Intracellular localization of ts53 PA. MDCK cells were infected with WSN/33 or ts53 virus at 34 or 39.5°C at an MOI of 10. At 3 (panels E to H and N) and 9 (panels I to L and O) h postinfection (h.p.i.), cells were fixed with 4% paraformaldehyde and indirect immunofluorescence assays were carried out using rat anti-PA antibody (panels A to L) and rat anti-PB1 antibody (panels M to O) as described in Materials and Methods. Mock-infected cells were also examined (panels A to D and M).
Synthesis of viral RNAs in ts53 virus-infected cells.
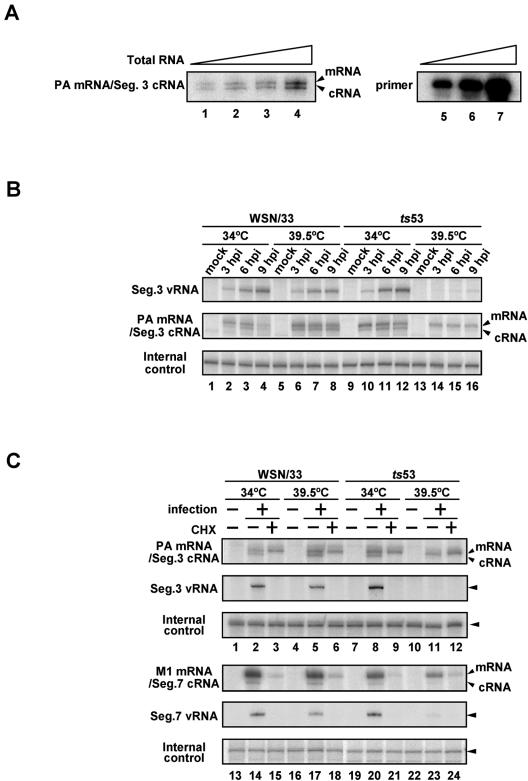
Previous reports showed that ts53 virus is most likely defective in synthesis of viral RNA (15). We examined the levels of vRNA, cRNA, and viral mRNA in ts53 virus-infected cells by quantitative primer extension assays (Fig. 4). Primer extension assays were performed with 4 μg of total RNAs and an excess amount of labeled primers. Under these conditions, the synthesis level of cDNA was proportional to increasing amounts of added total RNA (Fig. 4A). MDCK cells were infected with WSN/33 or ts53 virus at an MOI of 10. After incubation for 3 (Fig. 4B, lanes 2, 6, 10, and 14), 6 (Fig. 4B, lanes 3, 7, 11, and 15), and 9 (Fig. 4B, lanes 4, 8, 12, and 16) h at either 34 or 39.5°C, total RNAs were isolated and subjected to primer extension assays with 32P-labeled primers specific for segment 3 vRNA and both m- and cRNA (Fig. 4B). For an internal quality control, luciferase mRNA was added to each sample when preparation of RNA was initiated. Reverse-transcribed products were analyzed by electrophoresis on a 5% polyacrylamide gel containing 7 M urea and were detected by autoradiography. In cells infected with ts53 virus at 39.5°C, the levels of vRNA and cRNA synthesis were significantly reduced, while the mRNA level at 39.5°C was similar to that at 34°C. This result demonstrates that the RNA polymerase of ts53 virus is capable of synthesizing mRNA but not vRNA and cRNA. Essentially the same results were obtained for other RNA segments (data not shown).
FIG. 4.
Temperature sensitivity of viral RNA synthesis in ts53 virus-infected cells. Primer extension assays were performed with 4 μg of total RNAs and vRNA- or mRNA/cRNA-specific DNA primers labeled at their 5′ end with [γ-32P]ATP. Reverse-transcribed products were analyzed on a 5% polyacrylamide gels containing 7 M urea in TBE buffer and were detected by autoradiography. (A) Dose response in the primer extension assay. Total RNAs were purified from WSN/33 virus-infected MDCK cells at 6 h postinfection (hpi). Primer extension assays were performed with 1 (lane 1), 2 (lane 2), 4 (lane 3), and 8 μg (lane 4) of total RNA and a primer specific for segment (Seg.) 3 mRNA/cRNA as described in Materials and Methods. To show that the amount of the primer was in excess in the primer extension assay, 1/400 (lane 5), 1/200 (lane 6), and 1/100 (lane 7) of the input primers were also subjected to electrophoresis. (B) Synthesis of viral RNAs in ts53 virus-infected cells. MDCK cells were infectedwith WSN/33 or ts53 virus at an MOI of 10. At 3 (lanes 2, 6, 10, and 14), 6 (lanes 3, 7, 11, and 15), and 9 (lanes 4, 8, 12, and 16) h postinfection at 34°C (lanes 1 to 4 and 9 to 12) or 39.5°C (lanes 5 to 8 and 13 to 16), cells were collected and total RNAs were isolated. Primer extension assays were performed with primers specific for segment 3 vRNA or mRNA/cRNA. Total RNAs prepared from uninfected cells were also analyzed (lanes 1, 5, 9, and 13). As an internal control, luciferase mRNA (10 ng) was added to each sample, and the sample was then subjected to primer extension assays. (C) Viral mRNA synthesis in the presence of a protein synthesis inhibitor. MDCK cells were infected with WSN/33 or ts53 virus at an MOI of 10 in the presence or absence of 100 μg of cycloheximide (CHX)/ml. At 6 h postinfection in the presence (lanes 3, 6, 9, 12, 15, 18, 21, and 24) or absence (lanes 2, 5, 8, 11, 14, 17, 20, and 23) of CHX at 34 or 39.5°C, total RNAs were isolated. Primer extension assays were performed with primers specific for vRNA and mRNA/cRNA of segment 3 and segment 7. Total RNAs prepared from uninfected cells without CHX were also analyzed (lanes 1, 4, 7, 10, 13, 16, 19, and 22). luciferase mRNA was used as an internal control for the whole procedure.
