Abstract
Development of immunocompetent patient-like models that allow direct analysis of human adenovirus-based conditionally replicative adenoviruses (CRAds) would be beneficial for the advancement of these oncolytic agents. To this end, we explored the possibility of cross-species replication of human adenovirus type 5 (Ad5) in canine cells. With a panel of canine tumor cell lines of both epithelial and mesenchymal derivations, we demonstrate that human Ad5 can productively infect canine cells. Since the biological behavior and clinical presentation of certain dog tumors closely resemble those of their human counterparts, our results raise the possibility of exploiting canine models for preclinical analysis of candidate CRAd agents designed for human virotherapy.
Conditionally replicative adenovirus (CRAd) agents represent a promising new therapeutic approach for cancer. This strategy is based on the application of an adenovirus engineered to selectively replicate in tumor targets (1, 3). This tumor-selective replication forms the functional basis of the antineoplastic effect achieved by direct target cell killing in a process termed oncolysis (1, 16). The exceptional promise of these agents has predicated their rapid transition to human phase I clinical trials whereby the overall safety of this approach has been validated (15, 22, 28). Nonetheless, the very limited indications of efficacy to this point have established the requirement for further design advances to enhance CRAd antitumor potency (11, 15, 20, 23).
Critical to the derivation of advanced-generation CRAds is the development of model systems capable of delineating key therapeutic indices. For CRAds, the fact that human adenoviruses can accomplish only abortive replication in murine targets (7-9, 17) has restricted the preclinical toxicity information that may be derived from SCID-xenograft model systems typically used for efficacy analysis. Further, these immunologically incompetent models cannot provide useful information with respect to CRAd immunobiology and vector-host interactions. On this basis, it is clear that there exists a field-wide need for immunocompetent syngeneic models for full analysis of candidate CRAd agents.
Several recent reports have described model systems to achieve these goals. Hemminki et al. have exploited the availability of human-like canine models of cancer (31) for the evaluation of CRAd agents derived from canine adenoviruses (12, 15, 31). This approach potentially allows the study of vector-host interactions in an immunocompetent host in the context of a patient-like cancer model. CRAd constructs based on canine adenoviruses, however, may embody substantial biological differences from human adenovirus-based CRAds. Alternatively, an approach has been proposed whereby adapted murine cells permissive for limited human adenovirus replication are transplanted into an immunocompetent mouse host (10, 29). Whereas this approach may allow direct analysis of human adenovirus-based CRAds in a murine system, this transplant model does not represent a stringent patient-like context. Thus, although these approaches may potentially yield useful information with respect to CRAd agents, it is clear that there still exists the need for an immunocompetent patient-like model that allows direct analysis of human adenovirus-based CRAds.
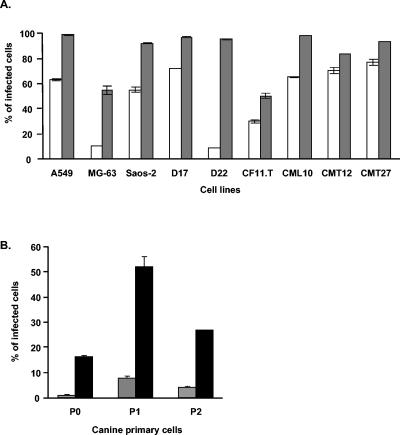
To this end, we explored the possibility of cross-species replication of human adenovirus in canine target cells. Our first analysis sought to establish the ability of human adenovirus to achieve genome delivery to canine cells. This was accomplished with human adenovirus type 5 (Ad5)-based replication-incompetent vectors expressing the green fluorescent protein (GFP) reporter (Ad5CMVGFP). Six established canine cell lines of both epithelial and mesenchymal cell derivations, three primary canine osteosarcoma cell lines, and three additional transformed human cell lines (as controls) were studied (Table 1). Infection of target cells with scoring of GFP positivity through flow cytometry demonstrated that while continuous canine tumor cell lines were efficiently infected with the human Ad5-based vector at a multiplicity of infection of 100 and 1,000 particles per cell (ppc), a higher dose was required for infection of primary canine osteosarcoma cell lines (Fig. 1). This finding suggests that human Ad5 can exploit an entry pathway in canine cells for successful transduction.
TABLE 1.
