Abstract
Ebola virus (EBOV) can cause a devastating hemorrhagic disease, leading to death in a short period of time. After infection, the resulting EBOV disease results in high levels of circulating cytokines, endothelial dysfunction, coagulopathy, and bystander lymphocyte apoptosis in humans and nonhuman primates. The VP40 matrix protein of EBOV is essential for viral assembly and budding from the host cell. Recent data have shown that VP40 exists in the extracellular environment, including in exosomes, and exosomal VP40 can impact the viability of recipient immune cells, including myeloid and T cells, through the regulation of the RNAi and endosomal sorting complexes required for transport pathways. In this study, we discuss the latest findings of the impact of exosomal VP40 on immune cells in vitro and its potential implications for pathogenesis in vivo.
Keywords: : Ebola, VP40, exosomes, ESCRT, RNAi, apoptosis
Introduction
Ebola virus (EBOV) is a single-stranded, enveloped, negative sense RNA virus of the Filoviridae family (Foster, 1999). EBOV infection can cause EBOV disease (EVD) in humans and nonhuman primates, resulting in an acute febrile illness (Singh et al., 2015). Cases of EVD can progress to hemorrhagic fever characterized by cytokine storm, coagulopathy, leaky vessels, bystander lymphocyte apoptosis, and high rates of mortality in those affected (Messaoudi and Basler, 2015; Messaoudi et al., 2015; Rougeron et al., 2015).
Recently, increasing numbers of studies of the survivors from Sierra Leone and Liberia have identified populations of seropositive individuals that either presented with or reported no symptoms of EVD (Leroy et al., 2000; Dean et al., 2016; Richardson et al., 2016). This could represent an important means of invisible transmission during outbreaks and has significant repercussions for the epidemiology and spread of the virus. In addition, those that recover from EVD do not always clear the virus completely.
Recent studies of survivors from Sierra Leone have shown that as many as one in four infected males can contain viral RNA in their semen for up to 6–9 months after disease onset (Christie et al., 2015; Deen et al., 2015; MacIntyre and Chughtai, 2016). Viral persistence has been documented in other compartments as well, including the eyes, brain, breast milk, and vaginal secretions (Rodriguez et al., 1999; Varkey et al., 2015; Billioux et al., 2016; Chancellor et al., 2016). The survival of EBOV in these localized tissues can result in delayed or transient sequelae, including ocular and neurological symptoms that endure long after the virus is undetectable (World Health Organization, 2016).
Viruses have evolved many strategies to evade host immune surveillance and enhance persistence in host tissues. One mechanism of recent focus has been the viral utilization of the exosomal pathway within infected host cells. Exosomes are small, membrane-bound microvesicles produced by host cells and originate from the late endosomal pathway. They can act as intercellular messengers through the delivery of proteins and nucleic acids from the parent cell to target recipient cells, and thereby can affect change in the recipient cell (Théry et al., 2002; Février and Raposo, 2004; Akers et al., 2013). In infected cells, viral proteins, mRNAs, and miRNAs can be packaged into exosomes to impact recipient neighboring cells (Fleming et al., 2014).
It has become clear in recent years that exosomes can play a significant role in the pathogenesis and progression of disease in viral infections, such as HIV-1, HTLV-1, and Rift Valley Fever virus (Lenassi et al., 2010; Narayanan et al., 2013; Jaworski et al., 2014a; Schwab et al., 2015; Ahsan et al., 2016; Sampey et al., 2016). In the case of Ebola, the viral matrix protein VP40 has recently been shown to be packaged into exosomes, which in turn can decrease the viability of recipient immune cells (Pleet et al., 2016). In this study, we review the recent findings regarding exosomal VP40 and the potential role of these exosomes in EVD pathogenesis.