To confirm that infecting RNA polymerases of ts53 by themselves are active in mRNA synthesis but are inactive in cRNA and vRNA synthesis, we utilized cycloheximide (CHX), a potent protein synthesis inhibitor (Fig. 4C). Previous reports showed that CHX suppresses viral protein synthesis and thereby represses vRNA replication (9, 33). Therefore, we could examine the viral transcription level without amplification of the viral genome in the presence of CHX. MDCK cells were infected with WSN/33 or ts53 viruses at an MOI of 10. After incubation at 34 or 39.5°C for 6 h in the presence (Fig. 4C, lanes 3, 6, 9, 12, 15, 18, 21, and 24) or absence (Fig. 4C, lanes 2, 5, 8, 11, 14, 17, 20, and 23) of 100 μg of CHX/ml, cells were collected. Total RNAs were isolated and subjected to primer extension assays with primers specific for vRNA and mRNA/cRNA of segments 3 and 7 as representatives of early and late genes, respectively. As expected, the synthesis of cRNA and vRNA of both segments 3 and 7 in ts53-infected cells at 39.5°C was severely inhibited. The level of segment 3 mRNA synthesis in ts53-infected cells at 39.5°C remained unaffected in the presence of CHX (lanes 11 and 12), whereas that of segment 7 mRNA was significantly reduced (lanes 23 and 24). Thus, it is likely that the genome replication process is not needed for segment 3 mRNA synthesis, while segment 7 mRNA synthesis is partially dependent on replication.
Viral RNA polymerase complexes present in nuclei of cells infected with ts53.
The viral polymerase consists of three subunit, PB2, PB1, and PA, among which PB2 and PA bind independently to PB1. Previously, it was shown that PB1 alone and PB2-PB1 binary complexes transcribe RNA templates, whereas PB1-PA complexes catalyzed de novo initiation of RNA synthesis either in vitro or in vivo (10, 14, 23, 24). However, other reports showed that all three polymerase subunits are required for efficient synthesis of all three viral RNA transcripts (5, 9). Therefore, the structural and functional identities of the ts53 polymerase in nuclei of infected cells are of interest. We tried to examine whether polymerase complexes of ts53 at the nonpermissive temperature are biochemically different from those at the permissive temperature by using glycerol density gradients.
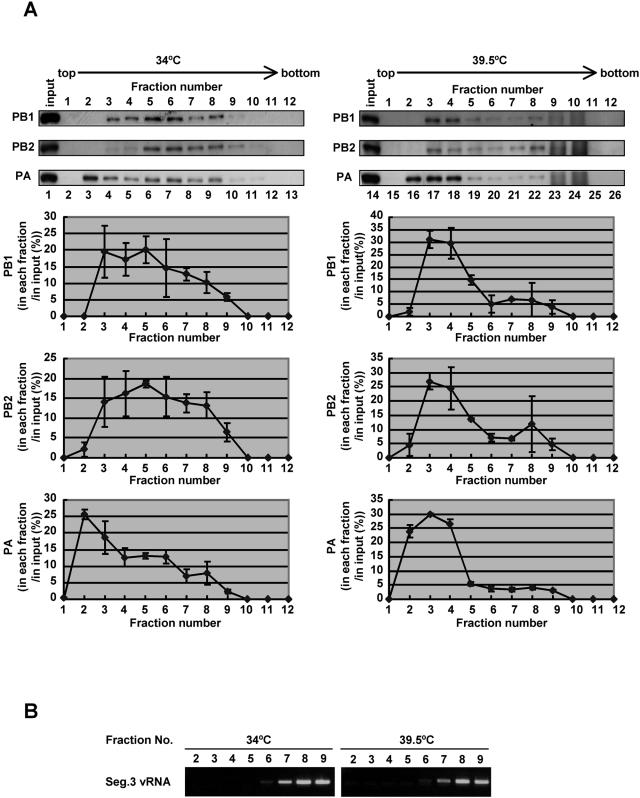
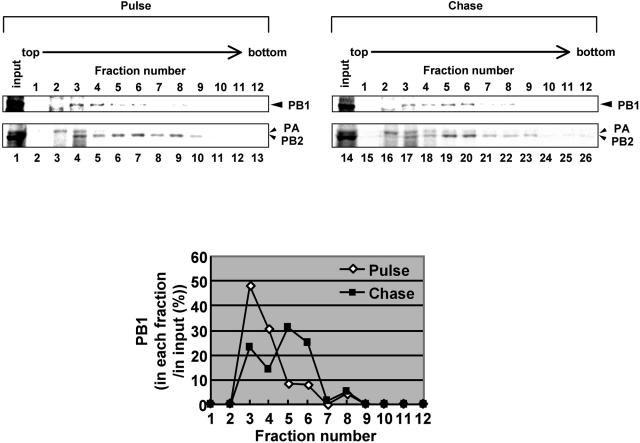
MDCK cells were infected with ts53 at an MOI of 10 and were incubated at 34 or 39.5°C for 6 h. Cells were collected and permeabilized with 100 μg of digitonin/ml. Resultant nuclei were disrupted in the IP buffer by using a 27-gauge needle. Lysates were subjected to centrifugation on a 30 to 60% (wt/vol) glycerol density gradient, and proteins in each fraction were analyzed by Western blotting with rabbit anti-PB1, anti-PB2, and anti-PA antibodies (Fig. 5A). Polymerase proteins in lysates prepared from ts53-infected cells at 39.5°C sedimented predominantly in fractions 2 to 4, whereas those at 34°C were widely recovered in fractions 2 to 8 (Fig. 5A and C). Polymerase proteins in lysates prepared from infected cells at 34°C seemed separated into three fractions, fractions 2 to 4 (designated light [L] fraction), fractions 5 and 6 (medium [M] fraction), and fractions 7 to 9 (heavy [H] fraction) (the lower left panel of Fig. 5A). Exactly the same result was