Abstract
Background: Myogenesis is positively regulated by thyroid hormone (triiodothyronine [T3]), which is amplified by the type 2 deiodinase (D2) activation of thyroxine to T3. Global inactivation of the Dio2 gene impairs skeletal muscle (SKM) differentiation and regeneration in response to muscle injury. Given that newborn and adult mice with late developmental SKM Dio2 disruption do not develop a significant phenotype, it was hypothesized that D2 plays an early role in this process.
Methods: This was tested in mice with SKM disruption of Dio2 driven by two early developmental promoters: MYF5 and MYOD.
Results: MYF5 myoblasts in culture differentiate normally into myotubes, despite loss of almost all D2 activity. Dio2 mRNA levels in developing SKM obtained from MYF5-D2KO embryos (E18.5) were about 54% of control littermates, but the expression of the T3-responsive genes Myh1 and 7 and Atp2a1 and 2 were not affected. In MYF5-D2KO and MYOD-D2KO neonatal hind-limb muscle, the expression of Myh1 and 7 and Atp2a2 remained unaffected, despite 60–70% loss in D2 activity and/or mRNA. Only in MYOD-D2KO neonatal muscle was there a 40% reduction in Atp2a1 mRNA. Postnatal growth of both mouse models and SKM function as assessed by exercise capacity and measurement of muscle strength were normal. Furthermore, an analysis of the adult soleus revealed no changes in the expression of T3-responsive genes, except for an about 18% increase in MYOD-D2KO SOL Myh7 mRNA.
Conclusion: Two mouse models of early developmental disruption of Dio2 in myocyte precursor exhibit no significant SKM phenotype.
Keywords: : myogenesis, thyroid hormone signaling, deiodinases, skeletal muscle function
Introduction
Skeletal muscle (SKM) is the most abundant tissue in human body mass (∼40%). It is an important site of adaptive thermogenesis and plays important roles in energy metabolism (1). A complex transcriptional network orchestrated by myogenic regulatory factors (MRF) that are downstream of the paired domain transcription factors in mice, Pax3 and Pax7, regulates myogenesis (2). MRF are basic helix–loop–helix transcriptional factors implicated in determination and differentiation of SKM precursors. The first MRF described to be able to induce a myogenic phenotype when transfected to non-myogenic cells (e.g., fibroblasts) was Myod (3). Subsequently, a distinct molecule, Myf5, was also observed to determine muscle cell lineage (4). Together with Myf6 (Mrf4) and myogenin (Myog), they form the MRF family. However, Myog is critical in muscle differentiation rather than in determination as the other members of the family are (5,6). Then, myoblasts differentiate into myocytes and ultimately fuse to form mature multinucleated myofiber expressing a set of myofibrillar proteins required for muscle function.
During embryogenesis, Myf5 is the first MRF to be expressed, followed by Myod, pointing to a hierarchy among the MRF (7). The fact that both MYF5-KO and MYOD-KO mice exhibit normal myogenesis (8,9) and the loss of myogenesis in the double MYF5/MYOD-KO mouse (9) suggest that (i) these transcription factors play a redundant role in the same precursor cell or (ii) they are expressed in different precursor cells capable of taking over in the absence of the other (10). Indeed, either the cre-MYOD or cre-MYF5 mouse when crossed with a floxed reporter gene induces expression in virtually all SKM fibers (11,12). However, the parallel model has been challenged recently, indicating that both Myf5 and Myod are expressed in the same precursor cell (13). Thus, cre-driven gene disruption using either Myf5 or Myod targets the muscle precursor cell.
Thyroid hormone (TH) affects myogenesis. The TH triiodothyronine (T3) enters SKM fibers through membrane transporters (MCT8 and MCT10) and diffuses to the nucleus to interact with TH receptors (THR; mainly THRα) and modulates the expression of specific sets of T3-responsive genes. T3 regulates muscle development, contractility, and metabolism (14,15). The first line of evidence supporting the role of T3 in SKM development came from studies where T3 treatment induced terminal differentiation and increased Myod expression in C2 myoblasts (16,17). T3-THR heterodimerizes with the retinoic receptors (RXR) to bind to the thyroid response element (TRE) located in the Myod gene (18). The T3-THR-RXR complex also directly regulates Myog expression (19). Thus, T3 ceases proliferation and stimulates differentiation in SKM precursor cell.
Normal serum levels of TH are essential to SKM function. Overt hypo- and hyperthyroid patients present SKM-related clinical symptoms characteristic of hypo- and hypermetabolic states, respectively (20–24). Also, subclinical thyroid disease impairs muscle function and/or exercise capacity (25,26). T3 upregulates the expression of fast-twitch SKM proteins, for example systemic hypothyroidism decreases the fast myosin heavy chain 1 and 2 (Myh1 and 2 genes that encode the MyHC 2X and 2A proteins, respectively) and increases the slow Myh7 (MyHC 1 protein) (27,28). Furthermore, T3 stimulates the expression of the fast reticulum endoplasmic calcium pump SERCA1a (29) and skeletal alpha-actin (30). In terms of metabolism, T3 positively regulates the Na+/K+-ATPase pump, GLUT4 (the major glucose transporter in SKM), peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC1a), malic enzyme 1, uncoupling protein 3 (UCP3), and glycerol phosphate dehydrogenase- alpha (αGPD) (31). In humans, >600 genes were modulated in patients under levothyroxine treatment, supporting a broad regulation of the muscle transcriptome by TH (32).
The presence of deiodinases in T3-responsive cells adds a mechanism through which TH action can be customized on a cell-specific fashion at a pre-receptor level (33). Deiodinases are homodimeric selenoenzymes that have a single trans-membrane segment connected to a cytosolic globular domain containing the active center embedded in a thioredoxin-like fold (34,35). The type 2 deiodinase (D2) catalyzes the conversion of the pro-hormone thyroxine (T4) into T3. Thus, its presence in cells strengthens the flow of T3 molecules reaching the nucleus, with the additional T3 produced locally. In contrast, the type 3 deiodinase (D3) attenuates the flow of T3 to the nucleus because it inactivates T3 to 3,5-diiodo-L-thyronine and prevents T4 utilization via inactivation to reverse T3 (14).
