Abstract
Previous study showed that expression of epithelial cell adhesion molecule (EpCAM) was significantly upregulated in porcine induced pluripotent stem cells (piPSCs). However, the regulatory mechanism and the downstream target genes of EpCAM were not well investigated. In this study, we found that EpCAM was undetectable in fibroblasts, but highly expressed in piPSCs. Promoter of EpCAM was upregulated by zygotic activated factors LIN28, and ESRRB, but repressed by maternal factors OCT4 and SOX2. Knocking down EpCAM by shRNA significantly reduced the pluripotent gene expression. Conversely, overexpression of EpCAM significantly increased the number of alkaline phosphatase positive colonies and elevated the expression of endogenous pluripotent genes. As a key surface-to-nucleus factor, EpCAM releases its intercellular domain (EpICD) by a two-step proteolytic processing sequentially. Blocking the proteolytic processing by inhibitors TAPI-1 and DAPT could reduce the intracellular level of EpICD and lower expressions of OCT4, SOX2, LIN28, and ESRRB. We noticed that increasing intracellular EpICD only was unable to improve activity of EpCAM targeted genes, but by blocking GSK-3 signaling and stabilizing beta-catenin signaling, EpICD could then significantly stimulate the promoter activity. These results showed that EpCAM intracellular domain required beta-catenin signaling to enhance porcine cell reprogramming.
The generation of porcine pluripotent stem cells may not only prove the concept of pluripotency in domestic animals, but also retain the enormous potential for animal reproduction and translational medicine. In last several years, porcine induced pluripotent stem cells (piPSCs) were generated in many research groups including our laboratory1,2,3,4,5,6,7,8. Because bona fide pig embryonic stem cells were not available yet, most of manipulation conditions for maintenance of piPSCs were consulted with the conditions for mouse iPS9 and human iPS cells10. Therefore, the reported piPSCs showed the divers morphology and biological features. Some piPSC lines were bFGF-dependent and showed mouse epiblast-derived stem cell like morphology2,11; other lines were LIF-dependence and showed mouse ESC-like morphology3. Thus, the optimal culture condition and regulatory circuitry for generation and maintenance of piPSCs are not standardized, and the generation and maintenance of naïve state piPSCs is still an important issue that has to be addressed. Previous reports were sure that signaling pathways used for maintaining human and mouse iPSCs did not sustain the self-renewal and pluripotency of porcine iPSCs12,13. The species-related regulatory signaling pathway as reported in mouse and human pluripotent stem cells (PSCs)14 is likely to be applied in pig and other animals, in which PI3K/AKT signaling and TGF-beta signaling pathways, instead of LIF and bFGF signaling pathways, may play key roles to maintain porcine stem cell pluripotency15.
The epithelial cell adhesion molecule (EpCAM) is a transmembrane glycoprotein encoded by the TACSTD1 gene, and is highly expressed in epithelia and epithelial-derived neoplasms16. In human and mouse iPSCs, EpCAM was also highly expressed and play a critical role in cell reprogramming17,18,19,20. Consistently, our previous study showed that EpCAM is highly expressed in porcine iPSCs13. Therefore, as a cell-to-cell adhesion molecule, EpCAM is involved in cell signaling, migration, proliferation, and differentiation19,20,21. Recent studies showed that EpCAM was a key surface receptor that was able to translocate to the nucleus and to regulate downstream target gene expression22. Through two-step proteolytic processing, EpCAM is sequentially cleaved by tumor necrosis factor-alpha converting enzyme (TACE/ADAM17) and presenilin 2 (PS-2), a protease component of gamma-secretase complex, and releases an N-terminal extracellular domain (EpEX) and a 5 kDa C-terminal intracellular domain (EpICD). The EpICD fragment, which is unstable in the cytoplasm, is able to translocate into nucleus and comes along with co-transcriptional activators to stimulate gene expression and cell proliferation23. The study showed that EpICD with FHL2, beta-catenin, and Lef-1 formed a nuclear complex, which contacted DNA at Lef-1 consensus sites, and stimulated c-Myc expression24. Consequently, the role of EpCAM in porcine cell proliferation and its association with reprogramming is worth to be investigated.
Studies have shown the fundamental function of EpCAM in regulation of human and mouse pluripotent stem cells17,18. In order to gain insight into the epigenetic regulation of porcine pluripotency, we comprehensively analyzed porcine EpCAM gene and investigated the regulation function of EpCAM for porcine cell reprogramming and maintenance of pluripotency. Our discoveries would be conducive to establish naïve state of porcine pluripotent stem cells.
Results
EpCAM Is Highly Expressed in Porcine Pluripotent Stem Cells
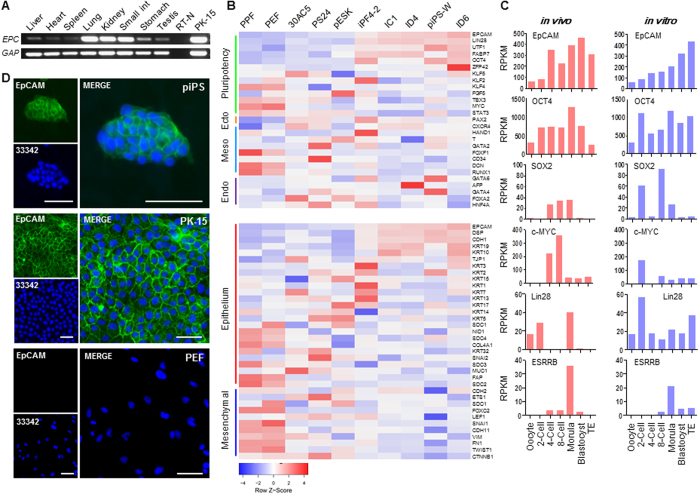
The expression profile of EpCAM in porcine tissues from newborn piglet was conducted by RT-PCR analysis. As described previously25,26, EpCAM is highly expressed in epithelial cells. In our study, EpCAM message was detectable in all tested samples, which may be due to the widespread epithelial cells in most of organs. In those epithelia enriched organs, for instance lung, kidney, and small intestine, EpCAM was relatively abundant than in other tissues (Fig. 1A). The heatmap of microarray data (note: NANOG and SOX2 genes were not included in the Affymetrix Pig GeneChipe13) of eight piPSC lines and two primary porcine skin fibroblasts showed that EpCAM and core pluripotent genes, such as OCT4, LIN28, UTF1, and ZFP42, were highly expressed, and the expression of lineage-specific genes were downregulated (Fig. 1B), suggesting that EpCAM might play an important role during porcine cell reprogramming. Additionally, the expression level of EpCAM and many epithelial genes were clearly higher in piPSCs than in fibroblasts, and the expression level of mesenchymal markers was clearly downregulated in piPSCs (Fig. 1B). These results indicated that a mesenchymal to epithelial transition was occurred during the porcine cell reprogramming, which was an important event during cell reprogramming as reported in mouse and human iPSCs27,28.
Figure 1. EpCAM expression in porcine tissues and iPSCs.
(A) EpCAM (EPC) expression in porcine tissues and epithelial cell PK-15. GAPDH (GAP) was used as internal control. (B) Expression profile of vital genes for the pluripotency and three germ layers (upper), and MET/EMT markers (lower) in porcine somatic and pluripotent cells. (C) Expression profile of EpCAM and pluripotent genes in porcine oocytes and early stage embryos maturated in vivo and in vitro. (D) Immunofluorescences of EpCAM (green) in piPS, PK-15, and PEF cells. Nuclei were stained with Hoechst 33342 (blue). Scale bar, 50 μm.