obtained from lysates prepared from WSN/33-infected cells at both 34 and 39.5°C (data not shown). vRNP complexes from purified virion sedimented to fractions corresponding to the H fraction (data not shown). Because vRNA was also predominantly present in H fraction (Fig. 5B), these fractions derived from infected nuclei could form RNP complexes similar to vRNP complexes from virions. To clarify the relationship between L and M fractions, we performed pulse-chase metabolic labeling experiments using [35S]methionine and [35S]cysteine. Pulse labeling was performed for 15 min at 5 h postinfection, and pulse-labeled cells were further incubated for 15 min or 1 h in MEM containing excess amounts of nonlabeled methionine and cysteine. Glycerol density gradient centrifugation experiments revealed that, after chasing for 15 min, more PB1 was found in the L fraction than in the M fraction, whereas after chasing for 1 h the level of PB1 in the M fraction was increased compared to that in pulse-labeled M fraction (Fig. 6). This was also the case for PB2 and PA. These findings indicate that polymerases in the L fraction could be converted into polymerases in the M fraction. Because polymerase proteins from lysates of ts53-infected cells at 39.5°C were mainly found in the L fraction, it is likely that ts53 polymerase complexes are somehow immature due to temperature-sensitive PA.
FIG. 5.
Fractionation of polymerase complexes in ts53-infected cells. MDCK cells were infected with ts53 virus at an MOI of 10 at 34 or 39.5°C for 6 h. Cells were collected and permeabilized in 100 μg of digitonin/ml. Soluble nuclear lysates prepared from permeabilized cells were loaded onto a 30 to 60% glycerol density gradient and centrifuged at 4°C in an SW28 rotor at 45,000 rpm for 5 h. Fractions (1 to 12) were recovered from the top of the gradient. (A) Western blotting analyses. For the upper panel, an aliquot of each fraction was separated in a SDS-7.5% PAGE and subjected to Western blotting using rabbit anti-PB1, anti-PB2, and anti-PA antibodies. Lanes 2 to 13 and lanes 15 to 25 correspond to glycerol gradient fractions of lysates prepared from cells infected at 34 and 39.5°C, respectively. Lysates loaded onto the glycerol density gradient are alsoshown in lanes 1 and 14. The lower panel shows results of the upper panel quantitatively analyzed by the NIH image analyzing system for determination of the amounts of PB1, PB2, and PA in each fraction. The ratio of the amount of PB1, PB2, or PA in each fraction relative to that in the input (the total amount of each polymerase protein) is indicated. (B) RNA analysis. RNAs were purified from glycerol density gradient fractions (fractions 2 to 9), and the segment (Seg.) 3 vRNA was semiquantitatively analyzed by reverse transcription-PCR. cDNA was synthesized by reverse transcriptase with PA-1356 as the primer. Single-stranded cDNA was then PCR amplified with two specific primers, PA-1683 primer (5′-GGCACTTCTTAGAAGCATATCTC-3′), corresponding to the PA coding region between nucleotide sequence positions 1661 and 1683, and PA-1356 primer (PCR cycles consisted of 20 cycles for samples obtained from 34°C assays and 25 cycles for those from 39.5°C assays). Amplified double-stranded DNAs were subjected to a 1% agarose gel electrophoresis and were visualized by ethidium bromide.
FIG. 6.