Role of VP40 in RNAi dysregulation and bystander lymphocyte apoptosis
During pathogenesis of EVD, the loss of T cell populations by bystander lymphocyte apoptosis has been well documented, despite the inability of EBOV to directly infect these cells (Geisbert et al., 2000; Bradfute et al., 2007; Wauquier et al., 2010). The molecular mechanisms of this bystander apoptosis are unknown, but a number of hypotheses including induction of cell death by dysfunctional cell–cell interactions, exposure to chemical mediators produced by infected cells (including TNF-α, NO species, and IL-1β), and interactions with viral proteins such as GP or sGP have been suggested (Geisbert et al., 2000; Bradfute et al., 2010).
Recently, we have found that 293T cells transfected with VP40-encoding plasmids generate exosomes containing VP40 protein. Furthermore, when these VP40-containing exosomes were placed on recipient immune cells (T cells and monocytes), cell death was readily observed (Pleet et al., 2016). This is similar to previous observations that exosomes from cells infected with HIV-1, HTLV-1, and Rift Valley Fever virus have been shown to negatively impact the viability of naive recipient immune cells (Lenassi et al., 2010; Jaworski et al., 2014a; Ahsan et al., 2016; Sampey et al., 2016). As exosomes from EBOV-infected cells contain VP40 and can cause apoptosis in recipient T cells in vivo, this could represent a novel mechanism of inducing apoptosis in uninfected lymphocytes.
Interestingly, it was observed that components of the miRNA machinery, including Dicer, Drosha, and Ago proteins, were downregulated in both donor 293T cells transfected with VP40 plasmids and naive recipient T cells (Pleet et al., 2016). Dicer, Drosha, and Ago are integral RNAi pathway proteins involved in the production of miRNAs and siRNAs for the silencing or degradation of target mRNA transcripts (Novina and Sharp, 2004). Previous studies have linked the downregulation of these components to the induction of apoptosis (Su et al., 2009; Han et al., 2013; Bian et al., 2014; Lombard et al., 2015). Combined, these studies suggest that EBOV VP40-laden exosomes from infected cells may be able to induce bystander lymphocyte apoptosis in uninfected, recipient immune cells, potentially through the modulation of RNAi components.
Exosomal VP40 and the endosomal sorting complexes required for transport pathway
The Endosomal Sorting Complexes Required for Transport (ESCRT) pathway consists of four complexes (ESCRT-0, -I, -II, and -III) that are largely responsible for the recognition and selective packaging of cargo into nascent exosomes (Henne et al., 2011). Previously, it has been shown that EBOV VP40 can recruit TSG101 and Alix proteins of the ESCRT pathway to aid in viral budding (Licata et al., 2003; Panchal et al., 2003; Timmins et al., 2003; Silvestri et al., 2007; Han et al., 2015). This is not unique to Ebola, as other viruses, including HIV-1, have likewise been shown to use ESCRT proteins such as TSG101 for this purpose (Garrus et al., 2001; Martin-Serrano et al., 2001). In addition, 293T cells transfected with VP40 increase in intracellular levels of TSG101 (ESCRT I), and EAP20 and EAP45 (ESCRT II) proteins (Pleet et al., 2016). Increases in ESCRT components may suggest an increase in exosomal biogenesis in the presence of VP40.
Along these lines, both intracellular and concentrated exosomal preparation levels of exosomal marker CD63 were also found to be increased in cells transfected with VP40 plasmid (Pleet et al., 2016). The upregulation of various host molecules derived from exosomes (i.e., CD81, CD63, and CD9) by several viruses and incorporation into the viral membrane for various purposes have been previously described (Dongen et al., 2016). For example, CD63 upregulation during HIV-1 infection has been suggested to mediate CD63 integration into HIV-1 membranes to aid in both viral fusion and replication (Li et al., 2011, 2014; Narayanan et al., 2013; Fu et al., 2015; Sampey et al., 2016). It is possible that EBOV VP40 may play a role in the upregulation of CD63 for a similar purpose.