Since the inner cell mass (ICM) derived embryo stem cells (ESCs) are the most extensively studied pluripotent stem cells29, transcriptome data of porcine oocyte and early state embryos maturated in vivo and in vitro were analyzed. The level of EpCAM expression was low in oocyte and 2-cell stages, but significantly increased in 4/8-cell stage till blastocyst stage (Fig. 1C), suggesting that EpCAM is a zygotic activated gene. We noticed that the high level expression of zygotic activated pluripotent genes LIN2830 and ESRRB31 was also presented at the morula stage in in vivo embryos though the expression profile of pluripotent genes displayed the slightly different expression patterns in in vitro embryos. Being consistent with the transcriptomic and RT-PCR analyses, immunofluorescence staining confirmed that EpCAM protein was expressed and translocated to the cellular membrane in piPSCs, but was undetectable in non-reprogrammed PEF cells (Fig. 1D). These results revealed that EpCAM could be a marker to monitor porcine somatic cell reprogramming.
EpCAM Is Activated by Zygotic Activated Pluripotent Factors
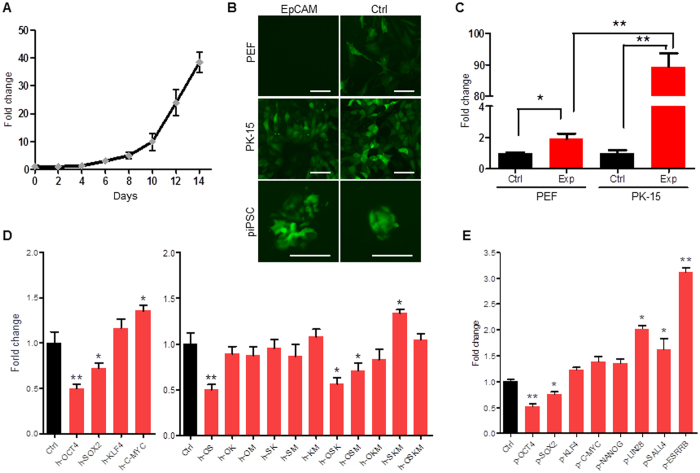
To further investigate the regulation of EpCAM in piPSCs, we detected the expression of EpCAM during porcine cell reprogramming. In the first four days post transfection of human OCT4, SOX2, KLF4, and c-MYC (hOSKM), the endogenous EpCAM was at very low level. After six days post transfection, EpCAM expression was significantly increased (Fig. 2A). To explore how EpCAM was activated, a 3.8 kb DNA fragment (GenBank accession number KY218795) that contains promoter region, 5′ UTR sequence, and ATG codon of EpCAM was cloned, confirmed by DNA sequencing, and subcloned into reporter vectors pTRIP-EGFP and pGL3-basic to form pTRIP-EpCAM-EGFP and pGL3-EpCAM-Pro constructs (Table S2). Results showed that a strong EGFP fluorescence was observed in PK-15 and piPSC cells, but the fluorescent signal was undetectable in control PEF cells (Fig. 2B). Luciferase assay showed that EpCAM promoter was significantly activated in epithelia PK-15 versus fibroblasts PEF (Fig. 2C), indicating the cell-specificity of cloned EpCAM promoter.
Figure 2. EpCAM promoter activation in reprogrammed cells.
. (A) The qRT-PCR analysis of EpCAM expression during porcine fibroblast reprogramming by hOSKM for 14 days. (B) Transduction of pTRIP-EpCAM-EGFP and pTRIP-CAGG-EGFP (Ctrl) into PEF, PK-15, and piPS cells, respectively, to determine activation of the EpCAM promoter. Scale bar, 100 μm. (C) Luciferase assay. Reporter pGL3-EpCAM-Pro (Exp) and pGL3-Basic (Ctrl) were transfected into PEF and PK-15 cells, respectively, for 36 h. (D) Luciferase assay of EpCAM promoter activation regulated by human pluripotent factor alone (left), or combination (right) in 293 T cells. Ctrl, cells were transfected with pGL3-EpCAM-Pro and pMXs-EGFP. (E) Luciferase assay of EpCAM promoter activation that was regulated by porcine pluripotent factors. Data are presented as mean ± S.D., *P < 0.05, **P < 0.01, n = 3.
To investigate whether EpCAM was directly regulated by pluripotent factors, the reporter pGL3-EpCAM-Pro with hOSKM vectors were co-transfected into 293 T cells, respectively. We noticed that EpCAM promoter activity was significantly repressed by OCT4 and SOX2 (Fig. 2D). Since OCT4 and SOX2 can form a complex, bind to downstream target genes, and maintain the pluripotency32, the multiple transduction of OSKM factors were applied. Results showed that OCT4-SOX2 complex as well as OSK and OSM combinants could significantly reduce EpCAM promoter activity, but combinations of OCT4-KLF4, OCT4-c-MYC, SOX2-KLF4, SOX2-c-MYC, and KLF4-c-MYC did not influence EpCAM activity. However, c-MYC and SKM combinant were able to enhance EpCAM promoter activity (Fig. 2D). We then detected whether EpCAM could be regulated by pig-oriented pluripotent factors. Similar to human factors, porcine OCT4 and SOX2 repressed EpCAM promoter activity (Fig. 2E). Additionally, zygotic activated pluripotent factors LIN28 and ESRRB could significantly upregulate EpCAM activity, suggesting that EpCAM is upregulated by zygotic activated pluripotent factors.
Knockdown EpCAM Reduces the Reprogramming Efficiency
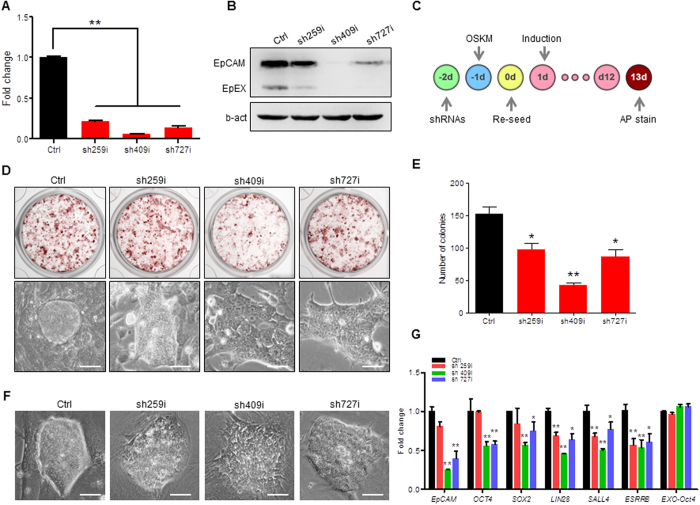
To knock down EpCAM expression, we designed three shRNAs that targeted to EpCAM extracellular domain sequence, and constructed three lentiviral shRNA vectors (Fig. S1). When PK-15 cells were transfected by lentiviral shRNA, the expression of endogenous EpCAM was down 70–90 percent compared to the control in both RNA level (Fig. 3A) and protein level (Fig. 3B). A diagram of cell reprogramming by four factors hOSKM and shRNA treatments was illustrated in Fig. 3C. PEF cells that were transfected with lentiviral shRNAs were reprogrammed by hOSKM (Fig. 3C). For two weeks induction and shRNA treatment, the reprogrammed cells showed the alkaline phosphatase (AP) staining positive colonies (Fig. 3D, upper), and the morphology of EpCAM knockdown cells showed much incompact (Fig. 3D lower). The quantification of AP-positive colonies showed that the reprogramming efficiency was significantly reduced when EpCAM expression was knocked down by shRNA (Fig. 3E). We then detected the effect of EpCAM in piPSCs. The shRNA constructs were transfected into DOX-iPSCs, a DOX induced piPSC line described previously11, for 60 h, respectively. The morphology of shRNA treated iPSC clones presented much looser and flat comparing to control clones (Fig. 3F). Quantitative RT-PCR analysis showed that in EpCAM-knockdown cells, the expression level of pluripotent genes including maternal factors OCT4, Sox2, and SALL4 and zygote activated factors LIN28 and ESRRB were significantly decreased (Fig. 3G), suggesting that EpCAM as an key surface-to-nucleus factor plays an important role to regulate porcine pluripotent gene expression.
Figure 3. Knockdown EpCAM expression in piPSCs.