Conversion of polymerase complexes. MDCK cells were infected with ts53 virus at an MOI of 10 at 34°C for 4.5 h. Cells were washed and incubated in methionine-free medium for 30 min. Infected cells were then pulse labeled at 34°C for 15 min in the presence of 10 μCi of [35S]methionine and [35S]cysteine mixture/ml. After being washed with MEM, cells were further incubated at 34°C for 15 (pulse) or 60 min (chase) in MEM containing excess amounts of nonlabeled methionine and cysteine. Lysates prepared from metabolically labeled cells were subjected to the glycerol density gradient centrifugation assay as described in the legend to Fig. 5. Upper panel, fractionation of pulse-labeled and pulse-chased polymerases. An aliquot of each fraction was separated in SDS-6% PAGE and then was visualized by autoradiography. PB1, PB2, and PA are indicated by arrowheads. We confirmed that these bands correspond to PB1, PB2, and PA by immunoprecipitation assay using antibodies specific for each polymerase subunit (data not shown). (Lower panel) To determine the amount of PB1 in each fraction, autoradiograms were quantitatively analyzed by the NIH image analyzing system, and the amount of PB1 in each fraction is shown as that relative to the total amount of PB1.
Differences in structure and activity of polymerase complexes.
It is likely that polymerase complexes in cells infected with ts53 at 39.5°C are not correctly assembled. In order to examine polymerase complexes in terms of their assembly status, we carried out immunoprecipitation assays using rabbit anti-PA antiserum (Fig. 3A). During the course of setting conditions for immunoprecipitation, we found an interesting specificity of the antibody. With this antibody, we could not immunoprecipitate the vRNP complexes prepared from purified virions that are functionally and structurally authentic. In contrast, the antibody could immunoprecipitate PA from SDS-disrupted vRNP complexes (Fig. 7A). This finding could be interpreted as meaning that a PA epitope(s) for the antibody is masked in correctly assembled polymerase complexes. Therefore, this antibody could be quite useful to discriminate correctly assembled polymerase complexes from unassembled polymerase complexes and/or aberrantly assembled ones. To characterize the polymerase complexes separated by glycerol density gradient centrifugation, fractions from lysates of ts53-infected cells at 34 and 39.5°C were subjected to immunoprecipitation assay using this antibody. Note that we could detect immunoprecipitated polymerase proteins only from fractions 2 to 4 (compare Fig. 7B to 5A). These results indicate that the conformation of polymerase complexes in the L fraction is different from those in other fractions. In the case of lysates prepared from ts53-infected cells at 39.5°C, polymerase subunits were immunoprecipitated from fractions 2 to 4 (Fig. 7B) only where polymerase subunits were present (Fig. 5). Thus, polymerase subunits in the L fraction prepared from cells kept at both 34 and 39.5°C do not seem to be assembled correctly. Besides, because the amount of PB1 coimmunoprecipitated with PA was far less than the amount of PB1 present in the fractions (Fig. 7B and 5A), the majority of PA may be free of PB1. This was also the case for PB2 (data not shown). Therefore, it is quite possible that the L fraction contains unassembled PA and/or aberrantly assembled polymerase complexes. Furthermore, the same results were obtained from lysates prepared from WSN/33-infected cells at either 34 or 39.5°C and those from ts53-infected cells at 39.5°C (data not shown).
FIG. 7.
Properties of polymerase complexes. (A) Recognition specificity of PA by the rabbit anti-PA antibody. vRNP obtained from purified virions was boiled at 100°C for 3 min with 2% SDS (lanes 1 to 3). The solution was then diluted with the IP buffer so that the final concentration of SDS was set at 0.04%. SDS-disrupted vRNP (lanes 1 to 3) and undisrupted vRNP (lanes 4 to 6) were subjected to immunoprecipitation with (lanes 1, 2, 4, and 5) or without (lanes 3 and 6) the rabbit anti-PA antibody as described in Materials and Methods. Immunoprecipitated proteins were separated in SDS-7.5% PAGE and were detected by Western blotting with the rat anti-PA antibody. Lane 7 shows the input vRNP (80 ng of PA). For lanes 1 and 4 and lanes 2, 3, 5, and 6, aliquots containing 400 and 800 ng of PA were used, respectively. (B) Immunoprecipitation of fractions obtained from the glycerol density gradient centrifugation. The fractions (50 μl) corresponding to those in Fig. 5A (lanes 3 to 10 and 16 to 23) were subjected to immunoprecipitation with the rabbit anti-PA antibody (lanes 1 to 8 and 9 to 16, respectively). Immunoprecipitated proteins were separated in SDS-7.5% PAGE and were detected by Western blotting by rat anti-PB1 and anti-PA antibodies. (C) In vitro RNA synthesis using fractions recovered from glycerol density gradient centrifugation. In vitro RNA synthesis was carried out with fraction 3 (lanes 1 and 2), fraction 5 (lanes 3 and 4), and vRNP (lanes 5 and 6; positive controls) prepared from purified virion as enzyme sources, essentially as described previously (20, 22, 38; for details, see the text). [α-32P]GTP-labeled products were separated on a 10% polyacrylamide gel containing 8 M urea and were visualized by autoradiography. Aliquots of fractions 3 and 5 containing 1.25 (lanes 1 and 3) and 5 ng (lanes 2 and 4) of PB1 and vRNP complexes containing 0.25 and 1 ng of PB1 (lanes 5 and 6, respectively) were used.
