Abstract
Severe and moderate traumatic brain injury (sTBI) often results in long-term cognitive deficits such as reduced processing speed and attention. The intraparietal sulcus (IPS) is a neocortical structure that plays a crucial role in the deeply interrelated processes of multi-sensory processing and top down attention. Therefore, we hypothesized that disruptions in the functional and structural connections of the IPS may play a role in the development of such deficits. To examine these connections, we used resting state magnetic resonance imaging (rsfMRI and diffusion kurtosis imaging (DKI) in a cohort of 27 patients with sTBI (29.3 ± 8.9 years) and 27 control participants (29.8 ± 10.3 years). Participants were prospectively recruited and received rsfMRI and neuropsychological assessments including the Automated Neuropsychological Assessment Metrics (ANAM) at greater than 6 months post-injury. A subset of participants received a DKI scan. Results suggest that patients with sTBI performed worse than control participants on multiple subtests of the ANAM suggesting reduced cognitive performance. Reduced resting state functional connectivity between the IPS and cortical regions associated with multi-sensory processing and the dorsal attention network was observed in the patients with sTBI. The patients also showed reduced structural integrity of the superior longitudinal fasciculus (SLF), a key white matter tract connecting the IPS to anterior frontal areas, as measured by reduced mean kurtosis (MK) and fractional anisotropy (FA) and increased mean diffusivity (MD). Further, this reduced structural integrity of the SLF was associated with a reduction in overall cognitive performance. These findings suggest that disruptions in the structural and functional connectivity of the IPS may contribute to chronic cognitive deficits experienced by these patients.
Keywords: : diffusion tensor imaging, functional connectivity, intraparietal sulcus, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of death and lifelong disability. Survivors of severe and moderate TBI (sTBI) often manifest cognitive deficits such as reduced processing speed and attention, resulting in a limited ability to return to work. The neurobiological basis for these impairments is not fully understood but may lie in subtle damage to the neural networks involved in attention and the integration and transfer of information across sensory modalities.
The ability to integrate stimuli from multiple individual sensory modalities present in the surrounding environment into a cohesive representation of the current situation is crucial for day-to-day life. Further, the creation of this unified perception of the surrounding environment relies on a complex interplay between top down attention and bottom up multi-sensory processing. This cortical processing of multi-sensory information includes the transfer of information between unique sensory modalities as well as the integration of information gathered from multiple senses, a phenomenon referred to as multi-sensory integration. In multi-sensory integration, inputs from multiple unisensory modalities are combined to yield a unified perceptual experience of multi-sensory events.1 This relationship between multi-sensory integration and attentional processes is multi-faceted and intimate.
The intraparietal sulcus (IPS), a neocortical area that integrates visual and somatosensory information, plays an important role in the maintenance of sustained attention and in the ability to select between competing external stimuli. It also plays a major role in mediating spatial working memory.2,3 The IPS is an anatomically constant structure in the human brain4 and is uniquely situated at the intersection of visual, somatosensory, and auditory association cortices making it ideally located for processing of multi-sensory attention.5 It contains topographically organized regions, similar to the retinotopic maps of the visual cortex. Moreover, the IPS is a heterogeneous region with the more anterior parts of the IPS connecting preferentially with prefrontal regions and the more posterior regions having stronger connections with retinotopically defined visual areas. 6,7
The IPS is recruited during tasks requiring attention and maintenance and manipulation of information in working memory, 8 which have been shown to be impaired after TBI. Structurally, the IPS connects with frontal areas through the superior longitudinal fasciculus (SLF), which can be divided further into multiple branches. The second branch of the SLF (SLF-II) originates in the anterior IPS and the angular gyrus (BA 39) and terminates in the posterior regions of the superior and middle frontal gyrus (BA 6, 8, 9). The third branch of the SLF (SLF-III) connects the IPS and inferior parietal lobule to the inferior frontal gyrus (BA 44, 45, 47).9
Both the IPS and the SLF play a crucial role in attention. Attention is an essential cognitive function that allows individuals to dynamically select relevant stimuli from all the available information in the external or internal environment, allowing for greater neural resources to be devoted to relevant processing.10 The control of attentional processes can be divided into a goal oriented “top down” network composed of parts of the frontal and parietal cortex and a “bottom up” stimulus driven network composed of various parts of the same network, but also encompassing the temporoparietal junction and subcortical areas such as the superior colliculus.
The top down network, of which the IPS is a part, is involved in preparing and applying goal-directed selection for stimuli and responses.11 Stimulus-driven, bottom up mechanisms induced by cross-modal interactions can automatically capture attention toward multi-sensory events. On the other hand, top down attention can facilitate the integration of multi-sensory inputs and lead to a spread of attention across sensory modalities. This is especially relevant when there is a high degree of competition between successive sensory inputs to the same modality or in the presence of concurrent inputs to different modalities.10,12,13
Previous research has provided evidence that patients with sTBI experience deficits in attentional processing that are exacerbated when simultaneous attention is required across multiple sensory modalities.14,15 Therefore, in this study we sought to investigate whether resting state magnetic resonance imaging (rsfMRI) and diffusion kurtosis imaging (DKI) are able to detect disruptions in the functional and structural connections between the IPS and regions associated with multi-sensory processing and attention after sTBI. Further, we hypothesized that the degree of functional or structural disruption would be associated with long-term functional outcome in these patients.
Methods
Participants
Twenty-seven patients with sTBI (29.3 ± 8.9, 21M:6F) were recruited prospectively from the R Adam Cowley Shock Trauma Center at the University of Maryland Medical Center. All participants were over the age of 18. Patients were screened and excluded for history of neurological and psychiatric illness, stroke, brain tumors, or seizures, and contraindications to MR. This population includes patients with a diagnosis of moderate or severe TBI based on an admission Glasgow Coma Scale (GCS) score of 3–12 and mechanism of injury consistent with trauma. Twenty-seven neurologically intact participants (29.8 ± 10.3 years, 16M:11F) served as the control population. Participant demographic information is shown in Table 1. This Health Insurance Portability and Accountability Act compliant study was approved by the International Review Board at the University of Maryland, and all participants provided informed consent.