Together, these observations could indicate that the presence of Ebola VP40 may upregulate the biogenesis of exosomes through manipulation of exosomal tetraspanins and the ESCRT pathway by an unknown mechanism. Increases in exosomes containing VP40 may then induce bystander apoptosis in immune cell populations and contribute to unregulated viral replication in infected hosts.
Potential therapeutic and diagnostic implications
Loss of T cell populations has been strongly associated with poor prognosis and fatality in EVD patients (Ryabchikova et al., 1999; Martines et al., 2015; Agrati et al., 2016). As previously described, exosomes containing VP40 from infected cells may contribute to this phenotype and potential mortality. Previous data have shown that treatment with Food and Drug Administration(FDA)-approved drugs such as oxytetracycline may be capable of downregulating the biogenesis and/or release of exosomes, as evidenced by a decrease in exosomal markers (i.e., CD63) in the extracellular milieu posttreatment (Pleet et al., 2016). Recipient cells incubated with supernatants (containing exosomes) from VP40-transfected cells that were treated with oxytetracycline demonstrated a recovery in cell viability in a dose-dependent manner.
Furthermore, treatment with oxytetracycline resulted in a decrease in CHMP6 (ESCRT-III) and VP40 protein levels within VP40-transfected cells (Pleet et al., 2016). This could indicate a possible mechanism for oxytetracycline inhibition of exosome production and a potential therapeutic option by repurposing of an FDA-approved drug. Although eukaryotic cells are not the normal target for antibiotics, other tetracycline-class drugs such as minocycline have demonstrated efficacy with other infections (Si et al., 2004; Zink et al., 2005; Szeto et al., 2010; Dutta and Basu, 2011). A true validation of the use of oxytetracycline would require animal predosing to potentially target the microbiome before observation of potential inhibition of exosome regulation in eukaryotic host cells in vivo.
Diagnostic methods in the field prove challenging for EBOV. Often, detection of virus by polymerase chain reaction or ELISA is the method of choice; however, samples should be shipped to a proper BSL-3 or -4 biocontainment facility for diagnostics to safely take place (Reusken et al., 2015; Kaushik et al., 2016). Several techniques geared toward better diagnostics are being developed, but few are capable of being simply and safely carried out at the point-of-care. Some potential methods for POC diagnosis, including an electrochemical immune-sensing approach, are highlighted by Kaushik et al. (2016), but another new method involves the use of nanoparticles.
Nanotrap (NT) particles are small hydrogel particles 700–800 nm in diameter with a bait core surrounded by a sieving shell. Pores in the shell allow for selective passage of particles attracted to the baits, and result in the concentration and protection from degradation of target molecules (Jaworski et al., 2014b). NTs have previously been used to reliably concentrate and capture exosomes from supernatants for downstream analyses (Jaworski et al., 2014a; Ahsan et al., 2016; Sampey et al., 2016). Along these lines, specific NTs have recently been explored as a method to detect Ebola viral proteins GP and VP40 from virus-like particles (VLPs) spiked into human samples with inactivating sodium dodecyl sulfate (SDS) buffer. Promisingly, NT219 particles (Cibacron Blue F3G-A affinity bait) have been successful in detecting VP40 protein from VLPs in human saliva and urine when using SDS buffer (Pleet et al., 2016).
This method of inactivating human samples with SDS buffer and capturing viral proteins (and potentially RNA molecules) with NTs could pose a safer alternative to other common methods, and represents a useful method to concentrate diagnostic targets and increase sensitivity as well as specificity for downstream assays. Another benefit of the use of these particles may be the potential to bypass a BSL-4 facility altogether; samples could be inactivated first with the SDS buffer and then subsequently utilized for diagnosis in BSL-1 or BSL-2 conditions with NTs. In summary, the development and exploration of novel therapeutics and diagnostics for EVD should be considered and encouraged.