It is important to examine whether polymerase complexes in the L fraction are also dysfunctional in polymerase activity. To determine the RNA synthesis activity of L and M fractions, fractions 3 and 5 prepared from ts53-infected cells at 34°C were examined in the in vitro RNA synthesis assay using the 53-mer exogenous viral model RNA template. It has been reported that in vitro RNA synthesis systems utilizing vRNP complexes are capable of supporting RNA synthesis from exogenously added viral model RNA (20, 22) consisting of the 5′- and 3′-terminal regions of the segment 8 vRNA that contains the cis-acting signals essential for transcription and replication (29, 47). This system was used to test the RNA-synthesizing activity of polymerase complexes separated by glycerol density gradient. RNA synthesis assays were performed with fractions 3 and 5, containing equal amounts of PB1 as enzyme sources. vRNP complexes prepared from purified virions were also used in the in vitro RNA synthesis. Figure 7C shows that polymerase complexes in fraction 5 are active in RNA synthesis, while those in fraction 3 do not synthesize RNA at all. By performing mixing experiments with fraction 3 and vRNP complexes purified from virions, we could not detect any inhibitory activity (such as RNase activity) or stimulatory activity in fraction 3 (data not shown). When fractions 3 and 5 were subjected to micrococcal nuclease treatment prior to the RNA synthesis assay, we could not find any significant difference of the RNA synthesis activity in fractions 3 and 5 (data not shown), suggesting that the difference in RNA synthesis activity is not due to RNA possibly present in fractions. Notably, fractions 3 and 5 contained all polymerase subunits, whereas there was less nucleoprotein (NP) in fraction 3 (data not shown). It was previously reported that the RNA synthesis system using the 53-mer exogenous vRNA template is not affected markedly by the presence or absence of NP (11, 16). Therefore, we could neglect the effects of the amount of NP in fractions on RNA synthesis.
DISCUSSION
We have identified a mutation point in the PA subunit of the ts53 virus that is causative of its temperature sensitivity. The mutation point, by which Leu at amino acid position 226 is changed to Pro, is located near a nuclear localization signal and putative phosphorylation sites possibly phosphorylated by CKII (Fig. 2A). Previous reports showed that the mutation at amino acid position 157 of PA (PA T157A), a potential site for phosphorylation, decreases the proteolytic induction activity of PA and the ability to synthesize cRNA through abnormal nuclear transport of PA (12, 31). ts53 PA was also defective in viral RNA replication, although the intracellular localization of ts53 PA in ts53-infected cells were indistinguishable from that of wild-type virus PA (Fig. 3B). We detected a phosphorylated form of ts53 PA by immunoprecipitation of lysates prepared from ts53 PA-transfected cells with anti-phosphoserine and anti-phosphothreonine antibodies in Western blotting analyses (data not shown). Therefore, the defect of ts53 in vRNA replication is caused by a mechanism distinct from that of PA T157A. Recent reports revealed that the region spanning amino acid positions 224 and 233 of PA is presented by major histocompatibility complex class I H-2Db (2). This region is highly conserved among different influenza A virus strains (Fig. 2B), although this region is exposed to selective pressure of cellular immune system. Nevertheless, it is possible that this region is important for the PA structure and/or its function.
Primer extension assays revealed that ts53 virus is defective in synthesis of vRNA and cRNA at the nonpermissive temperature, whereas mRNA synthesis was unaffected (Fig. 4B). These observations are in good agreement with a previously published notion (15). Because vRNA is amplified from cRNA, it is quite possible that a primary defect of ts53 virus is impairment of cRNA synthesis. The primary transcription level was unaffected in a gene(s) transcribed immediately after infection (early gene), even at the nonpermissive temperature (Fig. 4C). Early gene transcription is thought to be supported by infecting RNP complexes. In fact, the early gene mRNA was synthesized in the presence of CHX, a protein synthesis inhibitor, in both wild-type and ts53 virus-infected cells. In contrast, neither vRNA nor cRNA was synthesized in the presence of CHX. Therefore, it is possible that primary transcription is supported by infecting vRNP complexes, whereas newly synthesized vRNP complexes are required for replication. Further, ts53 polymerases, including tsPA, which have been once assembled at the permissive temperature, are sound or not inhibitory in viral mRNA synthesis even at the nonpermissive temperature. Taken together, tsPA synthesized at nonpermissive temperature, if assembled with other polymerase proteins, would be inactive in mRNA synthesis. However, at present it is not clear how replication but not transcription is affected by this mutation.