Table 1.
Demographics
| rsfMRI population | DKI population | |||||
|---|---|---|---|---|---|---|
| Control | sTBI | p value | Control | sTBI | p value | |
| N | 27 | 27 | NA | 22 | 19 | |
| Age | 29.8 ± 10.3 | 29.3 ± 8.9 | 0.86 | 30.8 ± 11.2 | 31.0 ± 9.0 | 0.96 |
| Education | 15 ± 2 | 13 ± 2 | 0.013 | 14 ± 2 | 13 ± 2.0 | 0.049 |
| Sex | 16M/11F | 21M/6F | 0.24 | 15M/7F | 15M/4F | 0.50 |
| GCS | NA | 5.7 ± 2.9 | NA | NA | 6.1 ± 2.9 | NA |
| Days post-injury | NA | 271 ± 175 | NA | NA | 258 ± 164 | NA |
rsfMRI, resting state magnetic resonance imaging; DKI, diffusion kurtosis imaging; sTBI, severe and moderate traumatic brain injury; GCS, Glasgow Coma Scale.
All patients with sTBI received a rsfMRI scan as well as a series of neuropsychological assessments during the chronic stage of injury (271 ± 175 days post-injury, range: 116–729 days). A subset of the sTBI patients (n = 19, 31.0 ± 9.0 years) and control participants (n = 22, 30.8 ± 11.2 years) also received a DKI scan during this visit (Table 1).
Neuropsychological assessment
Patients underwent neuropsychological assessment during this visit. The level of cognitive functioning was assessed by the administration of the Mini Mental State Examination (MMSE) at each visit. Patient outcome was assessed by the Glasgow Outcome Scale Extended (GOSE)16 at the chronic stage of injury.
In addition, participants completed the Automated Neuropsychological Assessment Metrics (ANAM) battery, which consists of seven subtests assessing processing speed, memory, and attention. Two sTBI participants and two control participants refused participation in the ANAM, resulting in 25 patients with sTBI and 25 control participants included in this behavioral analysis. The specific subtests included in the ANAM battery are the code substitution (CS), code substitution delayed (CSD), match to sample (MTS), math processing (MATH), procedural reaction time (PRT), simple reaction time (SRT), simple reaction time repeat (SRT2).17 From each subtest, an individual throughput score was calculated providing a single measure encompassing both accuracy and reaction time. Specifically, the throughput score is the number of correct responses per total amount of time a participant took to respond for each trial, expressed as the number of correct responses per minute.18 We opted to examine a weighted throughput score (WT-TH), which has previously been referred to as an Index of Cognitive Efficiency.19 The WT-TH was determined as an overall measure of performance on the ANAM and is given as:
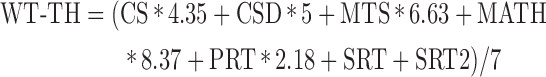 |
MR data acquisition
Imaging was performed on a 3T Siemens Tim Trio Scanner (Siemens Medical Solutions; Erlangen, Germany) using a 12-channel receiver only head coil. A high resolution T1-MPRAGE (echo time [TE] = 3.44 msec, repetition time [TR] = 2250 msec, inversion time [TI] = 900 msec, flip angle = 9 degrees, resolution = 256 × 256 × 96, field of view [FOV] = 220 mm, sl. thick. = 1.5 mm) was acquired for anatomic reference. For the rsfMRI scan, T2*-weighted images were acquired using a single-shot echo-planar imaging (EPI) sequence (TE = 30 ms, TR = 2000 ms, FOV = 230 mm, resolution = 64 × 64) with 36 axial slices (sl. thick. = 4 mm) over 5 min 42 sec that yielded 171 volumes. Before the acquisition of the resting state scan, participants were verbally instructed to rest peacefully with their eyes closed.
For the DKI acquisition, diffusion-weighted images were acquired with a twice refocused single shot, spin-echo EPI sequence. Thirty diffusion directions with two b-values (1000, 2000 sec/mm2), along with 4 b0 images and two repetitions were acquired. Parallel imaging was used with an acceleration factor of two at a TE/TR = 101 msec/6000 msec. The in-plane resolution was 2.7 mm2, with a 7/8 partial Fourier factor, at a slice thickness of 2.7 mm to cover the whole brain. The total image acquisition time was about 13 min.
rsfMRI data analysis
Data from two control participants were excluded from the resting state analysis results in a total of 25 control participants and 27 sTBI patients in this resting state analysis. Preprocessing of the imaging data was performed using SPM 8 (http://www.fil.ion.ucl.ac.uk/spm) and included motion correction of the time series, slice timing correction, band pass filtering (.009Hz < f < .08Hz), and registration of all the 171 volumes to the first volume of the time series. The resting state data were then registered to the individual's T1-MPRAGE images, spatially normalized to standard space using the Montreal Neurological Institute (MNI) template available in SPM 8, and resampled to a spatial resolution of 2.0 mm isotropic. Spatial smoothing was applied to the resting state data using a 5 mm Gaussian kernel. Individual T1-MPRAGE images in MNI space were segmented into white matter (WM), gray matter (GM), and cerebral spinal fluid (CSF). The mean Bold Oxygen Level Dependent (BOLD) time series extracted from the WM and CSF masks as well as the six motion correction parameters were included in the model as regressors to remove the variance related to non-neuronal contributions and motion.