(A,B) Quantitative RT-PCR (A) and western blotting (B) analyses of EpCAM expression knockdown by shRNAs in PK-15 cells for 60 h. b-act, beta-actin was used as internal control. (C) Schematic diagram of cell reprogramming by hOSKM and shRNA treatment. (D) Alkaline phosphatase staining of cells that were reprogrammed with shRNAs for 13 days (upper) and the morphology of reprogrammed cell colony (lower). (E) The number of AP positive colonies after shRNA treatment for 13 days. (F) Morphology of piPSCs that were transfected with shRNA. (G) qRT-PCR analysis of pluripotent gene expressions. DOX-iPSCs were transfected with shRNAs for 60 h. Ctrl, cells were treated without shRNA. Scale bar, 50 μm. Data are presented as mean ± S.D., *P < 0.05, **P < 0.01, n = 3.
Overexpression of EpCAM Enhances Reprogramming Efficiency
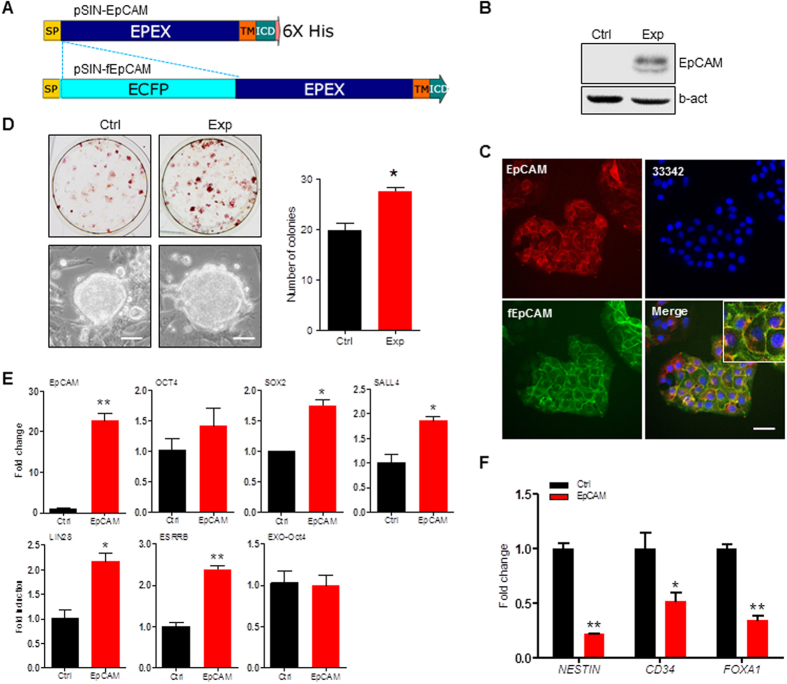
To further investigate EpCAM regulation function, we cloned porcine EpCAM coding DNA sequence (GenBank Accession No. KX904866), and constructed two overexpression vectors. The construct pSIN-EpCAM was derived from pSIN lentiviral plasmid with puromycin resistance, and the construct pSIN-fEpCAM was derived from pSIN-EpCAM with ECFP (enhanced cyan fluorescent protein) insertion in N-terminal of EpCAM protein (Fig. 4A). Western blotting analysis of PEF cells that were stably transfected by pSIN-EpCAM and selected by puromycin showed that cloned gene encoded EpCAM protein and overexpressed in transfected PEF cells (Fig. 4B). We then transfected pSIN-fEpCAM into PK-15 cells, and did immunofluorescence staining, which showed that fEpCAM fusion protein and endogenous EpCAM were co-localized in cell plasma membrane (Fig. 4C), indicating that the cloned EpCAM retained the biological function, and the constructs were able to be used for the following experiments.
Figure 4. Overexpression of EpCAM improves PEF cell reprogramming.
(A) Schematic diagram of lentiviral vectors for expressing EpCAM and ECFP-EpCAM fusion protein (fEpCAM). SP, signal peptide. TM, transmembrane domain. EpEX, extracellular domain. ICD, intracellular domain. (B) Western blotting analysis. Proteins were prepared from PEF (Ctrl) and PEF + EpCAM (Exp) cells that were transfected by pSIN-EpCAM lentivirus for 60 h. Anti-EpCAM antibody and anti-b-actin antibody were applied for immunoblotting. (C) Immunostaining of EpCAM by anti-EpCAM antibody (red) in PK-15 cells that were transfected with pSIN-fEpCAM (green). Nuclei were stained with Hoechst 33342 (blue). Scale bar, 25 μm. (D) AP staining of cells reprogrammed by OSKM for 13 days (upper) and the morphology of derived colonies (lower). AP positive colonies were counted (right panel). Scale bar, 50 μm. (E) qRT-PCR analysis of pluripotent gene expressions in DOX-iPSCs. Ctrl, cells were transfected with pSIN-EGFP. Exp, cells were transfected with pSIN-EpCAM. (F) qRT-PCR analysis of gene markers in piPS cells that were spontaneously differentiated into ectoderm (NESTIN), mesoderm (CD34), and endoderm (FOXA1). Data are presented as mean ± S.D., *P < 0.05, **P < 0.01, n = 3.
To determine EpCAM regulation during cell reprogramming, the pSIN-EpCAM was transfected into PEF cells, which were then induced by retroviral vectors carrying with hOSKM. For 13 days induction, the compact colonies were formed (Fig. 4D, lower) that showed AP-positive staining (Fig. 4D, upper). A quantification result showed that overexpression of EpCAM significantly increased the number of AP-positive colonies compared to the control group (Fig. 4D). Overexpression of EpCAM in DOX-iPSCs, which were maintained by addition of doxycycline, could also significantly elevate the expression of endogenous pluripotent genes, including SOX2, SALL4, LIN28, and ESRRB (Fig. 4E). We noticed that OCT4 expression was increased slightly, but not statistically significant, which may be due to the existence of high level DOX-induced OCT4 in DOX-iPSCs. We also detected the differentiation potential of piPS cells that were over expressed EpCAM. Results showed that overexpression of EpCAM maintained the self-renewal of piPS cells and impede cells to differentiate into the three germ layers (Fig. 4F).