Tumor cells examined for replication of human adenovirus
| Cell line | Species | Tissue of origin | Source |
|---|---|---|---|
| A549 | Human | Lung carcinoma | ATCCa |
| MG-63 | Human | Osteosarcoma | ATCC |
| Saos-2 | Human | Osteosarcoma | ATCC |
| D17 | Canine | Osteosarcoma | ATCC |
| D22 | Canine | Osteosarcoma | ATCC |
| CF11.T | Canine | Osteosarcoma | ATCC |
| CML10 | Canine | Melanoma | L. Wolfeb |
| CMT12 | Canine | Mammary carcinoma | L. Wolfe |
| CMT27 | Canine | Mammary carcinoma | L. Wolfe |
| P-0 | Canine | Primary osteosarcoma | Scott-Ritchey Research Centerb |
| P-1 | Canine | Primary osteosarcoma | Scott-Ritchey Research Center |
| P-2 | Canine | Primary osteosarcoma | Scott-Ritchey Research Center |
Manassas, Va.
Auburn University, Auburn Ala.
FIG. 1.
Transduction efficacy of Ad5CMVGFP vector in multiple human and canine cells. (A) Cell lines were infected with Ad5CMVGFP at 100 (white bars) or 1,000 (grey bars) ppc and harvested at 24 hpi. (B) Canine primary osteosarcoma cells were infected at 1,000 (gray bars) or 10,000 (black bars) ppc and harvested at 36 hpi. Efficiency of transduction was determined by fluorescence-activated cell sorter analysis for GFP expression. The data shown represent percentages of GFP-positive cells after gating of noninfected cells as background. Error bars indicate standard deviations for averages of two identical samples.
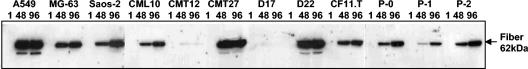
We then determined if Ad5 could express viral late genes in the context of canine target cell infection. In this regard, cross-species abortive infection frequently demonstrates only early adenoviral gene expression with an early-to-late transition block (8, 14, 17, 19, 25, 27, 30). Thus, demonstration of late gene expression would suggest viral genome replication and successful early-to-late transition of the viral life cycle. Ad5 late gene expression was evaluated by Western blotting with probe analysis for the adenovirus fiber late gene product. Protein lysates were obtained from cells harvested at 1, 48, and 96 h postinfection (hpi) with wild-type Ad5 at 1,000 ppc. Aliquots of 5 and 15 to 20 μg of total protein from human and canine cells, respectively, were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunoblotting with mouse monoclonal antifiber antibody 4D2 (provided by Jeffrey Engler, University of Alabama at Birmingham) (13), and then detection with the ECL Plus detection kit (Amersham Pharmacia Biotech). As shown in Fig. 2, high levels of human Ad5 fiber gene expression were detected in canine cells. Whereas the degree of expression was variable among the different cell lines, and low in CMT12 and D17, some level of late gene expression was uniformly observed. Of note, this phenomenon was also manifested in canine primary osteosarcoma tumor cells (P-0, P-1, P-2), a highly stringent in vitro substrate vis-à-vis viral replication (24) that most closely represents the cellular background from our proposed canine model.
FIG. 2.
Expression of fiber protein in cells infected with wild-type Ad5. Cells were infected with Ad5 at 1,000 ppc (cell lines in first nine lanes) or 10,000 ppc (primary cells in last three lanes) and harvested at 1, 48, and 96 hpi. Aliquots of cell lysates containing 5 μg (human cells) or 15 to 20 μg (canine cells) of total soluble protein were boiled and separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis. Fiber protein electroblotted onto a polyvinylidene difluoride membrane was probed with a 4D2 antifiber monoclonal antibody provided by Jeffrey Engler (University of Alabama at Birmingham) and detected with the ECL Plus detection kit (Amersham Pharmacia Biotech). The arrow indicates the fiber monomer (62 kDa).
