Abstract
Sequential infection with different strains of human immunodeficiency virus type 1 (HIV-1) is a rarely identified phenomenon with important implications for immunopathogenesis and vaccine development. Here, we identify an individual whose good initial control of viremia was lost in association with reduced containment of a superinfecting strain. Subject 2030 presented with acute symptoms of HIV-1 infection with high viremia and an incomplete seroconversion as shown by Western blotting. A low set point of viremia (∼1,000 HIV-1 copies/ml) was initially established without drug therapy, but a new higher set point (∼40,000 HIV-1 copies/ml) manifested about 5 months after infection. Drug susceptibility testing demonstrated a multidrug-resistant virus initially but a fully sensitive virus after 5 months, and an analysis of pol genotypes showed that these were two phylogenetically distinct strains of virus (strains A and B). Replication capacity assays suggested that the outgrowth of strain B was not due to higher fitness conferred by pol, and env sequences indicated that the two strains had the same R5 coreceptor phenotype. Delineation of CD8+-T-lymphocyte responses against HIV-1 showed a striking pattern of decay of the initial cellular immune responses after superinfection, followed by some adaptation of targeting to new epitopes. An examination of targeted sequences suggested that differences in the recognized epitopes contributed to the poor immune containment of strain B. In conclusion, the rapid overgrowth of a superinfecting strain of HIV-1 of the same subtype raises major concerns for effective vaccine development.
The global distribution of circulating recombinant forms of human immunodeficiency virus type 1 (HIV-1) M group strains and the high prevalence of intersubtype recombinants in some areas where multiple subtypes are common (reviewed in reference 35) demonstrate that dual infection with different strains of HIV-1 occurs. However, specific examples of dual infection have been documented clearly in only a few cases. Most of these examples have been observations of chronically infected patients with two different subtypes in countries where more than one subtype is prevalent (4, 7, 30, 37, 38), and a few examples of intrasubtype B dual infections have been described (14, 40, 41, 51). Whether these dual infections occurred as coinfections or superinfections is unknown, and the influence of cellular immunity on coexisting viruses has been largely undefined.
A few cases of superinfection, usually involving superinfecting strains that differ in antiretroviral drug susceptibility from the initial variant, have been identified. One individual was initially infected with a strain of HIV-1 belonging to clade CRF01_AE and, after a series of therapy interruptions, became superinfected with a B subtype strain (21). Another individual was initially infected with a drug-resistant strain but became superinfected in the absence of therapy with a drug-sensitive strain of the same subtype (23). One study explored the possible role of cellular immune responses in an individual who was undergoing structured treatment interruptions and had achieved relatively stable control of viremia, subsequently becoming superinfected with another B subtype strain and suffering an acute rise in viremia (2). However, data regarding the role of cellular immunity in superinfection in the absence of drug therapy have been lacking.
We have studied a subject who was initially infected with a multiply drug-resistant strain of HIV-1 subtype B. He controlled the initial infection well in the absence of antiretroviral therapy but became superinfected 4 months later with a second, drug-sensitive strain and showed a marked rise in viremia. Detailed genetic and immunological characterization showed that cytotoxic T-lymphocyte (CTL) responses against the first strain waned with outgrowth of the second strain. The failure of immune containment of the second strain despite early infection and an initially effective HIV-specific CTL response exemplifies a significant challenge to the development of effective vaccines.
MATERIALS AND METHODS
The subject.
Subject 2030 was a 33-year-old homosexual Asian-American male with no significant past medical history who presented after 10 days of an acute illness. He reported multiple ongoing unprotected sexual exposures as a risk factor for HIV-1 infection and was subsequently found to be seropositive. These studies were conducted with appropriate subject consent and approved by the Human Research Protections Program at the Harbor-UCLA Medical Center Research and Education Institute, University of California, Los Angeles, Calif.
HLA typing.
High resolution HLA typing was performed by Pel-Freez Biologicals.
HIV-1 phenotyping.
Antiretroviral susceptibility was determined by PhenoSense (ViroLogic, Inc.) as previously described (36). In this assay, a region extending from p7gag to amino acid 300 in reverse transcriptase (RT) is RT-PCR amplified from plasma viral RNA and inserted into the test vector. This construct, after transfection into the target cells, is used in a single cycle assay in the presence of drug for assaying drug susceptibility; similarly, in the absence of drug, it is used for assaying endogenous replication capacity (9). In both assays, the replication rate is compared to the replication rate of the control strain HIV-1 clone NL4-3.
HIV-1 genomic sequencing and phylogenetics.
