Abstract
Successful human immunodeficiency virus (HIV) vaccines will need to induce effective T-cell immunity. We studied immunodominant simian immunodeficiency virus (SIV) Gag-specific T-cell responses and their restricting major histocompatibility complex (MHC) class I alleles in pigtail macaques (Macaca nemestrina), an increasingly common primate model for the study of HIV infection of humans. CD8+ T-cell responses to an SIV epitope, Gag164-172KP9, were present in at least 15 of 36 outbred pigtail macaques. The immunodominant KP9-specific response accounted for the majority (mean, 63%) of the SIV Gag response. Sequencing from six macaques identified 7 new Mane-A and 13 new Mane-B MHC class I alleles. One new allele, Mane-A*10, was common to four macaques that responded to the KP9 epitope. We adapted reference strand-mediated conformational analysis (RSCA) to MHC class I genotype M. nemestrina. Mane-A*10 was detected in macaques presenting KP9 studied by RSCA but was absent from non-KP9-presenting macaques. Expressed on class I-deficient cells, Mane-A*10, but not other pigtail macaque MHC class I molecules, efficiently presented KP9 to responder T cells, confirming that Mane-A*10 restricts the KP9 epitope. Importantly, naïve pigtail macaques infected with SIVmac251 that respond to KP9 had significantly reduced plasma SIV viral levels (log10 0.87 copies/ml; P = 0.025) compared to those of macaques not responding to KP9. The identification of this common M. nemestrina MHC class I allele restricting a functionally important immunodominant SIV Gag epitope establishes a basis for studying CD8+ T-cell responses against AIDS in an important, widely available nonhuman primate species.
The urgent task of developing an effective human immunodeficiency virus (HIV) vaccine requires an understanding of HIV immunopathogenesis. CD8+ T-cell responses play a vital role in the control of natural HIV infection (35, 42, 45), and much vaccine development is focused on inducing these responses (6, 12, 41, 58). Detailed analysis of CD8+ T-cell responses requires knowledge of restricting major histocompatibility complex (MHC) class I molecules that present viral epitopes and determination of the specificity and breadth of the immune response.
In both human HIV infection and macaque models, a limited number of defined viral epitopes elicit CD8+ T-cell responses in any one subject (26, 44, 61, 63). Investigation of the strongest immunodominant T-cell responses facilitates a more detailed understanding of immune control of infection, guiding strategies for effective vaccination (2, 9, 60). Strong responses to immunodominant T-cell epitopes can, on their own, lead to protective immunity in some model viral infections (33). Conversely, T-cell responses can often select for HIV strains with mutations escaping T-cell control. Furthermore, mutations in T-cell epitopes can lead to AIDS, and it is likely that a protective HIV vaccine will need to elicit broadly directed responses against both dominant and subdominant T-cell epitopes (4, 11, 13, 17, 25, 28, 48).
Simian immunodeficiency virus (SIV) or chimeric SIV/HIV (SHIV) infection of Asian macaques, particularly the rhesus (Macaca mulatta), cynomolgus (M. fascicularis), and pigtail (M. nemestrina) macaques, are the most common and well-characterized animal models used in HIV research. The description of the rhesus macaque MHC class I allele, Mamu-A*01 (43), and the definition of its peptide binding motif (5) enabled detailed studies of specific CD8+ T-cell responses in that species. Unfortunately, there is now a very limited supply of Mamu-A*01-positive macaques (19, 46).
Many groups now utilize pigtail macaques as an alternative and reliable model for HIV vaccine (18, 22, 27) and pathogenesis (15, 16, 52, 57) studies. Unfortunately, the usefulness of this model has been hampered to date by a lack of detailed understanding of T-cell immunity and MHC genetics. The definition of immunodominant viral epitopes and their restricting MHC class I alleles in animal models such as pigtail macaques is an important and pressing concern in order to both confirm results in the rhesus model and widen the resources available to HIV researchers.
MHC class I alleles have not yet been comprehensively characterized in pigtail macaques. A limited number of M. nemestrina (Mane) MHC class I alleles have been sequenced (38), though T-cell responses restricted by these alleles have not been identified. In this study, we describe the identification of a common, immunodominant response to KP9, a 9-mer Gag epitope in vaccinated pigtail macaques, and the characterization of its restricting MHC class I molecule, Mane-A*10. In addition, study of naïve pigtail macaques infected with SIVmac251 demonstrates that KP9 responders have a significantly reduced viral load compared to that of KP9 nonresponders. Here we describe the use of multiple experimental strategies, including a conventional cloning and sequencing strategy, a novel adaptation of reference strand-mediated conformation analysis (RSCA), a fluorescence-based technique, and functional analyses to establish that Mane-A*10 binds the common and significant immunodominant CD8+ T-cell epitope KP9.
MATERIALS AND METHODS
Macaques and SIV Gag vaccine/challenge studies.
To generate and epitope map CD8+ T-cell responses to SIV Gag and subsequently determine their MHC restriction, we studied pigtail macaques (M. nemestrina) enrolled in SIV Gag vaccine and SHIV challenge studies. Macaques were immunized intramuscularly with combinations of DNA and fowlpoxvirus prime/boost vaccine regimens expressing SIVmac239 Gag as a modification of previously described methods (32). Macaques were challenged with either SHIVmn229 (21) or SHIVsf162P3 (29). A large increase in circulating SIV Gag-specific CD8+ T cells was detected in the first few weeks following virus exposure (20), as previously described for similar macaque SHIV vaccine/challenge experiments (6, 9, 58). To assess the effect of T-cell responses to SIV Gag164-172KP9, eight naïve macaques were infected intravenously with SIVmac251 (53). An SIVmac251 stock previously administered intrarectally (31) was diluted 1:200 in RPMI medium and was administered as a 1-ml dose. Presentation of KP9 following infection was elucidated by intracellular cytokine staining (described below). Plasma SIV RNA and peripheral CD4+ T-cell levels were assessed as previously described (20, 21). Macaques were housed under physical containment level 3 (PC3) conditions and were anesthetized with ketamine (10 mg/kg of body weight administered intramuscularly) prior to blood collection using either heparin or EDTA anticoagulants. The University of Melbourne and Commonwealth Scientific Industrial Research Organization (CSIRO) animal ethics committees approved all experiments.
Epitope mapping by IFN-γ ELISpot and intracellular cytokine staining.
Antigen-specific gamma interferon (IFN-γ) responses were measured in peripheral blood mononuclear cells (PBMC) or whole blood using ELISpot and intracellular cytokine staining (ICS) assays, respectively. The monkey IFN-γ ELISpot kit (U-CyTech, Utrecht, The Netherlands) was used as previously described (21). Briefly, PBMC were stimulated with a pool of 125 overlapping 15-mer SIVmac239 Gag peptides, individual 15-mer peptides (kindly supplied by the National Institutes of Health AIDS Research and Reference Reagent Program), or minimal 9- to 11-mer peptides (Chiron Mimotopes, Clayton, Australia) at 1 μg/ml for 18 h, washed, and transferred to anti-IFN-γ monoclonal antibody-coated plates and incubated for 5 h (37°C, 5% CO2). Cells were lysed and wells were incubated with biotinylated anti-IFN-γ polyclonal rabbit antibody, followed by incubation with a gold-labeled anti-biotin immunoglobulin G (IgG) antibody. IFN-γ spots were developed and counted on an automated reader (AID, Strassberg, Germany), and results were normalized to an antigen-specific IFN-γ-secreting precursor frequency per 106 PBMC. Cutoffs for positive responses were based on statistically defined thresholds for the ELISpot assay validated for clinical trials (54). This defined a positive response as meeting the following criteria: (i) ≥50 spot forming cells/106 PBMC after subtraction of spot numbers in negative controls, and (ii) nonspecific background of less than half the antigen-specific count.
Intracellular IFN-γ secretion was assessed by flow cytometry as previously described (21, 40). In short, 200 μl of whole blood was incubated with peptides as described above at 1 μg/ml along with costimulatory antibodies anti-CD28 (clone L293) and anti-CD49d (clone L25.3) (BD Biosciences, PharMingen, San Diego, Calif.) for 2 h at 37°C, 5% CO2. Brefeldin A (10 μg/ml; Sigma, St. Louis, Mo.), was then added, and blood was incubated for a further 4 to 5 h. The cells were then stained with anti-CD4-fluorescein isothiocyanate (clone M-T477), anti-CD3-phycoerythrin (clone SP34), and anti-CD8-PerCP (clone SK1) (BD Biosciences) for 30 min at 4°C. Erythrocytes were then lysed using fluorescence-activated cell sorter (FACS) lysing solution (BD) and washed with phosphate-buffered saline (PBS), and the remaining cells were permeabilized with FACS permeabilizing solution (BD). Cells were then incubated with anti-IFN-γ-allophycocyanin (APC; clone B27; BD) and formaldehyde-fixed before acquisition (BD FACScalibur). Antigen-specific CD8+ T-cell responses were assessed as the percentage of CD3+ CD8+ cells expressing IFN-γ above control-stimulated cultures. The cutoff for positive responses was determined to be 0.1% of all CD8+ T cells, provided the value was at least threefold greater than background IFN-γ production seen in dimethyl sulfoxide (DMSO)-stimulated cells.
MHC amplification and cloning.