Conclusion
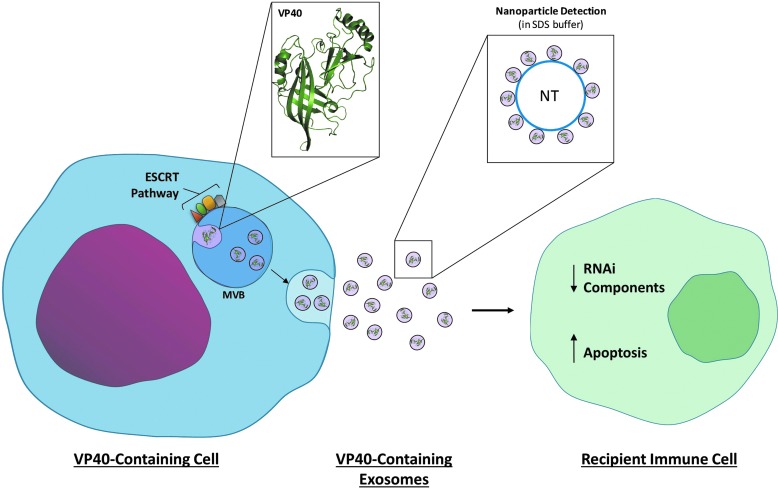
The overall role of exosomes containing VP40 in EVD pathogenesis may be quite complex (Fig. 1). Since exosomal membranes contain lipid rafts (Gassart et al., 2003; Janas et al., 2015), it is perhaps not surprising that VP40 can be integrated into exosomes, as VP40 normally oligomerizes in lipid rafts under the plasma membrane prior to viral budding (Bavari et al., 2002; Panchal et al., 2003). It is also therefore possible that in vivo exosomes from infected cells may package additional viral proteins such as GP and/or NP. It could be speculated that the combined effect of these viral proteins on recipient immune cells could be intensified, resulting in even more dramatic damage in vivo.
FIG. 1.
Impact of exosomes containing Ebola VP40 on recipient immune cells. Ebola VP40 protein becomes integrated into exosomes with the modulation of ESCRT pathway components. Upon release, these exosomes can be received by immune cells (i.e., T cells). RNAi components, including Drosha, Dicer, and Ago, in both exosome donor and recipient cells can become downregulated. Recipient immune cells can then take part in programmed cell death. Nanoparticles may be used for the concentration of exosomes to detect viral proteins (and potentially RNAs) from samples, including human material using SDS buffer. ESCRT, endosomal sorting complexes required for transport; SDS, sodium dodecyl sulfate.
During pathogenesis, EBOV can pass through the blood–brain barrier (Sagui et al., 2015; Billioux et al., 2016) and enter the brain. Cases of clinical latency or persistence of EBOV in ocular fluid or the brain may be due to migration of virally infected cells into these areas during this time. Once recovery takes place and the blood–brain barrier heals, infected cells may become trapped. When infected cells are no longer subjected to constant surveillance, they could be allowed to freely release exosomes containing Ebola proteins. These exosomes may originate from cells that are resistant to the cytopathic effects of EBOV, or it may be from cells with latent or integrated virus, which have been seen in other hosts (Belyi et al., 2010; Taylor et al., 2010, 2011). Then, these exosomes could migrate to distant compartments, such as lymph nodes, where immune cells could be inhibited or destroyed, thus allowing for unregulated replication or persistence of the hidden virus (Fig. 2).
FIG. 2.
Potential role of exosomes containing Ebola VP40 in viral persistence. Infected cells in immune privileged sites (i.e., ocular fluid) could release exosomes containing viral proteins such as VP40. These exosomes may then travel to distant areas such as lymph nodes, where they may induce apoptosis in immune and T cell populations. Increased cell death in surveilling immune cells could allow for increased viral replication and persistence in local compartments.
The potential impact of exosomes containing viral proteins in EVD could be substantial. Further in vivo research on this subject is needed to determine the true extent of the significance of exosomes containing Ebola VP40 during pathogenesis.
