Abstract
Objective:
To evaluate the effect of intra-articular injections of sodium bicarbonate with a single (SBCG1) or double dose (SBCG2) of calcium gluconate administered monthly compared with methylprednisolone (MP) for treatment of knee osteoarthritis.
Methods:
A 3-month, randomized, double-blind clinical trial with patients diagnosed with knee osteoarthritis (OA). The outcome variables were the Western Ontario-McMaster University Osteoarthritis Index (WOMAC) and the Lequesne functional index.
Results:
After 3 months, all treatments significantly improved in overall WOMAC and Lequesne scores. Mean changes (95% confidence interval) in WOMAC total score and the Lequesne index, respectively, for SBCG1 (−12.5 [−14.3, −10.7]; −9.0 [−11.4, −6.7]) and SBCG2 (−12.3 [−14.3, −10.4]; −8.9 [−10.4, −7.4]) were significantly greater than for MP (−5.0 [−7.2, −2.8]; −3.2 [−4.9, −1.5]) (P < .001).
Conclusions:
Intra-articular injections of sodium bicarbonate and calcium gluconate are useful for short-term relief of OA symptoms in patients with bilateral knee osteoarthritis. Both treatments are more effective than MP injections in the reduction of knee OA symptoms.
Trial Registration:
Keywords: osteoarthritis therapy, joint, knee, sodium bicarbonate, calcium gluconate
Introduction
Osteoarthritis (OA) is one of the most prevalent joint diseases due to progressive cartilage degradation. Hip and knee OA was ranked as the 11th highest contributor to global disability, and it has been estimated that the global age-standardized prevalence of knee OA is between 3.6% and 4.1%.1
The main goals of therapeutic management of OA are pain relief, increased range of motion, prevention of secondary functional disability, and protection against joint damage. Current therapeutic strategies for OA involve education, weight loss, counseling, use of assistive devices, analgesics, nutritional treatments such as glucosamine or chondroitin, nonsteroidal anti-inflammatory drugs, intra-articular injections of hyaluronate and corticosteroids, physical therapy, and surgery2; however, none of these interventions has been shown to stop the progression of the disease.
Initial studies conducted some time ago documented a beneficial effect of alkaline solutions of sodium bicarbonate on joint diseases3 and a potential effect of calcium gluconate on inflammatory diseases.4 Based on these initial clinical trials, a new pharmaceutical medication was developed combining the potential benefits of these 2 compounds. The efficacy of this combination in the reduction of the symptoms of knee OA was initially demonstrated in an 18-month longitudinal clinical trial.5 This study compared the short-term efficacy of intra-articular injection of a sodium bicarbonate solution combined with 2 different concentrations of calcium gluconate with that of methylprednisolone (MP) regarding knee OA symptoms. Methylprednisolone was chosen for comparison because it is, at present, the most medically accepted medication for the treatment of OA symptoms.
Materials and Methods
Subjects
Participants were recruited at the San José Hospital in Querétaro. By means of advertising, men and women were invited to participate if they were 40 years of age or above, with at least 1 year since diagnosis of OA of the knee, according to the American College of Rheumatology guidelines.6 Patients were enrolled in the study after radiological confirmation that they had grade II, III, or IV knee OA according to the Kellgren-Lawrence criteria.7 Participants were excluded from the study if they had had any intra-articular injection of corticosteroids or had undergone arthroscopic surgery within the 3 months prior to the commencement of the study. They were also excluded if they were pregnant or had been diagnosed with rheumatic disease, uncontrolled hypertension, active infection, or grade I radiographic OA according to the Kellgren and Lawrence classification. All radiographic examinations were performed by the same technician and were interpreted by 2 independent physicians, both certified in orthopedics and traumatology.
The study was conducted in compliance with International Committee on Harmonisation (ICH) Good Clinical Practice and the Declaration of Helsinki with its applicable revisions. The study was approved by the institutional review board for research involving human subjects of the University of Querétaro. All subjects voluntarily gave informed consent before being enrolled in the study.