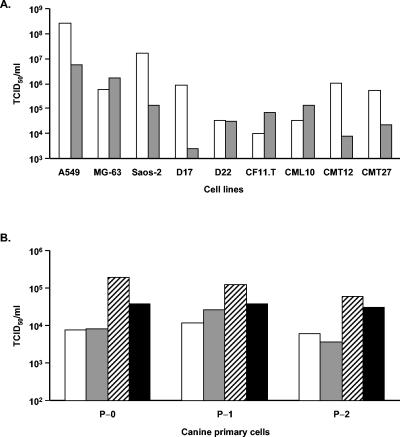
Direct analysis of productive replication was then carried out by performing a viral burst assay. Cells were infected at 1,000 ppc with wild-type Ad5 for 2 h in serum-free medium and incubated for another 48 (A549 cells) or 96 (all other cells) h in medium with 10% fetal bovine serum (FBS). At the specified time points, cells were collected and centrifuged at a low speed to separate cells from the supernatant. The cell pellets were resuspended in 1 ml of Dulbecco modified Eagle medium containing 2% FBS and freeze-thawed three times, and titers were determined on HEK293 cells with a 50% tissue culture infective dose method. Briefly, HEK293 cells were seeded in 96-well plates at 104 cells/well, and the next day harvested samples were diluted from 10−2 to 10−8 in FBS-free Dulbecco modified Eagle medium and used to infect the HEK293 cells in replicates of 10. The presence of a cytopathic effect was scored 10 days later, and the titer was calculated as T = 10(0.5 + S), where S is the sum of the ratios of all dilutions (AdEasy Application Manual; Qbiogene) (Fig. 3A). To examine if Ad5 could efficiently release its progeny from infected canine cells, the supernatant was also used for titer measurement (Fig. 3B). The results clearly demonstrate the ability of human Ad5 to replicate productively in a subset of canine cells.
FIG. 3.
Analysis of Ad5 replication in human and canine cells. (A) Cell lines were infected with wild-type Ad5 at 1,000 ppc. At 96 hpi, cells were collected and separated from the supernatant by low-speed centrifugation. The quantities of infectious viral particles present in the cell lysates (white bars) and supernatants (gray bars) were determined by titration on HEK293 cells by a 50% tissue culture infective dose (TCID50) method. (B) Canine primary cells were infected with Ad5 at 1,000 (white and gray bars) or 10,000 (striped and black bars) ppc. After 96 hpi, the infectious titers in the cell lysates (white and striped bars at 1,000 and 10,000 ppc, respectively) and supernatant (gray and black bars at 1,000 and 10,000 ppc, respectively) were determined as described above.
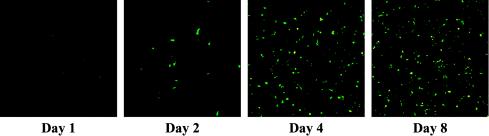
Further evidence of productive infection and lateralization was supported by direct monitoring of adenovirus replication and spread in primary dog osteosarcoma cells (Fig. 4). This experiment capitalized on our ability to directly incorporate the reporter protein enhanced GFP (EGFP) onto the Ad5 capsid pIX site, allowing direct visualization of increasing viral mass from an otherwise wild-type genome with intact E1 and E3 regions (18). By observing the same field of view on the dish, augmentation and spread of the pIX-EGFP signal could be seen, indicating productive replication in a canine host cell context.
FIG. 4.
Visualization of Ad5-wt-IX-EGFP replication and spread in primary canine osteosarcoma cells (P-0). pIX-EGFP expression from wild-type Ad5 was imaged on days 1, 2, 4, and 8 postinfection with 1,000 ppc with an inverted IX-70 microscope (Olympus, Melville, N.Y.) equipped with a Magnifire digital charge-coupled device camera (Optronics, Goleta, Calif.). Images were processed in Adobe Photoshop 7.0 (San Jose, Calif.) to discriminate the pIX-EGFP signal from the background.
Our report thus clearly demonstrates the ability of human Ad5 to productively infect canine target cells. The level of cross-species replication was about 2 log orders lower than the level of Ad5 replication in human target cells for a given tissue type. In this regard, the inability of human adenovirus to achieve cross-species infection has been amply documented for murine targets (2, 6-9, 17, 27). Indeed, the biology of such abortive infection has also been a subject of intensive studies in the context of the biology of human adenoviral infection of Syrian hamsters and monkeys (2, 7, 8, 17, 19, 25, 26). For development of CRAd agents, the human-mouse replication block has been particularly limiting, such that the available murine tumor models are of limited value vis-à-vis the ability to study replication-related toxicity and immunobiology. Alternatively, the availability of a range of canine models of human cancers (4, 5, 21, 31) potentially provides a stringent model system for CRAd analysis. Our documentation of the ability of human adenovirus to replicate in canine target cells extends earlier observations with respect to human-canine cross-species replication (30) and raises the possibility of exploiting these canine models for full and comprehensive preclinical analysis of candidate CRAd agents designed for human virotherapy.