Acknowledgments
We thank all members of the Kashanchi laboratory for assistance with the article. This study was supported by Department of Energy Grant DE-SC0001599, and the NIAID, National Institutes of Health Grants AI078859, AI074410, and AI043894 (to F.K.).
Disclosure Statement
No competing financial interests exist.
References
- Agrati C., Castilletti C., Casetti R., Sacchi A., Falasca L., Turchi F., Tumino N., Bordoni V., Cimini E., Viola D., Lalle E., Bordi L., Lanini S., Martini F., Nicastri E., Petrosillo N., Puro V., Piacentini M., Di Caro A., Kobinger G.P., Zumla A., Ippolito G., and Capobianchi M.R. (2016). Longitudinal characterization of dysfunctional T cell-activation during human acute Ebola infection. Cell Death Dis 7, e2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsan N.A., Sampey G.C., Lepene B., Akpamagbo Y., Barclay R.A., Iordanskiy S., et al. (2016). Presence of viral RNA and proteins in exosomes from cellular clones resistant to Rift Valley Fever Virus infection. Front Microbiol 7, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers J.C., Gonda D., Kim R., Carter B.S., and Chen C.C. (2013). Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol 113, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavari S., Bosio C.M., Wiegand E., Ruthel G., Will A.B., Geisbert T.W., et al. (2002). Lipid raft microdomains. J Exp Med 195, 593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyi V.A., Levine A.J., and Skalka A.M. (2010). Unexpected inheritance: multiple integrations of ancient Bornavirus and Ebolavirus/Marburgvirus sequences in vertebrate genomes. PLoS Pathog 6, e1001030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian X.-J., Zhang G.-M., Gu C.-Y., Cai Y., Wang C.-F., Shen Y.-J., et al. (2014). Down-regulation of Dicer and Ago2 is associated with cell proliferation and apoptosis in prostate cancer. Tumor Biol 35, 11571–11578 [DOI] [PubMed] [Google Scholar]
- Billioux B.J., Smith B., and Nath A. 2016. Neurological complications of Ebola virus infection. Neurotherapeutics 13, 461–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfute S.B., Brawn D.R., Shamblin J.D., Geisbert J.B., Paragas J., et al. (2007). Lymphocyte death in a mouse model of Ebola virus infection. J Infect Dis 196, S296–S304 [DOI] [PubMed] [Google Scholar]
- Bradfute S.B., Swanson P.E., Smith M.A., Watanade E., McDunn J.E., et al. (2010). Mechanisms and consequences of ebolavirus-induced lymphocyte apoptosis. J Immunol 184, 327–335 [DOI] [PubMed] [Google Scholar]
- Chancellor J.R., Padmanabhan S.P., Greenough T.C., Sacra R., Ellison R.T., Madoff L.C., et al. (2016). Uveitis and systemic inflammatory markers in convalescent phase of Ebola virus disease. Emerg Infect Dis 22, 295–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie A., Davies-Wayne G.J., Cordier-Lasalle T., Blackley D.J., Laney A.S., Williams D.E., et al. (2015). Possible sexual transmission of Ebola virus—Liberia, 2015. MMWR Morb Mortal Wkly Rep 64, 479–481 [PMC free article] [PubMed] [Google Scholar]
- Dean N.E., Halloran M.E., Yang Y., Longini I.M. (2016). Transmissibility and pathogenicity of Ebola virus: a systematic review and meta-analysis of household secondary attack rate and asymptomatic infection. Clin Infect Dis Off Publ Infect Dis Soc Am 62, 1277–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen G.F., Knust B., Broutet N., Sesay F.R., Formenty P., Ross C., et al. (2015). Ebola RNA persistence in semen of Ebola virus disease survivors—preliminary report. N Engl J Med 151014140118009; [Epub ahead of print], Doi: 10.1056/NEJMoa1511410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongen H.M. van Masoumi N., Witwer K.W., and Pegtel D.M. (2016). Extracellular vesicles exploit viral entry routes for cargo delivery. Microbiol Mol Biol Rev 80, 369–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta K., and Basu A. (2011). Use of minocycline in viral infections. Indian J Med Res 133, 467–470 [PMC free article] [PubMed] [Google Scholar]