Akkina et al. reported that in early phases of infection, PB1 and PB2 are detected in the nucleus, whereas PA is localized in the cytoplasm (1). All three polymerase proteins, including PA, appear in the nucleus in late phases of infection (1) (Fig. 3B). From these, it is assumed that PB2-PB1 heterocomplexes and PA exist as assembly intermediates in infected nuclei with or without an associated cellular protein(s). Lee et al. proposed that PA has a chaperone effect on PB1 or, alternatively, PA increases the ability of the PB1-RNA interaction, because efficient PB1 binding to the 5′ end of vRNA was shown to be dependent on the formation of a complex between PB1 and PA (16). The latter possibility is supported by an earlier work that found that PA is UV cross-linked to a 5′ vRNA molecule (6). It was reported that PA forms an active part of the cap-dependent endonuclease of PB1 (5). A suppressor mutation of a PB2 temperature-sensitive mutant was found in the PA gene (43). Therefore, it is quite likely that PA is involved in regulation of polymerase functions through the conformational change of polymerase complexes, including those involved not only in replication but also in transcription processes. Glycerol density gradient centrifugation assays showed that a large amount of polymerase was present as immature and nonfunctional polymerases in ts53-infected nuclei at nonpermissive temperature (Fig. 5, 6, and 7). By comparison of the PA amount by immunoprecipitation with that in input lysates (Fig. 7B), a majority of PA in the low-density fractions prepared from infected cells at the permissive temperature and the nonpermissive temperature was found to be present free of other polymerase proteins, while a small fraction of PA could be associated with other proteins. Based on these results, it is quite possible that ts53 PA inhibits the assembly of functional polymerase complexes, possibly from nonfunctional intermediates due to its functional and/or structural change at the nonpermissive temperature. Therefore, we propose that (wild-type) PA is important in the process of functional polymerase complex assembly.
We tried to assess ts53 PA polymerase activity by using a transient transfection system based on a minireplicon system with a model viral genome containing a reporter gene (26, 42, 44). It was previously reported that the expression of the reporter gene is replication dependent in this system (30). Indeed, ts53 PA caused temperature-sensitive expression (data not shown). In mixing transfection experiments with wild-type PA and ts53 PA, ts53 PA did not efficiently inhibit the function of wild-type PA, but an excess amount of ts53 PA did (data not shown). These results suggest that the binding activity of ts53 PA to PB1 is less than that of wild-type PA at the nonpermissive temperature, and ts53 PA is inactive even when interacted with PB1. These results further support the notion obtained from glycerol density gradient centrifugation assays (Fig. 7B).
Here we have proposed that PA could be involved in the assembly of functional viral RNA polymerases from nonfunctional intermediates. However, the molecular differences between intermediate and functional polymerases have not yet been defined. The process in which PA is involved is an open question. In addition, the exact nature of polymerase complexes involved in viral transcription and replication is also unclear. It was reported that PB1 alone catalyzes RNA synthesis, PB2-PB1 binary complexes appear to transcribe mRNA, and PB1-PA binary complexes catalyze vRNA replication in vitro and/or in vivo (10, 14, 23, 24). Because the extent of viral RNA synthesis by such partially assembled polymerase seems limited, for the maximal replication and transcription all three polymerase proteins could be required. Because replication and transcription take place in the nucleus, we should consider not only viral factors such as NP and RNA but also host factors. Indeed, it has been reported that polymerase proteins interact with hCLE and RAF-1/Hsp90 (13, 22). Precise analyses, including isolation of various forms of polymerase complexes and determination of their structures in the process of endonuclease cleavage, transcription, polyadenylation, and replication, are required.
Acknowledgments
We thank K. Tobita (Department of Virology, Jichi Medical School), Y. Kawaoka (Insititute of Medical Science, University of Tokyo), and T. Toyoda (Koyama Hospital) for the generous gifts of ts53 virus (K. Tobita) as well as pCAGGS-NP, pcDNA-PB1, pcDNA-PB2, pcDNA-PA, pPolI-WSN-PB2, pPolI-WSN-PB1, pPolI-WSN-PA, pPolI-WSN-HA, pPolI-WSN-NP, pPolI-WSN-NA, pPolI-WSN-M, pPolI-WSN-NS, and pHH21 (Y. Kawaoka) and rabbit anti-PB1 and anti-PB2 antisera (T. Toyoda).
This work was supported in part by a grant in aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to K.N.).