A sample size of 111 subjects was estimated from the Lequesne Functional Index (LFI) and Western Ontario-McMaster University Osteoarthritis Index (WOMAC) score changes in a pilot study in which 18 patients who had been diagnosed with OA received monthly intra-articular injections of sodium bicarbonate and calcium gluconate for up to 6 months. The sample size accounted for an expected clinically significant change of 30% between groups in the global LFI and WOMAC score, assuming a standard deviation of 50%, an alpha error of 5%, a beta error of 20%, and a dropout rate of 20%. With these assumptions, 36 subjects per treatment group were necessary for initial enrollment according to the study design.
Experimental design
A randomized, double-blind, active-controlled, parallel-group clinical trial was conducted in a 3-month intervention period. A 3-month intervention period complied with the recommended doses of MP, which was selected as the control treatment,8 and allowed for the evaluation of changes in OA symptoms. Subjects who met the inclusion criteria were randomly assigned to one of 3 treatments: (1) sodium bicarbonate and a single dose of calcium gluconate (SBCG1), (2) sodium bicarbonate and a double dose of calcium gluconate (SBCG2), or (3) MP. One researcher who had no direct contact with patients created a randomization list with randomly assorted digits. The list was delivered to the physicians who examined the patients, and treatment was assigned according to the randomization list order. Three experienced physicians scheduled several appointments with the participants for baseline assessment and 3 follow-up appointments for administration of the respective treatment in both knees (except for 2 patients with prosthesis who received treatment in 1 knee) and evaluation of disease progression. The same physician followed each patient throughout the study. At all 4 appointments, knee OA symptoms were evaluated with the WOMAC and Lequesne questionnaires, and adverse events were monitored. Concomitant treatment with analgesics and systemic corticosteroids was not allowed during the study.
Treatments
Treatments were identical in packaging, labeling, and appearance; thus, fieldwork personnel and patients were blinded to the treatment. All 3 treatments were suitable as ready-to-use aqueous solutions for injection, MP was administered via intra-articular injection at a dose of 8 mg/mL × 5 mL, SBCG1 was a solution containing 337.5 mg/5 mL sodium bicarbonate and 37.5 mg/5 mL calcium gluconate, and SBCG2 contained 337.5 mg/5 mL sodium bicarbonate and 75 mg/5 mL calcium gluconate. Both sodium bicarbonate and calcium gluconate (SBCG) treatments were claimed under patents.9,10
Outcome variables
The main outcome variables for assessment of the efficacy of the experimental treatments were the changes from baseline to final assessment in the WOMAC score and the LFI. As reported previously,5 the WOMAC OA index is a validated, multidimensional scale that consists of 24 questions in 3 separate subscales: pain, physical function, and stiffness. Each subscale score weighs 10 points, and the WOMAC global score is the sum of the 3 subscales and ranges from 0 to 30.11 The LFI is a 10-question interview-style survey that includes evaluation of pain or discomfort, maximum walking distance, and performance of daily activities. The total questionnaire is scored on a 24-point scale.12
The safety assessment was based on adverse events reported by the patient or observed by physicians during the study period. An adverse event was defined as any unfavorable and unintended sign, symptom, or disease temporally associated with the intervention treatment.
Data analysis
Baseline demographic variables and treatment compliance were analyzed with the chi-square test. The controlled trial was analyzed in 2 sets: one with participants who had at least 1 posttreatment evaluation (intention-to-treat basis) and another with subjects who finished the complete intervention and had no protocol deviations. Unadjusted analysis of variance (ANOVA) was used to compare mean changes from baseline to each posttreatment evaluation of WOMAC and LFI subscales and global scores. In addition, linear mixed models were used, including as terms the baseline values and the random effect of patients nested within the assessing doctor. Possible confounders, such as gender, age, and body mass index, had no effect on mean change and thus were not included in the analyses. Pairwise comparisons between treatments were made using the least significant difference (LSD) test.