Sequencing of viral genomic regions containing CTL epitopes was performed after RT-PCR amplification of plasma viral RNA (extracted with Finnzyme one-step RT-PCR kit, MJ Research, Waltham, Mass., or RNA isolation kit, QIAGEN, Valencia, Calif.). Primers to sequence HIV-1 were those employed by Altfeld et al. (2) (except those for Env), including 737-F (GCG ACT GGT GAG TAC GCC), 2095-R (TTC CCT AAA AAA TTA GCC TG), 1232-F (ACC TAG AAC TTT AAA TGC ATG GG), 1754-R (CAA CAA GGT TTC TGT CAT CC), 1816-F (TAG AAG ACA TGA TGA CAG CAT G), 3018-R (GGT GAT CCT TTC CAT CC), and 2422-R (TCT TAC TTT GAT AAA ACC TCC) (Gag); 5040-F (ATG GAA AAC AGA TGG CAG G), 6455-R (GGG TCT GTG GGT ACA CA), and 5579-R (GGT CTT CTG GGG CTT GTT CC) (Vif); 5692-F (TAT CTA TGA AAC TTA TGG GGA TAC) and 6229-R (CTT TCA TTG CCA CTG TCT TC) (Tat/Rev); 7361-F (TTA ATT GTG GAG GAG AAT TTT T), 9403-R (ACT CCG GAT GCA GCT CTC GGG C), 8804-F (ATG GGT GGC AAG TGG TC), and 9239-R (ACT GGT ACT AGC TTG AAG CAC C) (Nef); V3-F out (CAA AGG TAT CCT TTG AGC CAA T), V3-B out (ATT ACA GTA GAA AAA TTC CCC T), V3-B in (GCG TTA AAG CTT CTG GGT CCC CTC CTG AG), and V3-F in (GAA CAG GAC CAG GAT CCA ATG TCA GCA CAG TAC AAT) (Env). DNA fragments were then gel purified (gel purification kit; QIAGEN) and cloned into the TOPO TA vector (Invitrogen Corp., Carlsbad, Calif.). For each fragment, a minimum of three individual clones were sequenced to obtain consensus sequences for each time point. PCR products were sequenced with Prism Dye terminator kits (ABI, Foster City, Calif.) on an ABI 3100 Genetic Analyzer. Sequences were compiled, aligned, and edited by using Sequencher 4.0 (Genecodes, Ann Arbor, Mich.) and BioEdit (17), and phylogenetic analysis was performed by using MEGA2 (26). Neighbor-joining phylogenetic trees for the reverse transcriptase and protease coding region were obtained from a matrix of synonymous nucleotide distances. This method is most appropriate in regions where there is strong selection on nonsynonymous changes, which otherwise can lead to incorrect trees (demonstrated at http://www.hiv.lanl.gov/content/hiv-db/CONTAM/contam_conserved.html).
ELISPOT analysis of HIV-1-specific CD8+-T-cell responses.
Nonspecifically expanded CD8+ T cells were screened to define HIV-1-specific responses by using a standard gamma interferon enzyme-linked immunospot (ELISPOT) assay as previously described (49). Briefly, cryopreserved peripheral blood mononuclear cells from the indicated times were polyclonally expanded to yield CD3+/CD8+ lymphocytes by using a CD3:CD4-bispecific antibody (50) and evaluated for reactivity against overlapping HIV-1 subtype B 15-mer peptide sets (most of which are based on a consensus sequence) obtained from the NIH AIDS Research and Reference Reagent Repository (ARRRR; catalogue numbers 6869, 6208, 6451, 5189, 5138, 6445, 6446, 6447, and 6444). Initial screening was performed on pools of 16 or fewer peptides, followed by analysis of 4-by-4 matrix pools and confirmation using individual peptides. This method is reported to result in a small but quantitatively consistent bias in the expansion of antigen-specific CD8+ T lymphocytes (3, 20), in agreement with our own experience comparing ELISPOT responses with fresh and expanded CD8+ peripheral blood mononuclear cells (r2 = 0.58 for comparisons within nine chronically infected persons; data not shown).
RESULTS
Clinical presentation and course of subject 2030.
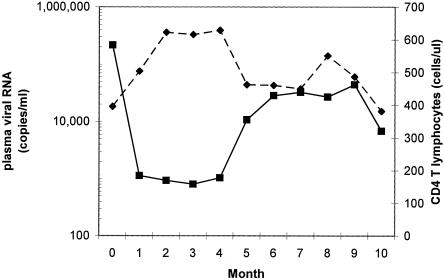
Subject 2030 presented with an acute syndrome of fever, rash, myalgia, cervical lymphadenopathy, oral ulcers, and headache of 10 days duration. Upon presentation, he had a positive serum ELISA for HIV-1 antibodies, a borderline Western blot for HIV-1 (bands for gp160 and p24 only), a peripheral blood CD4+-T-lymphocyte count of 396 cells/μl, and viremia of approximately 300,000 HIV-1 copies of HIV RNA/ml of plasma. Subsequently, the viremia decreased to an initial set point of approximately 1,000 copies/ml, and the CD4+-T-lymphocyte count rose to over 600 cells/μl (Fig. 1). However, after this period of relative stability following acute infection, plasma viremia rose sharply to a new plateau of approximately 30,000 to 40,000 copies/ml at 5 months after presentation, with a concomitant decline in the CD4+-T-lymphocyte count to about 450 cells/μl. Subject 2030 reported ongoing high-risk sexual exposures, raising the possibility that this acute change in set point could be due to HIV-1 superinfection.