REFERENCES
- 1.Akkina, R. K., T. M. Chambers, D. R. Londo, and D. P. Nayak. 1987. Intracellular localization of the viral polymerase proteins in cells infected with influenza virus and cells expressing PB1 protein from cloned cDNA. J. Virol. 61:2217-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belz, G. T., W. Xie, J. D. Altman, and P. C. Doherty. 2000. A previously unrecognized H-2D(b)-restricted peptide prominent in the primary influenza A virus-specific CD8(+) T-cell response is much less apparent following secondary challenge. J. Virol. 74:3486-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas, S. K., and D. P. Nayak. 1994. Mutational analysis of the conserved motifs of influenza A virus polymerase basic protein 1. J. Virol. 68:1819-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braam, J., I. Ulmanen, and R. M. Krug. 1983. Molecular model of a eucaryotic transcription complex: functions and movements of influenza P proteins during capped RNA-primed transcription. Cell 34:609-618. [DOI] [PubMed] [Google Scholar]
- 5.Fodor, E., M. Crow, L. J. Mingay, T. Deng, J. Sharps, P. Fechter, and G. G. Brownlee. 2002. A single amino acid mutation in the PA subunit of the influenza virus RNA polymerase inhibits endonucleolytic cleavage of capped RNAs. J. Virol. 76:8989-9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fodor, E., D. C. Pritlove, and G. G. Brownlee. 1994. The influenza virus panhandle is involved in the initiation of transcription. J. Virol. 68:4092-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fodor, E., B. L. Seong, and G. G. Brownlee. 1993. Photochemical cross-linking of influenza A polymerase to its virion RNA promoter defines a polymerase binding site at residues 9 to 12 of the promoter. J. Gen. Virol. 74:1327-1333. [DOI] [PubMed] [Google Scholar]
- 8.Fodor, E., and M. Smith. 2004. The PA subunit is required for efficient nuclear accumulation of the PB1 subunit of the influenza A virus RNA polymerase complex. J. Virol. 78:9144-9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gastaminza, P., B. Perales, A. M. Falcon, and J. Ortin. 2003. Mutations in the N-terminal region of influenza virus PB2 protein affect virus RNA replication but not transcription. J. Virol. 77:5098-5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honda, A., K. Mizumoto, and A. Ishihama. 2002. Minimum molecular architectures for transcription and replication of the influenza virus. Proc. Natl. Acad. Sci. USA 99:13166-13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honda, A., K. Ueda, K. Nagata, and A. Ishihama. 1988. RNA polymerase of influenza virus: role of NP in RNA chain elongation. J. Biochem. (Tokyo) 104:1021-1026. [DOI] [PubMed] [Google Scholar]
- 12.Huarte, M., A. Falcon, Y. Nakaya, J. Ortin, A. Garcia-Sastre, and A. Nieto. 2003. Threonine 157 of influenza virus PA polymerase subunit modulates RNA replication in infectious viruses. J. Virol. 77:6007-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huarte, M., J. J. Sanz-Ezquerro, F. Roncal, J. Ortin, and A. Nieto. 2001. PA subunit from influenza virus polymerase complex interacts with a cellular protein with homology to a family of transcriptional activators. J. Virol. 75:8597-8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi, M., T. Toyoda, and A. Ishihama. 1996. Influenza virus PB1 protein is the minimal and essential subunit of RNA polymerase. Arch. Virol. 141:525-539. [DOI] [PubMed] [Google Scholar]
- 15.Krug, R. M., M. Ueda, and P. Palese. 1975. Temperature-sensitive mutants of influenza WSN virus defective in virus-specific RNA synthesis. J. Virol. 16:790-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, M. T., K. Bishop, L. Medcalf, D. Elton, P. Digard, and L. Tiley. 2002. Definition of the minimal viral components required for the initiation of unprimed RNA synthesis by influenza virus RNA polymerase. Nucleic Acids Res. 30:429-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, M. L., P. Rao, and R. M. Krug. 2001. The active sites of the influenza cap-dependent endonuclease are on different polymerase subunits. EMBO J. 20:2078-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahy, B. W. 1983. Mutants of influenza virus, p. 192-254. In P. Palese (ed.), Genetics of influenza viruses. Springer-Verlag, New York, N.Y.
- 19.Markushin, S. G., and Y. Z. Ghendon. 1973. Genetic classification and biological properties of temperature-sensitive mutants of fowl plague virus. Acta Virol. 17:369-376. [PubMed] [Google Scholar]
- 20.Momose, F., C. F. Basler, R. E. O'Neill, A. Iwamatsu, P. Palese, and K. Nagata. 2001. Cellular splicing factor RAF-2p48/NPI-5/BAT1/UAP56 interacts with the influenza virus nucleoprotein and enhances viral RNA synthesis. J. Virol. 75:1899-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Momose, F., H. Handa, and K. Nagata. 1996. Identification of host factors that regulate the influenza virus RNA polymerase activity. Biochimie 78:1103-1108. [DOI] [PubMed] [Google Scholar]