Given that D2 is present in human SKM and considering the large muscle mass, it is reasonable to speculate that muscle D2 would play a major role in whole-body metabolism, muscle physiology, and/or development (36), albeit its specific activity is relatively low compared with other D2-expressing tissues such as the pituitary gland, brain, and brown adipose tissue (27). The development of a mouse model of global Dio2 inactivation (GLOB-D2KO) was the first step toward a better understanding of the role played by D2 in several tissues (37). Despite normal levels of T3, the GLOB-D2KO mouse is prone to obesity under thermoneutrality, a phenotype attributed to D2 disruption in BAT and/or SKM (38,39). However, disruption of Dio2 specifically in SKM fibers (SKM-D2KO) did not impact body metabolism, challenging the role played by D2 in SKM (40). Also, the SKM-D2KO mouse is systemically euthyroid, and the SKM does not exhibit evidence of hypothyroidism with preserved muscle performance (27). This contrasts with the severe muscle hypothyroidism observed in the GLOB-D2KO mice (41). An explanation has been the timing of Dio2 disruption: while the GLOB-D2KO mouse lacks Dio2 in all tissues since early in embryogenesis, the SKM-D2KO mouse experiences Dio2 disruption after the muscle fiber has differentiated (27). Indeed, primary differentiated myotubes and adult SKM of the GLOB-D2KO mouse exhibit signs of hypothyroidism and characteristics of a slow-twitch phenotype (i.e., decreased Myh2 expression) (41,42). In addition, cultures of GLOB-D2KO skeletal myocytes fail to differentiate into myotubes, indicating a phenotype of excessive proliferation and impaired differentiation (41).
This study reports that primary myoblasts from MYF5-D2KO mouse differentiate into myotubes, but after seven days of differentiation, the phenotype of the myotubes is shifted to a slow-twitch phenotype with a failure to express fully the fast Myh2 and upregulation of Myh7. However, the MYF5-D2KO and the MYOD-D2KO mice grow normally and exhibit normal exercise capacity and muscle strength. Therefore, muscle function is preserved in both MYF5-D2KO and MYOD-D2KO mice, indicating that the severe muscle hypothyroidism reported in the GLOB-D2KO mouse reflects the lack of D2 in other cell types within SKM, in addition to the myocytes.
Methods
Animals
All experimental procedures were planned following the American Thyroid Association guide to investigating TH economy and action in rodent and cell models (43) and approved by the local Institutional Animal Care and Use Committee (IACUC) at Rush University Medical Center. To eliminate Dio2 expression of SKM precursor cells, the floxed D2 mice were crossed with the cre-recombinase expression driven by Myf5 (MYF5-D2KO) (27) or Myod (MYOD-D2KO) (11). Myf5 and Myod are members of the muscle regulatory factors that determine myogenesis and are expressed in the same SKM precursor (13). Thus, the MYOD-D2KO mouse model was used to confirm results obtained with the MYF5-D2KO. Animals used in the experiments were hemizygous for the cre transgene expression, and the genetic background was C57/B6 for Floxed-D2 and Cre-MYF5, while the MYOD-D2KO mouse was generated in the 129SVB strain (11). Newborn (12–24 hours old) or male adult mice (16–24 weeks old) were used in the studies. Animals were kept on a standard chow diet (3.1 kcal/g; 2918 Teklan Global Protein rodent diet) or high-fat diet (HFD; 4.5 kcal/g; TD 95121; Harlan Teklad) for indicated weeks at room temperature (22°C), with a 12 hour dark/light cycle starting at 06:00 h, and were housed in standard plastic cages with four to five mice per cage. Cre-MLC littermates were used as controls.
Culture of murine primary SKM cells
Primary skeletal myoblasts of MYF5-D2KO mice (three weeks old) were obtained as previously described (27,44). Briefly, hind-limb muscles were digested in collagenase 0.2%, followed by dispase 2.4 IU/mL and trypsin (0.1%) digestion. After passing through a 70 mm cell strainer, cells were pre-plated in collagen-coated flasks in Dulbecco's modified Eagle's medium (DMEM) supplemented with 20% fetal bovine serum for 2 h to avoid fibroblast contamination. After that, the supernatant was recovered and re-plated. Myoblasts were kept in growth medium at 70% confluence. To evaluate myotube formation and gene expression, cells were differentiated for the indicated times in DMEM containing 2% horse serum. All cultures were supplemented with 10–7 M sodium selenite.
Deiodinase assays
SKM samples were sonicated in phosphate-EDTA buffer (PE) containing 10 mM of dithiotheitol (DTT), 0.25 M of sucrose and protease inhibitor cocktail (Roche). Protein was measured by Bio-Rad protein assay solution, and 200 μg were incubated for 3 h at 37°C in the presence of 20 mM of DTT, 1 mM of PTU, 10 nM of T3, 0.1 nM of T4, and 200 K cpm 125I-T4 (PerkinElmer Life and Analytical Sciences, Inc.; # NEX111H500UC). Assays were stopped with the addition of horse serum and 50% TCA and free 125I counted on the 2470 Automatic γ Counter Wizard2 (Perkin-Elmer), as described previously (27). This methodology was validated by Ultra Performance Liquid Chromatography (UPLC) (27).
Hypothyroidism
Hypothyroidism was induced by an iodine-deficient diet with 0.15% PTU (TD 95125; Harlan Teklad) and 0.05% of methiimadole in drinking water, as described previously (27).
Maximum exercise capacity test and physiological evaluation of muscle strength
Maximum exercise capacity and plantar flexors strength were evaluated in mice (20–24 weeks old), as previously described (27), and all tests were performed by personnel blind to the genotype. Mice were acclimatized to the treadmill (Columbus Instruments) for five to six consecutive days by running 5–10 min per day at 5–10 m/min. The test started at 10 m/min and the speed increased 2 m/min every 2 min until exhaustion. To assess muscle strength, electrodes were positioned to stimulate the plantar flexors (e.g., soleus and gastrocnemius muscles). The electric current applied to the muscle was progressively increased until the force developed plateaued at maximum level, which was then used to create a twitch response (1 Hz) and tetanus at 100 Hz. Force was recorded using the DMC software (Aurora Scientific v5.420), and the data were analyzed with DMA software (Aurora Scientific v5.220).
Indirect calorimetry
Animals were admitted to the comprehensive laboratory animal monitoring system (CLAMS; OXYMAX System 4.93; Columbus Instruments) with free access to food and water, as previously described (40). Animals were allowed to acclimatize in individual metabolic cages for 48 h before the 24 h measurements. This system allows for continuous measurement of oxygen consumption (VO2; mL/kg BW/h) and carbon-dioxide production (VCO2). These data were used to calculate the respiratory exchange ratio (RER; VCO2/VO2). The system is always calibrated against a standard gas mix containing defined quantities of O2 and CO2 before the experiment. Studies were performed at 22°C.
Gene expression analysis
Total RNA was extracted using RNeasy kits (Qiagen), according to the manufacturer's instructions. DNAse treatment (Qiagen) was performed to avoid genomic DNA contamination. The extracted RNA was quantified with a NanoDrop spectrophotometer, and 0.5–1.0 μg of total RNA was reverse transcribed into cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems). Genes of interest were measured by reverse transcription polymerase chain reaction (RT-PCR; StepOnePlus real-time PCR system, Applied Biosystems) using SYBR Green Supermix (Quanta Biosciences). Standard curves were performed for all gene expression analysis and consisted of four to five points of serially diluted cDNA. SKM PCR primers were designed to span exon–exon boundaries. The coefficient of correlation was >0.98 for all curves, and the amplification efficiency ranged between 80% and 110%. Amplicon specificity was assessed by the melting curve. Cyclophilin A or B was used as an internal control gene, and no significant changes in gene expression of either gene were observed between groups.