FIG. 1.
Subject 2030 plasma HIV-1 and CD4+-T-lymphocyte counts over the first 10 months of infection. Viremia (RNA genomes per ml of plasma; squares) and absolute CD4+-T-lymphocyte concentrations (cells/μl of blood; diamonds) for subject 2030 are plotted.
Dramatic shift in HIV-1 phenotype and genotype indicating infection with two distinct strains.
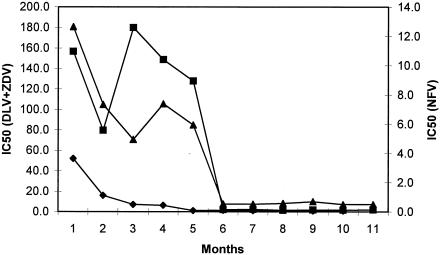
Phenotypic testing revealed that the initial infecting virus was resistant to multiple drugs (Fig. 2). The 50% inhibitory concentration of drug (IC50) of the baseline sample was 50-fold higher than the wild-type control HIV-1NL4-3 for zidovudine, approximately 160-fold higher for delavirdine, and 13-fold higher for nelfinavir. The pol genotype revealed multiple resistance-associated mutations in both protease (PR) and RT: V77I and L90 M in PR; and M41L, K103N, Y181C, T215F, and an insertion of two serine amino acids at position 69.
FIG. 2.
Subject 2030 plasma virus phenotypic susceptibility to delavirdine, zidovudine, and nelfinavir over the first 12 months of infection. Changes (n-fold) in the 50% inhibitory concentration (IC50) are plotted for delavirdine (DLV; squares), and zidovudine (ZDV; diamonds) using the y axis on the left and for nelfinavir (NFV; triangles) using the y axis on the right.
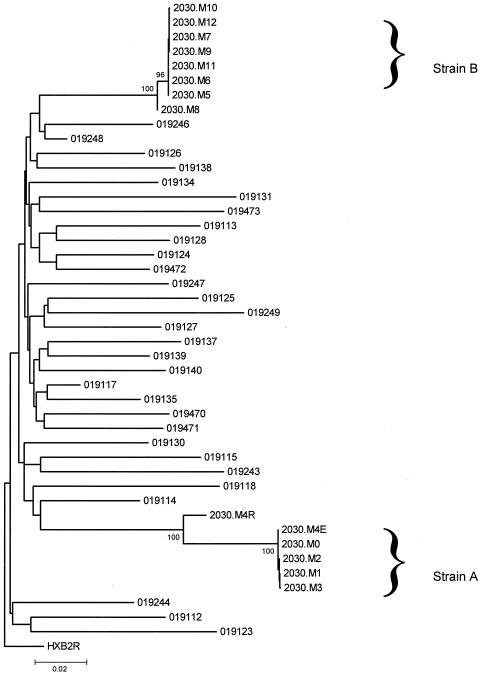
When viremia rose at month 5 (Fig. 1), this initially drug-resistant phenotype abruptly shifted to a phenotype that was susceptible to all tested drugs (Fig. 2). Genotyping at this point revealed none of the prior drug resistance mutations and showed distinct variations at polymorphic sites other than those associated with drug resistance. A phylogenetic analysis of protease and reverse transcriptase for this subject, and for control subjects in the same area with acute HIV-1 infection within the year, confirmed that the initial virus strain (A) and the subsequent strain (B) were phylogenetically distinct (Fig. 3). The genetic distance between strains A and B at synonymous sites within the sequenced gag-pol region was 15.42 ± 2.0%. This genetic distance was greater than that seen in 95% of comparisons among local acute HIV-1 infections involving subtype B (mean, 11.98%; maximum, 16.8%). The nef genes of the strains also were clearly distinct, although not as divergent as gag-pol. Within nef, nonsynonymous sites differed more, on average, between the two than did synonymous sites (dN = 5.7% and dS = 4.5%, as estimated by the modified Nei-Gojobori method with Jukes-Cantor correction for multiple hits). In the C2V3 region of env, however, there is no difference between dN and dS43, and a single overall rate was estimated. On the basis of 12 clonal sequences 409 bp in length from each of the two strains (strain A, month 3; strain B, month 7), the mean genetic distance was 19.2 ± 0.039% (Tamura-Nei model with γ-distributed rates; α value, 0.4). Overall, sequence data from across the genome clearly demonstrated infection of this individual with two distinct HIV-1 strains.