EpCAM Cleavage and EpICD Generation
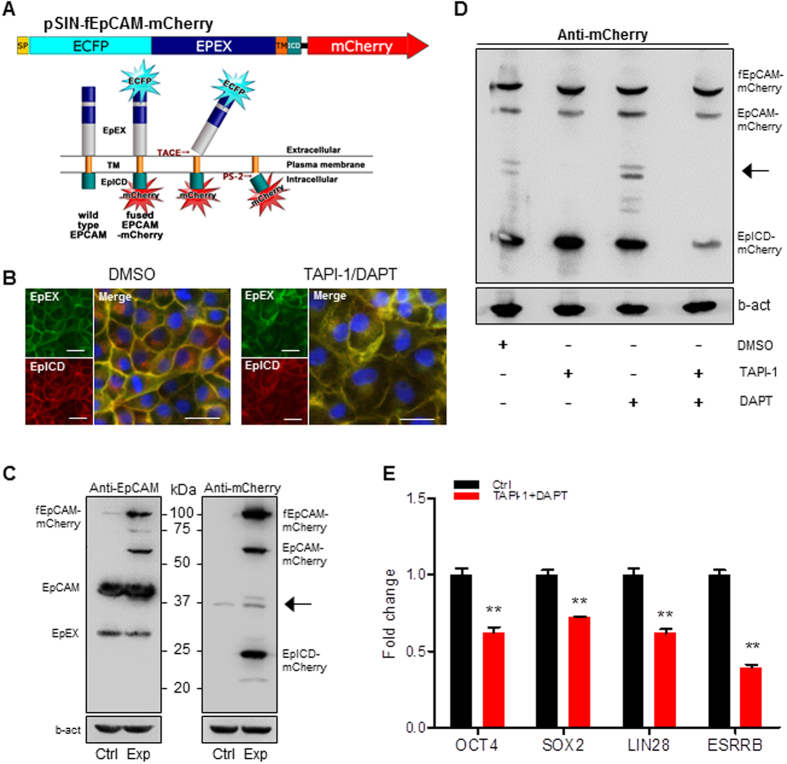
To monitor EpCAM protein processing, the protein was double labeled with ECFP in N-terminal and mCherry in C-terminal. The N-terminal domain EpEX (green) and C-terminal domain EpICD (red) can be cleavage sequentially by TACE/ADAM17 and PS-2 (Fig. 5A). The pSIN-fEpCAM-mCherry vector was transfected into pig (PEF and PK15), mouse (NIH3T3 and P19), and human (293 T and Hela) cells, respectively. The fEpCAM-mCherry fusion protein was highly expressed in epithelial and pluripotent cells, but low expression in fibroblasts (Fig. S2). Results of transfection of pSIN-fEpCAM-mCherry into PK-15 cells showed that EpICD (red) was released from cytoplasm membrane and translocated to nuclei in control (DMSO) cells. However, the cleavage of EpICD was blocked by addition of protease inhibitors TAPI-1 and DAPT since the fEpCAM-mCherry fusion protein was remained in cytoplasm membrane (yellow) (Fig. 5B). Western blotting assay showed that endogenous EpCAM and ectopically expressed fEpCAM-mCherry were detected by anti-EpCAM antibody presenting an approximate 39 kDa protein band of matured EpCAM and EpEX, and the ectopically expressed EpICD-mCherry was detected by anti-mCherry antibody (Fig. 5C). When fEpCAM-mCherry expressed PK-15 cells were treated with gamma-secretase complex inhibitor DAPT, the EpICD fraction was decreased and the partially degraded extracellular domain fraction was accumulated, which was undetectable in the treatment of TAPI-1, a TACE/ADAM17 inhibitor that prevents proteolytic cleavage of the EpCAM extracellular domain. However, when application of both TAPI-1 and DAPT, the level of EpICD in nuclear extract was significantly reduced, indicating that both factors block the cleavage of EpCAM (Fig. 5D). We then investigated the cleavage and function of EpICD in porcine iPS cells. Results of addition of TAPI-1 and DAPT in DOX-iPSCs cells showed that expression of pluripotent factors, including OCT4, SOX2, LIN28, and ESRRB, was significantly reduced (Fig. 5E), showing the similar result that EpCAM expression was knocked down by shRNAs (Fig. 3G). This observation indicates that EpICD is a core transcription activator to upregulate expression of porcine pluripotent factors.
Figure 5. EpCAM cleavage and EpICD generation.
(A) Schematic diagram of the lentiviral vector pSIN-fEPCAM-mCherry carrying with ECFP/mCherry double labeled EpCAM. The functional domains EpEX and EpICD can be released sequentially by TACE and PS-2. (B) Florescent images of EpEX (green) and EpICD (red) in PK-15 cells, which were transfected by pSIN-fEpCAM-mCherry and treated with or without TAPI-1/DAPT. Nuclei were stained by Hoechst 33342 (blue). Scale bar, 25 μm. (C) Western blotting was performed by anti-EpCAM and anti-mCherry antibodies, respectively. PK-15 cells were transfected by pSIN-EGFP (Ctrl) and pSIN-fEpCAM-mCherry (Exp), respectively. (D) Western blotting was performed by anti-mCherry antibody with the treatment of TAPI-1, DAPT, and DMSO in PK-15 cells. b-actin (b-act) was as internal control. The arrow indicates the partial degradation of extracellular domain fraction. (E) qRT-PCR analysis of pluripotent gene expression in piPSCs with or without addition of TAPI-1 and DAPT. Ctrl, cells were treated with DMSO. Data are presented as mean ± S.D., *p < 0.05, **p < 0.01, n = 3.
EpICD Regulates Pluripotent Gene Expression Requiring beta-catenin Signaling
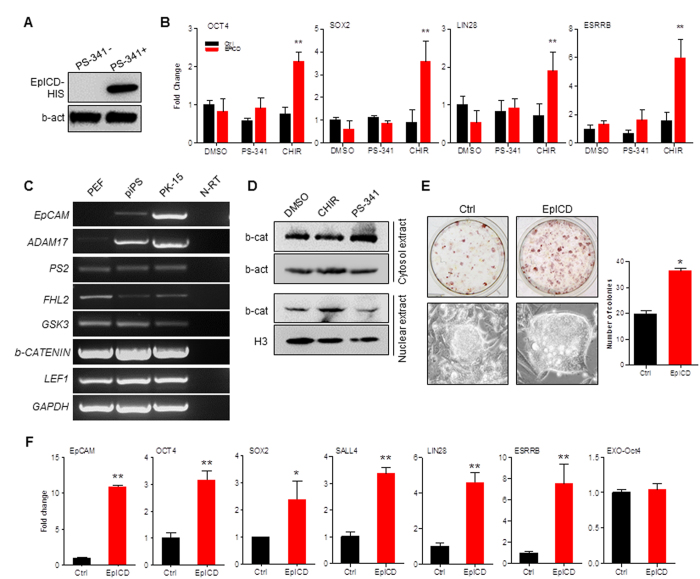
Because the size of EpICD-mCherry fusion protein is extremely larger than EpICD and causes the difficulty to translocation into nuclei, as seen in Fig. S2 that EpICD-mCherry was accumulated around nucleus (Figs S2, 5B), we then constructed pSIN-EpICD vector that contained an EpICD peptide fused with His tag. As previously reported that EpICD could be degraded by proteasome33, the proteasome inhibitor PS-341 was used when overexpression of EpICD in PEF, in which most of ectopically expressed EpICD was degraded if without addition of PS-341 (Fig. 6A), indicating that EpICD was unstable in PEF cells. To determine EpICD regulation function to enhance the transcriptional activation, promoter sequences of OCT4 (4.2 kb, KY218798), SOX2 (3.0 kb, KY218799), LIN28 (3.3 kb, KY218797), and ESRRB (3.3 kb, KY218796) were subcloned into pGL3-Basic reporter vector. When pSIN-EpICD was co-transfected with reporter vectors into PEFs, the promoter activity was not activated. To avoid EpICD degradation by proteasome, the inhibitor PS-341 was added in culture medium to elevate EpICD peptide level, but the promoter activity was still not activated (Fig. 6B), suggesting that quantity of EpICD was not essential for the activation of pluripotent genes. We then added inhibitor CHIR99021 into medium, which inhibits GSK-3 activity and therefore stabilize intracellular beta-catenin34. Results of promoter activity showed that expressions of OCT4 (2.8 fold), SOX2 (4.0 fold), LIN28 (2.6 fold), and ESRRB (3.4 fold) were significantly increased (Fig. 6B), indicating that EpICD required beta-catenin signaling to stimulate pluripotent gene expression. We then investigated EpCAM and beta-catenin signaling associated gene expressions in fibroblast, epithelia, and piPS cells. Except for EpCAM and a regulated intramembrane proteolysis initiator gene ADAM17, which were very lowly expressed in PEFs, the other regulated intramembrane proteolysis initiator gene PS2, the ICD binding partner gene FHL2, GSK3, beta-Catenin, and LEF1, an enhancer-binding factor formed a complex with beta-Catenin, were stably expressed in all three cell lines (Fig. 6C). This observation suggested that different cleavage patterns and signaling pathways might exist among cell types. The western blotting assay confirmed that there was more active beta-catenin in nuclear fraction when GSK3 inhibitor CHIR99021 was applied in PEFs, while the protein level of inactive beta-catenin in cytosol was basically consistent among three treatments (Fig. 6D). These results pointed out that GSK3 inhibitor CHIR99021 might be required as an essential ingredient in medium to maintain porcine iPSC self-renewal.
Figure 6. EpICD regulates cell reprogramming through b-catenin signaling.