Serum hormone measurements
Blood samples were processed for thyrotropin (TSH), T3, and T4 analysis using a MILLIPLEX rat TH panel kit following the instructions of the manufacturer (Millipore Corp.) and read on a BioPlex (Bio-Rad).
Statistical analysis
All data are expressed as the mean ± standard error of the mean (SEM). Student's t-test was used to compare the differences between knockout and control mice in D2 activity, gene expression, exercise capacity, and muscle strength. One-way analysis of variance (ANOVA) was used to compare more than two groups (body weight), and Tukey's correction for multiple comparisons was applied as a post hoc test. Oxygen consumption (VO2) and RER over time were analyzed by two-way ANOVA followed by Bonferroni's correction. Statistical significance was set at p ≤ 0.05.
Results
MYF5-D2KO and MYOD-D2KO animals exhibit normal postnatal growth
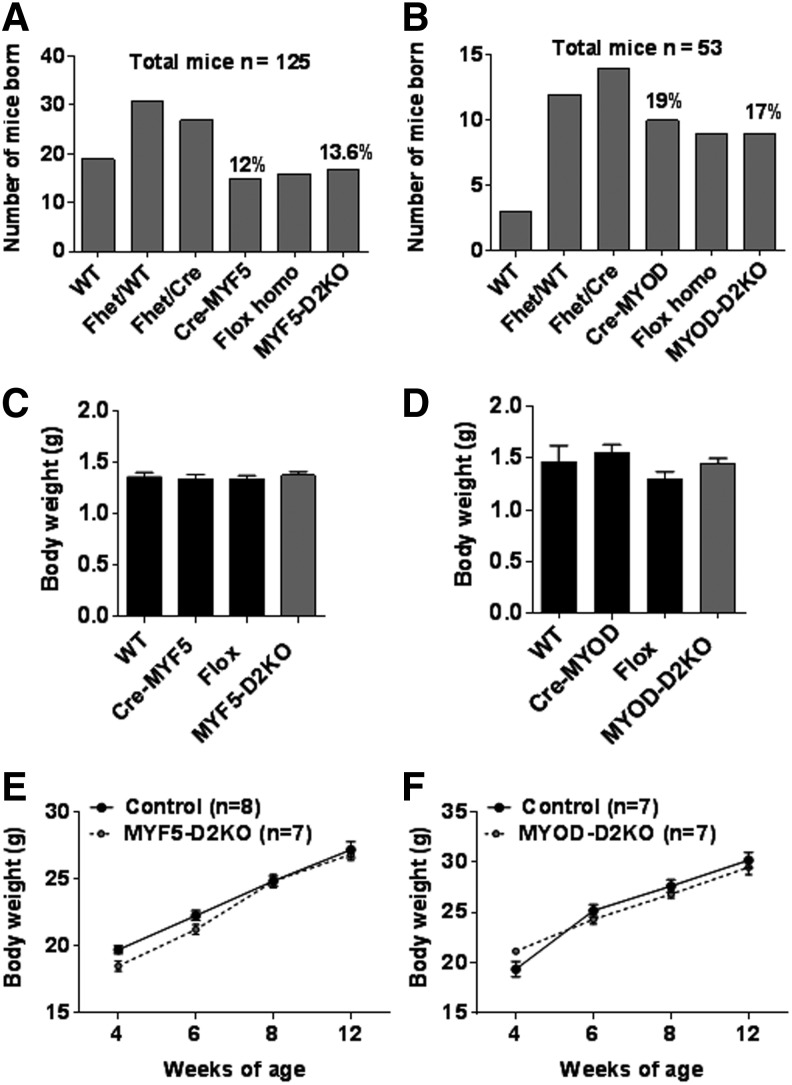
The contrasting results obtained from the GLOB-D2KO (41) and the SKM-D2KO (27,40) led to the hypothesis that D2 plays a major role during SKM development. Two mouse models with Dio2 inactivation specifically in SKM precursors were studied: MYF5-D2KO and MYOD-D2KO. Mice were born to the expected Mendelian distribution (Fig. 1A and B), and no differences in body weight were noticed (Fig. 1C and D). Also, they gained weight similarly to control littermate mice over a 12-week period (Fig. 1E and F).
FIG. 1.
MYF5-D2KO and MYOD-D2KO mice grow normally. (A) Number of mice born of each genotype after crossing a floxed Dio2 heterozygous/Cre-MYF5-negative female mouse with a floxed Dio2 heterozygous/Cre-MYF5-positive male mouse. (B) Same as (A) but a floxed Dio2 heterozygous/Cre-MYOD-negative female mouse was crossed with a floxed Dio2 heterozygous/Cre-MYOD-positive male mouse. Numbers above bars denote the relative number of mice born. (C) Body weight of neonatal MYF5-D2KO mice; and wild-type floxed Dio2 and Cre-MYF5 littermates (n = 13–18). (D) Body weight of neonatal (12–24 h) MYOD-D2KO mice, and wild-type floxed Dio2 and Cre-MYF5 littermates (n = 3–9). (E) Body weight growth curve of MYF5-D2KO and Cre-MYF5 mice (n = 7–9). (F) Same as (E) but MYOD-D2KO and Cre-MYOD mice were used (n = 6–8). Values are the mean ± standard error of the mean (SEM).
MYF5-D2KO myoblasts fuse into myotubes but exhibit a slow-twitch phenotype
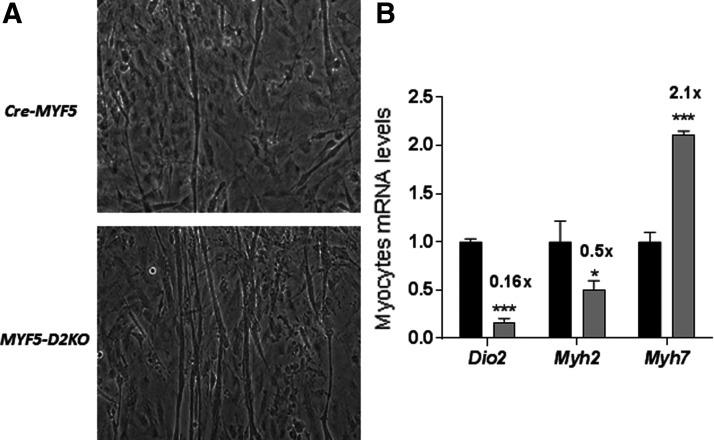
MYF5-D2KO myoblasts were obtained from the hind limb of three-week-old MYF5-D2KO mice. To understand the role played by D2 specifically in myocyte differentiation, primary MYF5-D2KO myoblasts were cultured for seven days. The findings indicated a milder phenotype compared with GLOB-D2KO. Despite an 80% loss in Dio2 expression (Fig. 2B), myotubes were formed in both Cre-MYF5 and MYF5-D2KO differentiated myoblasts (Fig. 2A). However, the phenotype regarding the myosin expression is shifted to a slow-contracting phenotype in the MYF5-D2KO myotubes: Myh2 mRNA levels were about 50% lower, while Myh7 were 100% increased (Fig. 2B), indicating impaired T3 signaling.