FIG. 3.
Phylogeny of pol sequences for subject 2030 and other HIV-1 infected persons in the Los Angeles area. Serial pol sequences from subject 2030 were compared to those of other HIV-1-infected persons presenting with acute infection in the Los Angeles area from 1998 to 2000. Consensus sequences of PR and 315 codons of RT were used to construct a neighbor-joining tree on synonymous sites by using a modified Nei-Gojobori model, rooted on HIV-1HXB2R (25). The percentage of bootstrap resamples (out of 1,000) in which a clade was identified are shown when above 70%. Sequences from subject 2030 are distinguished by month (2030.M0 to 2030.M12) and identified as strain A and strain B.
Recombination between strains A and B.
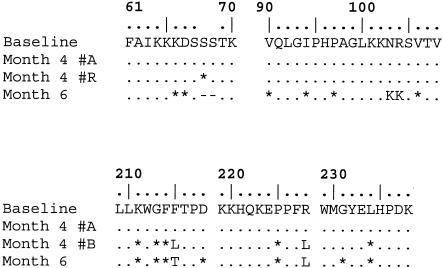
Very little viral sequence diversity accumulated in the first three months of infection, with the exception of amino acid position 215 in RT. At month 4, the consensus sequence contained a series of mixtures at both synonymous and nonsynonymous positions, clustered between amino acids 190 and 300 in RT. These mixtures generally contained one nucleotide identical to the consensus sequence from the first 3 months (strain A) and another nucleotide corresponding to the subsequently distinct consensus sequence at 6 months (strain B; Fig. 4). The most parsimonious reconstruction of the month 4 pol genotype therefore was a mixture of two distinct sequences, one from strain A and a second recombinant of strains A and B. Only at amino acid 215 in RT, where one of the parental alternatives (T) was not detected, was this reconstruction ambiguous. This was the first evidence of the presence of strain B in Subject 2030 and the only evidence of recombination between the two infecting strains detected in this study.
FIG. 4.
Recombinant RT sequence detected in month 4 sample. ., same amino acid as in baseline sequence; *, difference from baseline at synonymous nucleotide site in that codon; -, codon deletion; #A, reconstructed month 4 sequence of strain A type; #R, reconstructed month 4 sequence of recombinant type. The month 4 sequence showed a series of ambiguity codes at sites towards the carboxy-terminal end of the sequenced region, which when resolved always included the nucleotide observed with strain A. This result is most readily interpreted as indicating that a recombinant sequence reached significant frequencies at this time point. No recombinant sequence was observed at any other time point.
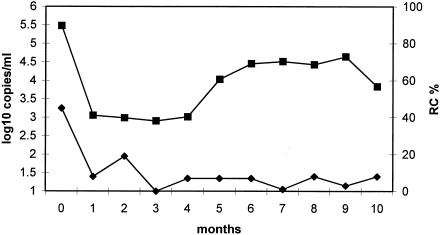
Reduced replicative capacity of pol in strain A compared to strain B.
The phenotypes of the pol genes from the two strains were compared also by using the ViroLogic replication capacity assay (Fig. 5). This assay correlates significantly with in vitro fitness and usually, in the context of drug resistance, with plasma viremia (9). The assay compares the number of infective particles produced (in the absence of drug) by recombinant viruses containing subject-derived gagp7-RT segments in an NL4-3 backbone. Normalized to the average of subject-derived viruses, the mean replicative capacity for strain A (i.e., before month 4) was 35% of wild-type subject-derived virus isolates (range, 64 to 12%), while that for strain B (after month 4) was 8% (12 to 1.5%) of wild-type subject-derived virus isolates. The generally lower value for strain B was consistent with the observation of protease hypersusceptibility of this strain (data not shown), which has been associated with low replicative capacity (29). These data show that the in vivo overgrowth of strain A by strain B was not a result of lower intrinsic replicative ability of strain A associated with drug resistance mutations in pol, raising the question of differential immune containment of the two viruses.
FIG. 5.
Replicative capacity of subject 2030 pol and plasma viremia over time. Replication capacity (as determined by the PhenoSense assay of pol) and plasma viremia are shown over the period of shift from viremia with strain A (months 0 to 3) to that with strain B (months 5 to 10). Squares, viremia (RNA genomes per ml of plasma); diamonds, replicative capacity (RC; percentage of median wild-type value).
Coreceptor usage of strains A and B.