First-strand cDNA was created from total RNA (extracted using a QIAamp RNA Blood Mini kit; QIAGEN, Hilden, Germany) in a reaction mixture containing oligo(dT)12-18 (Invitrogen, Carlsbad, Calif.), 2.5 mM each deoxynucleoside triphosphate (Promega, Madison, Wis.), dithiothreitol (Invitrogen), RNasin (Promega), 5× first-strand buffer, and SuperScript III RNase H− Reverse Transcriptase (Invitrogen). The reaction conditions were 65°C for 5 min, 42°C for 2 min (pause), 42°C for 50 min, and 70°C for 15 min, using a GeneAmp 9700 PCR Thermal Cycler (Applied Biosystems, Foster City, Calif.).
Full-length M. nemestrina MHC class I was amplified using 25 μM each primer sets 5′ALoci (5′-TCACACTTTACAAGCCGTGAGAGACAC-3′) and 3′ALoci (5′-ATGGCGCCCCGAACCCTC-3′) (A loci) or 5′BLoci (5′-TCATGGCGCCCCGAACCCTC-3′) and 3′BLoci (5′-TCAAGCCGTGAGAGACWCATCAGAGCC-3′) (B loci), designed based on rhesus macaque MHC class I sequences (47). Amplicons were generated by using Platinum Pfx DNA polymerase (Invitrogen) for 20 cycles (94°C for 1 min, 65°C for 1 s, 69°C for 90 s). The amplified MHC class I 1.1-kbp cDNA was purified from a 1% agarose gel using the QIAquick gel extraction kit (QIAGEN) according to manufacturer's instructions. Individual MHC class I amplicons were then ligated into the pCR vector (Invitrogen) and were transformed into competent Escherichia coli. Miniprep DNA extractions (QIAGEN QIAprep) were prepared from 48 colonies of each of the A loci and B loci transformants. Both strands of each clone were sequenced by using BigDye Terminator chemistry v1.1 (Applied Biosystems) and then were run on a capillary sequencer (3730 DNA Analyzer; Applied Biosystems). Amplification from one of the six macaques sequenced (4247) was performed using a modified method with 20 pmol of each 5′RSCA (5′-GCTACGTGGAYGAYACGC-3′) and 3′RSCA (5′-CARAAGGCACMWCCACAGC-3′) primers (J. Weinfurter et al., unpublished data), utilizing Amplitaq Gold (Applied Biosystems) for 35 cycles (initial cycle 95°C for 10 min, followed by 94°C for 30 s, 57°C for 1 min, 72°C for 1 min), generating a ∼700-bp fragment. Sequences were analyzed using Sequencher software (Gene Codes Corporation, Ann Arbor, Michigan). Identical sequence with contiguous forward and reverse sequences from three or more clones were considered likely to be true MHC class I alleles.
RSCA.
The RSCA method to characterize M. nemestrina MHC molecules was adapted from similar methods to detect rhesus macaque (Weinfurter et al., unpublished) and human MHC alleles (7). Approximately 700 bp of MHC class I cDNA spanning exons two and three (encoding the peptide-binding regions) were amplified from pigtail macaque cDNA, using the 5′RSCA and 3′RSCA primers as described above, for 35 cycles with Taq Hi Fi DNA polymerase (Invitrogen). Reference strands Mamu-B*07 and Mamu-B*60 were PCR amplified with the same conditions, except that 5′RSCA was replaced with 5′-end-labeled Cy5-5′RSCA. RSCA reactions to form heteroduplexes between the reference strands and alleles from amplified cDNA with unknown MHC alleles were performed as follows: 95°C for 4 min, 55°C for 5 min, 15°C for 5 min. The product was then run on a nondenaturing polyacrylamide gel for 7 h along with external size standards on an AlfExpress DNA Analyzer (Amersham Biosciences, Piscataway, N.J.) according to the manufacturer's instructions. An allele's migration rate in the gel was dictated by the conformation of the allele with a particular reference strand. Identical alleles shared between macaques have the same mobility when heteroduplexed to the same reference strand. Occasionally, the conformational heteroduplex of distinct alleles will have identical mobility, so each cDNA sample is hybridized separately to multiple reference strands to verify RSCA results.
Generation of MHC class I/GFP fusion constructs.
Full-length MHC class I amplicons were generated from macaque 4247 cDNA by using the primers ManeMHCF (5′-RWKSYGDTCRTGGCGCYC-3′) and ManeMHCR (5′-GAKCCRTGAGAGACACATC-3′) and then were ligated into the pcDNA3.1-CT-GFP-TOPO vector (Invitrogen), creating a C-terminal fusion product. MHC/green fluorescent protein (GFP) fusion plasmids were transformed into TOP10 E. coli, and DNA was extracted using a FastPlasmid Mini kit (Eppendorf, Hamburg, Germany). Transformants were screened for correct insert orientation by sequencing from the 3′ end using the GFP reverse primer (5′-GGGTAAGCTTTCCGTATGTAGC-3′) and DYEnamic ET Terminator cycle sequencing chemistry (Amersham) run on an ABI 3100 Genetic Analyzer (Applied Biosystems). Inserts in the correct orientation were then fully sequenced from the 5′ end by using the T7 primer (5′-TAATACGACTCACTATAGGG-3′).
Stable transfection into C1R cells.
The MHC class I-deficient human B-lymphoblastoid cell line, C1R (64), was maintained in RPMI 1640 medium (Invitrogen) supplemented with 100 U of penicillin G sodium/ml, 100 μg of streptomycin sulfate/ml, 292 μg of l-glutamine (PSG) (Invitrogen)/ml, and 10% fetal calf serum (FCS) (CSL, Lenexa, Kans.) (RF-10). Five MHC/GFP constructs were selected for stable transfection into C1R cells: Mane-A*10 (C1R-A*10), Mane-B*02 (C1R-B*02), a further A-like construct, 4247-09/G (C1R-09/G), a further B-like construct, 4247-02/G (C1R-02/G), and a control construct with the insert in the reverse orientation, 4247-11/G (C1R-11/G). The transfected Mane-A*10 construct inadvertently contained an A-to-G mutation at nucleotide position 656, corresponding to an E-to-G substitution at amino acid position 198. Lying within the α3 domain, this substitution is unlikely to affect the peptide-binding specificity of the molecule and is thought to have arisen by random PCR error. Ten micrograms of sterile plasmid was electroporated at 220 V, 950 μF into 107 C1R cells by using a Gene Pulser II (Bio-Rad Laboratories, Richmond, Calif.) with Capacitance Extender (Bio-Rad). Cells were cultured in RF-10 for 48 h and were selected in RF-10 plus 1 mg of Geneticin (Invitrogen)/ml and 2 mM HEPES in 96-well U-bottomed plates (Nunc, Roskilde, Denmark). Media were changed every 3 to 4 days for 3 weeks, and wells containing growing cells were screened for GFP and MHC class I expression by using the primary pan-class I antibody W6/32 (8, 51), a secondary sheep-anti-mouse Ig-phycoerythrin antibody (Chemicon), and flow cytometry (BD FACScalibur).
Generation of KP9-specific T-cell lines.
Frozen PBMC were thawed rapidly from macaques 4247 and 4296 and were washed twice in RPMI. PBMC (5 × 106) were designated stimulator cells, and 107 PBMC were designated responder cells (2:1 responder-to-stimulator ratio). Responder cells were resuspended in 1 ml of RF-15 supplemented with 50 U of human interleukin-2 (IL-2) (Sigma)/ml and 10 ng of human IL-7 (Sigma)/ml and were placed in a 24-well plate (TPP, Trasadingen, Switzerland) at 37°C, 5% CO2. Stimulator cells were resuspended in 1 ml of RPMI plus 1 μg of KP9 peptide/ml and incubated at 37°C for 30 min and then washed in RPMI. The stimulator cells were then irradiated using a 60Co source at 3,000 rad, washed again in RPMI, resuspended in 1 ml of RF-15 plus IL-2 and IL-7, and added to the responder cells. Cells were split every 3 to 4 days and were fed with fresh RF-15 plus 50 U of IL-2/ml as required. Cells were restimulated with peptide pulsed irradiated PBMC feeder cells at days 10 to 12 and 23 and were assayed either at day 17 or 27.
MHC restriction of KP9-specific T cells.
A modified intracellular IFN-γ staining assay was used to assess antigen presentation by the transfected C1R clones. A total of 2 × 105 expanded T cells (responders) were washed in RPMI, resuspended in 100 μl of a solution containing RPMI/PSG plus 5% FCS (RF-5), added to wells of a U-bottomed 96-well plate (Nunc), and placed at 37°C. A volume of 105 C1R-A*10, C1R-B*02, C1R-02/G, C1R-09/G, and C1R-11/G (stimulators) was washed twice in RPMI. The stimulator cells were then resuspended in 500 μl of RPMI and pulsed with 1 μg of KP9 peptide/ml for 30 min at 37°C. The cells were then washed twice in RPMI, resuspended in 100 μl of RF-5, and added to the responder cells in the 96-well plate (2:1 responder-to-stimulator ratio). The mixed cells were incubated at 37°C, 5% CO2 for 2 h, and then 10 μg of Brefeldin A (Sigma)/ml was added. A further 4-h incubation followed, and then the cells were stained with anti-CD4-fluorescein isothiocyanate, anti-CD3-phycoerythrin, and anti-CD8-PerCP (BD) for 30 min at 4°C. The cells were washed, fixed in 1% paraformaldehyde for 20 min and then were washed again. A combination of 0.3% Saponin and anti-IFN-γ-APC (BD) was then added to cells for 40 min at 4°C. Cells were washed once and were transferred to 5-ml polystyrene FACS tubes for acquisition (BD FACScalibur) and analysis using CellQuest Pro (BD).