FIG. 2.
MYF5-D2KO primary myoblasts differentiation. (A) Primary myoblasts of Cre-MYF5 and MYF5-D2KO mice after seven days of differentiation. Note the presence of elongated myotubes in both cultures. (B) Dio2 and Myh2 mRNA levels of cells shown in (A) (n = 3); Cre-MYF5 values were set as 1.0. Values are the mean ± SEM. *p ≤ 0.05; ***p ≤ 0.001 versus Cre.
Dio2 disruption in developing SKM does not impair muscle-related gene expression
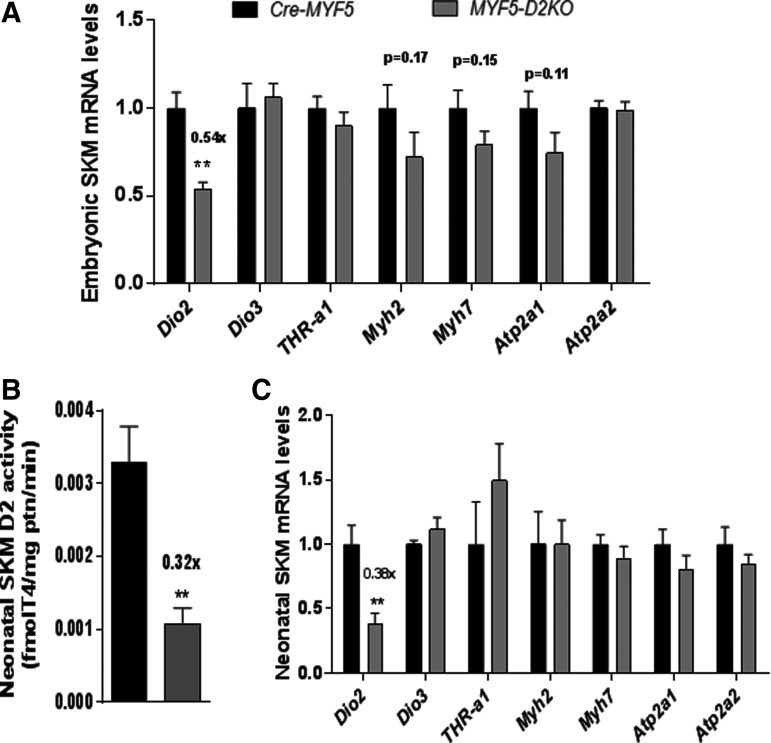
In order to test whether D2 plays a role during embryogenesis, SKM was obtained from MYF5-D2KO embryos at day 18.5 of development. Dio2 mRNA levels were about 54% of the control littermate expression (Fig. 3A). The expression of the TH inactivating enzyme D3 (Dio3 gene) and the main muscle TRH receptor TRHα1 were normally expressed in the MYF5-D2KO muscles (Fig. 3A). The loss of D2 did not impair the expression of T3-resposive genes Myh2/7 and Atp1a1/a2 (which encode the sarcoplasmic reticulum calcium ATPase 1 and 2—SERCA 1/2, respectively).
FIG. 3.
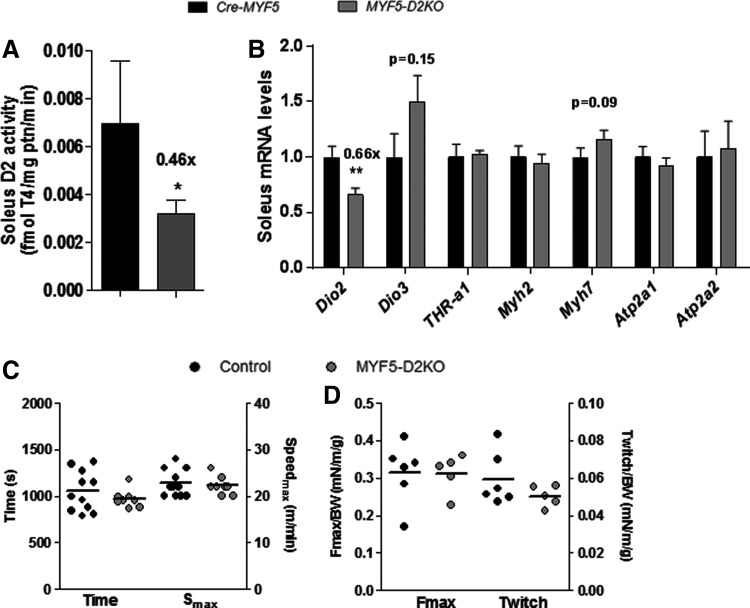
Embryonic and neonatal muscle of MYF5-D2KO mice. (A) mRNA levels of indicated genes in skeletal muscle of Cre-MYF5 and MYF5-D2KO embryos (E18.5; n = 6–8). (B) D2 activity of neonatal skeletal muscle of Cre-MYF5 and MYF5-D2KO mice (n = 3–8). (C) mRNA levels in neonatal muscle of Cre-MYF5 and MYF5-D2KO mice (n = 3–8). Values are the mean ± SEM. **p ≤ 0.01.
TH signaling is preserved in MYF5-D2KO and MYOD-D2KO neonatal SKM
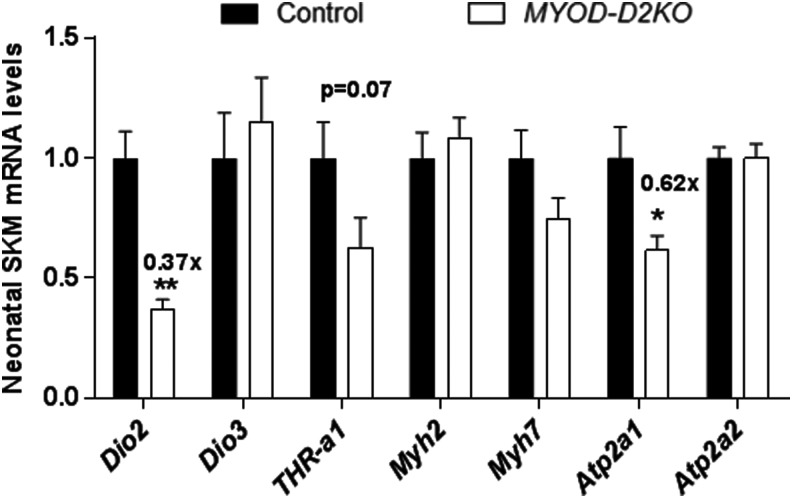
SKM D2 activity peaks at birth and decreases with age, suggesting a role in postnatal development (27,41). To test whether T3 signaling is affected by neonatal SKM D2 expression, the expression of T3-responsive genes was studied in the neonatal hind-limb muscles of the MYF5-D2KO mice. SKM D2 activity was decreased by 70% (Fig. 3B), and Dio2 expression by 60% (Fig. 3C) in MYF5-D2KO muscles. However, Myh2, Atp2a1, Myh7, and Atp2a2 gene expression was not influenced by the low levels of D2 (Fig. 2D). To confirm the results in the MYF5-D2KO, the MYOD-D2KO mice were also studied. Similarly, Dio2 mRNA was diminished by about 60% in neonatal MYOD-D2KO hind-limb muscles, and Atp2a1 was 40% reduced (Fig. 4).