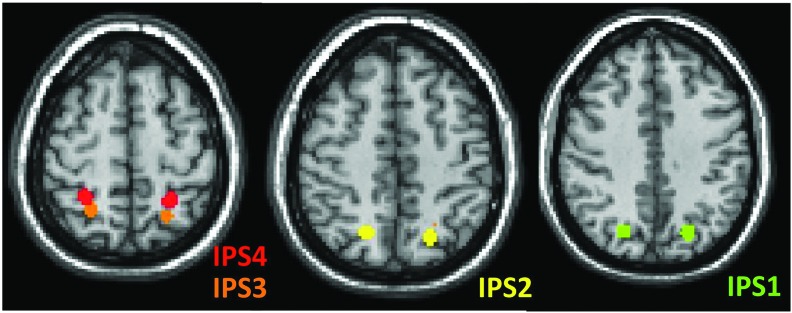
Seed based functional connectivity (FC) analysis was performed using the CONN-fMRI Functional Connectivity toolbox v13.h (http://www.nitrc.org/projects/conn). The IPS was divided into four bilateral regions using 5 mm spherical regions of interest (ROIs) placed along the length of the IPS (Fig. 1).6 For each ROI (IPS1, IPS2, IPS3, IPS4), the mean BOLD time series was extracted and correlated with the time series of each voxel within the entire brain. Correlations were converted to normalized z-scores within the CONN-fMRI Functional Connectivity toolbox (http://www.nitrc.org/projects/conn) before further analysis.
FIG. 1.
Visualization of the four intraparietal sulcus (IPS) regions of interest (ROIs) based on Bray and colleagues.20 The ROIs are overlaid on the Montreal Neurological Institute template. Color image is available online at www.liebertpub.com/neu
Within group rs-FC maps of the four IPS ROIs were created using SPM8. Positive FC maps were thresholded at a voxel-wise p value of 0.001 (uncorrected) and cluster extent threshold of p value of 0.05 using a false discovery rate (FDR) correction for multiple comparisons. Individual maps were made for the control group and the sTBI group. In addition, between group contrast maps were created and were thresholded at voxel-wise p value of 0.025 (uncorrected) and cluster extent threshold p value of 0.05 using a FDR correction for multiple comparisons.
DKI data analysis
Diffusion weighted images were motion and eddy current corrected using SPM8 co-registration. Three dimensional Gaussian smoothing with full width at half maximum (FWHM) = 3.0 mm was applied to improve the signal to noise ratio (SNR). The DKI reconstruction was performed using in-house MATLAB program with constrained linear least squares fitting20 (for a more complete discussion of DKI processing, please see Stokum and associates21). The DKI processing resulted in maps of various diffusion parameters including mean diffusivity (MD), fractional anisotropy (FA), and mean kurtosis (MK). Voxel-wise statistical analysis of DKI data was performed using Tract Based Statistics (TBSS).22
The FA, MD, and MK data were projected onto a mean FA tract skeleton, before applying voxel-wise cross-subject statistics (p < 0.01 corrected). To investigate the association between altered diffusion parameters and cognitive performance, whole brain skeletons of MD, FA, and MK were correlated voxel-wise to the ANAM WT-TH scores for both TBI patients and control group while adjusting for age using General Linear Model and the Randomise command within FSL.23 Multiple comparisons were corrected by TFCE (Threshold-Free Cluster Enhancement).
The ROI analysis was performed for the right and left superior longitudinal fasciculi (SLF), the main WM tract connecting the IPS to the frontal lobe based attention networks. The ROIs were created based on the overlap of the Johns Hopkins University WM atlas24 and the mean FA tract skeleton from the TBSS results. For each participant, the average WM FA, MD, and MK were extracted separately from the right and left SLF ROIs.
Statistical analysis
Group differences in demographic data between the sTBI and controls groups were determined using t-tests for age and years of education. Differences in sex between the two groups were determined using a chi-square test. Given the likely influence of age and level of education on cognitive performance, differences in neuropsychological assessments were determined using an analysis of covariance (ANCOVA) including age and years of education as covariates. Because previous work has suggested that age has a large impact on structural and functional connectivity, group differences in imaging measures (rs fMRI and DKI) were determined using an ANCOVA including age as a covariate. Potential associations between imaging measures and neuropsychological assessments were investigated using the Pearson partial correlations considering age as a covariate.
Results
Participants
There were no differences in age (p = 0.86) or sex (p = 0.24) between the control group and the sTBI group; however, there were significant differences in years of education (p = 0.013). For the subset of participants who received DKI scans, there was no significant difference in age (p = 0.96) or sex (p = 0.50); however, there was a significant difference in education (p = 0.049) (Table 1). At the time of injury, a total of nine of the 27 (33%) patients had imaging findings compatible with diffuse axonal injury (DAI) based on clinical imaging (computed tomography and conventional MR imaging). In addition, a board certified neuroradiologist (PR) reviewed all chronic MR imaging scans and determined that no discrete imaging abnormalities or microbleeds were present within the chronic stage imaging sequences acquired as part of this study.
Neuropsychological assessment
All behavioral results are shown in Table 2. After controlling for age and level of education, the sTBI patients have an overall reduction in cognitive performance on the ANAM WT-TH score (F = 9.63; p = 0.003). Further, the sTBI group performed worse than the control group on multiple subtests of the ANAM including PRT (F = 4.67; p = 0.036), CS (F = 8.88; p = 0.005), CSD (F = 7.90; p = 0.007), MTS (F = 4.08; p = 0.049), MATH (F = 4.28; p = 0.044), and SRT2 (F = 5.52; p = 0.023). No differences were noted in the first SRT subtest (F = 0.84; p = 0.366) between the control and sTBI groups.
Table 2.
Behavioral Results
| sTBI mean | sTBI SD | Control mean | Control SD | Statistics* | |
|---|---|---|---|---|---|
| ANAM | |||||
| CS | 40.8 | 15.3 | 54.5 | 14.1 | F = 8.88; p = 0.005 |
| CSD | 32.1 | 17.7 | 47.9 | 19.5 | F = 7.90; p = 0.007 |
| MTS | 26.4 | 13.1 | 35.0 | 10.6 | F = 4.08; p = 0.049 |
| MATH | 16.6 | 6.9 | 23.2 | 8.4 | F = 4.28; p = 0.044 |
| PRT | 87.0 | 28.6 | 103.3 | 16.3 | F = 4.67; p = 0.036 |
| SRT | 209.2 | 54.2 | 231.0 | 41.6 | F = 0.84; p = 0.366 |
| SRT2 | 200.8 | 53.8 | 231.5 | 32.8 | F = 5.52; p = 0.023 |
| WT-TH | 178.2 | 54.4 | 227.2 | 42.4 | F = 9.63; p = 0.003 |
| Other | |||||
| GOSE Total | 5.59 | 1.95 | NA | NA | NA |
SD, standard deviation; ANAM, Automated Neurophsychlogical Assessment Metrics; CS, code substitution; CSD, code substitution delayed; MTS, match to sample; MATH, processing; PRT, procedural reaction time; SRT, simple reaction time; SRT2, simple reaction time repeat; WT-TH, weighted throughput score; GOSE, Glasgow Outcome Scale Extended.