Nucleotide sequence accession numbers.
The sequences for the 20 novel MHC class I alleles determined in this study have been deposited in the GenBank database under accession numbers AY557348 to AY557367.
RESULTS
Immunodominant SIV Gag epitopes in pigtail macaques.
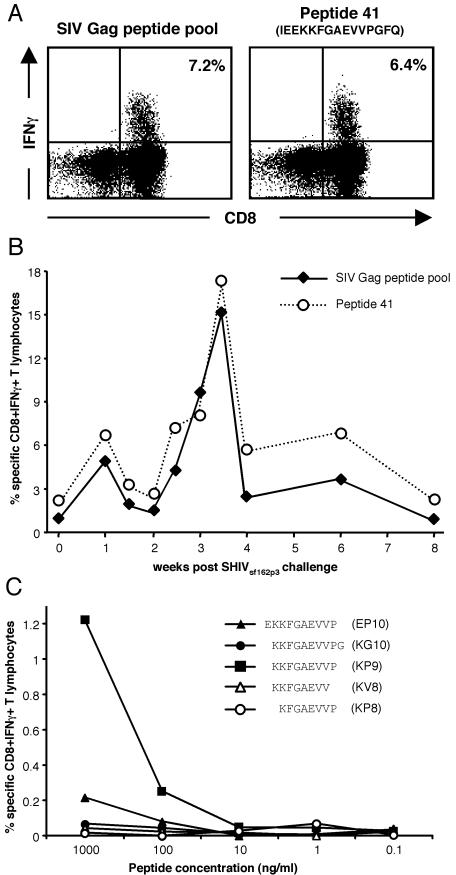
No previous studies have described CD8+ T-cell epitopes or the MHC restriction of these responses in pigtail macaques, an increasingly widespread animal model for HIV. In this study, we initially characterized commonly recognized SIV Gag epitopes in a large cohort of pigtail macaques. T-cell responses to SIV Gag were analyzed in detail by IFN-γ ELISpot and ICS assays in 36 pigtail macaques enrolled in SHIV vaccine trials involving immunization with various combinations of DNA and fowlpoxvirus vaccines and challenge with either SHIVmn229 or SHIVsf162P3 (20, 21, 29). Strong SIV Gag CD8+ T-cell responses, involving 0.6 to 34.7% of all CD8+ T cells, were detected postchallenge in 23 of 36 macaques (63.9%) by using a peptide pool containing 125 15-mer peptides overlapping by 11 amino acids spanning the entire SIV Gag region. These responses were mapped using 5- or 10-peptide pools, followed by individual 15-mer peptides. A response to the 15-mer Gag161-175 peptide 41 was detected in 15 of the 23 macaques responding to Gag, representing 41.7% of all macaques and 65.2% of Gag responders (Table 1). No responses were detected to the immediately adjacent overlapping peptides 40 or 42, suggesting that the minimal epitope was likely to be the 9-mer spanning the centre of peptide 41. On average, the CD8+ T-cell response to peptide 41 accounted for 63% of the response to Gag (Fig. 1A and B; Table 1), although this may be somewhat of an overestimate, given that in a few macaques the peptide 41 response exceeded that of the total Gag pool. The higher response to peptide 41 compared to that to Gag in these animals suggests possible competition for MHC binding from other peptides within the pool. The kinetics of the peptide 41-specific CD8+ T-cell response paralleled the total SIV Gag response following SHIV challenge (Fig. 1B).
TABLE 1.
Immunodominance of SIV Gag peptide 41
| Macaque | 15-mer peptidea | Sequence | % CD8+ T cells expressing IFN-γb
|
|
|---|---|---|---|---|
| Peptide specific | Gag specific | |||
| 4246 | 36-40 | 0.0 | 0.8 | |
| 4277 | 40 | WVKLIEEKKFGAEVV | 0.0 | 0.6 |
| 3790 | 41 | IEEKKFGAEVVPGFQ | 1.7 | 1.4 |
| 4241 | 41 | IEEKKFGAEVVPGFQ | 0.3 | 0.4 |
| 4246 | 41 | IEEKKFGAEVVPGFQ | 2.1 | 0.8 |
| 4247 | 41 | IEEKKFGAEVVPGFQ | 8.5 | 5.0 |
| 4277 | 41 | IEEKKFGAEVVPGFQ | 0.7 | 0.6 |
| 4290 | 41 | IEEKKFGAEVVPGFQ | 4.3 | 8.6 |
| 4292 | 41 | IEEKKFGAEVVPGFQ | 1.9 | 1.5 |
| 4295 | 41 | IEEKKFGAEVVPGFQ | 5.9 | 34.7 |
| 4296 | 41 | IEEKKFGAEVVPGFQ | 1.2 | 14.0 |
| 4380 | 41 | IEEKKFGAEVVPGFQ | 0.7 | 1.7 |
| 4382 | 41 | IEEKKFGAEVVPGFQ | 0.1 | 2.6 |
| 4386 | 41 | IEEKKFGAEVVPGFQ | 0.9 | 3.4 |
| 4664 | 41 | IEEKKFGAEVVPGFQ | 9.8 | 5.3 |
| 4668 | 41 | IEEKKFGAEVVPGFQ | 6.4 | 7.2 |
| H8x | 41 | IEEKKFGAEVVPGFQ | 4.2 | 2.1 |
| 4246 | 42 | KFGAEVVPGFQALSE | 0.0 | 0.6 |
| 4277 | 42-45 | 0.0 | 0.8 | |
| 4293 | 41 | 0.0 | 11.7 | |
| 4523 | 41 | 0.0 | 0.9 | |
| H20x | 41 | 0.0 | 5.2 | |
| H21x | 41 | 0.0 | 1.0 | |
Peptides were numbered sequentially from 1 to 125 from a pool of 125 15-mer peptides overlapping by 11 amino acids and spanning the entire SIVmac239 Gag region.
Measured by ICS following SHIV challenge.
FIG. 1.
Mapping the minimal immunodominant SIV Gag epitope in peptide 41. (A) Whole blood from SIV Gag-immunized pigtail macaque 4668 3 weeks following SHIVmn229 challenge was stimulated either with a pool of 125 SIV Gag 15-mers or only the 41st peptide in this pool. CD3+ T lymphocytes were studied for the percentage of CD8+ T cells expressing IFN-γ by ICS. (B) SIV Gag and peptide 41 responses from macaque 4246 were monitored in the 8 weeks following SHIV challenge. (C) The minimal epitope within SIV Gag 15-mer peptide 41 was mapped by generating a T-cell line from animal 4296 and studying the T-cell reactivity to the central 10-, 9-, and 8-mer peptides within peptide 41 and not overlapping with 15-mer peptides 40 and 42 (see Table 1).
The minimum epitope contained within the peptide 41 15-mer was determined by intracellular IFN-γ staining using antigen-specific T cells and peptide titrations of the 8-, 9-, and 10-mers derived from the central region of peptide 41 (Fig. 1C). This titration clearly demonstrated the superior T-cell reactivity to the 9-mer KP9 compared to that of the other adjacent 8-mer and 10-mer peptides. These titration results defining KP9 as the minimal epitope were confirmed by ELISpot analyses on fresh PBMC samples, with KP9 being the only peptide still stimulating a response at 10 ng/ml (data not shown).
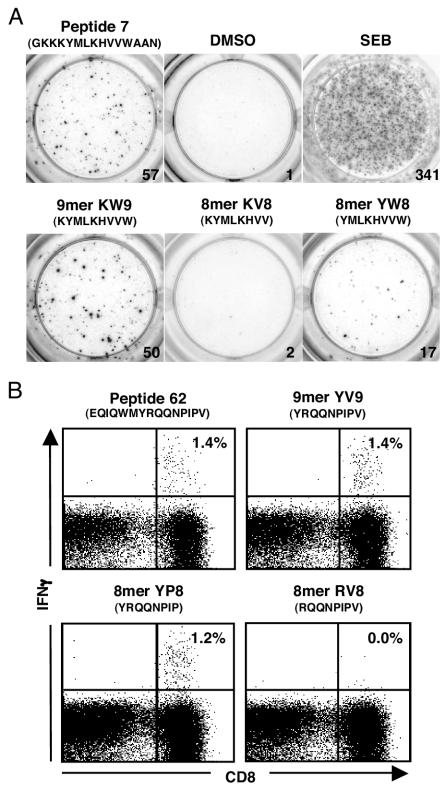
In addition to the response detected to KP9 within peptide 41, subdominant CD8+ T-cell responses in more than one pigtail macaque were detected to peptide 7 (GKKKYMLKHVVWAAN), peptide 62 (EQIQWMYRQQNPIPV), and peptide 63 (WMYRQQNPIPVGNIY). A similar process of defining the minimal epitope through the use of truncated peptides in ELISpot and ICS assays was performed, with the peptide 7 response mapped to the 9-mer KW9 (KYMLKHVVW) (Fig. 2A) and the peptide 62/63 response mapped to the 8-mer YP8 (YRQQNPIP) (Fig. 2B).
FIG. 2.