Adverse events were classified and quantified to evaluate treatment safety. Analyses were performed with SPSS for Windows version 18.0 (IBM)
Results
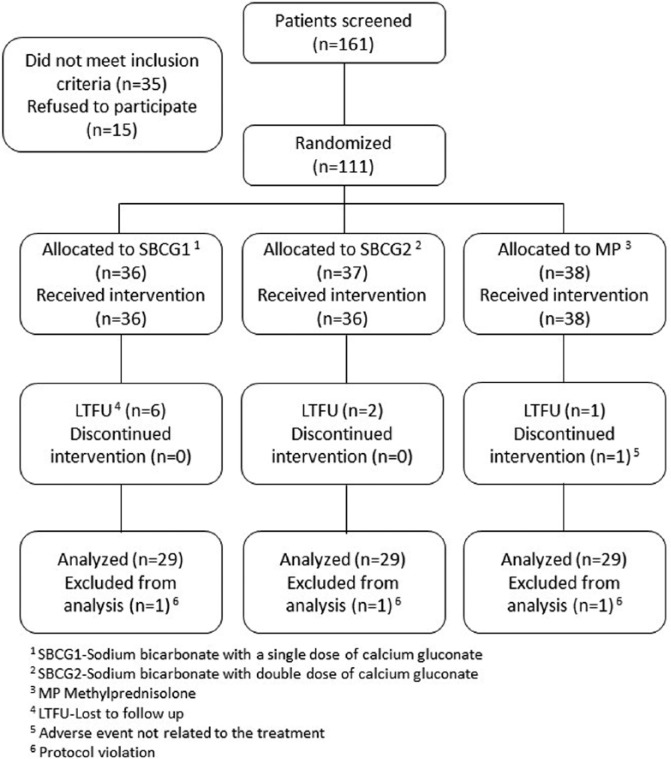
Of the 161 patients who were screened for the study, 111 met the inclusion criteria and agreed to participate. Participants were randomly assigned to one of the 3 study medications. During the 3-month study, 1 refused to receive the allocated intervention, 9 refused to continue due to personal reasons, 1 developed rashes and stopped the treatment according to the physician’s recommendation, and 4 patients had protocol violations. Thus, 97 patients were included in the “per-protocol” data set analyses (Figure 1).
Figure 1.
Flow of patients through the study.
The demographic characteristics at baseline of the patients who completed the study were statistically similar among groups (Table 1). Radiographs taken at baseline did not show significant differences among treatment groups as to the patients’ joint-space and marginal osteophyte formation in both knee compartments. A greater proportion of the patients were women (74 of 97), the mean age (±SD) was 54.7 ± 8.9 years, and the mean body mass index was classified as obese (31.7 ± 4.8 kg/m2). Most patients (78 of 97) reported pain intensity in the target knee from moderate to severe.
Table 1.
Baseline demographic characteristics of patients with osteoarthritis of the knee, by study group.a,b
| Characteristics | SBCG1 | SBCG2 | MP |
|---|---|---|---|
| N | 29 | 34 | 34 |
| Female, % | 89.7 | 76.5 | 64.7 |
| Male, % | 10.3 | 23.5 | 35.3 |
| Age, y | 54.4 (9.6) | 54.2 (9.0) | 55.3 (8.5) |
| BMI, kg/m2 | 31.8 (5.0) | 32.1 (4.7) | 31.1 (4.9) |
| BMI > 30 | 89.7 | 76.5 | 64.7 |
| Location and grade of OA | |||
| Left knee grade II | 19.2 | 22.6 | 20.0 |
| Left knee grade III | 46.2 | 51.6 | 56.7 |
| Left knee grade IV | 34.6 | 25.8 | 23.3 |
| Right knee grade II | 23.1 | 18.8 | 16.1 |
| Right knee grade III | 42.3 | 56.3 | 58.1 |
| Right knee grade IV | 34.6 | 25.0 | 25.8 |
Abbreviations: ANOVA, analysis of variance; BMI, body mass index; MP, methylprednisolone; OA, osteoarthritis; SBCG1, sodium bicarbonate and a single dose of calcium gluconate; SBCG2, sodium bicarbonate and a double dose of calcium gluconate.