Because the coreceptor usage of HIV-1 determines the cells that can serve as targets of infection and is associated with disease pathogenesis, the usages of strains A and B were determined by sequencing of the C2V3 region of Env. Clonal sequences obtained from the plasma were assessed for amino acids at positions 11 and 25 of V3, because CXCR4 coreceptor usage is determined by specific amino acid substitutions in the V3 region of env, notably basic amino acid substitutions (16). Although the V3 loop sequences of the strains were distinct (12 clonal sequences each from the month 3 and month 12 time points, data not shown), 12 of 12 clones from strain A (month 3) and 11 of 12 clones from strain B (month 12) had serine at position 11. Similarly, 12 of 12 strain A and 11 of 12 strain B sequences had an acidic residue at position 25 (glutamic acid in strain A and aspartic acid in strain B). One strain B clone had asparagine at position 11 and another had asparagine at position 25, but no clone had negatively charged amino acids at both positions. Thus, there was no significant difference between the two strains with respect to predicted coreceptor usage, with both strains being the R5 phenotype.
Evolution of CD8+ cellular immune responses suggesting differential recognition of the viruses.
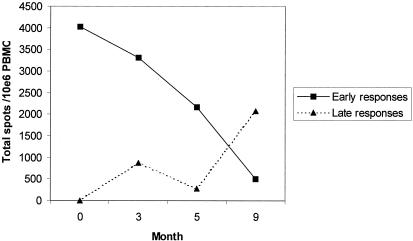
The initially low set point of viremia followed by the subsequent sharp increase (in the absence of antiretroviral therapy) suggested a change in immune control over time. We therefore analyzed the HIV-1-specific CD8+-T-lymphocyte responses by gamma interferon ELISPOT assays (Table 1). Recognition of epitopes in Gag, Nef, and Vif was observed as early as 17 days after the onset of symptoms of acute infection. These early responses generally persisted during the subsequent 3 months, accompanied by broadening to recognize additional epitopes in Gag and Tat. However, after the fifth month, the early responses waned, and new responses were noted in Nef and Rev by the ninth month. Classifying CTL responses as those detected at the first time point (“early responses”) or only afterwards (“late responses”) demonstrated a clear pattern of evolution (Fig. 6), with the magnitude of early responses declining consistently starting in the third month and the magnitude of late responses rising sharply starting in the fifth month. In the context of superinfection and overgrowth of strain A by strain B, these data strongly suggest that the initial CTL responses were specific to strain A and poorly recognized strain B; while there was adaptive retargeting of responses, this retargeting was inadequate to contain strain B to the degree that the initial responses had contained strain A.
TABLE 1.
CTL responses over timea
| Peptide (ARRRR no.) | Location | SFC count on month indicated
|
|||
|---|---|---|---|---|---|
| 0 | 3 | 5 | 9 | ||
| LKETINEEAAEWDRV (5035) | Gag 201-215 (p24) | 0 | 143 | 0 | 0 |
| INEEAAEWDRVHPVH (5036) | Gag 205-219 (p24) | 321 | 253 | 138 | 0 |
| AAEWDRVHPVHAGPI (5037) | Gag 209-223 (p24) | 899 | 341 | 328 | 0 |
| DRVHPVHAGPIAPGQ (5038) | Gag 213-227 (p24) | 45 | 0 | 0 | 0 |
| GPIAPGQMREPRGSD (5040) | Gag 221-235 (p24) | 42 | 0 | 0 | 0 |
| PGQMREPRGSDIAGT (5041) | Gag 225-239 (p24) | 238 | 0 | 0 | 0 |
| EPIDKELYPLTSLRS (5104) | Gag 477-491 (p6) | 0 | 270 | 208 | 0 |
| KIATESIVIWGKTPK (5593) | Pol 529-543 (RT) | 0 | 0 | 65 | 0 |
| WPTVRERMRRAEPAA (5142) | Nef 13-27 | 0 | 0 | 0 | 60 |
| EVGFPVRPQVPLRPM (5155) | Nef 65-79 | 0 | 255 | 0 | 448 |
| PGPGIRYPLTFGWCF (5171) | Nef 129-143 | 231 | 927 | 463 | 401 |
| IRYPLTFGWCFKLVP (5172) | Nef 133-147 | 704 | 1,185 | 559 | 0 |
| KCCFHCQVCFTTKGL (5120) | Tat 29-43 | 0 | 148 | 0 | 0 |
| DEELLKTVRLIKFLY (5993) | Rev 9-23 | 0 | 0 | 0 | 764 |
| LKTVRLIKFLYQSNP (5994) | Rev 13-27 | 0 | 0 | 0 | 789 |
| YWGLHTGERDWHLGQ (6035) | Vif 69-83 | 704 | 215 | 268 | 43 |
| HTGERDWHLGQGVSI (6036) | Vif 73-87 | 831 | 361 | 376 | 62 |
CTL responses identified by ELISPOT screening against overlapping peptides (obtained from the NIH ARRRR) are shown. The locations of the peptides (in relationship to the HXB2 sequence) are given. Values are SFC per 106 CD8+ T lymphocytes (means of results of duplicate experiments).