(A) Western blotting of EpICD expression in PEFs with or without proteasome inhibitor PS-341. (B) Luciferase assay. The pSIN-EpICD with pGL3-OCT4-Pro, pGL3-SOX2-Pro, pGL3-LIN28-Pro, and pGL3-ESRRB-Pro were co-transfected into PEFs for 36 h in medium with PS-341 and CHRI99021 (CHIR), respectively. DMSO was used as control. (C) RT-PCR analysis of genes associated with EpCAM signaling. GAPDH was used as control. (D) Western blotting of b-catenin (b-cat) protein in cytoplasm and nucleus. PEF cells were treated with DMSO, CHRI99021 (CHIR) and PS-341, respectively. Beta-actin (b-act) and histone H3 (H3) were determined as internal controls. (E) PEF cells were reprogrammed by hOSKM for 13 days with (Ctrl) or without (EpICD) co-transfection of pSIN-EpICD. AP staining (upper), the morphology of derived colonies (lower), and counts of AP positive colonies (right panel). (F) qRT-PCR analysis of pluripotent gene expressions in piPSCs, which were transfected by pSIN-EpICD. Ctrl, cells were transfected by pSIN-EGFP. Data are presented as mean ± S.D., *p < 0.05, n = 3, **p < 0.01, n = 3.
To confirm whether EpICD and CHIR99021 could enhance cell reprogramming, PEFs were induced by retroviral vectors carrying with hOSKM and EpICD for 13 days in the medium supplemented with CHIR99021. The AP staining and the number of AP-positive colonies were significantly increased in EpICD-expressed group than in control group (Fig. 6E). Quantitative RT-PCR analysis showed that EpICD significantly increased endogenous pluripotent gene expression including OCT4 (3.2 fold), SOX2 (2.4 fold), SALL4 (3.3 fold), LIN28 (4.6 fold), and ESRRB (7.5 fold) (Fig. 6F).
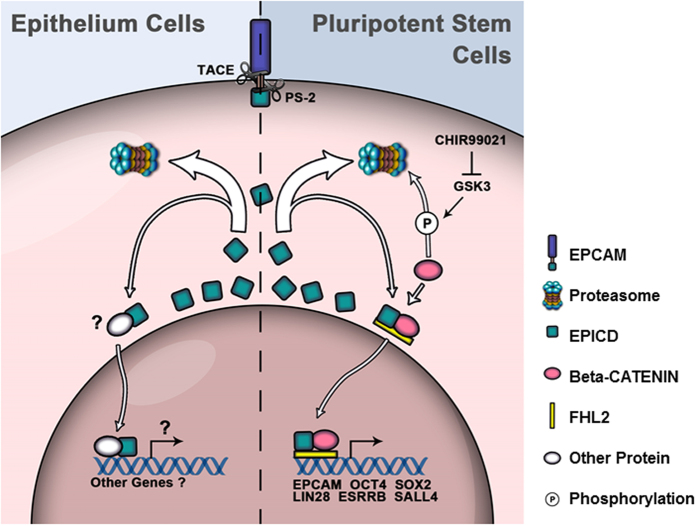
Based on this study and other laboratory’s reports24,35, we indicate that in epithelial cells EpICD molecule released from EpCAM is immediately removed by proteasome degradation, while in reprogrammed cells EpICD with beta-catenin and FHL2 forms a complex, translocates into nucleus, and subsequently promotes downstream pluripotent gene expression (Fig. 7).
Figure 7. Diagram of EpCAM signaling in epithelial cells and pluripotent stem cells.
Discussion
As a single-transmembrane glycoprotein, EpCAM is one core tumor-associated antigens36 and is the diagnosis and therapeutic target for various cancers37. However, recent study showed that high-level expression of EpCAM was required for the maintenance of self-renewal in mouse ES cells, especially under the differentiation condition in the absence of LIF, ectopic expression of EpCAM could partially compensate and retain ES phenotype33. Further study showed that in partially reprogrammed stem cells EpCAM did not activated, but in fully reprogrammed mouse iPSC cells EpCAM was highly expressed, indicating that EpCAM could be identified as a new surface marker for efficiently identifying fully reprogrammed iPSCs20. High-level and selective expression of EpCAM was also reported in undifferentiated, rather than differentiated, hES cells18. When knocked down endogenous EpCAM, hESC decreased cell growth and increased gene expression in the endoderm and mesoderm lineages19. In our previously study, we found that no matter what induction conditions were used, EpCAM was upregulated and highly expressed in porcine iPSCs13. Further analysis of transcriptome data from early state embryos maturated in vivo and in vitro showed that EpCAM expression was low in oocyte and 2-cell stages, but significantly increased in 4/8-cell stage (Fig. 1), which is the time of the maternal to zygotic transition in porcine embryogenesis38,39. We found in this study that the expression level of EpCAM was correlated with the expression of pluripotent genes. Down regulation of EpCAM expression by shRNA interference or ectopic expression EpCAM could significantly influence endogenous pluripotent gene expression and the formation of AP-positive colonies (Figs 3 and 4). In accord with the findings in mouse and human18,20, we confirmed that in OSKM mediated induced pluripotent stem cells EpCAM could be a critical marker that monitors pluripotent state of reprogrammed cells.
Studies in mouse and human pluripotent stem cells showed that EpCAM and its intercellular domain could directly regulate the promoter activation of pluripotent genes including Oct4, Sox2, and Nanog etc.17,18. In porcine iPS cells, overexpression of EpCAM significantly enhanced the promoter activation of OCT4 and SOX2. Furthermore, the promoter activity of LIN28, which is used as one of the reprogramming factors in hiPSCs10 and is an essential factor of nucleologenesis during early embryonic development30, and ESRRB, which plays the key role in regulating the naïve pluripotent state40, were also significantly stimulated by EpCAM. On the other hand, the activation of EpCAM promoter was also regulated by pluripotent factors. We noticed that zygotic activated pluripotent factors LIN2830 and ESRRB40 significantly stimulated EpCAM promoter activation. Unexpectedly, the maternal pluripotent factors OCT4 and SOX2, as well as OS, OSK, and OSM combinants, significantly suppressed EpCAM promoter activation. This founding indicates that as a zygotic activated gene EpCAM with other zygotic activated pluripotent factors forms a positive feedback regulatory circuitry to activate pluripotent gene expression and to maintain pluripotency of iPS cells. In hOSKM mediated PEF reprogramming, EpCAM expression did not immediately upregulated, but was delayed till 6 days post induction. This observation indicated that EpCAM could be used as a reprogramming selection marker to monitor porcine cell reprogramming.
By two-step proteolytic processing through adequate cell-to-cell contact EpCAM is sequentially cleaved and plays the function of surface-to-nucleus signaling22. Upon TACE/ADAM17 cleavage, the extracellular domain EpEX is released as a soluble ligand, and gamma-secretase complex cleavage releases the C-terminal intracellular domain EpICD, which is in combination with four-and-a-half LIM domains protein 2 and beta-catenin and drives cell proliferation24. We found that EpICD fragment was unstable in the cytoplasm due to the proteasome degradation; however, EpICD degradation could be diminished by application of the proteasome inhibitor (Fig. 6). This observation might partially explain why the high level endogenous EpCAM found in epithelial cells did not activate pluripotent gene expression.
The signaling pathway of EpCAM includes members of EpEX, EpICD, E-cadherin, Cldn7, beta-catenin, presenilin-2 (PS2), and ADAM1717,41. We found that by just increasing the quantity of EpICD in cytoplasm, EpCAM signaling was not functional because the promoter of EpCAM downstream target genes were not activated (Fig. 6). However, when stabilizing the intracellular beta-catenin by blocking GSK-3 activity, EpCAM signaling could promote the activation of pluripotent genes OCT4, SOX2, LIN28, and ESRRB and enhance reprogramming efficiency. Thus, EpICD did require beta-catenin to play a role in the regulation of pluripotency of piPSCs, and preservation of Wnt/beta-catenin signaling by inhibition of GSK3 activity was necessitated with addition of CHIR99021 as an essential ingredient in culture medium. Furthermore, there were Tcf binding elements found in EpCAM promoter region. The Tcf/beta-catenin complex could bind to EpCAM promoter and regulate EpCAM gene expression, indicating that EpCAM is a Wnt/beta-catenin targeted gene35. Therefore, in addition to LIF and bFGF pathways, the Wnt/beta-catenin signaling pathway might be a key pluripotency-maintaining pathway for porcine pluripotent stem cells.