Analysis of covariance controlling for age and education.
Resting state fMRI
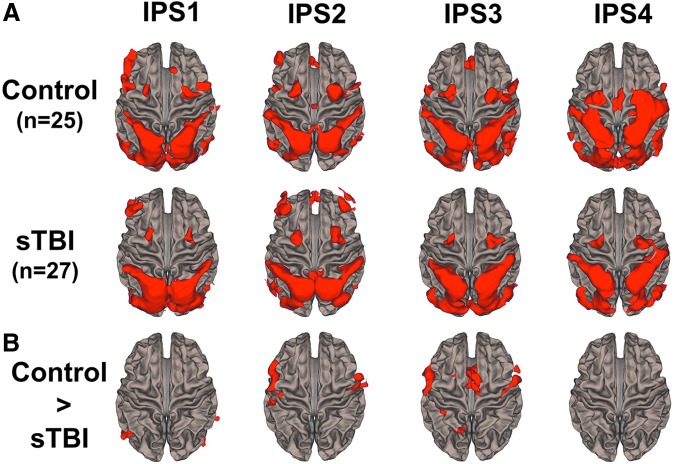
Results from the seed based rs-FC analysis for three of the IPS ROIs (IPS1, IPS2, IPS3) are shown for the control and sTBI groups in Figure 2A. The control group demonstrated FC between the IPS ROIs and cortical regions associated with attentional control (i.e., premotor cortex and dorsolateral prefrontal cortex) and with regions associated with visual and somatosensory processing. The posterior IPS ROIs (IPS1 and IPS2) demonstrated greater connectivity with frontal ROIs than the anterior IPS in both the control and sTBI populations. The sTBI group had an overall reduced FC across four IPS ROIs with cortical regions associated with attentional control, visual, and somatosensory processing (Fig. 2A). Reduced connectivity between the IPS and extrastriate visual areas and the dorsolateral prefrontal cortex was observed in the sTBI group compared with the control group (Fig. 2B). Specifically, reduced connectivity of extrastriate areas with the subregions of the IPS was noted in the sTBI group while reduced connectivity with the dorsolateral prefrontal cortex was only noted with IPS2 and IPS 3 sub-regions. There were no regions of increased FC in the sTBI group compared with the control group for any of the four IPS ROIs.
FIG. 2.
Functional connectivity results of the four intraparietal sulcus (IPS) regions of interest (ROIs). (A) Average group functional connectivity maps of the IPS1, IPS2, IPS3, and IPS4 ROIs for the control group (n = 26) and the severe and moderate traumatic brain injury (sTBI) group (n = 27). Results are thresholded at a voxel-wise p value of 0.001 (uncorrected) and cluster extent threshold of p value of 0.05 using a false discovery rate (FDR) correction for multiple comparisons. (B) Between group contrast maps showing regions with greater functional connectivity in the control group compared with the sTBI group. Results are thresholded at voxel-wise p value of 0.025 (uncorrected) and cluster extent threshold of p value of 0.05 using a FDR correction for multiple comparisons. Color image is available online at www.liebertpub.com/neu
No group differences in motion parameters for maximum rotation or translation were noted between the control and sTBI groups (all p values >0.05). Therefore, we believe that findings were not confounded by differences in motion between the two groups.
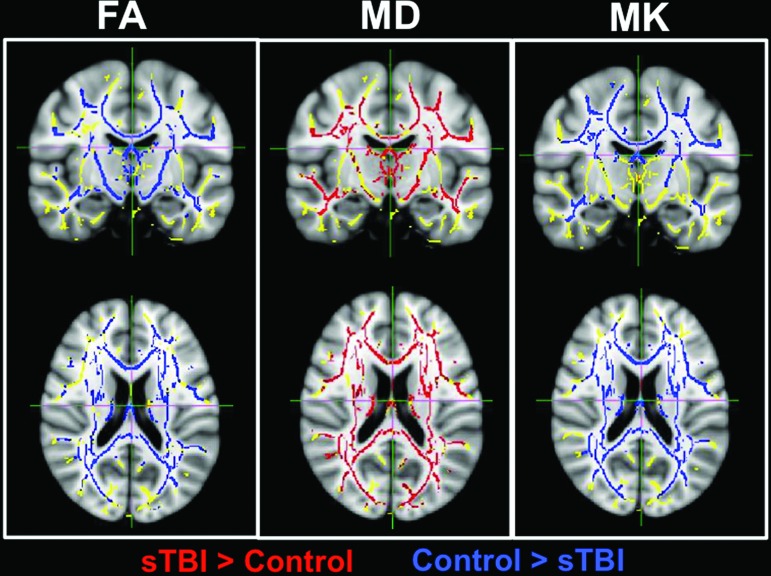
DKI results
Results from the TBSS analysis of the DKI data are shown in Figure 3. A diffuse pattern of reductions in MK and FA and diffuse areas of reduction in MD were observed in the sTBI group compared with the control group suggestive of widespread structural damage (Fig. 3). No group differences in motion parameters for maximum rotation or translation were noted between the control and sTBI groups (all p values >0.05).
FIG. 3.