- Février B., and Raposo G. (2004). Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 16, 415–421 [DOI] [PubMed] [Google Scholar]
- Fleming A., Sampey G., Chung M.C., Bailey C., Hoek ML. van Kashanchi F., et al. (2014). The carrying pigeons of the cell: exosomes and their role in infectious diseases caused by human pathogens. Pathog Dis 71, 109–120 [DOI] [PubMed] [Google Scholar]
- Foster J.R. (1999). Marburg and Ebola viruses. Br J Biomed Sci 56, 237 [Google Scholar]
- Fu E., Pan L., Xie Y., Mu D., Liu W., Jin F., et al. (2015). Tetraspanin CD63 is a regulator of HIV-1 replication. Int J Clin Exp Pathol 8, 1184–1198 [PMC free article] [PubMed] [Google Scholar]
- Garrus J.E., von Schwedler U.K., Pornillos O.W., Morham S.G., Zavitz K.H., Wang H.E., et al. (2001). Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107, 55–65 [DOI] [PubMed] [Google Scholar]
- Gassart A. de Géminard C., Février B., Raposo G., and Vidal M. (2003). Lipid raft-associated protein sorting in exosomes. Blood 102, 4336–4344 [DOI] [PubMed] [Google Scholar]
- Geisbert T.W., Hensley L.E., Gibb T.R., Steele K.E., Jaax N.K., and Jahrling P.B. (2000). Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Lab Invest 80, 171–186 [DOI] [PubMed] [Google Scholar]
- Han Y., Liu Y., Gui Y., and Cai Z. (2013). Inducing cell proliferation inhibition and apoptosis via silencing Dicer, Drosha, and Exportin 5 in urothelial carcinoma of the bladder. J Surg Oncol 107, 201–205 [DOI] [PubMed] [Google Scholar]
- Han Z., Madara J.J., Liu Y., Liu W., Ruthel G., Freedman B.D., et al. (2015). ALIX rescues budding of a double PTAP/PPEY L-domain deletion mutant of Ebola VP40: a role for ALIX in Ebola virus egress. J Infect Dis 212, S138–S145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne W.M., Buchkovich N.J., and Emr S.D. (2011). The ESCRT pathway. Dev Cell 21, 77–91 [DOI] [PubMed] [Google Scholar]
- Jaworski E., Narayanan A., Van Duyne R., Shabbeer-Meyering S., Iordanskiy S., Saifuddin M., et al. (2014a). Human T-lymphotropic virus type 1-infected cells secrete exosomes that contain Tax protein. J Biol Chem 289, 22284–22305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski E., Saifuddin M., Sampey G., Shafagati N., Van Duyne R., Iordanskiy S., et al. (2014b). The use of nanotrap particles technology in capturing HIV-1 virions and viral proteins from infected cells. PLoS One 9, e96778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik A., Tiwari S., Dev Jayant R., Marty A., and Nair M. (2016). Towards detection and diagnosis of Ebola virus disease at point-of-care. Biosens Bioelectron 75, 254–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenassi M., Cagney G., Liao M., Vaupotič T., Bartholomeeusen K., Cheng Y., et al. (2010). HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 11, 110–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy E., Baize S., Volchkov V., Fisher-Hoch S., Georges-Courbot M-C, Lansoud-Soukate J., et al. (2000). Human asymptomatic Ebola infection and strong inflammatory response. Lancet 355, 2210–2215 [DOI] [PubMed] [Google Scholar]