In summary, we confirmed that EpCAM was an important surface-to nucleus signaling molecule in piPSCs. Through releasing the intercellular domain, EpCAM complex proteins activated pluripotent gene expression and promoted cell reprogramming. This study is conducive to understand the regulatory mechanism of porcine cell reprogramming and to facilitate the generation of naïve porcine iPSCs.
Methods
Cell culture
Cell lines HEK-293, HEK-293T, Hela, PK-15 cells, and NIH3T3 cells were cultured in DMEM supplemented with 10% FBS, 1 mM L-glutamine (Gibco, USA), 0.1 mM nonessential amino acids (NEAA, Gibico, USA). Porcine embryonic fibroblast (PEF) and mouse embryonic fibroblast (MEF) cells were cultured in DMEM medium supplemented with 15% FBS. Mouse embryonic carcinoma cell P19 was cultured in α-MEM supplemented with 2.5% FBS. Cells were cultured at 37 °C with 5% CO2 in a humidified atmosphere. To maintain porcine DOX-iPSCs, a tetracycline operator (TetO)-inducible porcine induced pluripotent cell line reported in this laboratory previously11, cells were plated on MEF feeders, which were derived from C57 mice and mitotically inactivated, and cultured in an iPS medium consisted of DMEM, 15% FBS, 1mM L-glutamine, 0.1 mM NEAA, 0.1 mM β-mercaptoethanol, 10 ng/mL human LIF (LIF1050, Millipore), 10 ng/mL human basic FGF (100-18B, PeproTech), 3 μM CHIR99021 (S1263, Selleck Chemicals), 2 μM SB431542 (S1067, Selleck Chemicals), and 4 μg/mL doxycycline (D9891-25G-9, Sigma Aldrich) at 37 °C, 5% CO2. To inhibit protease activity, 50 μM TAPI-1 (an inhibitor of TACE/ADAM17, S7434, Selleck Chemicals), 50 μM DAPT (an inhibitor of the gamma-secretase complex, S2215, Selleck Chemicals), and 20 μM PS-341 (an inhibitor of proteasome, S1013, Selleck Chemicals) were applied in media where relevant experiment needs, respectively.
RNA Extraction and RT-PCR
Total RNAs from porcine cells and tissues were extracted by Trizol Reagent (Invitrogen). RNAs were examined by measuring OD260/280 ratio, and samples with a ratio around 2.0 were used for reverse transcription. Two microgram total RNAs were reverse-transcribed with oligo-dT primer (Thermo Fisher) using RevertAidTM reverse transcriptase (Thermo Fisher). RT-PCRs were performed using 2 × Es Taq MasterMix, 2 × Taq MasterMix (CW Biotech, China) for 32 cycles at 94 °C 30 s, 57 °C 30 s, and 72 °C 45 s. The negative control was done by directly performing PCR with total RNAs to check the contamination of genome DNA. Quantitative RT-PCRs (qRT-PCR) were performed using a 10-fold dilution of cDNA with SYBR Green PCR Master Mix (Takara Biotechnology, China), and detected with StepOnePlus Real-Time PCR System (Applied Biosystems). Reactions were performed for 40 cycles at 95 °C 15 s and 60 °C 60 s, and measurements were performed on three biological replicates and each reaction was performed in triplicate. The expression level of target gene was normalized to the expression level of beta-actin. Melting curve analysis was conducted to confirm the specificity. Primers used in this study are listed in Supplementary Table S1.
Vector construction
To clone porcine EpCAM cDNA, total RNA was extracted from PK-15 cells. EpCAM coding DNA sequence (CDS) was amplified by RT-PCR using CloneAmp™ HiFi PCR Premix (#639298, Clontech). PCR fragment was cloned into pGEM-T Easy vector (A1360, Promega) and confirmed by DNA sequencing. Porcine EpCAM cDNA sequence was submitted to GenBank (Accession No. KX904866). To construct expression vector, EpCAM and EpICD fragments were subcloned into BamHI/EcoRI linearized pSIN-EF2-PuroR vector using In-Fusion® HD Cloning kit (#639642, Clontech), respectively, to form pSIN-EpCAM and pSIN-EpICD vectors. To construct expression vector of pSIN-fEpCAM that contains ECFP-EpCAM fusion protein, ECFP gene was inserted into the downstream of EpCAM signal peptide and fused with EpCAM extracellular domain (EpEX) in vector pSIN-EpCAM. To construct expression vector pSIN-fEpCAM-mCherry that contains a mCherry protein in C-terminal downstream of EpICD, the fEpCAM was subcloned into pmCherry-N1, and the fragment of fEpCAM-mCherry was then subcloned into pSIN-EF2- PuroR vector. Expression vectors of OCT4, SOX2, KLF4, c-MYC, NANOG, LIN28, SALL4, and ESRRB from porcine iPSCs were constructed by pMXs vector, which were reported previously9. All constructs used in this study were listed in Table S2.
Cell Reprogramming
For preparing retroviral particles, 2 × 106 293 T cells were planted on a 60 mm cultural dish. When cells reach to 80% confluence, pMXs vectors (4 μg/plasmid) carrying human OCT4, SOX2, KLF4, and c-MYC were transfected into 293 T cells together with 4 μg pCL-Eco and 2 μg pCMV-VSV-G at a ratio of 5:3:2 using Lipofectamine 2000 (Invitrogen). For preparing lentiviral particles, 4 μg shRNA constructs of pSIH (−), pSIH259i, pSIH409i, and pSIH727i and 4 μg EpCAM constructs of pSIN-EpCAM and pSIN-EpICD were transfected into 293 T cells with 4 μg pSPAX2 and 2 μg pMD 2.G, respectively. After 48 hours post-transduction, media with viral particles were collected and filtered through 0.45 μm filter (Millipore). To induce cell reprogramming, the equal amount of hOSKM viral supernatants mixed with 8 mg/mL Polybrene were infected into PEF cells for 12 h. To increase infection efficiency, a second infection was applied depending on the experimental needs. The infected PEF cells were then reseed at 2 × 104 cell/cm2 and cultured in the iPS medium for 13 days.
piPS Cell Differentiation
To detect the differentiation potential when knockdown or overexpression of EpCAM, DOX-iPSCs were transfected with viral particles that carrying either pSIN-EpCAM or pSIH-409i. After one passage, cells were detached from feeders by 1 mg/mL collagenase type IV and transferred to an ultra-low-attachment culture dish with the medium without growth factors and small molecules. For 3 days of suspension culturing, the embryoid bodies were formed, which were then transferred to a gelatine-coated culture dish and continually cultured in the regular medium (DMEM + 15% FBS) for 5 days. The spontaneously differentiated cells were harvested and used for RT-PCR analysis.
Luciferase Assay
For the EpCAM promoter activity assay, PK-15 and PEF cells were planted on a 96-well plate. When cells reach to 80% confluence, 1 μg pGL3-EpCAM-pro and pGL3-Basic with 0.01 μg pRL-TK vector that is an internal control were co-transfected into cells, respectively. To determine EpCAM promoter activation, which was activated by human OSKM and porcine OCT4, SOX2, KLF4, c-MYC, NANOG, LIN28, SALL4, and ESRRB, 293 T cells were planted on a 96-well plate. When cells reach to 80% confluence, the mixed pMXs vectors (0.5 μg) with 0.5 μg pGL3-EpCAM-pro and 0.01 μg pRL-TK were co-transfected into cells, respectively.
To determine EpICD regulation function, PEF cells were planted on a 96-well plate. When cells reach to 80% confluence, 0.5 μg pGL3-OCT4-pro, pGL3-SOX2-pro, pGL3-LIN28-pro, and pGL3-ESRRB-pro with pSIN-EpICD, pSIN-EGFP, and 0.01 μg pRL-TK were co-transfected into PEFs, respectively. Transfection was performed using Lipofectamine 2000 (Invitrogen). To confirm EpICD signaling pathway, 3 μM CHIR99021 (a GSK3 inhibitor), 20 μM PS-341 (a proteasome inhibitor), and control DMSO were added to media at 12 hours post transfection, respectively. Cells were harvested at 36 h post transfection and lysed at room temperature for 10 min using passive lysis buffer (E1941, Promega). Luciferase activity was detected by luciferase assay reagents (E1960, Promega) in a 96-well solid white flat bottom microplate (#3917, Corning) using BHP9504 microplate luminometer (D04407H, Hamamatsu, China). Triplicates were measured for each treatment, and the average values of the ratio of firefly luciferase units to Renilla luciferase units were used for data analysis.