Results of Tract Based Statistics (TBSS) analysis for fractional anisotropy (FA), mean diffusivity (MD), and mean kurtosis (MK) showing group differences between the severe and moderate traumatic brain injury (sTBI) group (n = 19) and the control group (n = 22). Voxels in red represent regions with greater values in the sTBI group while voxels in blue represent regions with greater values in the control group. The TBSS skeleton is shown in yellow. Results are overlaid on the Montreal Neurological Institute template. Color image is available online at www.liebertpub.com/neu
Associations between imaging and behavior
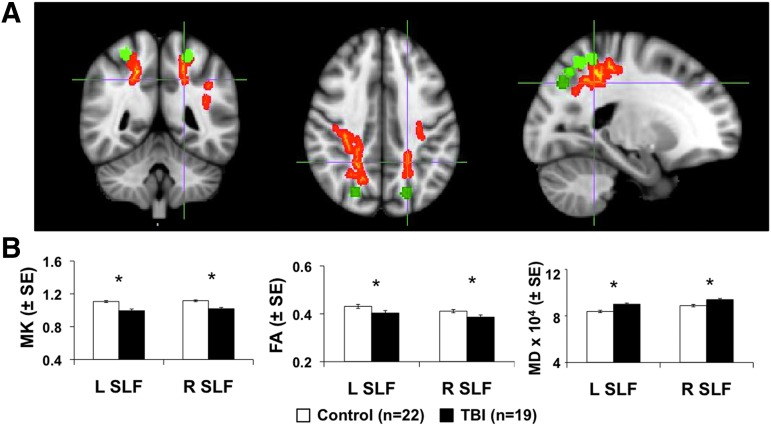
While the results of the imaging analysis demonstrate reduced structural and functional connectivity of the IPS in patients with sTBI, we were interested in determining the association of the reduced connectivity with alterations in cognitive performance among these patients. After adjusting for age, we found evidence that increased FA and MK and reduced MD within diffuse fiber tracts were significantly associated with higher cognitive performance within the sTBI population (Supplementary Figure 1; see online supplementary material at ftp.liebertpub.com). No voxel-wise correlations between MD, FA, or MD and cognitive performance were noted in the control group. The overlap between the maps representing voxels that demonstrated significant correlation with the ANAM WT-TH for all DKI parameters (MD, FA, and MK) is shown in Figure 4A. These tracts were identified as the SLF and posterior corona radiata, which are WM tracts that are known to be associated with multi-sensory processing that involves the IPS.
FIG. 4.
(A) Tract Based Statistics (TBSS) map for disrupted white matter regions that showed significant correlations for the overlap of the three diffusion kurtosis imaging parameters (fractional anisotropy [FA], mean diffusivity [MD], and mean kurtosis [MK]) with the cognitive score Automated Neuropsychological Assessment Metrics weighted throughput score (ANAM WT-TH). Voxels in red represent the TBSS results while the green regions of interest (ROIs) shown represent the intraparietal sulcus ROIs used in the resting state magnetic resonance imaging analysis. (B) Results of the ROI analysis of the superior longitudinal fasciculus (SLF) ROIs for the right (R) and left (L) hemispheres for the control population (n = 22) and the severe and moderate traumatic brain injury (sTBI) population (n = 19). Diffusion parameters shown represent FA, MD, and MK. *p < 0.05 based on analysis of covariance including age as a covariate. SE, standard error. Color image is available online at www.liebertpub.com/neu
Led by the results noted in Figure 4A as well as our specific interest in the communication between the IPS and the frontal lobe attention network, diffusion parameters were extracted from the right and left SLF. In addition to the diffuse alterations in diffusion parameters noted by the sTBI patients also demonstrate specific reductions in the MK and FA of the bilateral SLF and increases in MD (Figure 4B) suggesting weakened connectivity between the IPS and the frontal lobe association network.
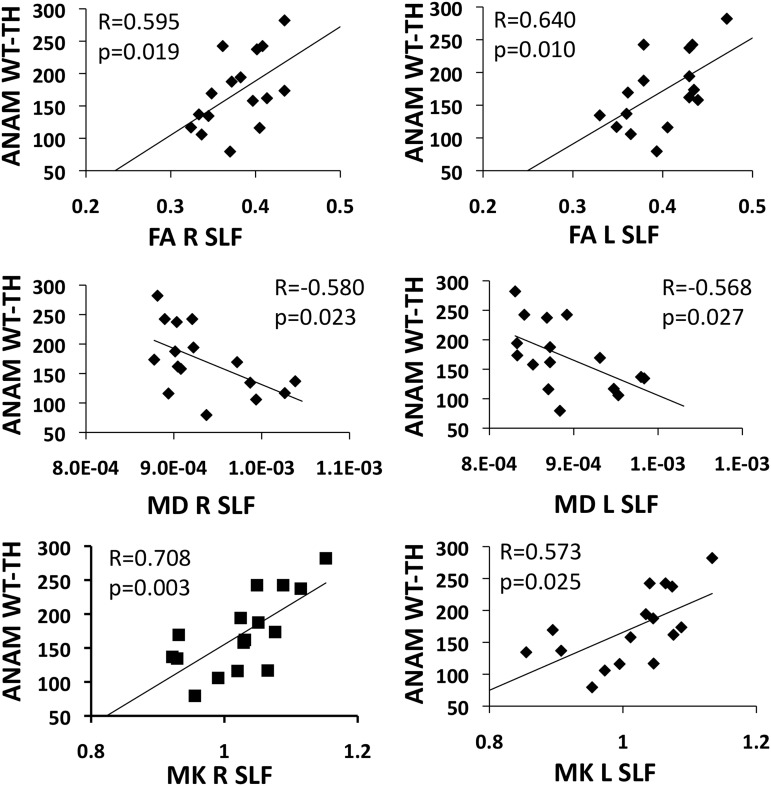
After controlling for the influence of age, correlations were noted between the DKI parameters (MK, FA, and MD) within the right and left SLF and the ANAM WT-TH in the sTBI group (Fig. 5). This finding is indicative of an association between reduced axonal integrity within the major WM tract connecting the posterior IPS to the anterior frontal lobe and deficits in overall cognitive performance. No significant correlations were noted between FC measures and behavioral performance.
FIG. 5.