FIG. 6.
Dynamics of early and subsequent CTL responses for subject 2030. The sum of CTL frequencies for responses detected upon initial testing (month 0) are plotted over time, in comparison to the sum of frequencies of CTL responses detected only after initial testing. PBMC, peripheral blood mononuclear cells.
Differences in strain A and strain B CTL epitopes.
To examine whether sequence differences might account for differential immune control of the two viruses, regions targeted by CTL were sequenced from strains A and B (Table 2). The in vivo viral sequences corresponding to the recognized screening peptides were compared in the context of the HLA type of the subject (A*03, A*24, B*35, and B*40). Of responses that exceeded 100 spot-forming cells (SFC)/106 CD8+ T lymphocytes, the majority that waned after superinfection contained potentially significant differences in strain B compared to strain A. These differences included an insertion of four amino acids flanking a predicted epitope (Table 2, row 4) and changes within the TCR-binding region (Table 2, rows 6 and 8) or HLA-binding motif (Table 2, row 9) of the predicted epitopes in four of six examples. Two of these six examples (Table 2, rows 1 and 2) showed no difference between the strains. However, it is clear that this waning of the initial responses was not due to generalized immune deficiency, because a vigorous new response against Rev was generated after superinfection (Table 2, row 8). The recognized region of Rev contained two amino acid differences in the TCR-binding region of the predicted epitope in strain A compared to strain B, and the screening peptide was identical to strain B in this sequence. Furthermore, the persistence of an early response recognizing a predicted epitope that was identical in strains A and B (Table 2, row 5) indicated that the decay of the early responses was selective. Overall, these findings are consistent with significant epitope differences in the two viruses having a central role in differential immune containment by CTL and persistence of the CTL response.
TABLE 2.
Epitope sequence comparisons of strain A and strain Ba
| Recognized peptide | Presumed epitope (HLA) | Epitope location | Sequence for strain A and strain B | ΔCTL |
|---|---|---|---|---|
| LKETINEEAAEWDRV | EEAAEWDRV (B*40)c | Gag 207-215 (p24) | EEAAEWDRL | ↓ |
| EEAAEWDRL | ||||
| INEEAAEWDRVHPVH ....AAEWDRVHPVHAGPI | AEWDRVHPV (B*40)b | Gag 210-218 (p24) | AEWDRLHPV | ↓ |
| AEWDRLHPV | ||||
| PGQMREPRGSDIAGT | Unknown | (Gag 225-239 [p24]) | (PGQMREPRGSDIAGT) | ± |
| (PGQMREPRGSDIAGT) | ||||
| EPIDKELYPLTSLRS | KELYPLTSL (B*40)b | Gag 481-489 (p6) | KELYPLASL | ↓ |
| RGIDKELYPLASLd | ||||
| EVGFPVRPQVPLRPM | FPVRPQVPL (B*35)b | Nef 68-76 | FPVRPQVPL | ↔ |
| FPVRPQVPL | ||||
| PGPGIRYPLTFGWCF ....IRYPLTFGWCFKLVP | YPLTFGWCF (B*35)b or RYPLTFGWCF (A*24)b | Nef 135-143 or 134-143 | YPLTFGWCF or RYPLTFGWCF | ↓ |
| YPLCFGWCF or RYPLCFGWCF | ||||
| KCCFHCQVCFTTKGL | Unknown | (Tat 29-43) | (OCCFHCQVCFITKGL) | ↓ |
| (KCCLHCQVCFTRKGL) | ||||
| DEELLKTVRLIKFLY ....LKTVRLIKFLYQSNP | TVRLIKFLY (A*03)c | Rev 15-23 | TVKIIKFLY | ↑ |
| TVRLIKFLY | ||||
| YWGLHTGERDWHLGQ ....HTGERDWHLGQGVSI | HTGERDWHL (B*35)c | Vif 73-81 | HPGERDWHL | ↓ |
| HTGERDWHL |
For peptides recognized in the ELISPOT assay at a frequency of at least 100 SFC/106 CD8+ T lymphocytes, the following are listed: the presumed epitope and its location, the sequence of the epitope in strain A (top) and strain B (bottom; differences from the screening sequence are shown in underlined boldface type), and the change of the CTL response following superinfection. Putative locations and sequences are shown in parentheses. ↓, Present before superinfection and waned after superinfection; ↑, present only after superinfection; ↔, persisting before and after superinfection; ±, present early but waned before superinfection.
Previously reported epitope consistent with subject's known HLA type (A*03, A*24, B*35, B*40).
Inferred from the HLA binding motifs of the subject's known HLA type.