FIG. 4.
Neonatal muscle of MYOD-D2KO mice. mRNA levels of indicated genes in neonatal muscle of Cre-MYF5 and MYF5-D2KO mice (n = 3–8). Values are the mean ± SEM. *p ≤ 0.05; **p ≤ 0.01.
SKM function is preserved in adult MYF5-D2KO mice, despite low D2 expression
Soleus muscle gene expression is dramatically affected by systemic hypothyroidism (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/thy), with decreased expression of Myh1, Myh2, Atp2a1, and Myod, and increased expression of Myh7 (Supplementary Fig. S1B). Adult MYF5-D2KO mice were euthyroid (Table 1), and their soleus exhibited 40–60% reduction in D2 activity and expression (Fig. 5A and B), but there was no change in the expression of T3-responsive genes (Fig. 5B). In addition, MYF5-D2KO mice exhibited normal exercise capacity (Fig. 5C) and plantar flexor muscle strength in response to 1–100 Hz stimulation (Fig. 5D). When admitted to the comprehensive laboratory animal monitoring system, the knockout mice exhibited similar VO2 and RER compared to control mice (Supplementary Fig. S2A–C).
Table 1.
Serum Levels of T3, T4, and TSH of Cre-MYF5 and MYF5-D2KO Mice
| T3 (ng/mL) | T4 (ng/mL) | TSH (ng/mL) | |
|---|---|---|---|
| Cre-MYF5-D2KO | 8.94 ± 0.68 | 85.94 ± 25.63 | 0.72 ± 0.17 |
| MYF5-D2KO | 10.38 ± 0.44 | 63.08 ± 8.2 | 0.67 ± 0.15 |
Values are the mean ± standard error of the mean.
T3, triiodothyronine; T4, thyroxine; TSH, thyrotropin.
FIG. 5.
Adult soleus (SOL) D2 activity, gene expression and skeletal muscle function of Cre-MYF5 and MYF5-D2KO mice. (A) D2 activity of adult SOL muscle (n = 5). (B) Dio2 and selected triiodothyronine (T3)-responsive genes mRNA levels in SOL (n = 9–11). (C) Exercise time and maximum speed (Smax) achieved on the treadmill maximal exercise test. (D) Maximal force (100 Hz; Fmax) and twitch force (1 Hz) of electrically stimulated plantar flexors (e.g., soleus and gastrocnemius muscles) normalized by body weight. Values are the mean ± SEM. *p ≤ 0.05; **p ≤ 0.01 vs. Cre-MYF5.
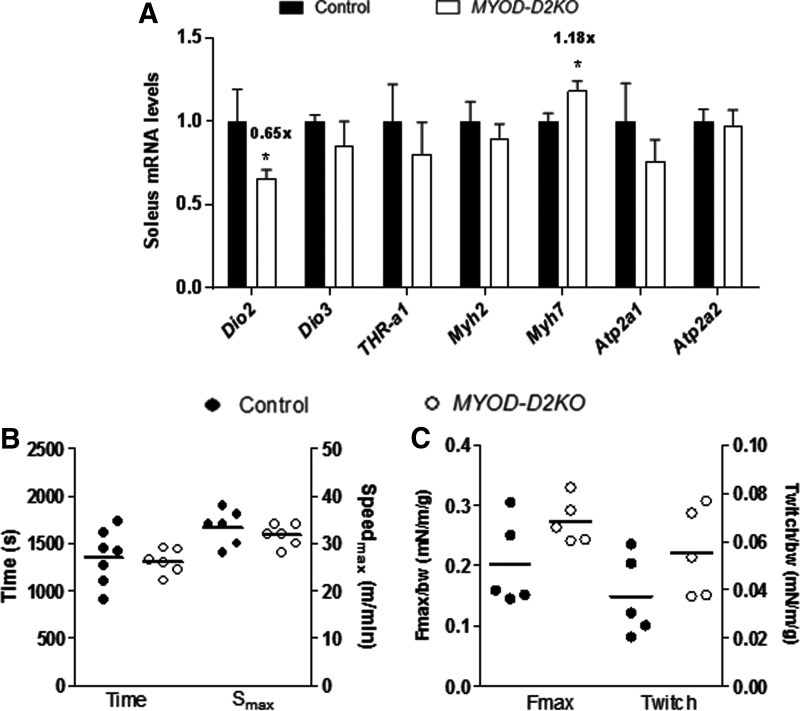
Adult MYOD-D2KO mouse exhibits mild SKM hypothyroidism but normal performance
MYOD-D2KO mice have normal levels of TSH (0.31 ± 0.02 vs. 0.37 ± 0.07 ng/mL) in blood, indicating a systemic euthyroid state. In MYOD-D2KO soleus, Dio2 expression was ∼40% decreased compared with control littermates (Fig. 6A). Myh7 expression was upregulated by 20%, whereas Myh2, Atp2a1/a2 mRNA levels were not affected (Fig. 6A). The MYOD-D2KO mouse performed similarly in the maximal exercise test as the control littermates (Fig. 6B). Moreover, when the plantar flexor muscles were stimulated at 1–100 Hz, the resulting force was not statistically different between MYOD-D2KO and control animals, although there was a trend toward increased strength in MYOD-D2KO mice (p = 0.08; Fig. 6C).
FIG. 6.
Adult SOL gene expression and skeletal muscle function of Cre-MYOD and MYOD-D2KO mice. (A) Dio2 and selected T3-responsive genes mRNA levels in SOL muscle (n = 6). (B) Exercise time and maximum speed (Smax) achieved on the treadmill maximal exercise test. (C) Maximal force (100 Hz; Fmax) and twitch force (1 Hz) of electrically stimulated plantar flexors (e.g., soleus and gastrocnemius muscles) normalized by body weight. Values are the mean ± SEM. *p ≤ 0.05 vs. Cre-MYOD.