Scatter plots showing the association between the Automated Neuropsychological Assessment Metrics weighted throughput (ANAM WT-TH) and diffusion weighted values of the right and left superior longitudinal fasciculus (SLF). FA, fractional anisotropy; MD, mean diffusivity; ML, mean kurtosis. The p values are based on the Pearson partial correlations controlling for age.
Discussion
We examined changes in FC and structural connectivity of the IPS in the chronic stage in a population of patients with sTBI. Our results provide preliminary evidence that in the chronic stages of injury, patients with sTBI demonstrate reduced FC between the IPS and cortical regions associated with multi-sensory processing and the dorsal attention network (Fig. 2) as well reduced structural integrity of the SLF, a key WM tract connecting the IPS to the anterior frontal lobe areas (Fig. 4B). Further, our results indicate that this reduced structural integrity of the SLF is associated with a reduction in global cognitive performance (Fig. 4A, 5). While the structural damage to the SLF as measured by DKI showed significant correlations with the overall performance on the ANAM battery, no such correlations were observed between FC parameters and ANAM performance.
Structural connectivity
Given the complexity of the pathological changes induced by axonal damage after trauma, it is difficult to determine the precise nature of the microstructural changes. As has been consistently reported in the literature, however, our results demonstrate a diffuse reduction in axonal integrity across major WM tracts in the chronic stages of sTBI as evidenced by reduced FA and elevated MD (Fig. 3). In addition, the disperse reduction in MK is indicative of a reduction in diffusion heterogeneity that suggests a less complex tissue microstructure. This reduced complexity may be the result of subtle DAI and subsequent Wallerian degeneration or loss of myelin integrity within major WM tracts.25,26 These findings are further replicated within the bilateral SLF within this population, again demonstrating reductions in FA and MK and elevated MD, which together provide evidence for specific axonal damage to the SLF (Fig. 4B).
Further, these measures of structural integrity of the SLF also showed strong associations with cognitive performance as measured by the WT-TH on the ANAM, suggesting that structural damage to the SLF may contribute to reduced global cognitive performance (Fig. 4A, 5). These findings are in keeping with other investigations of the impact of WM integrity on cognitive outcome after TBI.27,28
The ANAM has multiple subtests that assess various cognitive domains, but one cognitive aspect that each subtest requires is attention. Attention deficits are often included in the cognitive sequelae of TBI and can be considered a consequence of “a non-specific slowing of perceptual, motor and cognitive process, approximately equal to task difficulty.”29 Large and significant deficits in information processing speed, attention span, selective and sustained attention, as well as supervisory attentional control have been demonstrated after severe TBI that are independent of age, education, and post-injury interval.15 Further, similar to what our group has shown previously in the population with mild TBI reporting high post-concussive symptoms,30 the patients with sTBI did not perform worse than the control group on the first SRT tests, but did perform worse on the SRT2 test. This reduction in processing speed noted only at the end of computer assessment is suggestive of increased cognitive fatigue over the course of the assessment in the patient population.
There is no doubt that attention and multi-sensory integration/processing are deeply intertwined, interact at multiple stages in the brain, and utilize similar neural resources.10 The IPS, although only one of other heteromodal cortical areas that participate in both of these processes, was chosen because it plays a major role in visuotactile integration, coordination of visuomotor action and numerical cognition among others, all of which we believe are integral to performance on several components of the ANAM.
While the ANAM does not specifically assess multi-sensory processing, the findings can be extrapolated to previous behavioral studies investigating multi-sensory integration after TBI. For example, Sarno and coworkers14 investigated multi-sensory integration using both simple and choice RT tests that paired visual, auditory, and tactile stimuli in a population of 35 patients with TBI and 35 matched controls. Results showed that the RTs were strongly influenced by sensory-specific and cross-modal factors. Patients with TBI demonstrated prolonged simple and choice RTs throughout all tasks; difficulty with integrating tactile stimuli with visual and auditory stimuli was disproportionately higher compared with controls. The authors postulated that diffuse axonal damage to multi-sensory neurons in the parietal lobes and their subsequent projections may underlie this phenomenon.14
The damage to the structural and functional projections from parietal lobes observed in this present analysis in patients with TBI lends further support to this hypothesis. When viewed in conjunction with the reduction in IPS FC noted in the population with sTBI, these findings provide a neuroanatomical basis for the prolonged RTs observed by Sarno and colleagues14 when combinations of sensory stimuli were presented to patients with TBI. In addition, the IPS has been linked to ideomotor praxis, which is associated with the ability to use tools that involve the integration of visual and sensory systems.31 Because apraxia or the inability to make purposeful movements is often noted after severe TBI and is one of the many challenges these patients face in returning to independent living, the damage to the structural connections of the IPS that are noted in this population may be one of the contributing factors to this deficit.
Reductions in FC
Our results reveal a pattern of decreased FC between the IPS and regions associated with multi-sensory integration (i.e., associative visual cortex, somatosensory association areas, supramarginal gyrus) and attention (i.e., premotor cortex and dorsolateral prefrontal cortex) in patients with severe TBI (Fig. 2). There has been great discrepancy in the field regarding whether sTBI results in increases or reductions in FC,32–34 and the variability in reported findings is likely because of specific neural networks investigated, techniques used, and time since injury. The findings of reduced FC between the IPS and frontal lobe cortical regions, however, are supported by the reductions in structural integrity of the SLF that are noted from the DKI analysis.
Yet, while significant correlations between cognitive performance and DKI values of the SLF were noted, the reductions in FC failed to demonstrate associations with cognitive performance. The lack of correlation of ANAM scores with FC changes may be explained by the fact that the performance of the various subtests within the ANAM battery depends on multiple neural networks in addition to those networks that include the IPS as a node. These alternative networks may not demonstrate the same degree of damage as the IPS. Further, because the results presented are collected in the chronic stages of injury, there likely have been various degrees of functional recovery within the individuals that have been able to compensate for the specific damage to the IPS as well as its structural projections. Because the alternate pathways are highly variable across the populations because of the heterogeneity of injury, the patterns of FC across the patient population are likely inconsistent, and this variability may contribute to the lack of an association between FC and behavioral performance.