Acknowledgments
This work was supported by grants from the NIH (P30 AR41031, RO1 CA93796, RO1 CA940840, and 1P50 CA83591), the Department of Defense (W81XWH-04-1-0025), and the Haley's Hope Memorial Support Fund for Osteosarcoma Research.
REFERENCES
- 1.Alemany, R., C. Balague, and D. T. Curiel. 2000. Replicative adenoviruses for cancer therapy. Nat. Biotechnol. 18:723-727. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, K. P., and D. F. Klessig. 1984. Altered mRNA splicing in monkey cells abortively infected with human adenovirus may be responsible for inefficient synthesis of the virion fiber polypeptide. Proc. Natl. Acad. Sci. USA 81:4023-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bischoff, J. R., D. H. Kirn, A. Williams, C. Heise, S. Horn, M. Muna, L. Ng, J. A. Nye, A. Sampson-Johannes, A. Fattaey, and F. McCormick. 1996. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274:373-376. [DOI] [PubMed] [Google Scholar]
- 4.Cornell, K. K., D. G. Bostwick, D. M. Cooley, G. Hall, H. J. Harvey, M. J. Hendrick, B. U. Pauli, J. A. Render, G. Stoica, D. C. Sweet, and D. J. Waters. 2000. Clinical and pathologic aspects of spontaneous canine prostate carcinoma: a retrospective analysis of 76 cases. Prostate 45:173-183. [DOI] [PubMed] [Google Scholar]
- 5.Dickerson, E. B., S. Fosmire, M. L. Padilla, J. F. Modiano, and S. C. Helfand. 2002. Potential to target dysregulated interleukin-2 receptor expression in canine lymphoid and hematopoietic malignancies as a model for human cancer. J. Immunother. 25:36-45. [DOI] [PubMed] [Google Scholar]
- 6.Duncan, S. J., F. C. Gordon, D. W. Gregory, J. L. McPhie, R. Postlethwaite, R. White, and H. N. Willcox. 1978. Infection of mouse liver by human adenovirus type 5. J. Gen. Virol. 40:45-61. [DOI] [PubMed] [Google Scholar]
- 7.Eggerding, F. A., and W. C. Pierce. 1986. Molecular biology of adenovirus type 2 semipermissive infections. I. Viral growth and expression of viral replicative functions during restricted adenovirus infection. Virology 148:97-113. [DOI] [PubMed] [Google Scholar]
- 8.Eron, L., H. Westphal, and G. Khoury. 1975. Posttranscriptional restriction of human adenovirus expression in monkey cells. J. Virol. 15:1256-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman, L. A., J. S. Butel, and F. Rapp. 1966. Interaction of a simian papovavirus and adenoviruses. I. Induction of adenovirus tumor antigen during abortive infection of simian cells. J. Bacteriol. 91:813-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallden, G., R. Hill, Y. Wang, A. Anand, T. C. Liu, N. R. Lemoine, J. Francis, L. Hawkins, and D. Kirn. 2003. Novel immunocompetent murine tumor models for the assessment of replication-competent oncolytic adenovirus efficacy. Mol. Ther. 8:412-424. [DOI] [PubMed] [Google Scholar]
- 11.Hamid, O., M. L. Varterasian, S. Wadler, J. R. Hecht, A. Benson III, E. Galanis, M. Uprichard, C. Omer, P. Bycott, R. C. Hackman, and A. F. Shields. 2003. Phase II trial of intravenous CI-1042 in patients with metastatic colorectal cancer. J. Clin. Oncol. 21:1498-1504. [DOI] [PubMed] [Google Scholar]
- 12.Hemminki, A., A. Kanerva, E. J. Kremer, G. J. Bauerschmitz, B. F. Smith, B. Liu, M. Wang, R. A. Desmond, A. Keriel, B. Barnett, H. J. Baker, G. P. Siegal, and D. T. Curiel. 2003. A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol. Ther. 7:163-173. [DOI] [PubMed] [Google Scholar]
- 13.Hong, J. S., and J. A. Engler. 1991. The amino terminus of the adenovirus fiber protein encodes the nuclear localization signal. Virology 185:758-767. [DOI] [PubMed] [Google Scholar]
- 14.Johnston, J. M., K. P. Anderson, and D. F. Klessig. 1985. Partial block to transcription of human adenovirus type 2 late genes in abortively infected monkey cells. J. Virol. 56:378-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirn, D. 2001. Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Ther. 8:89-98. [DOI] [PubMed] [Google Scholar]