- Li G., Dziuba N., Friedrich B., Murray J.L., and Ferguson M.R. (2011). A post-entry role for CD63 in early HIV-1 replication. Virology 412, 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Endsley M.A., Somasunderam A., Gbota S.L., Mbaka M.I., Murray J.L., et al. (2014). The dual role of tetraspanin CD63 in HIV-1 replication. Virol J 11, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata J.M., Simpson-Holley M., Wright N.T., Han Z., Paragas J., and Harty R.N. (2003). Overlapping motifs (PTAP and PPEY) within the Ebola virus VP40 proteins function independently as late budding domains: involvement of host proteins TSG101 and VPS-4. J Virol 77, 1812–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard A.P., Lim R.M., Nakagawa R.M., Vidallo K.D., Libertini S.J., Platero A.J., et al. (2015). Dicer ablation promotes a mesenchymal and invasive phenotype in bladder cancer cells. Oncol Rep 34, 1526–1532 [DOI] [PubMed] [Google Scholar]
- MacIntyre C.R., and Chughtai A.A. (2016). Recurrence and reinfection—a new paradigm for the management of Ebola virus disease. Int J Infect Dis 43, 58–61 [DOI] [PubMed] [Google Scholar]
- Martin-Serrano J., Zang T., and Bieniasz P.D. (2001). HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat Med 7, 1313–1319 [DOI] [PubMed] [Google Scholar]
- Martines R.B., Ng D.L., Greer P.W., Rollin P.E., and Zaki S.R. (2015). Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J Pathol 235, 153–174 [DOI] [PubMed] [Google Scholar]
- Messaoudi I., Amarasinghe G.K., and Basler C.F. (2015). Filovirus pathogenesis and immune evasion: insights from Ebola virus and Marburg virus. Nat Rev Microbiol 13, 663–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi I., and Basler C.F. (2015). Immunological features underlying viral hemorrhagic fevers. Curr Opin Immunol 36, 38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan A., Iordanskiy S., Das R., Van Duyne R., Santos S., Jaworski E., et al. (2013). Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J Biol Chem 288, 20014–20033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novina C.D., and Sharp P.A. (2004). The RNAi revolution. Nature 430, 161–164 [DOI] [PubMed] [Google Scholar]
- Panchal R.G., Ruthel G., Kenny T.A., Kallstrom G.H., Lane D., Badie S.S., et al. (2003). In vivo oligomerization and raft localization of Ebola virus protein VP40 during vesicular budding. Proc Natl Acad Sci U S A 100, 15936–15941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleet M.L., Mathiesen A., DeMarino C., Akpamagbo Y., Barclay R.A., Schwab A., Iordanskiy S., Sampey G.C., Lepene B., Nekhai S., Aman M.J., and Kashanchi F. (2016). Ebola VP40 in exosomes can cause immune cell dysregulation. Front Microbiol 7, 1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C., Niedrig M., Pas S., Anda P., Baize S., Charrel R., et al. (2015). Identification of essential outstanding questions for an adequate European laboratory response to Ebolavirus Zaire West Africa 2014. J Clin Virol 62, 124–134 [DOI] [PubMed] [Google Scholar]
- Richardson E.T., Kelly J.D., Barrie M.B., Mesman A.W., Karku S., Quiwa K., et al. (2016). Minimally symptomatic infection in an Ebola “hotspot”: a cross-sectional serosurvey. PLoS Negl Trop Dis 10, e0005087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez L.L., Roo A.D., Guimard Y., Trappier S.G., Sanchez A., Bressler D., et al. (1999). Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis 179, S170–S176 [DOI] [PubMed] [Google Scholar]
- Rougeron V., Feldmann H., Grard G., Becker S., and Leroy E.M. (2015). Ebola and Marburg haemorrhagic fever. J Clin Virol 64, 111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabchikova E.I., Kolesnikova L.V., and Luchko S.V. (1999). An analysis of features of pathogenesis in two animal models of Ebola virus infection. J Inf Dis 179, (Suppl 1):S199–S202 [DOI] [PubMed] [Google Scholar]