Values are % or mean (SD).
No significant differences were found among treatment groups with ANOVA or chi-square tests.
Both sets of analyses produced similar results; thus, “per-protocol” analysis is presented throughout this study. Baseline, final values, and mean changes of the WOMAC subscale scores are shown in Table 2. At the end of the study, the 3 treatment groups showed significant improvement in the WOMAC overall and subscale scores. The adjusted and unadjusted mean changes in the SBCG1 and SBCG2 groups were significantly greater than those of the MP group. Changes in the WOMAC global score after each month of treatment are shown in Figure 2.
Table 2.
WOMAC subscale and global scores at baseline and after 3 months of treatment.a
| WOMAC subscales | SBCG1 | SBCG2 | MP | P value |
|---|---|---|---|---|
| N | 29 | 34 | 34 | |
| Pain | ||||
| Baseline | 5.77 (5.15 to 6.39) | 5.78 (5.14 to 6.42) | 5.32 (4.56 to 6.08) | |
| After 3 mo | 1.87 (1.32 to 2.42)c | 2.04 (1.44 to 2.63)c | 3.18 (2.30 to 4.06)c | |
| Unadjusted change | −3.90 (−4.66 to −3.14)d | −3.75 (−4.45 to −3.05)d | −2.14 (−2.92 to −1.35)e | 0.001 |
| Adjusted changeb | −3.82 (−4.50 to −3.15)d | −3.66 (−4.28 to −3.04)d | −2.29 (−2.92 to −1.67)e | 0.002 |
| Stiffness | ||||
| Baseline | 5.93 (5.09 to 6.77) | 6.44 (5.73 to 7.15) | 5.12 (4.06 to 6.18) | |
| After 3 mo | 1.71 (1.02 to 2.39)c | 2.04 (1.28 to 2.81)c | 3.74 (2.69 to 4.78)c | |
| Unadjusted change | −4.22 (−5.07 to −3.38)d | −4.40 (−5.32 to −3.47)d | −1.38 (−2.46 to −0.31)e | <0.001 |
| Adjusted changeb | −4.11 (−4.99 to −3.24)d | −3.96 (−4.80 to −3.11)d | −1.73 (−2.59 to −0.87)e | <0.001 |
| Physical functioning | ||||
| Baseline | 6.01 (5.33 to 6.69) | 6.25 (5.48 to 7.03) | 5.12 (4.34 to 5.89) | |
| After 3 mo | 1.62 (1.21 to 2.03)c | 2.05 (1.50 to 2.61)c | 3.07 (2.24 to 3.91)c | |
| Unadjusted change | −4.39 (−5.06 to −3.72)d | −4.20 (−4.92 to −3.48)d | −2.04 (−2.78 to −1.30)e | <0.001 |
| Adjusted changeb | −4.22 (−4.84 to −3.60)d | −3.86 (−4.46 to −3.25)d | −2.35 (−2.97 to −1.73)e | <0.001 |
| Global score | ||||
| Baseline | 17.71 (15.90 to 19.53) | 18.48 (16.63 to 20.32) | 15.55 (13.16 to 17.94) | |
| After 3 mo | 5.20 (3.75 to 6.64)c | 6.13 (4.42 to 7.85)c | 10.52 (7.75 to 13.28)c | |
| Unadjusted change | −12.52 (−14.30 to −10.74)d | −12.34 (−14.33 to −10.36)d | −5.03 (−7.23 to −2.84)e | <0.001 |
| Adjusted changeb | −11.89 (−13.94 to −9.84)d | −11.18 (−13.20 to −9.16)d | −5.22 (−7.34 to −3.09)e | <0.001 |
Abbreviations: MP, methylprednisolone; SBCG1, sodium bicarbonate and a single dose of calcium gluconate; SBCG2, sodium bicarbonate and a double dose of calcium gluconate; WOMAC, Western Ontario and McMaster University.