Although the overall patterns of connectivity of the IPS in the control group are in agreement with the current literature, we observed slightly increased connectivity between the more posterior IPS regions and frontal areas compared with the more anterior IPS ROIs. This finding is at variance with those of Bray and associates6 and Uddin and coworkers,7 and we believe its basis may lie in either the sizes of the ROIs or the spatial smoothing that was applied as part of the preprocessing.
Limitations
One noted shortcoming to the current study is the limited sample size, which may influence the ability to generalize the findings to the greater TBI population as a whole. An additional limitation to this current study is that the findings regarding altered functional communication of the IPS are specific to resting state conditions because our study design did not include task based functional MRI to determine how these networks respond under task based conditions. We believe that future studies combining advanced MR imaging modalities with behavioral tests to specifically measure neural network communication during multi-sensory processing after severe TBI are needed.
In addition, while we are aware that the SLF is not the only WM pathway connecting the IPS with unisensory and multi-sensory cortical areas,35 we opted to focus our analysis on this tract because of our particular interest in the connectivity of the IPS with frontal attention areas; this is a commonly noted deficit after TBI. While the control group used in this study is comparable in demographic features to the patients with TBI, future studies would benefit from a more effective control group such as orthopedically injured or general trauma patients without head injury, because it would control for risk factors associated with traumatic injury that could also affect attention.
Finally, although our patients were all imaged at least three months after their injury, there was some variability in the time points at which they were imaged (271 ± 175 days post-injury). This may have resulted in some heterogeneity in the degree of recovery of the IPS after injury across individual patients.
Conclusion
Overall, these novel findings suggest that disruptions in the FC and structural connectivity of the IPS may contribute to chronic cognitive deficits and reduction in processing speed experienced by these patients. Further, these results provide preliminary evidence that the neural networks that support multi-sensory processes are altered after TBI and that future work is greatly needed to determine the association between disrupted IPS connectivity and specific assessments of multi-sensory integration and visuospatial attention to further elucidate these findings. Recognizing the neural basis of impaired cognitive processing including those associated with the ability to process multi-sensory inputs after TBI, however, will likely have important implications for rehabilitation.
Currently used strategies that employ the mechanisms of multi-sensory processing to improve cognitive performance, including proprioceptive muscle stimulation, motor imagery, action observation, training with a mirror or in a virtual environment, and various kinds of music therapy,36,37 have made great progress in mitigating the effects of various neural injuries. The logical next step in this line of research includes the use of advanced MR imaging modalities to identify specific neuroanatomic substrates, such as those provided within this study, which would potentially provide targets for non-invasive brain stimulation techniques that can be personalized to a person's anatomical injury or specific behavioral deficit.
Supplementary Material
Acknowledgments
The authors would like to thank Joshua Betz, Jacqueline Janowich, Teodora Stoica, and Joseph Rosenberg for their help with patient recruitment and enrollment, and George Makris for his help with acquiring the data. Support for this work was in part provided by the Department of Defense (W81XWH-08-1-0725 & W81XWH-12-1-0098 to RPG) and the National Institutes of Health (1R03NS088014-01).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Stein B.E., Stanford T.R., Ramachandran R., Perrault T.J., Jr, and Rowland B.A. (2009). Challenges in quantifying multisensory integration: alternative criteria, models, and inverse effectiveness. Exp. Brain Res. 198, 113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silk T.J., Bellgrove M.A., Wrafter P., Mattingley J.B., and Cunnington R. (2010). Spatial working memory and spatial attention rely on common neural processes in the intraparietal sulcus. Neuroimage 53, 718–724 [DOI] [PubMed] [Google Scholar]
- 3.Goltz D., Gundlach C., Nierhaus T., Villringer A., Muller M., and Pleger B. (2015). Connections between intraparietal sulcus and a sensorimotor network underpin sustained tactile attention. J. Neurosci. 35, 7938–7949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grefkes C., and Fink G.R. (2005). The functional organization of the intraparietal sulcus in humans and monkeys. J. Anat. 207, 3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson J.S., Ferguson M.A., Lopez-Larson M., and Yurgelun-Todd D. (2010). Topographic maps of multisensory attention. Proc. Natl. Acad. Sci. U. S. A. 107, 20110–20114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray S., Arnold A.E., Iaria G., and MacQueen G. (2013). Structural connectivity of visuotopic intraparietal sulcus. Neuroimage 82, 137–145 [DOI] [PubMed] [Google Scholar]
- 7.Uddin L.Q., Supekar K., Amin H., Rykhlevskaia E., Nguyen D.A., Greicius M.D., and Menon V. (2010). Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb. Cortex 20, 2636–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bray S., Almas R., Arnold A.E., Iaria G., and MacQueen G. (2015). Intraparietal sulcus activity and functional connectivity supporting spatial working memory manipulation. Cereb. Cortex 25, 1252–1264 [DOI] [PubMed] [Google Scholar]
- 9.Wang X., Pathak S., Stefaneanu L., Yeh F.C., Li S., and Fernandez-Miranda J.C. (2016). Subcomponents and connectivity of the superior longitudinal fasciculus in the human brain. Brain Struct. Funct. 221, 2075–2092 [DOI] [PubMed] [Google Scholar]
- 10.Talsma D., Senkowski D., Soto-Faraco S., and Woldorff M.G. (2010). The multifaceted interplay between attention and multisensory integration. Trends Cogn. Sci. 14, 400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbetta M., and Shulman G.L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215 [DOI] [PubMed] [Google Scholar]