Discussion
The present studies of selective Dio2 inactivation in skeletal myocytes (i.e., MYF5-D2KO and MYOD-D2KO mice) indicate that early inactivation of the Dio2 gene during development only mildly affects SKM when assessed in cultured skeletal myocytes and does not cause a notable phenotype in mice. This is unexpected given previous data in mice with global Dio2 inactivation (41). It is conceivable that, as in the brain (45), residual D2 expression in other SKM cells act paracrinally and supply sufficient T3 to the developing skeletal myocyte, minimizing Dio2 inactivation.
D2 activity is very low in primary cultures of myoblasts but increases during differentiation (42). Similar findings were observed in C2C12 myoblasts and muscle-derived stem cells (41), suggesting that the surge in D2-generated T3 drives muscle differentiation. However, data in cells obtained from different mouse models led to conflicting results. Muscle-derived stem cells of GLOB-D2KO mice do not differentiate in myotubes, resulting in no Myod, Myog, and Myh2 expression (41). Furthermore, silencing Dio2 in C2C12 myoblasts arrested cells in a proliferative state (41). In contrast, previous studies have observed normal myotube formation in GLOB-D2KO myoblasts and upregulation of slow Troponin I and Myh7 (42). Even knockdown of THRα in C2C12 and primary myoblasts only reduces (by 50–60%) but does not abolish the myotubes fusion index and Serca1 expression (46). It is not clear whether this is a consequence of impaired cell proliferation and/or differentiation seen in THRαKO cells. In agreement, the present study reports that primary MYF5-D2KO myoblasts fuse normally into myotubes. However, the phenotype is shifted from a fast-twitch footprint to a slow-twitch phenotype with down regulation of Myh2 and upregulation of Myh7 (Fig. 2A and B). Thus, decreasing T3 signaling by disrupting intracellular T4 to T3 conversion or THR expression impairs differentiation and gene expression to a certain extent but does not seem to affect myotube formation.
In mice, TH positively regulates the expression of two MRFs, Myod and Myog, suggesting that T3 promotes SKM differentiation. Disrupting both THRα1 and β results in a typical hypothyroid phenotype, with an increase in the expression of Myh7concomitantly with a reduction in the fast myosins (47), and the THRα1PV mice expressing a dominant negative TRα1 exhibit smaller Myh2+myofibers (fast-twitch fibers). Studies in the GLOB-D2KO mouse indicate that Dio2 inactivation also causes important disruption of TH signaling in SKM, given that GLOB-D2KO neonatal (P1) mice exhibit decreased levels of T3-regulated genes in SKM (41). This is likely to take place during early phases of SKM differentiation, given that selective disruption of Dio2 in the SKM-D2KO mouse resulted in normal neonatal and mature SKM fibers (27). To analyze the role played by Dio2 in early SKM differentiation, it seemed logical in the present investigation to disrupt Dio2 in SKM precursors driven by the expression of early MRFs (i.e., Myf5 and Myod) (11,12). Unexpectedly, no major changes in the expression of key T3-responsive genes were observed in SKM of newborn or adult mice of either strain (Figs. 3–6).
SKM contractile capacity is a key endpoint when studying muscle function. SKM capacity was assessed at the age of 20–24 weeks through a maximal exercise capacity protocol, and muscle twitch force (1 Hz) and maximal strength (Tetanic force at 100 Hz) were also measured. In both approaches, MYF5-D2KO and MYOD-D2KO mice performed similarly to control littermates, indicating normal SKM function (Figs. 5 and 6). Young adult (10–12 weeks old) GLOB-D2KO mice exhibited a normal motor phenotype (48,49), but older GLOB-D2KO mice (24 weeks old) exhibited abnormal locomotion patterns (49). It is not clear whether this was caused by reduced brain T3 content and abnormal neural control of locomotion. In contrast, disruption of TRα1 (main isoform found in SKM) induced 20–40% longer contraction and relaxation times of twitches and tetani in soleus muscles compared with wild-type controls, which is explained by the reduced expression of the fast-type sarcoplasmic reticulum Ca-ATPase (SERCa1) (50).
The differences in the role of D2 in vitro and in vivo could be explained by the nature of the two experimental approaches. In the cell culture setup, D2-generated T3 binds to THR and/or effluxes to the culture medium. This increases T3 concentration in the medium, which can enter back into the myocytes and promote T3 signaling. Thus, the cell culture setting lends itself to maximize the role played by the D2 pathway. This is not the case in vivo, given that D2-generated T3 equilibrates with plasma and the systemic circulation before it can re-enter the skeletal myocytes and promote TH signaling. Therefore, it is not surprising that a more significant phenotype is observed in the cell culture system.
The present results indicate that D2 is not a critical factor for SKM development and/or function. This is in agreement with previous observations that T3 signaling is not affected by Dio2 inactivation in the adult SKM (27). However, a number of physiological stimuli and conditions are known to regulate D2 expression and activity in SKM such as physical exercise, IGF-1, insulin and insulin sensitizers, fasting, muscle injury, and inflammation, which could be associated with physiological consequences (41,42,51–54). For example, disruption of Dio2 in SKM fibers (SKM-D2KO mouse) impairs the acute and chronic exercise-induced PGC1a expression and mitochondria content (51).
In conclusion, Dio2 inactivation in differentiating myoblasts changes its phenotype but does not interfere with myoblast fusion. In addition, disruption of D2-mediated T3 production in SKM precursor cell does not impair muscle development and muscle function in vivo.
Supplementary Material
Acknowledgments
This work was supported by NIDDK (R01 65055—A.C.B.), Brazilian National Research Council (CNPq; 202189/2011-2—J.P.W.C. and fellowship to D.L.I.), American Thyroid Association (ATA; M1301627—J.P.W.C.), and Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (FAPERJ—J.P.W.C.). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Disclosure Statement
All authors declare that no competing financial interests exist.