Values are mean (95% confidence interval).
Change adjusted for baseline value and the patient-nested-in-doctor random effect.
Significantly different from baseline value in paired t-test (P < .05).
Different letters indicate significant differences according to the least significant difference test for pairwise comparisons.
Figure 2.
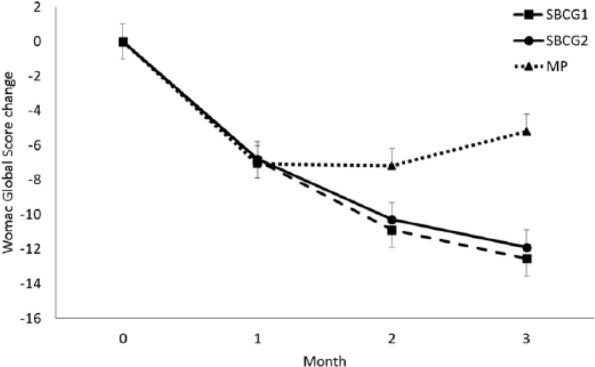
Monthly changes in Western Ontario-McMaster University Osteoarthritis Index (WOMAC) pain index by treatment group. Mean values (±SEM) are adjusted for baseline values.
Baseline, final values, and mean changes of the LFI subscale scores are shown in Table 3. After 3 months of treatment, patients in all 3 groups showed significant improvement in the subscale scores. The adjusted and unadjusted mean changes in both SBCG groups were significantly greater than the change in the MP group in at least 2 of the 3 subscales.
Table 3.
Lequesne functional index subscale and global scores at baseline and after 3 months of treatment.a
| Lequesne subscales | SBCG1 | SBCG2 | MP | P |
|---|---|---|---|---|
| N | 29 | 34 | 34 | |
| Pain | ||||
| Baseline | 5.28 (4.72 to 5.84) | 5.50 (5.07 to 5.93) | 4.71 (4.08 to 5.34) | |
| After 3 mo | 2.45 (1.89 to 3.00)c | 2.32 (1.76 to 2.89)c | 3.91 (3.17 to 4.66)c | |
| Unadjusted change | −2.83 (−3.61 to −2.05)d | −3.18 (−3.76 to −2.59)d | −0.79 (−1.44 to −0.15)e | <0.001 |
| Adjusted changeb | −2.77 (−3.39 to −2.15)d | −3.00 (−3.58 to −2.42)d | −1.02 (−1.60 to −0.44)e | <0.001 |
| Maximum walking distance | ||||
| Baseline | 4.21 (3.35 to 5.07) | 4.15 (3.49 to 4.81) | 3.47 (2.71 to 4.23) | |
| After 3 mo | 2.17 (1.35 to 2.99)c | 1.68 (1.08 to 2.28)c | 3.15 (2.43 to 3.87) | |
| Unadjusted change | −2.03 (−2.86 to −1.21)d | −2.47 (−3.17 to −1.77)d | −0.32 (−1.22 to 0.57)e | <0.001 |
| Adjusted changeb | −1.87 (−2.56 to −1.18)d | −2.34 (−2.98 to −1.70)d | −0.59 (−1.24 to 0.05)e | 0.001 |
| Daily activities | ||||
| Baseline | 8.07 (6.80 to 9.34) | 7.26 (6.49 to 8.03) | 7.03 (6.07 to 7.99) | |
| After 3 mo | 3.90 (3.29 to 4.51)c | 4.06 (3.33 to 4.79)c | 5.00 (4.06 to 5.94)c | |
| Unadjusted change | −4.17 (−5.60 to −2.74)d | −3.21 (−4.06 to −2.35) | −2.03 (−2.91 to −1.15)e | 0.017 |
| Adjusted changeb | −3.75 (−4.62 to −2.87)d | −3.37 (−4.26 to −2.48) | −2.35 (−3.22 to −1.47)e | 0.028 |
| Global score | ||||
| Baseline | 17.55 (15.29 to 19.81) | 16.91 (15.72 to 18.10) | 15.21 (13.45 to 16.97) | |
| After 3 mo | 8.52 (6.85 to 10.18)c | 8.03 (6.46 to 9.60)c | 12.06 (10.06 to 14.06)c | |
| Unadjusted change | −9.03 (−11.40 to −6.67)d | −8.88 (−10.37 to −7.40)d | −3.15 (−4.85 to −1.45)e | <0.001 |
| Adjusted changeb | −8.51 (−10.18 to −6.85)d | −8.68 (−10.21 to −7.15)d | −3.79 (−5.34 to −2.25)e | <0.001 |
Abbreviations: MP, methylprednisolone; SBCG1, sodium bicarbonate and a single dose of calcium gluconate; SBCG2, sodium bicarbonate and a double dose of calcium gluconate.