Insertion of four amino acids.
DISCUSSION
Previous studies of the evolution of the HIV-1 quasispecies within infected individuals led to the widespread belief that superinfection is a rare event (5, 10-13, 18, 27, 28, 31, 33, 34, 39, 42-48). While dual infection was demonstrated to be possible (51), only recently have clear examples of superinfection emerged (2, 21). These two cases demonstrated superinfection in subjects who underwent structured treatment interruptions with partial control of viremia, presumed to be immunologically mediated. Other recent studies, including a third case in which coinfection or superinfection occurred in the absence of therapy (23), suggest that it can occur in the setting of natural infection without alteration of immunity by pharmacologic intervention. The case described here extends these novel findings and clearly documents superinfection after an initial infection reaching a stable set point in the absence of antiretroviral treatment as a confounding factor.
It is impossible to entirely exclude the possibility that a superinfecting strain was present as an initial coinfection. In the case of subject 2030, however, overgrowth by strain B occurred rapidly and completely once it was detected, showing that its overall growth advantage in vivo was great. If present during acute infection, this overgrowth should have occurred much earlier than 4 months after infection. This example is striking because superinfection was clearly documented after a stable viremia set point was established, in contrast to most published reports. The subject had developed a broad and stable CTL response to the first virus and reached an equilibrium of viremia, suggesting efficient immune suppression of viral replication. Strain B, which was highly genetically distinct from strain A, was detectable only at 4 months after primary infection, but it rapidly overgrew strain A with a substantial increase in viremia set point. A fitness assessment of the pol genes showed a lower replication capacity for strain B than strain A, suggesting that substantially greater intrinsic replicative fitness due to differences in pol was not the mechanism of this overgrowth. Although fitness differences due to genes other than pol have not been excluded, the high level of viremia during primary infection suggested that strain A was not generally defective in replicative capacity. Furthermore, an evaluation of coreceptor usage revealed that both strains were predominately the R5 phenotype, making it unlikely that a difference in tropism could account for differences in replication in vivo. This finding indicates that differential immune containment of strain B versus strain A could be the key factor.
Consistent with the report on immune responses of a subject who had superinfection after structured treatment interruption (2), we found that our subject had developed a stable and broadening repertoire of CTL responses against the initial virus, which failed to prevent or contain infection with the second virus. Sampling of epitope sequences revealed significant differences between the viruses, suggesting that the mechanism of immune failure was nonrecognition of the second strain. Strikingly, the majority of CTL responses decayed rapidly after superinfection, similar to the observed loss of CTL after epitope mutation and escape occurring in chronically infected persons (19). Furthermore, while new CTL responses were detected after superinfection, these were fewer and failed to contain the second virus. This result could have been a consequence of the divergent sequence of the superinfecting strain or might imply a role for a mechanism, such as original antigenic sin (22), limiting the adaptability of CTL responses after initial infection.
The marked rise in viremia after superinfection is consistent with another detailed study of superinfection in the setting of immune control achieved by structured treatment interruption therapy in early infection (2). This phenomenon is also reminiscent of the increased viremia that has been observed with escape occurring during natural infection (15, 32) and reinfusion of an ex vivo expanded CTL clone (24) and associated with SIV vaccine failure in macaques (6). The ability of CTL to recognize the challenging viral sequences thus appears to be a crucial determinant of immune control.
Superinfection and loss of immune control in this subject, despite apparently effective CTL responses to the initial strain, may have serious implications for vaccine design. The determinants of an effective CTL response against HIV-1 remain unknown, and attempts to correlate the magnitude or breadth of the response (8) to control of viremia generally have been disappointing (1). Our data, in agreement with those of Altfeld et al. (2), indicate that protective CTL targeting against HIV-1 may vary not only by subtype of virus but even by individual strains. Given the great variability of HIV-1, this phenomenon may pose a significant obstacle to generating protective immunity with a fixed vaccine sequence.
Acknowledgments
This work was supported by grants AI27670, AI38858, AI43638, AI43203, AI41224, AI27660, AI055276, UCSD Center for AIDS Research grant AI36214, and grant AI29164 from the National Institutes of Health, the Research Center for AIDS and HIV Infection of the San Diego Veterans Affairs Healthcare System, and General Clinical Research Center, National Center for Research Resources grant M01-RR00425.