- 16.Kirn, D. 2000. Replication-selective oncolytic adenoviruses: virotherapy aimed at genetic targets in cancer. Oncogene 19:6660-6669. [DOI] [PubMed] [Google Scholar]
- 17.Klessig, D. F., and C. W. Anderson. 1975. Block to multiplication of adenovirus serotype 2 in monkey cells. J. Virol. 16:1650-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le, L. P., M. Everts, I. Dmitriev, J. Davidova, M. Yamamoto, and D. Curiel. 2004. Fluorescently labeled adenovirus with pIX-EGFP for vector detection. Mol. Imaging 3:105-116. [DOI] [PubMed] [Google Scholar]
- 19.Lucher, L. A. 1995. Abortive adenovirus infection and host range determinants. Curr. Top. Microbiol. Immunol. 199(Pt. 1):119-152. [DOI] [PubMed] [Google Scholar]
- 20.Makower, D., A. Rozenblit, H. Kaufman, M. Edelman, M. E. Lane, J. Zwiebel, H. Haynes, and S. Wadler. 2003. Phase II clinical trial of intralesional administration of the oncolytic adenovirus ONYX-015 in patients with hepatobiliary tumors with correlative p53 studies. Clin. Cancer Res. 9:693-702. [PubMed] [Google Scholar]
- 21.Mutsaers, A. J., W. R. Widmer, and D. W. Knapp. 2003. Canine transitional cell carcinoma. J. Vet. Intern. Med. 17:136-144. [DOI] [PubMed] [Google Scholar]
- 22.Nemunaitis, J., C. Cunningham, A. Buchanan, A. Blackburn, G. Edelman, P. Maples, G. Netto, A. Tong, B. Randlev, S. Olson, and D. Kirn. 2001. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther. 8:746-759. [DOI] [PubMed] [Google Scholar]
- 23.Nemunaitis, J., F. Khuri, I. Ganly, J. Arseneau, M. Posner, E. Vokes, J. Kuhn, T. McCarty, S. Landers, A. Blackburn, L. Romel, B. Randlev, S. Kaye, and D. Kirn. 2001. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J. Clin. Oncol. 19:289-298. [DOI] [PubMed] [Google Scholar]
- 24.Preuss, M. A., J. T. Lam, M. Wang, C. A. Leath III, M. Kataram, P. J. Mahasreshti, R. D. Alvarez, and D. T. Curiel. 2004. Transcriptional blocks limit adenoviral replication in primary ovarian tumor. Clin. Cancer Res. 10:3189-3194. [DOI] [PubMed] [Google Scholar]
- 25.Ross, D., and E. Ziff. 1994. Defective processing of human adenovirus 2 late transcription unit mRNAs during abortive infections in monkey cells. Virology 202:107-115. [DOI] [PubMed] [Google Scholar]
- 26.Silverman, L., V. Cleghon, and D. F. Klessig. 1989. Increased permissivity of monkey cells to human adenovirus multiplication is affected by culturing conditions and correlates with both synthesis of virion fiber protein and altered splicing of its mRNA. Virology 173:109-119. [DOI] [PubMed] [Google Scholar]
- 27.Silverstein, G., and W. A. Strohl. 1986. Restricted replication of adenovirus type 2 in mouse Balb/3T3 cells. Arch. Virol. 87:241-264. [DOI] [PubMed] [Google Scholar]
- 28.Vasey, P. A., L. N. Shulman, S. Campos, J. Davis, M. Gore, S. Johnston, D. H. Kirn, V. O'Neill, N. Siddiqui, M. V. Seiden, and S. B. Kaye. 2002. Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J. Clin. Oncol. 20:1562-1569. [DOI] [PubMed] [Google Scholar]
- 29.Wang, Y., G. Hallden, R. Hill, A. Anand, T. C. Liu, J. Francis, G. Brooks, N. Lemoine, and D. Kirn. 2003. E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nat. Biotechnol. 21:1328-1335. [DOI] [PubMed] [Google Scholar]
- 30.Winters, W. D., and R. J. Young. 1980. Incomplete human adenovirus replication in canine tumour cells and serological evidence of infections in tumour-bearing canine pets. J. Comp. Pathol. 90:197-207. [DOI] [PubMed] [Google Scholar]
- 31.Withrow, S. J., B. E. Powers, R. C. Straw, and R. M. Wilkins. 1991. Comparative aspects of osteosarcoma. Dog versus man. Clin. Orthop. 270:159-168. [PubMed] [Google Scholar]