Immunofluorescence Assay and Alkaline Phosphatase Staining
For immunofluorescence staining, cells were fixed with 4% paraformaldehyde in PBS for 15 min and blocked by 1% BSA at room temperature for 1 h. Cells were then incubated with primary anti-EpCAM antibody (1:200, ab71916, Abcam) at 4 °C overnight. After washed three times with PBS, cells were incubated with FITC conjugated secondary anti-rabbit antibody for 1 h. Nuclei were stained with 10 ug/mL Hoechst 33342 for 2 min. Images were captured under a Nikon fluorescence microscope. The alkaline phosphatase (AP) staining was determined by AST Fast Red TR and α-Naphthol AS-MX Phosphate (Sigma Aldrich) as our previously described4. Images were captured by a Pentax K-50 digital camera.
Western Blotting
To determine endogenous EpCAM expression, PK-15 cells treated by shRNA for 60 h and PEF cells transfected with pSIN-EpCAM were lysed by RIPA buffer (Thermo Scientific) for 10 min on ice, resuspended in 5 × SDS-PAGE loading buffer (50 mM Tris-HCl pH 6.8, 2% SDS, 10% glycerol, 2% β-mercaptoethanol and 0.05% bromophenol blue), and heated at 100 °C for 5 min. A 15 μL cell lysate was loaded onto 10% SDS-PAGE gel. After electrophoresis, proteins were transferred to a PVDF membrane (LC2002, Invitrogen) by semidry electrophoretic transfer (Bio-Rad) for 45 min at 15 V. The membrane was blocked with blocking buffer (20 mM Tris/HCl pH7.6, 137 mM NaCl, 0.1% Tween 20, and 8% skim milk) at 25 °C for 2 h, and then incubated with the primary anti-EpCAM antibody (1: 1000, ab71916, Abcam) in the blocking buffer at 4 °C overnight. After washing three times with TBS-T buffer (20 mM Tris/HCl pH 7.6, 137 mM NaCl, 0.1% Tween 20), the membrane was incubated with HRP-conjugated secondary anti-rabbit antibody (1:2000) at room temperature for 1 h. After washing three times in TBS-T at room temperature, the membrane was incubated in the enhanced chemiluminescent substrate (#32106, Pierce) and detected with a Chemiluminescent Imaging System (ZY058176, Tanon-4200, China). To monitor the fEpCAM-mCherry fusion protein cleavage, PK-15 cells were transduced with pSIN-fEpCAM-mCherry for 12 h, and then TAPI-1 and DAPT was added to media. At 48 h post transduction, cells were directly lysed by RIPA buffer for 10 min on ice, and protein blotting were performed as mentioned above with anti-mCherry antibody (1:1000, KM8017, Sungene Biotech, China) and anti-EpCAM antibody (1:1000), respectively. The HRP-conjugated secondary anti-mouse antibody (1:2000) was applied following with the incubation with the enhanced chemiluminescent substrate. To verify the EpICD expression, PEF cells were transduced with pSIN-EpICD for 12 h, and then PS-341 was added to medium. Cells were harvested at 60 h post transduction. The anti-His antibody (1:1000, #12698, Cell Signaling) were used to detect EpCAM-His fusion protein. To dissect the beta-catenin and EpICD signaling, PEF cells treated with 3 μM CHIR99021 or 20 μM PS-341 for 24 h were harvested, and nuclear proteins were extracted by the Nuclear Protein Extraction kit (R0050, Solarbio, China). The anti-beta-catenin antibody (1:1000, ab23671, Abcam) was applied following above procedure. The anti-beta-actin antibody (1:2000, KM9001, Sungene Biotech, China) and anti-histone H3 antibody (1:1000, #5192 Cell Signaling) were used as internal controls for the total proteins and nuclear proteins, respectively.
Bioinformatic Analysis
The microarray data were acquired from GEO database as previously described13, and GEO numbers are: GSE48434 for PEF4,42, PS244, 30 AC54, iPF4-242, and piPSCs-w2, GSE26369 for pESK7, and GSE15472 for IC1, ID4, and ID643. Pluripotent genes, lineage specific genes, and MET (mesenchymal-epithelial transition)/EMT (epithelial–mesenchymal transition) markers were chosen for this study. R software for windows was used to draw the heatmap. Sequencing data of mRNA from porcine early stage embryo were acquired from the Lab Archive (www.ncbi.nlm.nih.gov/sra) under accession number SRA076823 as described previously44, and the pluripotency related genes were chosen and charted using GraphPad Prism™ 5.0 for windows.
Statistical analysis
Statistical analyses were performed with the SPSS 16.0 for windows. Two-way ANOVA were used to study differences between grouped data, Student’s T tests were performed with one way analysis. Statistical significance was accepted at P < 0.05.
Additional Information
How to cite this article: Yu, T. et al. EpCAM Intracellular Domain Promotes Porcine Cell Reprogramming by Upregulation of Pluripotent Gene Expression via Beta-catenin Signaling. Sci. Rep. 7, 46315; doi: 10.1038/srep46315 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31571521 and 31371505).
Footnotes
The authors declare no competing financial interests.
Author Contributions Conceived and designed the experiments: H.W. Performed the experiments: Y.T., Y.M. Analyzed the data: Y.T., H.W. Contributed reagents/materials/analysis tools: Y.T., Y.M. Wrote the paper: H.W., Y.T.