Minimal epitope mapping of additional Gag responses. (A) The response to peptide 7 was mapped to the minimal 9-mer KW9 by ELISpot. DMSO and staphylococcal enterotoxin B (SEB) responses are shown as negative and positive controls, respectively. (B) The responses to overlapping peptides 62 and 63 were mapped to the 8-mer YP8 by ICS.
Sequencing of 20 novel pigtail macaque MHC class I alleles.
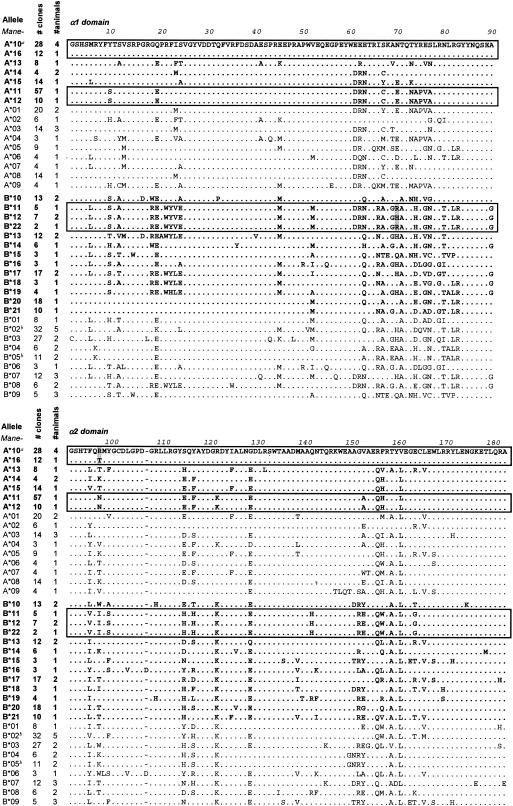
The use of conserved primers designed for rhesus macaque MHC class I alleles enabled the amplification of multiple pigtail macaque sequences. A cloning and sequencing strategy generated multiple sequences from macaques 4241, 4247, 4293, 4295, 4296, and 4664. A conservative approach for the definition of novel alleles was taken, with at least three identical, independent contiguous sequences required. Using this approach, 20 novel MHC class I alleles were identified in pigtail macaques, 7 Mane-A alleles and 13 Mane-B (GenBank accession numbers AY557348 to AY557367). An alignment of the predicted amino acid sequences of the peptide-binding domains of these alleles is shown in Fig. 3, with the previously published Mane sequences provided for comparison. Up to two Mane-A and four Mane-B alleles were identified in each macaque, consistent with the observation that duplication of the Mane-B locus has occurred in this species (38), and similar to observations with rhesus macaques and baboons (1, 14). It is likely that a less conservative method of allele definition would have resulted in more than two Mane-A alleles being detected in a single macaque, because this locus has previously been shown to be duplicated in M. nemestrina (38). Surprisingly, of the 18 pigtail macaque MHC class I alleles previously described (38), only two were detected in our cohort of macaques: Mane-B*02 in macaque 4247 and Mane-B*05 in macaque 4664.
FIG. 3.
Amino acid alignment of α1 and α2 domains of 20 novel pigtail macaque MHC class I alleles. Up to 96 MHC class I cDNA clones from six pigtail macaques underwent forward and reverse sequencing, generating 700- to 1,000-bp fragments. Alleles were defined where at least three identical clones with full contiguous sequence were detected (Mane-B*22 was defined due to its close relationship to Mane-B*11 and Mane-B*12 despite only being present in two clones). Predicted amino acid translations of the α1 and α2 domains, the peptide binding regions, are shown. The leader, α3, transmembrane, and cytoplasmic sequences were also identical within each defined allele (data not shown; see GenBank accession numbers AY557348 to AY557367). Closely related alleles are boxed together (see the text and Table 2). Boldface alleles represent newly described alleles. Alleles not in boldface were previously described (38) and are provided for comparison. We confirmed the presence of Mane-B*02 and Mane-B*05 in pigtail macaques studied in this report (marked with a superscript letter b), with the additional clones and numbers of animals consolidated.
Variation between the newly defined alleles occurs in distinct regions of the peptide-binding (α1 and α2) domains, particularly the regions spanning residues 9 to 25, 62 to 83, and 152 to 170. These variations correspond with the location of known pocket residues that are important in determining the peptide-binding specificity of the alleles (55, 62). Some of the novel allele sequences described are very closely related. Mane-A*10 and Mane-A*16 differ by a total of five nucleotides, resulting in just two differences at the amino acid level (positions −9 and 97), one of these in the leader sequence which is cleaved from the mature MHC class I molecule. Mane-A*11 and Mane-A*12 differ by a single nucleotide, resulting in an amino acid substitution at position 309, towards the C terminus of the transmembrane region. Mane-B*11, Mane-B*12, and Mane-B*22 are all closely related, with Mane-B*11 and Mane-B*22 differing by just one nucleotide (and by one amino acid) in the cytoplasmic tail. Mane-B*12 differs from Mane-B*11 and Mane-B*22 by two nucleotides, one causing a nonsynonymous R-to-H amino acid change at position 70 in the α1 domain.
MHC class I alleles shared by macaques presenting SIV epitopes.
Following MHC class I sequencing, the detected immune responses to KP9, YP8, and KW9 were correlated with the presence of particular alleles to identify potential restricting alleles for those epitopes (Table 2). Of the six macaques that had their MHC class I alleles sequenced, five responded to KP9: 4241, 4247, 4295, 4296, and 4664. The Mane-A*10 allele was detected in four of these macaques, and the closely related allele, Mane-A*16, was detected in the weak KP9-responder 4241. Neither Mane-A*10 nor Mane-A*16 was detected in the non-KP9 responder 4293. This strongly suggests that the Mane-A*10 molecule is involved in the presentation of the KP9 epitope. Two macaques, 4295 and 4293, both responded to the Gag251-258YP8 epitope. Mane-A*12 was detected in macaque 4295, and the closely related allele, Mane-A*11 (differing by only one nucleotide), was detected in 4293. Further investigation will be required to determine if one or both of these alleles can present YP8. Macaques 4296 and 4293 both responded to the Gag164-172KW9 epitope. These two macaques share the expression of two molecules, Mane-A*14 and Mane-B*10, that could potentially bind KW9. In addition, 4296 expresses the Mane-B*22 molecule and 4293 expresses the very closely related Mane-B*11 molecule. The third molecule in the related cluster, Mane-B*12, is expressed by 4241 and 4295, and it has no response to the KW9 epitope detected in those macaques, making it unlikely that any of the B*11/B*12/B*22 cluster binds KW9. Two further alleles are shared between macaques, though their expression does not correlate with any of the Gag CD8+ T-cell responses detected to date. Macaques 4241 and 4295 share the Mane-B*13 allele, and 4296 and 4664 share Mane-B*18. Further investigations will be necessary to determine the SIV epitopes bound by Mane-B*13 and Mane-B*18.
TABLE 2.
Correlation of common peptide-specific T-cell responses with shared MHC class I alleles
| Peptide or shared MHC class I allele | T-cell response or allele detection for macaque no:
|
|||||
|---|---|---|---|---|---|---|
| 4241 | 4247 | 4295 | 4296 | 4664 | 4293 | |
| Peptide | ||||||
| KP9 | ×a | × | × | × | × | |
| YP8 | × | × | ||||
| KW9 | × | × | ||||
| Allele | ||||||
| Mane-A*10b | + (12) | + (2)c | + (6) | + (14) | + (6) | |
| Mane-A*16 | + (12) | |||||
| Mane-A*11 | + (57) | |||||
| Mane-A*12 | + (10) | |||||
| Mane-A*14 | + (2) | + (2) | ||||
| Mane-B*10 | + (7) | + (6) | ||||
| Mane-B*11 | + (5) | |||||
| Mane-B*12 | + (4) | + (3) | ||||
| Mane-B*22 | + (2) | |||||
| Mane-B*13 | + (9) | + (3) | ||||
| Mane-B*18 | + (7) | + (10) | ||||
An × indicates the detection of a T-cell response by ELISpot of >50 antigen-specific IFN-γ spot-forming cells/106 PBMC to the epitopes listed.
MHC class I alleles possessing <5 nucleotide differences are shown grouped together (see the text).
A plus sign indicates detection of the allele, with the number of clones derived from each macaque shown in parentheses.
RSCA of macaques presenting the SIV Gag KP9 epitope.
Cloning and sequencing of MHC class I cDNAs is a difficult method for screening larger numbers of subjects for multiple unknown MHC class I transcripts. Developed in humans, and adapted more recently to rhesus macaques, RSCA is a highly sensitive technique for the characterization of multiple expressed MHC class I cDNAs in a single sample (7). The technique hybridizes approximately 700 bp of MHC class I cDNA with fluorescent-labeled reference class I clones. The various expressed MHC class I cDNA molecules from one macaque heteroduplex with the fluorescently labeled clone and run with particular mobilities on nondenaturing gels. Heteroduplexing unknown MHC class I cDNAs against more than one fluorescent reference strand characterizes a unique pattern of mobility for particular MHC class I transcripts. Previous studies have utilized allogeneic reference MHC class I alleles. This does not allow detection of the MHC class I allele identical to the reference strand, as the homoduplex between the fluorescent and nonfluorescent allele is obscured by homoduplexes between the fluorescent allele alone. The use of xenogeneic reference strands to overcome this problem has not previously been reported.