Values are mean (95% confidence interval).
Change adjusted for baseline value and the patient-nested-in-doctor random effect.
Significantly different from baseline value in paired t-test (P < .05).
Different letters indicate significant differences according to the least significant difference test for pairwise comparisons.
A greater mean change was observed in the global LFI in both SBCG groups compared with the mean change in the MP group (Figure 3).
Figure 3.
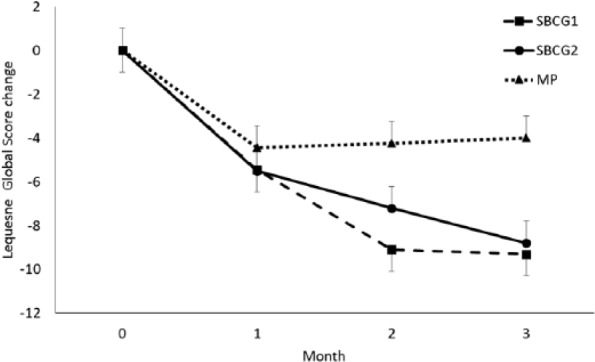
Monthly changes in the Lequesne functional index by treatment group. Mean values (±SEM) are adjusted for baseline values.
Knee pain was the most common adverse event reported in the 3 treatment groups. None of the reported adverse events was classified as severe.
Discussion
The results of this study confirm that intra-articular injections of sodium bicarbonate and calcium gluconate into the knee joint are significantly more effective in the reduction of knee OA symptoms than intra-articular injections of MP, which is a widely accepted corticosteroid treatment. Both SBCG groups showed a lower WOMAC total score and LFI by approximately 70% and 50%, respectively, compared with baseline values. The improvement based on these indices was significantly different from that of MP, which reduced OA symptoms by about 30% and 20%, respectively. This controlled trial had a duration of 3 months because 3 to 4 months have been recommended as the maximum dose of MP.8 In contrast, monthly SBCG intra-articular injections have been administered safely for 12 months, their beneficial effects continuing for another 6 months.5
An effective OA treatment has been defined as a 30% reduction in pain and functionality symptoms.13,14 Few intra-articular interventions have remarkably surpassed this threshold.15
A recent clinical trial showed reductions with plasma rich in growth factors of 42% and 39% in WOMAC and LFI, respectively, compared with baseline values after 24 months.14 The treatments evaluated in this study, SBCG1 and SBCG2, in only 3 months reduced pain and functionality scores even more than the effective plasma rich in growth factors. Although it has been assumed that plasma rich in growth factors plays an important role in the regeneration of damaged cartilage, there is no conclusive clinical evidence that supports its mechanism of action. Research evidence indicates that the beneficial effect of plasma rich in growth factors might be due to an anti-inflammatory effect and an inhibition of pain sensation.15
The mechanism of action of the evaluated combination of sodium bicarbonate and calcium gluconate has not been entirely explained, either; the conceptualization and fundamentals of the development of the formulation have been explained previously.5 Briefly, the facts that elucidate its mechanism of action are based on the alkaline pH of sodium bicarbonate, which promotes recovery of intracellular chondrocyte pH, normalizing intracellular metabolic activities,16 leading to a rearrangement of the extracellular matrix.17-19 Calcium gluconate protects the linkage between chondral and bone proteins from the hyperosmotic and acid conditions of the extracellular matrix, allowing for the recovery of the homeostatic mechanisms of the cartilage.20 The effect of both compounds administered together may modify cartilage metabolism by stimulating anabolic activities and decreasing catabolic activities.