References
- 1.Salvatore D, Simonides WS, Dentice M, Zavacki AM, Larsen PR. 2014. Thyroid hormones and skeletal muscle—new insights and potential implications. Nat Rev Endocrinol 10:206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moncaut N, Rigby PW, Carvajal JJ. 2013. Dial M(RF) for myogenesis. FEBS J 280:3980–3990 [DOI] [PubMed] [Google Scholar]
- 3.Davis RL, Weintraub H, Lassar AB. 1987. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51:987–1000 [DOI] [PubMed] [Google Scholar]
- 4.Braun T, Buschhausen-Denker G, Bober E, Tannich E, Arnold HH. 1989. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J 8:701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Megeney LA, Rudnicki MA. 1995. Determination versus differentiation and the MyoD family of transcription factors. Biochem Cell Biol 73:723–732 [DOI] [PubMed] [Google Scholar]
- 6.Kassar-Duchossoy L, Gayraud-Morel B, Gomes D, Rocancourt D, Buckingham M, Shinin V, Tajbakhsh S. 2004. Mrf4 determines skeletal muscle identity in Myf5:Myod double-mutant mice. Nature 431:466–471 [DOI] [PubMed] [Google Scholar]
- 7.Ott MO, Bober E, Lyons G, Arnold H, Buckingham M. 1991. Early expression of the myogenic regulatory gene, myf-5, in precursor cells of skeletal muscle in the mouse embryo. Development 111:1097–1107 [DOI] [PubMed] [Google Scholar]
- 8.Rudnicki MA, Braun T, Hinuma S, Jaenisch R. 1992. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell 71:383–390 [DOI] [PubMed] [Google Scholar]
- 9.Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R. 1993. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75:1351–1359 [DOI] [PubMed] [Google Scholar]
- 10.Haldar M, Karan G, Tvrdik P, Capecchi MR. 2008. Two cell lineages, myf5 and myf5-independent, participate in mouse skeletal myogenesis. Dev Cell 14:437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JC, Mortimer J, Marley J, Goldhamer DJ. 2005. MyoD-cre transgenic mice: a model for conditional mutagenesis and lineage tracing of skeletal muscle. Genesis 41:116–121 [DOI] [PubMed] [Google Scholar]
- 12.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. 2008. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comai G, Sambasivan R, Gopalakrishnan S, Tajbakhsh S. 2014. Variations in the efficiency of lineage marking and ablation confound distinctions between myogenic cell populations. Dev Cell 31:654–667 [DOI] [PubMed] [Google Scholar]
- 14.Arrojo EDR, Fonseca TL, Werneck-de-Castro JP, Bianco AC. 2013. Role of the type 2 iodothyronine deiodinase (D2) in the control of thyroid hormone signaling. Biochim Biophys Acta 1830:3956–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brent GA. 2012. Mechanisms of thyroid hormone action. J Clin Invest 122:3035–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carnac G, Albagli-Curiel O, Vandromme M, Pinset C, Montarras D, Laudet V, Bonnieu A. 1992. 3,5,3′-Triiodothyronine positively regulates both MyoD1 gene transcription and terminal differentiation in C2 myoblasts. Mol Endocrinol 6:1185–1194 [DOI] [PubMed] [Google Scholar]
- 17.Hughes SM, Taylor JM, Tapscott SJ, Gurley CM, Carter WJ, Peterson CA. 1993. Selective accumulation of MyoD and myogenin mRNAs in fast and slow adult skeletal muscle is controlled by innervation and hormones. Development 118:1137–1147 [DOI] [PubMed] [Google Scholar]
- 18.Muscat GE, Mynett-Johnson L, Dowhan D, Downes M, Griggs R. 1994. Activation of myoD gene transcription by 3,5,3′-triiodo-L-thyronine: a direct role for the thyroid hormone and retinoid X receptors. Nucl Acids Res 22:583–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downes M, Griggs R, Atkins A, Olson EN, Muscat GE. 1993. Identification of a thyroid hormone response element in the mouse myogenin gene: characterization of the thyroid hormone and retinoid X receptor heterodimeric binding site. Cell Growth Differ 4:901–909 [PubMed] [Google Scholar]
- 20.Khaleeli AA, Gohil K, McPhail G, Round JM, Edwards RH. 1983. Muscle morphology and metabolism in hypothyroid myopathy: effects of treatment. J Clin Pathol 36:519–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan MD, Coenen-Schimke JM, Bigelow ML, Nair KS. 2006. Changes in skeletal muscle protein metabolism and myosin heavy chain isoform messenger ribonucleic acid abundance after treatment of hyperthyroidism. J Clin Endocrinol Metab 91:4650–4656 [DOI] [PubMed] [Google Scholar]
- 22.Khaleeli AA, Griffith DG, Edwards RH. 1983. The clinical presentation of hypothyroid myopathy and its relationship to abnormalities in structure and function of skeletal muscle. Clin Endocrinol 19:365–376 [DOI] [PubMed] [Google Scholar]
- 23.Mastaglia FL, Ojeda VJ, Sarnat HB, Kakulas BA. 1988. Myopathies associated with hypothyroidism: a review based upon 13 cases. Aust N Z J Med 18:799–806 [DOI] [PubMed] [Google Scholar]
- 24.Erkintalo M, Bendahan D, Mattei JP, Fabreguettes C, Vague P, Cozzone PJ. 1998. Reduced metabolic efficiency of skeletal muscle energetics in hyperthyroid patients evidenced quantitatively by in vivo phosphorus-31 magnetic resonance spectroscopy. Metabolism 47:769–776 [DOI] [PubMed] [Google Scholar]
- 25.Caraccio N, Natali A, Sironi A, Baldi S, Frascerra S, Dardano A, Monzani F, Ferrannini E. 2005. Muscle metabolism and exercise tolerance in subclinical hypothyroidism: a controlled trial of levothyroxine. J Clin Endocrinol Metab 90:4057–4062 [DOI] [PubMed] [Google Scholar]
- 26.Monzani F, Caraccio N, Siciliano G, Manca L, Murri L, Ferrannini E. 1997. Clinical and biochemical features of muscle dysfunction in subclinical hypothyroidism. J Clin Endocrinol Metab 82:3315–3318 [DOI] [PubMed] [Google Scholar]
- 27.Werneck-de-Castro JP, Fonseca TL, Ignacio DL, Fernandes GW, Andrade-Feraud CM, Lartey LJ, Ribeiro MB, Ribeiro MO, Gereben B, Bianco AC. 2015. Thyroid hormone signaling in male mouse skeletal muscle is largely independent of D2 in myocytes. Endocrinology 156:3842–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiaffino S, Reggiani C. 2011. Fiber types in mammalian skeletal muscles. Physiol Rev 91:1447–1531 [DOI] [PubMed] [Google Scholar]
- 29.van der Linden CG, Simonides WS, Muller A, van der Laarse WJ, Vermeulen JL, Zuidwijk MJ, Moorman AF, van Hardeveld C. 1996. Fiber-specific regulation of Ca(2+)-ATPase isoform expression by thyroid hormone in rat skeletal muscle. Am J Physiol 271:C1908–1919 [DOI] [PubMed] [Google Scholar]
- 30.Collie ES, Muscat GE. 1992. The human skeletal alpha-actin promoter is regulated by thyroid hormone: identification of a thyroid hormone response element. Cell Growth Differ 3:31–42 [PubMed] [Google Scholar]