- Sagui E., Janvier F., Baize S., Foissaud V., Koulibaly F., Savini H., et al. (2015). Severe Ebola virus infection with encephalopathy: evidence for direct virus involvement. Clin Infect Dis 61, 1627–1628 [DOI] [PubMed] [Google Scholar]
- Sampey G.C., Saifuddin M., Schwab A., Barclay R., Punya S., Chung M-C, et al. (2016). Exosomes from HIV-1-infected cells stimulate production of pro-inflammatory cytokines through trans-activating response (TAR) RNA. J Biol Chem 291, 1251–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab A., Meyering S.S., Lepene B., Iordanskiy S., van Hoek M.L., Hakami R.M., et al. (2015). Extracellular vesicles from infected cells: potential for direct pathogenesis. Front Microbiol 6, 1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Q., Cosenza M.A., Kim M.-O., Zhao M.-L., Brownlee M., Goldstein H., et al. (2004). A novel action of minocycline: inhibition of human immunodeficiency virus type 1 infection in microglia. J Neurovirol 10, 284–292 [DOI] [PubMed] [Google Scholar]
- Silvestri L.S., Ruthel G., Kallstrom G., Warfield K.L., Swenson D.L., Nelle T., Iversen P.L., Bavari S., and Aman M.J. (2007). Involvement of vacuolar protein sorting pathway in Ebola virus release independent of TSG101 interaction. J Infect Dis 196, Suppl 2:S264–S270 [DOI] [PubMed] [Google Scholar]
- Singh G., Kumar A., Singh K., and Kaur J. (2015). Ebola virus: an introduction and its pathology. Rev Med Virol 26, 49–56 [DOI] [PubMed] [Google Scholar]
- Su H., Trombly M.I., Chen J., and Wang X. (2009). Essential and overlapping functions for mammalian Argonautes in microRNA silencing. Genes Dev 23, 304–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto G.L., Brice A.K., Yang H.-C., Barber S.A., Siliciano R.F., and Clements J.E. (2010). Minocycline attenuates HIV infection and reactivation by suppressing cellular activation in human CD4+ T cells. J Infect Dis 201, 1132–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D.J., Dittmar K., Ballinger M.J., and Bruenn J.A. (2011). Evolutionary maintenance of filovirus-like genes in bat genomes. BMC Evol Biol 11, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D.J., Leach R.W., and Bruenn J. (2010). Filoviruses are ancient and integrated into mammalian genomes. BMC Evol Biol 10, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C., Zitvogel L., and Amigorena S. (2002). Exosomes: composition, biogenesis and function. Nat Rev Immunol 2, 569–579 [DOI] [PubMed] [Google Scholar]
- Timmins J., Schoehn G., Ricard-Blum S., Scianimanico S., Vernet T., Ruigrok R.W.H., et al. (2003). Ebola virus matrix protein VP40 interaction with human cellular factors Tsg101 and Nedd4. J Mol Biol 326, 493–502 [DOI] [PubMed] [Google Scholar]
- Varkey J.B., Shantha J.G., Crozier I., Kraft C.S., Lyon G.M., Mehta A.K., et al. (2015). Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med 372, 2423–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauquier N., Becquart P., Padilla C., Baize S., and Leroy E.M. (2010). Human fatal Zaire Ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis. PLoS Negl Trop Dis 4, e837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2016). Interim guidance: clinical care for survivors of Ebola virus disease. Geneva: Available at www.who.int/csr/resources/publications/ebola/guidance-survivors/en Last accessed December12, 2016 [Google Scholar]
- Zink M.C., Uhrlaub J., DeWitt J., Voelker T., Bullock B., Mankowski J., et al. (2005). Neuroprotective and anti–human immunodeficiency virus activity of Minocycline. JAMA 293, 2003–2011 [DOI] [PubMed] [Google Scholar]