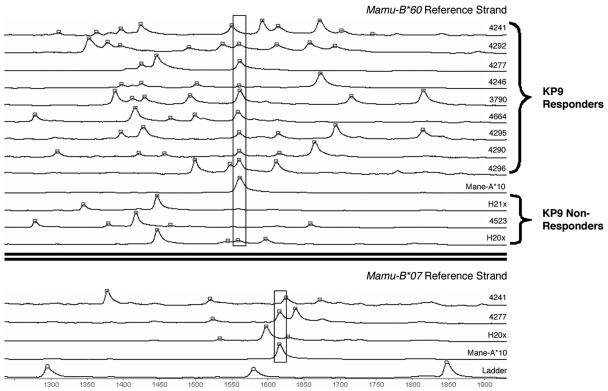
To generate a pattern of MHC class I alleles shared by M. nemestrina capable of responding to the KP9 SIV Gag epitope, we hybridized MHC class I cDNA from nine macaques presenting KP9 and three macaques not capable of presenting KP9 with fluorescent M. mulatta Mamu-B*60 and Mamu-B*07 alleles and analyzed the mobility of the alleles on a nondenaturing gel. To identify the mobility of Mane-A*10, the likely KP9-presenting allele identified by sequencing, we analyzed the mobility of individual Mane-A*10 clones from macaques 4295 and 4664.
This RSCA technique successfully delineated up to eight separate MHC class I alleles from an individual macaque (Fig. 4). Eight of the nine macaques capable of responding to KP9 had one MHC class I allele that migrated with identical mobility when heteroduplexed to Mamu-B*60. This mobility was identical to that of the Mane-A*10 clones from macaques 4295 and 4664 heteroduplexed to Mamu-B*60. The other KP9-responding macaque (4241) had an allele with mobility marginally slower than that of the other KP9-responding macaques. Interestingly, one macaque (H20x) in which we could not detect an immune response to KP9 (despite a vigorous CD8-mediated response to SIV Gag) had an allele with mobility identical to that of Mane-A*10. To resolve these issues, MHC cDNA from these macaques was heteroduplexed with another M. mulatta allele, Mamu-B*07. In this case, an allele with migration identical to that of Mane-A*10 was not detected in cDNA from macaque H20x. This suggests that the MHC class I allele from H20x with migration similar to that of Mane-A*10 when heteroduplexed with Mamu-B*60 is not Mane-A*10, consistent with the lack of CD8+ T-cell response to KP9. When the MHC class I cDNAs from nine KP9 responders were heteroduplexed with Mamu-B*07, eight of the nine KP9 responders had an allele with mobility identical to that of Mane-A*10, with the outlier, macaque 4241, again having an allele with slightly slower mobility. Interestingly, macaque 4241 expresses Mane-A*16, an allele that differs from Mane-A*10 by just one nucleotide in the ∼700-bp RSCA fragment. We and others have previously observed the remarkable sensitivity of the RSCA technique, whereby a single nucleotide is usually sufficient to alter the mobility of heteroduplexed clones (data not shown).
FIG. 4.
Mane-A*10 is usually expressed in cDNA from pigtail macaques responding to the KP9 epitope by RSCA. MHC class I cDNA from 12 pigtail macaques and cloned Mane-A*10 were heteroduplexed to fluorescent rhesus Mamu-B*60 or Mamu-B*07 alleles, and their mobility was assessed on a nondenaturing gel. The number of minutes each Mane MHC class I allele takes to run out is shown on the x axis. From total MHC class I cDNA samples, each peak represents a single MHC class I allele. Alleles comigrating with the Mane-A*10 clone are boxed. Occasionally, different alleles may have the same mobility as one reference strand (e.g., animal H20x; see the text). However, this can be resolved by using multiple separate reference strands. The bottom section shows representative samples heteroduplexed with labeled Mamu-B*07.
Mane-A*10 binds the KP9 epitope.
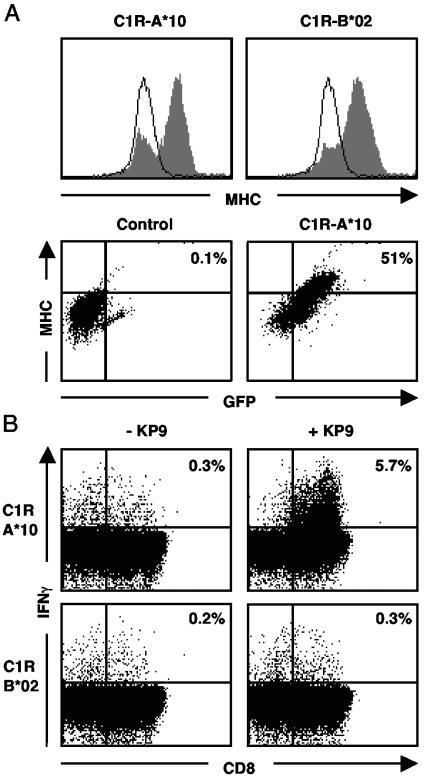
To definitively identify the pigtail macaque MHC class I molecule most commonly presenting the immunodominant KP9 epitope, we selectively transfected a human cell line with full-length M. nemestrina MHC class I cDNA clones. Five MHC class I cDNAs obtained from macaque 4247 (a KP9 responder) were cloned into a GFP-expressing vector and were stably transfected into the MHC class I-deficient cell line, C1R (64). Fluorescence microscopy of the transfected cells demonstrated a surface-associated pattern of GFP expression, suggesting that the transfected MHC class I cDNAs were correctly processed and transported to the cell surface (data not shown). MHC class I-transfected cells expressing GFP demonstrated an increased level of surface MHC class I expression, while cells transfected with a control construct (11/G) showed no such increase (Fig. 5A).
FIG. 5.
Mane-A*10 binds the KP9 epitope. (A) Mane-A*10, Mane-B*02, and reverse-oriented control MHC class I clone (11/G) from animal 4247 were cloned into a GFP-expressing vector, and stable transfectants were generated in C1R cells. MHC class I and GFP expression were characterized by flow cytometry. Histograms show the majority of Mane-A*10 or Mane-B*02 transfectants expressing high-level MHC class I (gray-filled graph) compared to that of control-transfected cells (single line). The dot plots show that in the MHC class I-transfected cells (C1R-A*10), increased MHC class I expression is associated with GFP fluorescence compared to that of control-transfected cells (C1R-11/G). (B) A KP9-specific T-cell line was generated from animal 4296 and was mixed with C1R cells expressing either Mane-A*10 or Mane-B*02 unpulsed with peptide (−KP9) or loaded with the KP9 epitope (+KP9) for 6 h. High specific stimulation of IFN-γ expression in CD8+ T cells was detected by flow cytometry when Mane-A*10 presented KP9.
PBMC from macaques 4296 and 4247 were expanded into KP9-specific T-cell lines over the course of 3 weeks and were used in a modified intracellular IFN-γ staining assay to assess MHC class I restriction of the KP9 response. In two separate experiments, results clearly demonstrated that C1R-A*10 pulsed with KP9 was able to stimulate a significant KP9-specific CD8+ T-cell IFN-γ response in the 4296 T-cell line (Fig. 5B). No specific IFN-γ production was seen when C1R-A*10 cells were not pulsed with the KP9 peptide or when the C1R-B*02 transfectant was pulsed with the KP9 peptide. Similar results were obtained with the 4247 T-cell line (data not shown). In addition, no peptide-specific IFN-γ production was seen in either the 4247 or 4296 T-cell line exposed to KP9-pulsed C1R clones transfected with two other MHC alleles or a control allele in the reverse orientation (data not shown).
KP9-responding macaques have lower viral loads following SIVmac251 infection.
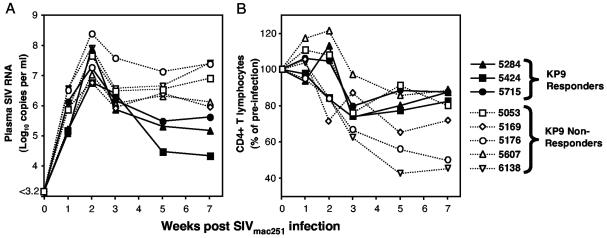
To evaluate whether responses to the KP9 epitope had an effect on the course of SIV infection, eight naïve macaques (three KP9 responders and five nonresponders) were infected with SIVmac251 and were assessed during acute infection for plasma SIV viral load and peripheral CD4+ T-cell levels. Over the first 7 weeks of infection, comparison of plasma SIV viral load and analysis by the Wilcoxon rank-sum test demonstrated that KP9 responders had a significantly lower viral load (log10 5.57 copies/ml) than KP9 nonresponders (log10 6.44 copies/ml; P = 0.025) (Fig. 6A). A similar analysis of peripheral CD4+ T cells did not demonstrate any significant difference between KP9 responders and nonresponders (Fig. 6B). Although the analysis was done early (up to week 7 postinfection), the KP9-presenting animals were among the animals with the greatest retention of CD4+ T cells, and significant differences would likely not appear until later in infection.
FIG. 6.
Presentation of KP9 results in significantly lower viral load following SIV challenge in unvaccinated macaques. (A) Plasma SIV RNA viral loads were calculated serially for 7 weeks following acute SIVmac251 infection. Open shapes depict KP9 nonresponding macaques, and closed shapes depict KP9 responders. The difference in viral load between KP9 responders and KP9 nonresponders (log10 0.87) is statistically significant (P = 0.025). (B) Peripheral CD4+ lymphocyte counts were observed over the same period of acute SIV infection and are displayed here as the percentage of change over time. Differences between KP9 responders and nonresponders are not significant up to week 7 postinfection (P > 0.05).