- 31.Simonides WS, van Hardeveld C. 2008. Thyroid hormone as a determinant of metabolic and contractile phenotype of skeletal muscle. Thyroid 18:205–216 [DOI] [PubMed] [Google Scholar]
- 32.Visser WE, Heemstra KA, Swagemakers SM, Ozgur Z, Corssmit EP, Burggraaf J, van Ijcken WF, van der Spek PJ, Smit JW, Visser TJ. 2009. Physiological thyroid hormone levels regulate numerous skeletal muscle transcripts. J Clin Endocrinol Metab 94:3487–3496 [DOI] [PubMed] [Google Scholar]
- 33.Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeold A, Bianco AC. 2008. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29:898–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callebaut I, Curcio-Morelli C, Mornon JP, Gereben B, Buettner C, Huang S, Castro B, Fonseca TL, Harney JW, Larsen PR, Bianco AC. 2003. The iodothyronine selenodeiodinases are thioredoxin-fold family proteins containing a glycoside hydrolase clan GH-A-like structure. J Biol Chem 278:36887–36896 [DOI] [PubMed] [Google Scholar]
- 35.Sagar GD, Gereben B, Callebaut I, Mornon JP, Zeold A, Curcio-Morelli C, Harney JW, Luongo C, Mulcahey MA, Larsen PR, Huang SA, Bianco AC. 2008. The thyroid hormone-inactivating deiodinase functions as a homodimer. Mol Endocrinol 22:1382–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salvatore D, Bartha T, Harney JW, Larsen PR. 1996. Molecular biological and biochemical characterization of the human type 2 selenodeiodinase. Endocrinology 137:3308–3315 [DOI] [PubMed] [Google Scholar]
- 37.Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. 2001. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol 15:2137–2148 [DOI] [PubMed] [Google Scholar]
- 38.Castillo M, Hall JA, Correa-Medina M, Ueta C, Won Kang H, Cohen DE, Bianco AC. 2011. Disruption of thyroid hormone activation in type 2 deiodinase knockout mice causes obesity with glucose intolerance and liver steatosis only at thermoneutrality. Diabetes 60:1082–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsili A, Aguayo-Mazzucato C, Chen T, Kumar A, Chung M, Lunsford EP, Harney JW, Van-Tran T, Gianetti E, Ramadan W, Chou C, Bonner-Weir S, Larsen PR, Silva JE, Zavacki AM. 2011. Mice with a targeted deletion of the type 2 deiodinase are insulin resistant and susceptible to diet induced obesity. PloS One 6:e20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fonseca TL, Werneck-De-Castro JP, Castillo M, Bocco BM, Fernandes GW, McAninch EA, Ignacio DL, Moises CC, Ferreira AR, Gereben B, Bianco AC. 2014. Tissue-specific inactivation of type 2 deiodinase reveals multilevel control of fatty acid oxidation by thyroid hormone in the mouse. Diabetes 63:1594–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dentice M, Marsili A, Ambrosio R, Guardiola O, Sibilio A, Paik JH, Minchiotti G, DePinho RA, Fenzi G, Larsen PR, Salvatore D. 2011. The FoxO3/type 2 deiodinase pathway is required for normal mouse myogenesis and muscle regeneration. J Clin Invest 120:4021–4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grozovsky R, Ribich S, Rosene ML, Mulcahey MA, Huang SA, Patti ME, Bianco AC, Kim BW. 2009. Type 2 deiodinase expression is induced by peroxisomal proliferator-activated receptor-gamma agonists in skeletal myocytes. Endocrinology 150:1976–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bianco AC, Anderson G, Forrest D, Galton VA, Gereben B, Kim BW, Kopp PA, Liao XH, Obregon MJ, Peeters RP, Refetoff S, Sharlin DS, Simonides WS, Weiss RE, Williams GR. 2014. American thyroid association guide to investigating thyroid hormone economy and action in rodent and cell models. Thyroid 24:88–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gharaibeh B, Lu A, Tebbets J, Zheng B, Feduska J, Crisan M, Peault B, Cummins J, Huard J. 2008. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc 3:1501–1509 [DOI] [PubMed] [Google Scholar]
- 45.Freitas BC, Gereben B, Castillo M, Kallo I, Zeold A, Egri P, Liposits Z, Zavacki AM, Maciel RM, Jo S, Singru P, Sanchez E, Lechan RM, Bianco AC. 2010. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J Clin Invest 120:2206–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milanesi A, Lee JW, Kim NH, Liu YY, Yang A, Sedrakyan S, Kahng A, Cervantes V, Tripuraneni N, Cheng SY, Perin L, Brent GA. 2016. Thyroid hormone receptor alpha plays an essential role in male skeletal muscle myoblast proliferation, differentiation, and response to injury. Endocrinology 157:4–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu F, Gothe S, Wikstrom L, Forrest D, Vennstrom B, Larsson L. 2000. Effects of thyroid hormone receptor gene disruption on myosin isoform expression in mouse skeletal muscles. Am J Physiol Regul Integr Comp Physiol 278:R1545–1554 [DOI] [PubMed] [Google Scholar]
- 48.Galton VA, Wood ET, St Germain EA, Withrow CA, Aldrich G, St Germain GM, Clark AS, St Germain DL. 2007. Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient rodent brain during development. Endocrinology 148:3080–3088 [DOI] [PubMed] [Google Scholar]
- 49.Barez-Lopez S, Bosch-Garcia D, Gomez-Andres D, Pulido-Valdeolivas I, Montero-Pedrazuela A, Obregon MJ, Guadano-Ferraz A. 2014. Abnormal motor phenotype at adult stages in mice lacking type 2 deiodinase. PLOS ONE 9:e103857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johansson C, Lannergren J, Lunde PK, Vennstrom B, Thoren P, Westerblad H. 2000. Isometric force and endurance in soleus muscle of thyroid hormone receptor-alpha(1)- or -beta-deficient mice. Am J Physiol Regul Integr Comp Physiol 278:R598–603 [DOI] [PubMed] [Google Scholar]
- 51.Bocco BM, Louzada RA, Silvestre DH, Santos MC, Anne-Palmer E, Rangel IF, Abdalla S, Ferreira AC, Ribeiro MO, Gereben B, Carvalho DP, Bianco AC, Werneck-de-Castro JP. 2016. Thyroid hormone activation by type-2 deiodinase mediates exercise-induced PGC-1a expression in skeletal muscle. J Physiol 594:5255–5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lartey LJ, Werneck-de-Castro JP, O'Sullivan I, Unterman TG, Bianco AC. 2015. Coupling between nutrient availability and thyroid hormone activation. J Biol Chem 290:30551–30561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marsili A, Tang D, Harney JW, Singh P, Zavacki AM, Dentice M, Salvatore D, Larsen PR. 2011. Type II iodothyronine deiodinase provides intracellular 3,5,3′-triiodothyronine to normal and regenerating mouse skeletal muscle. Am J Physiol Endocrinol Metab 301:E818–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bloise FF, van der Spek AH, Surovtseva OV, Ortiga-Carvalho TM, Fliers E, Boelen A. 2016. Differential effects of sepsis and chronic inflammation on diaphragm muscle fiber type, thyroid hormone metabolism, and mitochondrial function. Thyroid 26:600–609 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.