Clinical evidence from monthly intra-articular injections of SBCG2, which has a higher dose of calcium gluconate, has shown, on average, a slight increase in intra-articular joint space after 12 months of intervention.5 This might suggest that cartilage regeneration, not pain inhibition or anti-inflammatory effect, is the mechanism that improves pain and functionality scores. However, to confirm this effect, further studies evaluating magnetic resonance imaging outcomes are needed to support the effectiveness of SBCG in regenerating knee cartilage.
The use of SBCG therapy may produce similar or even greater effects than other promising treatments, but the advantage of this novel combination is the nature of its elemental compounds, guaranteeing safe administration in patients with OA for more than 3 injections. Repeated administration of SBCG may help ensure its effectiveness.
The results of this study are of particular significance for patients with knee OA, whose mobility and quality of life can be severely impaired. Improving a patient’s ability to work and physical functioning is important, as this helps to maintain independence in activities of daily living, and the functional independence of older adults is associated with decreased mortality and decreased admission into nursing homes and hospitals.21
This clinical trial had some limitations that should be considered. (1) For ethical reasons, the trial design did not include a control group with placebo; thus, the psychologic influence when measuring pain could not be assessed. (2) According to our design, it was not possible to compare the experimental treatments for a longer amount of time due to the adverse effects that the control treatment might have produced. However, in a previous publication, we demonstrated the effectiveness of the combination of sodium bicarbonate and calcium gluconate in the reduction of knee OA symptoms for up to 18 months.5 (3) Because the number of participants studied was small, patient data were not analyzed on the basis of OA severity. Thus, the study was not able to determine whether or not the formulation is more effective in patients with more severe symptoms. (4) The effectiveness of the SBCG solutions was not compared with additional treatments in this study. However, given the highly effective response observed in both the LFI and the WOMAC scores, much higher than that which has been reported for other treatments, we can anticipate that the study combination has a higher efficacy than most reported treatments.
Conclusions
Monthly intra-articular injections into the knee joint of sodium bicarbonate combined with calcium gluconate significantly reduce pain and improve physical function after the first monthly intervention. After 3 months, this treatment is significantly more effective than MP in reducing the WOMAC total score and the LFI score. The administration of both SBCG combinations represents a highly effective and safe alternative for the treatment of knee OA.
Footnotes
Peer Review:Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 950 words, excluding any confidential comments to the academic editor.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by Nucitec S.A. de C.V. and by Conacyt, Mexico, grant number C0003-2009-1-110016.
Declaration of Conflicting Interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This study was partially funded by Nucitec S.A. de C.V. Dr Miguel Ángel Duarte is an employee at this company and did not receive any compensation for this study. Dr Jorge L Rosado owns shares in this company. They participated in the conception and design of the study and in the revision of the final draft of the manuscript. They were not involved in the fieldwork and did not participate in the data analysis. The other authors are employed at Querétaro State University and/or Cindetec A.C. where the study and data analyses were conducted.
Author Contributions: MADV participated in the design and provided important intellectual content. KEGR coordinated the fieldwork and was responsible for quality assurance. SGP supervised the fieldwork and contributed to the interpretation of the results. MCP performed the data analysis and contributed to the interpretation of the results and the revision of the manuscript. JLR participated in the conception and design of the study and in the revision of the manuscript. All authors read and approved the final manuscript.