DISCUSSION
This report describes a very common immunodominant SIV Gag epitope, KP9, in vaccinated pigtail macaques and its restricting MHC class I allele, Mane-A*10. We also describe two subdominant epitopes and their likely restricting MHC class I alleles. A novel RSCA method for defining shared MHC class I alleles, using xenogeneic rhesus macaque reference strands, facilitated this analysis. Nineteen additional novel A- and B-like MHC class I alleles and two additional shared CD8+ T-cell epitopes are also described for M. nemestrina. We also show that presentation of KP9 has a beneficial effect on acute SIV viral levels. These experiments represent the first correlation of immunodominant SIV responses, MHC class I restriction, and outcome of SIV infection in pigtail macaques. This is an important advance for the increasing number of studies using pigtail macaques as an animal model for HIV infection, facilitating the development of MHC class I tetramers and SIV or SHIV vaccine studies with pigtail macaques selected for the presence of Mane-A*10.
The study of immunodominant T-cell epitopes yields important information about the characteristics and dynamics of effective (and not so effective) immune responses. CD8+ T-cell responses have been shown to play a crucial role in the control of both acute and chronic HIV and SIV infection (30, 42, 49, 56). The identification of the dominant SIV Gag epitope in pigtail macaques and its restricting allele permits more detailed analysis of the role of this CD8+ T-cell response in control of SIV, similar to work done on the Gag CM9 epitope in Mamu-A*01-positive rhesus macaques (2, 3, 24, 37). This significantly broadens the resources available to model HIV immunopathogenesis and evaluate candidate vaccines.
This report supplements the single previous report on sequencing pigtail macaque MHC class I (38). In that study, no epitope presentation was linked to the 19 Mane alleles sequenced from five animals. Interestingly, few of the previously characterized M. nemestrina MHC class I alleles were detected in the cohort of macaques studied here, and the Mane-A*10 allele that we found to be expressed in 8 of 11 macaques we studied by sequencing and RSCA had not been previously identified. Interestingly, KP9-specific CD8+ T-cell responses were identified in 15 of 36 animals (41.7%) responding to SIV Gag, almost certainly reflecting an exceedingly high frequency of Mane-A*10 or closely related alleles. We have detected the Mane-A*10 allele in pigtail macaques from both Australian and Indonesian breeding colonies, as well as from the University of Washington (data not shown), suggesting that Mane-A*10 may be common and widespread. The lack of detection of Mane-A*10 in the five pigtail macaques studied in the previous report (38) either could reflect a founder effect in the colony from which the five macaques were obtained or could be due to the small number of animals studied in that report for which low-throughput sequencing techniques were used. The Gag amino acid sequence across the KP9 epitope is conserved in most SIV strains derived from macaques and also appears conserved in many HIV-2 strains (36).
Multiple convergent technologies were utilized to define the M. nemestrina molecule restricting the KP9 epitope: epitope mapping using ELISpot and ICS, cloning and sequencing, transfection of isolated alleles, antigen presentation assays on T-cell lines, and a novel xenogeneic RSCA method. While a rigorous but low-throughput cloning and sequencing strategy was required for the initial characterization of alleles, the RSCA technology permitted sensitive and rapid screening of MHC class I alleles in multiple pigtail macaques. Throughput could potentially be increased by using liquid-phase capillary-based technologies now utilized for high-throughput sequencing (23, 59). Using a capillary sequencer, multiple reference alleles labeled with different fluorescent dyes can be used, eliminating the need to run multiple nondenaturing gels with different reference alleles.
Of the 20 MHC class I alleles described here, 7 of the alleles are closely related to one or two others, as outlined in Fig. 2. The possibility of detecting spurious MHC class I alleles from PCR amplification errors or recombination events was minimized by the use of a high-fidelity enzyme, a low number of amplification cycles, and a conservative method of allele definition. The observation that macaque 4241 could respond weakly to the KP9 epitope while expressing the Mane-A*16 allele and not Mane-A*10 suggests that these closely related alleles share a common peptide-binding specificity. Further experiments with C1R cells transfected with the Mane-A*16 allele could demonstrate the functional ability of this allele to present the KP9 epitope.
There is now a body of information on a total of 38 M. nemestrina classical MHC class I alleles to draw upon for further study. Sequence-specific primers can now be designed to detect important alleles such as Mane-A*10 by PCR, similar to assays developed for rhesus macaques (34, 39). PCR-based assays, which can frequently detect closely related alleles, could subsequently be validated by RSCA, which is sensitive to single-nucleotide changes. The existence of closely related alleles was demonstrated by the Mane-A*10 and Mane-A*16 alleles identified in this report, although both of these alleles are likely to present KP9. Sequence-specific primers would have to be able to distinguish between Mane-A*10 and Mane-A*16. A Mane-A*10-KP9 tetramer reagent can also be developed, enabling more detailed study of the role of KP9-specific CD8+ T cells, contributing to our understanding of T-cell immune pressure applied following vaccination and during the course of infection. Furthermore, the selection of Mane-A*10-positive macaques for vaccine trials will enable a more robust comparison of vaccine regimens and facilitate a better understanding of the characteristics of species-specific immune responses to SIV and SHIV. The presentation of the KP9 epitope alone confers an advantage on pigtail macaques in controlling acute SIV infection. A similar effect has been observed in Mamu-A*01-positive rhesus macaques (47, 50, 65), but this is the first observation in an additional nonhuman primate species. Further analysis should clarify the mechanism and immunological advantage provided by the KP9-specific CD8+ T-cell response during both acute and chronic SIV infection.
Dominant T-cell responses, while often effective, can be undermined by the emergence and maintenance of viral escape at immunodominant epitopes, resulting in disease progression (10, 11, 13, 28). We have recently identified Mane-A*10 and Mane-A*16-positive macaques infected with SHIV strains containing mutations at positions 2 and 9 of the KP9 epitope, which represent escape mutations (C. S. Fernandez et al., submitted for publication). The definition of subdominant epitopes in this study, such as YP8 (restricted by Mane-A*11/Mane-A*12) and KW9 (restricted by Mane-A*14 or Mane-B*10), now permits a dissection of the role of subdominant T-cell responses. Of particular interest is when KP9 responders also present these subdominant epitopes (such as in animals 4295, 4296, and 4293) and where escape occurs at the dominant epitope and the subdominant responses become critical. We have recently mapped an additional 12 CD8+ T-cell epitopes in M. nemestrina and are now focused on determining the MHC class I restriction of these epitopes and attempting to correlate these responses with outcome of SIV or SHIV infection.
In summary, we have identified an immunodominant SIV Gag CD8+ T-cell epitope, KP9, and its restricting MHC class I allele, Mane-A*10, present in a large proportion of pigtail macaques. In unvaccinated macaques, CD8+ T-cell responses to KP9 result in lower viral loads during acute SIV infection, demonstrating that this immunodominant response alone has a significant effect on the outcome of SIV infection. This has important implications for future HIV-related studies of M. nemestrina. Investigation of the development, kinetics, and specificity of CD8+ T-cell responses, along with further study of viral escape at both immunodominant and subdominant epitopes in pigtail macaques, will lead to refinements in vaccine design and contribute to our understanding of protective immunity to SIV and SHIV.
Acknowledgments
We thank Matthew Law for assistance with statistical analysis and Socheata Chea for general assistance and CD4+ lymphocyte counts.
This work was supported by Australian National Health and Medical Research Council grants 251653, 251654, and 299907 and NIH awards R24-RR-15371, R24-RR-016038, and P51-RR-00167643.