- 12.Talsma D., and Woldorff M.G. (2005). Selective attention and multisensory integration: multiple phases of effects on the evoked brain activity. J. Cogn. Neurosci. 17, 1098–1114 [DOI] [PubMed] [Google Scholar]
- 13.van Ee R., van Boxtel J.J., Parker A.L., and Alais D. (2009). Multisensory congruency as a mechanism for attentional control over perceptual selection. J. Neurosci. 29, 11641–11649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarno S., Erasmus L.P., Lipp B., and Schlaegel W. (2003). Multisensory integration after traumatic brain injury: a reaction time study between pairings of vision, touch and audition. Brain Inj. 17, 413–426 [DOI] [PubMed] [Google Scholar]
- 15.Tombaugh T.N., Rees L., Stormer P., Harrison A.G., and Smith A. (2007). The effects of mild and severe traumatic brain injury on speed of information processing as measured by the computerized tests of information processing (CTIP). Arch. Clin. Neuropsychol. 22, 25–36 [DOI] [PubMed] [Google Scholar]
- 16.Teasdale G.M., Pettigrew L.E., Wilson J.T., Murray G., and Jennett B. (1998). Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow Outcome Scale. J. Neurotrauma 15, 587–597 [DOI] [PubMed] [Google Scholar]
- 17.Reeves D.L., Bleiberg J., Roebuck-Spencer T., Cernich A.N., Schwab K., Ivins B., Salazar A.M., Harvey S.C., Brown F.H., Jr, and Warden D. (2006). Reference values for performance on the Automated Neuropsychological Assessment Metrics V3.0 in an active duty military sample. Mil. Med. 171, 982–994 [DOI] [PubMed] [Google Scholar]
- 18.Ivins B.J., Kane R., and Schwab K.A. (2009). Performance on the Automated Neuropsychological Assessment Metrics in a nonclinical sample of soldiers screened for mild TBI after returning from Iraq and Afghanistan: a descriptive analysis. J. Head Trauma Rehabil. 24, 24–31 [DOI] [PubMed] [Google Scholar]
- 19.Reich S., Short P., Kane R., Weiner W., Shulman L., and Anderson K. (2005). Validation of the ANAM Test Battery in Parkinson's Disease. http://www.dtic.mil/cgi-bin/GetTRODOC?AD=ADA452204 (last accessed October1, 2016)
- 20.Tabesh A., Jensen J.H., Ardekani B.A., and Helpern J.A. (2011). Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn. Reson. Med. 65, 823–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stokum J.A., Sours C., Zhuo J., Shanmuganathan K., and Gullapalli R.P. (2015). A longitudinal evaluation of diffusion kurtosis imaging in patients with mild traumatic brain injury. Brain Inj. 29, 47–57 [DOI] [PubMed] [Google Scholar]
- 22.Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., and Behrens T.E. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505 [DOI] [PubMed] [Google Scholar]
- 23.Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., and Nichols T.E. (2014). Permutation inference for the general linear model. Neuroimage 92, 381–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakana S., Caprihan A., Panzenboeck M.M., Fallon J.H., Perry M., Gollub R.L., Hua K., Zhang J., Jiang H., Dubey P., Blitz A., van Zijl P., and Mori S. (2007). Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36, 630–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dodd A.B., Epstein K., Ling J.M., and Mayer A.R. (2014). Diffusion tensor imaging findings in semi-acute mild traumatic brain injury. J. Neurotrauma 31, 1235–1248 [DOI] [PubMed] [Google Scholar]
- 26.Shenton M.E., Hamoda H.M., Schneiderman J.S., Bouix S., Pasternak O., Rathi Y., Vu M.A., Purohit M.P., Helmer K., Koerte I., Lin A.P., Westin C.F., Kikinis R., Kubicki M., Stern R.A., and Zafonte R. (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 6, 137–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farbota K.D., Bendlin B.B., Alexander A.L., Rowley H.A., Dempsey R.J., and Johnson S.C. (2012). Longitudinal diffusion tensor imaging and neuropsychological correlates in traumatic brain injury patients. Front. Hum. Neurosci. 6, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraus M.F., Susmaras T., Caughlin B.P., Walker C.J., Sweeney J.A., and Little D.M. (2007). White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 130, 2508–2519 [DOI] [PubMed] [Google Scholar]
- 29.van Zomeren A.H., and Brouwer W.H. (1994). Clinical Neuropsychology of Attention. Oxford University Press: New York, NY [Google Scholar]
- 30.Sours C., Rosenberg J., Kane R., Roys S., Zhuo J., Shanmuganathan K., and Gullapalli R.P. (2015). Associations between interhemispheric functional connectivity and the Automated Neuropsychological Assessment Metrics (ANAM) in civilian mild TBI. Brain Imaging Behav. 9, 190–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makuuchi M., Kaminaga T., and Sugishita M. (2005). Brain activation during ideomotor praxis: imitation and movements executed by verbal command. J. Neurol. Neurosurg. Psychiatry 76, 25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hillary F.G., Slocomb J., Hills E.C., Fitzpatrick N.M., Medaglia J.D., Wang J., Good D.C., and Wylie G.R. (2011). Changes in resting connectivity during recovery from severe traumatic brain injury. Int. J. Psychophysiol. 82, 115–123 [DOI] [PubMed] [Google Scholar]
- 33.Tang C.Y., Eaves E., Dams-O'Connor K., Ho L., Leung E., Wong E., Carpenter D., Ng J., Gordon W., and Pasinetti G. (2012). Diffuse Disconnectivity in tBi: a resting state fMri and Dti study. Transl. Neurosci. 3, 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venkatesan U.M., Dennis N.A., and Hillary F.G. (2015). Chronology and chronicity of altered resting-state functional connectivity after traumatic brain injury. J. Neurotrauma 32, 252–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeatman J.D., Weiner K.S., Pestilli F., Rokem A., Mezer A., and Wandell B.A. (2014). The vertical occipital fasciculus: a century of controversy resolved by in vivo measurements. Proc. Natl. Acad. Sci. U. S. A. 111, E5214–E5223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller S.V., von Schweder A.J., Frank B., Dengler R., Munte T.F., and Johannes S. (2002). The effects of proprioceptive stimulation on cognitive processes in patients after traumatic brain injury. Arch. Phys. Med. Rehabil. 83, 115–121 [DOI] [PubMed] [Google Scholar]
- 37.Johansson B.B. (2012). Multisensory stimulation in stroke rehabilitation. Front. Hum. Neurosci. 6, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.