References
- 1. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323-1330. [DOI] [PubMed] [Google Scholar]
- 2. Pelletier JP, Choquette D, Haraoui B, et al. Pharmacologic therapy of osteoarthritis. Curr Rheumatol Rep. 1999;1:54-58. [DOI] [PubMed] [Google Scholar]
- 3. Schweiz KR. Intra-articular alkali therapy (Die intra-artikuläre alkali-therapie). Med Wschr. 1948;78:80. [PubMed] [Google Scholar]
- 4. Walter R. Corticosteroid calcium compositions and treatment of rheumatic diseases therewith. US patent 4,252,797. February 24, 1981. [Google Scholar]
- 5. García-Padilla S, Duarte-Vázquez MA, Gonzalez-Romero KE, Caamaño MeC, Rosado JL. Effectiveness of intra-articular injections of sodium bicarbonate and calcium gluconate in the treatment of osteoarthritis of the knee: a randomized double-blind clinical trial. BMC Musculoskelet Disord. 2015;16:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039-1049. [DOI] [PubMed] [Google Scholar]
- 7. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ. 2004;328:869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amador R, Rosado JL, García-Padilla S, Duarte Vázquez MÁ. Compositions and methods for treatment and prevention of osteoarthritis. US patent 8,779,006 B2. July 15, 2014. [Google Scholar]
- 10. Amador R, Rosado JL, García-Padilla S, Duarte Vázquez MÁ. Compositions and methods for treatment and prevention of osteoarthritis. US patent 8,790,714 B2. July 29, 2014. [Google Scholar]
- 11. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833-1840. [PubMed] [Google Scholar]
- 12. Faucher M, Poiraudeau S, Lefevre-Colau MM, Rannou F, Fermanian J, Revel M. Algo-functional assessment of knee osteoarthritis: comparison of the test-retest reliability and construct validity of the WOMAC and Lequesne indexes. Osteoarthritis Cartilage. 2002;10:602-610. [DOI] [PubMed] [Google Scholar]
- 13. Lequesne MG. The algofunctional indices for hip and knee osteoarthritis. J Rheumatol. 1997;24:779-781. [PubMed] [Google Scholar]
- 14. Vaquerizo V, Plasencia MÁ, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PRGF-Endoret) versus Durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy. 2013;29:1635-1643. [DOI] [PubMed] [Google Scholar]
- 15. Anitua E, Sánchez M, Aguirre JJ, Prado R, Padilla S, Orive G. Efficacy and safety of plasma rich in growth factors intra-articular infiltrations in the treatment of knee osteoarthritis. Arthroscopy. 2014;30:1006-1017. [DOI] [PubMed] [Google Scholar]
- 16. Simpkin VL, Murray DH, Hall AP, Hall AC. Bicarbonate-dependent pH(i) regulation by chondrocytes within the superficial zone of bovine articular cartilage. J Cell Physiol. 2007;212:600-609. [DOI] [PubMed] [Google Scholar]
- 17. Waldman SD, Couto DC, Omelon SJ, Kandel RA. Effect of sodium bicarbonate on extracellular pH, matrix accumulation, and morphology of cultured articular chondrocytes. Tissue Eng. 2004;10:1633-1640. [DOI] [PubMed] [Google Scholar]
- 18. Browning JA, Wilkins JR. Mechanisms contributing to intracellular pH homeostasis in a immortalized human chondrocyte cell line. Comp Biochem Physiol A Mol Integr Physiol. 2004;137:409-418. [DOI] [PubMed] [Google Scholar]
- 19. Wilkins RJ, Hall AC. Control of matrix synthesis in isolated bovine chondrocytes by extracellular and intracellular pH. J Cell Physiol. 1995;164:474-481. [DOI] [PubMed] [Google Scholar]
- 20. Dascalu A, Nevo Z, Korenstein R. The control of intracellular pH in cultured avian chondrocytes. J Physiol. 1993;461:583-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharkey NA, Williams NI, Guerin JB. The role of exercise in the prevention and treatment of osteoporosis and osteoarthritis. Nurs Clin North Am. 2000;35:209-221. [PubMed] [Google Scholar]