REFERENCES
- 1.Adams, E. J., and P. Parham. 2001. Species-specific evolution of MHC class I genes in the higher primates. Immunol. Rev. 183:41-64. [DOI] [PubMed] [Google Scholar]
- 2.Allen, T. M., P. Jing, B. Calore, H. Horton, D. H. O'Connor, T. Hanke, M. Piekarczyk, R. Ruddersdorf, B. R. Mothe, C. Emerson, N. Wilson, J. D. Lifson, I. M. Belyakov, J. A. Berzofsky, C. Wang, D. B. Allison, D. C. Montefiori, R. C. Desrosiers, S. Wolinsky, K. J. Kunstman, J. D. Altman, A. Sette, A. J. McMichael, and D. I. Watkins. 2002. Effects of cytotoxic T lymphocytes (CTL) directed against a single simian immunodeficiency virus (SIV) Gag CTL epitope on the course of SIVmac239 infection. J. Virol. 76:10507-10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, T. M., B. R. Mothe, J. Sidney, P. Jing, J. L. Dzuris, M. E. Liebl, T. U. Vogel, D. H. O'Connor, X. Wang, M. C. Wussow, J. A. Thomson, J. D. Altman, D. I. Watkins, and A. Sette. 2001. CD8(+) lymphocytes from simian immunodeficiency virus-infected rhesus macaques recognize 14 different epitopes bound by the major histocompatibility complex class I molecule Mamu-A*01: implications for vaccine design and testing. J. Virol. 75:738-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 5.Allen, T. M., J. Sidney, M. F. del Guercio, R. L. Glickman, G. L. Lensmeyer, D. A. Wiebe, R. DeMars, C. D. Pauza, R. P. Johnson, A. Sette, and D. I. Watkins. 1998. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J. Immunol. 160:6062-6071. [PubMed] [Google Scholar]
- 6.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 7.Arguello, J. R., A. M. Little, E. Bohan, J. M. Goldman, S. G. Marsh, and J. A. Madrigal. 1998. High resolution HLA class I typing by reference strand mediated conformation analysis (RSCA). Tissue Antigens 52:57-66. [DOI] [PubMed] [Google Scholar]
- 8.Barnstable, C. J., W. F. Bodmer, G. Brown, G. Galfre, C. Milstein, A. F. Williams, and A. Ziegler. 1978. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell 14:9-20. [DOI] [PubMed] [Google Scholar]
- 9.Barouch, D. H., A. Craiu, S. Santra, M. A. Egan, J. E. Schmitz, M. J. Kuroda, T. M. Fu, J. H. Nam, L. S. Wyatt, M. A. Lifton, G. R. Krivulka, C. E. Nickerson, C. I. Lord, B. Moss, M. G. Lewis, V. M. Hirsch, J. W. Shiver, and N. L. Letvin. 2001. Elicitation of high-frequency cytotoxic T-lymphocyte responses against both dominant and subdominant simian-human immunodeficiency virus epitopes by DNA vaccination of rhesus monkeys. J. Virol. 75:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barouch, D. H., J. Kunstman, J. Glowczwskie, K. J. Kunstman, M. A. Egan, F. W. Peyerl, S. Santra, M. J. Kuroda, J. E. Schmitz, K. Beaudry, G. R. Krivulka, M. A. Lifton, D. A. Gorgone, S. M. Wolinsky, and N. L. Letvin. 2003. Viral escape from dominant simian immunodeficiency virus epitope-specific cytotoxic T lymphocytes in DNA-vaccinated rhesus monkeys. J. Virol. 77:7367-7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. [DOI] [PubMed] [Google Scholar]
- 12.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 13.Borrow, P., H. Lewiki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. A. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205-211. [DOI] [PubMed] [Google Scholar]
- 14.Boyson, J. E., C. Shufflebotham, L. F. Cadavid, J. A. Urvater, L. A. Knapp, A. L. Hughes, and D. I. Watkins. 1996. The MHC class I genes of the rhesus monkey. Different evolutionary histories of MHC class I and II genes in primates. J. Immunol. 156:4656-4665. [PubMed] [Google Scholar]
- 15.Buch, S. J., F. Villinger, D. Pinson, Y. Hou, I. Adany, Z. Li, R. Dalal, R. Raghavan, A. Kumar, and O. Narayan. 2002. Innate differences between simian-human immunodeficiency virus (SHIV) (KU-2)-infected rhesus and pig-tailed macaques in development of neurological disease. Virology 295:54-62. [DOI] [PubMed] [Google Scholar]
- 16.Chen, Z., X. Zhao, Y. Huang, A. Gettie, L. Ba, J. Blanchard, and D. D. Ho. 2002. CD4+ lymphocytopenia in acute infection of Asian macaques by a vaginally transmissible subtype-C, CCR5-tropic simian/human immunodeficiency virus (SHIV). J. Acquir. Immune Defic. Syndr. 30:133-145. [DOI] [PubMed] [Google Scholar]
- 17.Chen, Z. W., A. Craiu, L. Shen, M. J. Kuroda, U. C. Iroku, D. I. Watkins, G. Voss, and N. L. Letvin. 2000. Simian immunodeficiency virus evades a dominant epitope-specific cytotoxic T lymphocyte response through a mutation resulting in the accelerated dissociation of viral peptide and MHC class I. J. Immunol. 164:6474-6479. [DOI] [PubMed] [Google Scholar]
- 18.Cho, M. W., Y. B. Kim, M. K. Lee, K. C. Gupta, W. Ross, R. Plishka, A. Buckler-White, T. Igarashi, T. Theodore, R. Byrum, C. Kemp, D. C. Montefiori, and M. A. Martin. 2001. Polyvalent envelope glycoprotein vaccine elicits a broader neutralizing antibody response but is unable to provide sterilizing protection against heterologous simian/human immunodeficiency virus infection in pigtailed macaques. J. Virol. 75:2224-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen, J. 2000. AIDS research. Vaccine studies stymied by shortage of animals. Science 287:959-960. [DOI] [PubMed] [Google Scholar]
- 20.Dale, C. J., R. De Rose, I. Stratov, S. Chea, D. Montefiori, S. A. Thomson, I. A. Ramshaw, B. E. Coupar, D. B. Boyle, M. Law, and S. J. Kent. Efficacy of DNA and fowlpoxvirus prime/boost vaccines for SHIV. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 21.Dale, C. J., X. S. Liu, R. De Rose, D. F. Purcell, J. Anderson, Y. Xu, G. R. Leggatt, I. H. Frazer, and S. J. Kent. 2002. Chimeric human papilloma virus-simian/human immunodeficiency virus virus-like-particle vaccines: immunogenicity and protective efficacy in macaques. Virology 301:176-187. [DOI] [PubMed] [Google Scholar]
- 22.Doria-Rose, N. A., C. Ohlen, P. Polacino, C. C. Pierce, M. T. Hensel, L. Kuller, T. Mulvania, D. Anderson, P. D. Greenberg, S. L. Hu, and N. L. Haigwood. 2003. Multigene DNA priming-boosting vaccines protect macaques from acute CD4+-T-cell depletion after simian-human immunodeficiency virus SHIV89.6P mucosal challenge. J. Virol. 77:11563-11577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drake, G. J., L. J. Kennedy, H. K. Auty, R. Ryvar, W. E. Ollier, A. C. Kitchener, A. R. Freeman, and A. D. Radford. 2004. The use of reference strand-mediated conformational analysis for the study of cheetah (Acinonyx jubatus) feline leucocyte antigen class II DRB polymorphisms. Mol. Ecol. 13:221-229. [DOI] [PubMed] [Google Scholar]
- 24.Egan, M. A., M. J. Kuroda, G. Voss, J. E. Schmitz, W. A. Charini, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Use of major histocompatibility complex class I/peptide/β2M tetramers to quantitate CD8(+) cytotoxic T lymphocytes specific for dominant and nondominant viral epitopes in simian-human immunodeficiency virus-infected rhesus monkeys. J. Virol. 73:5466-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans, D. T., D. H. O'Connor, P. Jing, J. L. Dzuris, J. Sidney, J. da Silva, T. M. Allen, H. Horton, J. E. Venham, R. A. Rudersdorf, T. Vogel, C. D. Pauza, R. E. Bontrop, R. DeMars, A. Sette, A. L. Hughes, and D. I. Watkins. 1999. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat. Med. 5:1270-1276. [DOI] [PubMed] [Google Scholar]
- 26.Frahm, N., B. T. Korber, C. M. Adams, J. J. Szinger, R. Draenert, M. M. Addo, M. E. Feeney, K. Yusim, K. Sango, N. V. Brown, D. SenGupta, A. Piechocka-Trocha, T. Simonis, F. M. Marincola, A. G. Wurcel, D. R. Stone, C. J. Russell, P. Adolf, D. Cohen, T. Roach, A. St. John, A. Khatri, K. Davis, J. Mullins, P. J. Goulder, B. D. Walker, and C. Brander. 2004. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J. Virol. 78:2187-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorelick, R. J., R. E. Benveniste, J. D. Lifson, J. L. Yovandich, W. R. Morton, L. Kuller, B. M. Flynn, B. A. Fisher, J. L. Rossio, M. Piatak, Jr., J. W. Bess, Jr., L. E. Henderson, and L. O. Arthur. 2000. Protection of Macaca nemestrina from disease following pathogenic simian immunodeficiency virus (SIV) challenge: utilization of SIV nucleocapsid mutant DNA vaccines with and without an SIV protein boost. J. Virol. 74:11935-11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goulder, P. J., R. E. Phillips, R. A. Colbert, S. N. McAdam, G. S. Ogg, M. A. Nowak, P. Giangrande, G. Luzzi, B. Morgan, A. Edwards, A. J. McMichael, and S. L. Rowland-Jones. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3:212-217. [DOI] [PubMed] [Google Scholar]
- 29.Harouse, J. M., A. Gettie, R. C. Tan, J. Blanchard, and C. Cheng-Mayer. 1999. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 284:816-819. [DOI] [PubMed] [Google Scholar]
- 30.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kent, S. J., C. J. Dale, S. Preiss, J. Mills, D. Campagna, and D. F. Purcell. 2001. Vaccination with attenuated simian immunodeficiency virus by DNA inoculation. J. Virol. 75:11930-11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kent, S. J., A. Zhao, S. J. Best, J. D. Chandler, D. B. Boyle, and I. A. Ramshaw. 1998. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J. Virol. 72:10180-10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klavinskis, L. S., J. L. Whitton, and M. B. Oldstone. 1989. Molecularly engineered vaccine which expresses an immunodominant T-cell epitope induces cytotoxic T lymphocytes that confer protection from lethal virus infection. J. Virol. 63:4311-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knapp, L. A., E. Lehmann, M. S. Piekarczyk, J. A. Urvater, and D. I. Watkins. 1997. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens 50:657-661. [DOI] [PubMed] [Google Scholar]
- 35.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuiken, C. L., B. Foley, E. Freed, B. Hahn, B. Korber, M. P. A., F. McCutchan, J. W. Mellors, and S. Wolinksy (ed.). 2002. HIV sequence compendium. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.M.