- 22.Momose, F., T. Naito, K. Yano, S. Sugimoto, Y. Morikawa, and K. Nagata. 2002. Identification of Hsp90 as a stimulatory host factor involved in influenza virus RNA synthesis. J. Biol. Chem. 277:45306-45314. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa, Y., N. Kimura, T. Toyoda, K. Mizumoto, A. Ishihama, K. Oda, and S. Nakada. 1995. The RNA polymerase PB2 subunit is not required for replication of the influenza virus genome but is involved in capped mRNA synthesis. J. Virol. 69:728-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakagawa, Y., K. Oda, and S. Nakada. 1996. The PB1 subunit alone can catalyze cRNA synthesis, and the PA subunit in addition to the PB1 subunit is required for viral RNA synthesis in replication of the influenza virus genome. J. Virol. 70:6390-6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neumann, G., T. Watanabe, H. Ito, S. Watanabe, H. Goto, P. Gao, M. Hughes, D. R. Perez, R. Donis, E. Hoffmann, G. Hobom, and Y. Kawaoka. 1999. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl. Acad. Sci. USA 96:9345-9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumann, G., A. Zobel, and G. Hobom. 1994. RNA polymerase I-mediated expression of influenza viral RNA molecules. Virology 202:477-479. [DOI] [PubMed] [Google Scholar]
- 27.Nieto, A., S. de la Luna, J. Barcena, A. Portela, and J. Ortin. 1994. Complex structure of the nuclear translocation signal of influenza virus polymerase PA subunit. J. Gen. Virol. 75:29-36. [DOI] [PubMed] [Google Scholar]
- 28.Ohtsu, Y., Y. Honda, Y. Sakata, H. Kato, and T. Toyoda. 2002. Fine mapping of the subunit binding sites of influenza virus RNA polymerase. Microbiol. Immunol. 46:167-175. [DOI] [PubMed] [Google Scholar]
- 29.Parvin, J. D., P. Palese, A. Honda, A. Ishihama, and M. Krystal. 1989. Promoter analysis of influenza virus RNA polymerase. J. Virol. 63:5142-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perales, B., and J. Ortin. 1997. The influenza A virus PB2 polymerase subunit is required for the replication of viral RNA. J. Virol. 71:1381-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perales, B., J. J. Sanz-Ezquerro, P. Gastaminza, J. Ortega, J. F. Santaren, J. Ortin, and A. Nieto. 2000. The replication activity of influenza virus polymerase is linked to the capacity of the PA subunit to induce proteolysis. J. Virol. 74:1307-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poch, O., I. Sauvaget, M. Delarue, and N. Tordo. 1989. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 8:3867-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pons, M. W. 1973. The inhibition of influenza virus RNA synthesis by actinomycin D and cycloheximide. Virology 51:120-128. [DOI] [PubMed] [Google Scholar]
- 34.Ritchey, M. B., and P. Palese. 1977. Identification of the defective genes in three mutant groups of influenza virus. J. Virol. 21:1196-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanz-Ezquerro, J. J., S. de la Luna, J. Ortin, and A. Nieto. 1995. Individual expression of influenza virus PA protein induces degradation of coexpressed proteins. J. Virol. 69:2420-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanz-Ezquerro, J. J., J. Fernandez Santaren, T. Sierra, T. Aragon, J. Ortega, J. Ortin, G. L. Smith, and A. Nieto. 1998. The PA influenza virus polymerase subunit is a phosphorylated protein. J. Gen. Virol. 79:471-478. [DOI] [PubMed] [Google Scholar]
- 37.Scholtissek, C., and A. L. Bowles. 1975. Isolation and characterization of temperature-sensitive mutants of fowl plague virus. Virology 67:576-587. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu, K., H. Handa, S. Nakada, and K. Nagata. 1994. Regulation of influenza virus RNA polymerase activity by cellular and viral factors. Nucleic Acids Res. 22:5047-5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson, R. W., and G. K. Hirst. 1968. Temperature-sensitive mutants of influenza A virus: isolation of mutants and preliminary observations on genetic recombination and complementation. Virology 35:41-49. [DOI] [PubMed] [Google Scholar]
- 40.Sugiura, A., K. Tobita, and E. D. Kilbourne. 1972. Isolation and preliminary characterization of temperature-sensitive mutants of influenza virus. J. Virol. 10:639-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugiura, A., M. Ueda, K. Tobita, and C. Enomoto. 1975. Further isolation and characterization of temperature-sensitive mutants of influenza virus. Virology 65:363-373. [DOI] [PubMed] [Google Scholar]
- 42.Toyoda, T., K. Hara, and Y. Imamura. 2003. Ser624 of the PA subunit of influenza A virus is not essential for viral growth in cells and mice, but required for the maximal viral growth. Arch. Virol. 148:1687-1696. [DOI] [PubMed] [Google Scholar]
- 43.Treanor, J., M. Perkins, R. Battaglia, and B. R. Murphy. 1994. Evaluation of the genetic stability of the temperature-sensitive PB2 gene mutation of the influenza A/Ann Arbor/6/60 cold-adapted vaccine virus. J. Virol. 68:7684-7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turan, K., M. Mibayashi, K. Sugiyama, S. Saito, A. Numajiri, and K. Nagata. 2004. Nuclear MxA proteins form a complex with influenza virus NP and inhibit the transcription of the engineered influenza virus genome. Nucleic Acids Res. 32:643-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ulmanen, I., B. A. Broni, and R. M. Krug. 1981. Role of two of the influenza virus core P proteins in recognizing cap 1 structures (m7GpppNm) on RNAs and in initiating viral RNA transcription. Proc. Natl. Acad. Sci. USA 78:7355-7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamanaka, K., A. Ishihama, and K. Nagata. 1990. Reconstitution of influenza virus RNA-nucleoprotein complexes structurally resembling native viral ribonucleoprotein cores. J. Biol. Chem. 265:11151-11155. [PubMed] [Google Scholar]
- 47.Yamanaka, K., N. Ogasawara, H. Yoshikawa, A. Ishihama, and K. Nagata. 1991. In vivo analysis of the promoter structure of the influenza virus RNA genome using a transfection system with an engineered RNA. Proc. Natl. Acad. Sci. USA 88:5369-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]