References
- Zhang S. et al. Generation of intermediate porcine iPS cells under culture condition favorable for mesenchymal-to-epithelial transition. Stem cell reviews 11, 24–38, doi: 10.1007/s12015-014-9552-x (2015). [DOI] [PubMed] [Google Scholar]
- West F. D. et al. Porcine induced pluripotent stem cells produce chimeric offspring. Stem cells and development 19, 1211–1220, doi: 10.1089/scd.2009.0458 (2010). [DOI] [PubMed] [Google Scholar]
- Fujishiro S. H. et al. Generation of naïve-like porcine-induced pluripotent stem cells capable of contributing to embryonic and fetal development. Stem cells and development 22, 473–482, doi: 10.1089/scd.2012.0173 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D. et al. Porcine induced pluripotent stem cells require LIF and maintain their developmental potential in early stage of embryos. PloS one 7, e51778, doi: 10.1371/journal.pone.0051778 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montserrat N. et al. Generation of pig iPS cells: a model for cell therapy. Journal of cardiovascular translational research 4, 121–130, doi: 10.1007/s12265-010-9233-3 (2011). [DOI] [PubMed] [Google Scholar]
- Telugu B. P., Ezashi T. & Roberts R. M. Porcine induced pluripotent stem cells analogous to naïve and primed embryonic stem cells of the mouse. The International journal of developmental biology 54, 1703–1711, doi: 10.1387/ijdb.103200bt (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telugu B. P. et al. Leukemia inhibitory factor (LIF)-dependent, pluripotent stem cells established from inner cell mass of porcine embryos. The Journal of biological chemistry 286, 28948–28953, doi: 10.1074/jbc.M111.229468 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- West F. D. et al. Brief report: chimeric pigs produced from induced pluripotent stem cells demonstrate germline transmission and no evidence of tumor formation in young pigs. Stem cells 29, 1640–1643 (2011). [DOI] [PubMed] [Google Scholar]
- Takahashi K. & Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676, doi: 10.1016/j.cell.2006.07.024 (2006). [DOI] [PubMed] [Google Scholar]
- Yu J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920, doi: 10.1126/science.1151526 (2007). [DOI] [PubMed] [Google Scholar]
- AnQin D., Ning W., Tong Y. & HuaYan W. A porcine (Sus scrofa domesticus) somatic cell line of tetracycline operator (TetO)-inducible system for reprogramming. Journal of Agricultural Biotechnology 22, 1065–1073 (2014). [Google Scholar]
- Ezashi T., Telugu B. P. & Roberts R. M. Induced pluripotent stem cells from pigs and other ungulate species: an alternative to embryonic stem cells? Reproduction in domestic animals = Zuchthygiene 47 Suppl 4, 92–97, doi: 10.1111/j.1439-0531.2012.02061.x (2012). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. Comparative gene expression signature of pig, human and mouse induced pluripotent stem cell lines reveals insight into pig pluripotency gene networks. Stem cell reviews 10, 162–176, doi: 10.1007/s12015-013-9485-9 (2014). [DOI] [PubMed] [Google Scholar]
- Ying Q. L. et al. The ground state of embryonic stem cell self-renewal. Nature 453, 519–523, doi: 10.1038/nature06968 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevini T., Pennarossa G., Maffei S. & Gandolfi F. Pluripotency network in porcine embryos and derived cell lines. Reproduction in domestic animals = Zuchthygiene 47 Suppl 4, 86–91, doi: 10.1111/j.1439-0531.2012.02060.x (2012). [DOI] [PubMed] [Google Scholar]
- Herlyn D., Herlyn M., Steplewski Z. & Koprowski H. Monoclonal antibodies in cell-mediated cytotoxicity against human melanoma and colorectal carcinoma. European journal of immunology 9, 657–659, doi: 10.1002/eji.1830090817 (1979). [DOI] [PubMed] [Google Scholar]
- Huang H. P. et al. Epithelial cell adhesion molecule (EpCAM) complex proteins promote transcription factor-mediated pluripotency reprogramming. The Journal of biological chemistry 286, 33520–33532, doi: 10.1074/jbc.M111.256164 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T. Y. et al. Epithelial cell adhesion molecule regulation is associated with the maintenance of the undifferentiated phenotype of human embryonic stem cells. The Journal of biological chemistry 285, 8719–8732, doi: 10.1074/jbc.M109.077081 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng V. Y., Ang S. N., Chan J. X. & Choo A. B. H. Characterization of Epithelial Cell Adhesion Molecule as a Surface Marker on Undifferentiated Human Embryonic Stem Cells. Stem cells 28, 29–35, doi: 10.1002/stem.221 (2010). [DOI] [PubMed] [Google Scholar]
- Chen H. F. et al. Surface marker epithelial cell adhesion molecule and E-cadherin facilitate the identification and selection of induced pluripotent stem cells. Stem Cell Rev Rep 7, 722–735, doi: 10.1007/s12015-011-9233-y (2011). [DOI] [PubMed] [Google Scholar]
- Munz M., Baeuerle P. A. & Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer research 69, 5627–5629, doi: 10.1158/0008-5472.CAN-09-0654 (2009). [DOI] [PubMed] [Google Scholar]
- Carpenter G. & Red Brewer M. EpCAM: another surface-to-nucleus missile. Cancer cell 15, 165–166, doi: 10.1016/j.ccr.2009.02.005 (2009). [DOI] [PubMed] [Google Scholar]
- Johannessen M., Moller S., Hansen T., Moens U. & Van Ghelue M. The multifunctional roles of the four-and-a-half-LIM only protein FHL2. Cellular and molecular life sciences: CMLS 63, 268–284, doi: 10.1007/s00018-005-5438-z (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maetzel D. et al. Nuclear signalling by tumour-associated antigen EpCAM. Nature cell biology 11, 162–U117, doi: 10.1038/ncb1824 (2009). [DOI] [PubMed] [Google Scholar]
- Litvinov S. V., Velders M. P., Bakker H. A., Fleuren G. J. & Warnaar S. O. Ep-CAM: a human epithelial antigen is a homophilic cell-cell adhesion molecule. The Journal of cell biology 125, 437–446 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzar M., Winter M. J., de Boer C. J. & Litvinov S. V. The biology of the 17-1A antigen (Ep-CAM). Journal of molecular medicine 77, 699–712 (1999). [DOI] [PubMed] [Google Scholar]
- Li R. et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell stem cell 7, 51–63 (2010). [DOI] [PubMed] [Google Scholar]
- Polo J. M. & Hochedlinger K. When Fibroblasts MET iPSCs. Cell Stem Cell 7, 5–6, doi: 10.1016/j.stem.2010.05.018 (2010). [DOI] [PubMed] [Google Scholar]
- Niwa H. How is pluripotency determined and maintained? Development 134, 635–646, doi: 10.1242/dev.02787 (2007). [DOI] [PubMed] [Google Scholar]
- Vogt E. J., Meglicki M., Hartung K. I., Borsuk E. & Behr R. Importance of the pluripotency factor LIN28 in the mammalian nucleolus during early embryonic development. Development 139, 4514–4523, doi: 10.1242/dev.083279 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N. J. et al. Supplementation With Cell-Penetrating Peptide-Conjugated Estrogen-Related Receptor beta Improves the Formation of the Inner Cell Mass and the Development of Vitrified/Warmed Mouse Embryos. Reproductive sciences 23, 1509–1517, doi: 10.1177/1933719116643594 (2016). [DOI] [PubMed] [Google Scholar]
- Pan X. et al. Site-specific Disruption of the Oct4-Sox2 Interaction Reveals Coordinated Mesendodermal Differentiation and the Epithelial-Mesenchymal Transition. The Journal of biological chemistry, doi: 10.1074/jbc.M116.745414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez B., Denzel S., Mack B., Conrad M. & Gires O. EpCAM is involved in maintenance of the murine embryonic stem cell phenotype. Stem cells 27, 1782–1791, doi: 10.1002/stem.97 (2009). [DOI] [PubMed] [Google Scholar]
- Ikeda S. et al. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. The EMBO journal 17, 1371–1384, doi: 10.1093/emboj/17.5.1371 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Budhu A., Forgues M. & Wang X. W. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer research 67, 10831–10839, doi: 10.1158/0008-5472.CAN-07-0908 (2007). [DOI] [PubMed] [Google Scholar]
- Herlyn M., Steplewski Z., Herlyn D. & Koprowski H. Colorectal carcinoma-specific antigen: detection by means of monoclonal antibodies. Proceedings of the National Academy of Sciences of the United States of America 76, 1438–1442 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle P. A. & Gires O. EpCAM (CD326) finding its role in cancer. British journal of cancer 96, 417–423, doi: 10.1038/sj.bjc.6603494 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. et al. Dynamics of TET family expression in porcine preimplantation embryos is related to zygotic genome activation and required for the maintenance of NANOG. Developmental biology 386, 86–95, doi: 10.1016/j.ydbio.2013.11.024 (2014). [DOI] [PubMed] [Google Scholar]
- Hu J. et al. Vitamin C enhances the in vitro development of porcine pre-implantation embryos by reducing oxidative stress. Reproduction in domestic animals = Zuchthygiene 47, 873–879, doi: 10.1111/j.1439-0531.2011.01982.x (2012). [DOI] [PubMed] [Google Scholar]
- Martello G. et al. Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell 11, 491–504, doi: 10.1016/j.stem.2012.06.008 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warneke V. S. et al. Members of the EpCAM signalling pathway are expressed in gastric cancer tissue and are correlated with patient prognosis. British journal of cancer 109, 2217–2227, doi: 10.1038/bjc.2013.536 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z. et al. Generation of pig induced pluripotent stem cells with a drug-inducible system. Journal of molecular cell biology 1, 46–54, doi: 10.1093/jmcb/mjp003 (2009). [DOI] [PubMed] [Google Scholar]
- Ezashi T. et al. Derivation of induced pluripotent stem cells from pig somatic cells. Proceedings of the National Academy of Sciences of the United States of America 106, 10993–10998, doi: 10.1073/pnas.0905284106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S. et al. Specific gene-regulation networks during the pre-implantation development of the pig embryo as revealed by deep sequencing. BMC genomics 15, 4, doi: 10.1186/1471-2164-15-4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.