- 37.Kuroda, M. J., J. E. Schmitz, D. H. Barouch, A. Craiu, T. M. Allen, A. Sette, D. I. Watkins, M. A. Forman, and N. L. Letvin. 1998. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J. Exp. Med. 187:1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lafont, B. A., A. Buckler-White, R. Plishka, C. Buckler, and M. A. Martin. 2003. Characterization of pig-tailed macaque classical MHC class I genes: implications for MHC evolution and antigen presentation in macaques. J. Immunol. 171:875-885. [DOI] [PubMed] [Google Scholar]
- 39.Lobashevsky, A. L., and J. M. Thomas. 2000. Six mamu-A locus alleles defined by a polymerase chain reaction sequence specific primer method. Hum. Immunol. 61:1013-1020. [DOI] [PubMed] [Google Scholar]
- 40.Maecker, H. T., H. S. Dunn, M. A. Suni, E. Khatamzas, C. J. Pitcher, T. Bunde, N. Persaud, W. Trigona, T. M. Fu, E. Sinclair, B. M. Bredt, J. M. McCune, V. C. Maino, F. Kern, and L. J. Picker. 2001. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J. Immunol. Methods 255:27-40. [DOI] [PubMed] [Google Scholar]
- 41.McConkey, S. J., W. H. Reece, V. S. Moorthy, D. Webster, S. Dunachie, G. Butcher, J. M. Vuola, T. J. Blanchard, P. Gothard, K. Watkins, C. M. Hannan, S. Everaere, K. Brown, K. E. Kester, J. Cummings, J. Williams, D. G. Heppner, A. Pathan, K. Flanagan, N. Arulanantham, M. T. Roberts, M. Roy, G. L. Smith, J. Schneider, T. Peto, R. E. Sinden, S. C. Gilbert, and A. V. Hill. 2003. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat. Med. 9:729-735. [DOI] [PubMed] [Google Scholar]
- 42.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 43.Miller, M. D., H. Yamamoto, A. L. Hughes, D. I. Watkins, and N. L. Letvin. 1991. Definition of an epitope and MHC class I molecule recognized by gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys. J. Immunol. 147:320-329. [PubMed] [Google Scholar]
- 44.Mothé, B. R., H. Horton, D. K. Carter, T. M. Allen, M. E. Liebl, P. Skinner, T. U. Vogel, S. Fuenger, K. Vielhuber, W. Rehrauer, N. Wilson, G. Franchini, J. D. Altman, A. Haase, L. J. Picker, D. B. Allison, and D. I. Watkins. 2002. Dominance of CD8 responses specific for epitopes bound by a single major histocompatibility complex class I molecule during the acute phase of viral infection. J. Virol. 76:875-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musey, L., J. Hughes, T. Schacker, T. Shea, L. Corey, and M. J. McElrath. 1997. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 337:1267-1274. [DOI] [PubMed] [Google Scholar]
- 46.O'Connor, D. H., T. M. Allen, and D. I. Watkins. 2001. Where have all the monkeys gone?: evaluating SIV-specific CTL in the post-Mamu-A*01 era. In B. Korber, C. Brander, B. Haynes, R. A. Koup, C. Kuiken, J. P. Moore, B. D. Walker, and D. I. Watkins (ed.), HIV molecular immunology. Los Alamos National Laboratory, Theoretical Biology and Biophysics, Los Alamos, N.M.
- 47.O'Connor, D. H., B. R. Mothe, J. T. Weinfurter, S. Fuenger, W. M. Rehrauer, P. Jing, R. R. Rudersdorf, M. E. Liebl, K. Krebs, J. Vasquez, E. Dodds, J. Loffredo, S. Martin, A. B. McDermott, T. M. Allen, C. Wang, G. G. Doxiadis, D. C. Montefiori, A. Hughes, D. R. Burton, D. B. Allison, S. M. Wolinsky, R. Bontrop, L. J. Picker, and D. I. Watkins. 2003. Major histocompatibility complex class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. J. Virol. 77:9029-9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Connor, D. H., and D. I. Watkins. 1999. Houdini's box: towards an understanding of AIDS virus escape from the cytotoxic T-lymphocyte response. Immunogenetics 50:237-241. [DOI] [PubMed] [Google Scholar]
- 49.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 50.Pal, R., D. Venzon, N. L. Letvin, S. Santra, D. C. Montefiori, N. R. Miller, E. Tryniszewska, M. G. Lewis, T. C. VanCott, V. Hirsch, R. Woodward, A. Gibson, M. Grace, E. Dobratz, P. D. Markham, Z. Hel, J. Nacsa, M. Klein, J. Tartaglia, and G. Franchini. 2002. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J. Virol. 76:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parham, P., C. J. Barnstable, and W. F. Bodmer. 1979. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J. Immunol. 123:342-349. [PubMed] [Google Scholar]
- 52.Patel, P. G., M. T. Yu Kimata, J. E. Biggins, J. M. Wilson, and J. T. Kimata. 2002. Highly pathogenic simian immunodeficiency virus mne variants that emerge during the course of infection evolve enhanced infectivity and the ability to downregulate CD4 but not class I major histocompatibility complex antigens. J. Virol. 76:6425-6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romano, J. W., R. N. Shurtliff, E. Dobratz, A. Gibson, K. Hickman, P. D. Markham, and R. Pal. 2000. Quantitative evaluation of simian immunodeficiency virus infection using NASBA technology. J. Virol. Methods 86:61-70. [DOI] [PubMed] [Google Scholar]
- 54.Russell, N. D., M. G. Hudgens, R. Ha, C. Havenar-Daughton, and M. J. McElrath. 2003. Moving to human immunodeficiency virus type 1 vaccine efficacy trials: defining T-cell responses as potential correlates of immunity. J. Infect. Dis. 187:226-242. [DOI] [PubMed] [Google Scholar]
- 55.Saper, M. A., P. J. Bjorkman, and D. C. Wiley. 1991. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6Å resolution. J. Mol. Biol. 219:277-319. [DOI] [PubMed] [Google Scholar]
- 56.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 57.Shen, Y., D. Zhou, L. Chalifoux, L. Shen, M. Simon, X. Zeng, X. Lai, Y. Li, P. Sehgal, N. L. Letvin, and Z. W. Chen. 2002. Induction of an AIDS virus-related tuberculosis-like disease in macaques: a model of simian immunodeficiency virus-mycobacterium coinfection. Infect. Immun. 70:869-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shiver, J. W., T. M. Fu, L. Chen, D. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 59.Turner, D., S. Akpe, J. Brown, C. Brown, A. McWhinnie, A. Madrigal, and C. Navarrete. 2001. HLA-B typing by reference strand mediated conformation analysis using a capillary-based semiautomated genetic analyzer. Hum. Immunol. 62:414-418. [DOI] [PubMed] [Google Scholar]
- 60.Vogel, T. U., B. E. Beer, J. zur Megede, H. G. Ihlenfeldt, G. Jung, S. Holzammer, D. I. Watkins, J. D. Altman, R. Kurth, and S. Norley. 2002. Induction of anti-simian immunodeficiency virus cellular and humoral immune responses in rhesus macaques by peptide immunogens: correlation of CTL activity and reduction of cell-associated but not plasma virus load following challenge. J. Gen. Virol. 83:81-91. [DOI] [PubMed] [Google Scholar]
- 61.Yamamoto, H., M. D. Miller, H. Tsubota, D. I. Watkins, G. P. Mazzara, V. Stallard, D. L. Panicali, A. Aldovini, R. A. Young, and N. L. Letvin. 1990. Studies of cloned simian immunodeficiency virus-specific T lymphocytes. gag-specific cytotoxic T lymphocytes exhibit a restricted epitope specificity. J. Immunol. 144:3385-3391. [PubMed] [Google Scholar]
- 62.Young, A. C., S. G. Nathenson, and J. C. Sacchettini. 1995. Structural studies of class I major histocompatibility complex proteins: insights into antigen presentation. FASEB J. 9:26-36. [DOI] [PubMed] [Google Scholar]
- 63.Yu, X. G., M. M. Addo, E. S. Rosenberg, W. R. Rodriguez, P. K. Lee, C. A. Fitzpatrick, M. N. Johnston, D. Strick, P. J. Goulder, B. D. Walker, and M. Altfeld. 2002. Consistent patterns in the development and immunodominance of human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T-cell responses following acute HIV-1 infection. J. Virol. 76:8690-8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zemmour, J., A. M. Little, D. J. Schendel, and P. Parham. 1992. The HLA-A,B “negative” mutant cell line C1R expresses a novel HLA-B35 allele, which also has a point mutation in the translation initiation codon. J. Immunol. 148:1941-1948. [PubMed] [Google Scholar]
- 65.Zhang, Z. Q., T. M. Fu, D. Casimiro, M. E. Davies, X. Liang, W. A. Schleif, L. Handt, L. Tussey, M. Chen, A. Tang, K. A. Wilson, W. L. Trigona, D. C. Freed, C. Y. Tan, M. Horton, E. A. Emini, and J. W. Shiver. 2002. Mamu-A*01 allele-mediated attenuation of disease progression in simian-human immunodeficiency virus infection. J. Virol. 76:12845-12854. [DOI] [PMC free article] [PubMed] [Google Scholar]