REFERENCES
- 1.Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altfeld, M., T. M. Allen, X. G. Yu, M. N. Johnston, D. Agrawal, B. T. Korber, D. C. Montefiori, D. H. O'Connor, B. T. Davis, P. K. Lee, E. L. Maier, J. Harlow, P. J. Goulder, C. Brander, E. S. Rosenberg, and B. D. Walker. 2002. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature 420:434-439. [DOI] [PubMed] [Google Scholar]
- 3.Altfeld, M., J. van Lunzen, N. Frahm, X. G. Yu, C. Schneider, R. L. Eldridge, M. E. Feeney, D. Meyer-Olson, H. J. Stellbrink, and B. D. Walker. 2002. Expansion of pre-existing, lymph node-localized CD8+ T cells during supervised treatment interruptions in chronic HIV-1 infection. J. Clin. Investig. 109:837-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artenstein, A. W., T. C. VanCott, J. R. Mascola, J. K. Carr, P. A. Hegerich, J. Gaywee, E. Sanders-Buell, M. L. Robb, D. E. Dayhoff, S. Thitivichianlert, et al. 1995. Dual infection with human immunodeficiency virus type 1 of distinct envelope subtypes in humans. J. Infect. Dis. 171:805-810. [DOI] [PubMed] [Google Scholar]
- 5.Balfe, P., P. Simmonds, C. A. Ludlam, J. O. Bishop, and A. J. Leigh Brown. 1990. Concurrent evolution of human immunodeficiency virus type 1 in patients infected from the same source: rate of sequence change and low frequency of inactivating mutations. J. Virol. 64:6221-6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. [DOI] [PubMed] [Google Scholar]
- 7.Becker-Pergola, G., J. L. Mellquist, L. Guay, F. Mmiro, C. Ndugwa, P. Kataaha, J. B. Jackson, and S. H. Eshleman. 2000. Identification of diverse HIV type 1 subtypes and dual HIV type 1 infection in pregnant Ugandan women. AIDS Res. Hum. Retrovir. 16:1099-1104. [DOI] [PubMed] [Google Scholar]
- 8.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell, T. B., K. Schneider, T. Wrin, C. J. Petropoulos, and E. Connick. 2003. Relationship between in vitro human immunodeficiency virus type 1 replication rate and virus load in plasma. J. Virol. 77:12105-12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheynier, R., S. Henrichwark, F. Hadida, E. Pelletier, E. Oksenhendler, B. Autran, and S. Wain-Hobson. 1994. HIV and T cell expansion in splenic white pulps is accompanied by infiltration of HIV-specific cytotoxic T lymphocytes. Cell 78:373-387. [DOI] [PubMed] [Google Scholar]
- 11.Delassus, S., R. Cheynier, and S. Wain-Hobson. 1991. Evolution of human immunodeficiency virus type 1 nef and long terminal repeat sequences over 4 years in vivo and in vitro. J. Virol. 65:225-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delassus, S., R. Cheynier, and S. Wain-Hobson. 1992. Nonhomogeneous distribution of human immunodeficiency virus type 1 proviruses in the spleen. J. Virol. 66:5642-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delwart, E. L., H. W. Sheppard, B. D. Walker, J. Goudsmit, and J. I. Mullins. 1994. Human immunodeficiency virus type 1 evolution in vivo tracked by DNA heteroduplex mobility assays. J. Virol. 68:6672-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz, R. S., E. C. Sabino, A. Mayer, J. W. Mosley, M. P. Busch, and The Transfusion Safety Study Group. 1995. Dual human immunodeficiency virus type 1 infection and recombination in a dually exposed transfusion recipient. J. Virol. 69:3273-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feeney, M. E., Y. Tang, K. A. Roosevelt, A. J. Leslie, K. McIntosh, N. Karthas, B. D. Walker, and P. J. Goulder. 2004. Immune escape precedes breakthrough human immunodeficiency virus type 1 viremia and broadening of the cytotoxic T-lymphocyte response in an HLA-B27-positive long-term-nonprogressing child. J. Virol. 78:8927-8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fouchier, R. A., M. Groenink, N. A. Kootstra, M. Tersmette, H. G. Huisman, F. Miedema, and H. Schuitemaker. 1992. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule. J. Virol. 66:3183-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis suite. North Carolina State University, Raleigh, N.C.
- 18.Holmes, E. C., L. Q. Zhang, P. Simmonds, C. A. Ludlam, and A. J. Leigh Brown. 1992. Convergent and divergent sequence evolution in the surface envelope glycoprotein of human immunodeficiency virus type 1 within a single infected patient. Proc. Natl. Acad. Sci. USA 89:4835-4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamieson, B. D., O. O. Yang, L. Hultin, M. A. Hausner, P. Hultin, J. Matud, K. Kunstman, S. Killian, J. Altman, K. Kommander, B. Korber, J. Giorgi, and S. Wolinsky. 2003. Epitope escape mutation and decay of human immunodeficiency virus type 1-specific CTL responses. J. Immunol. 171:5372-5379. [DOI] [PubMed] [Google Scholar]
- 20.Jones, N., D. Agrawal, M. Elrefaei, A. Hanson, V. Novitsky, J. T. Wong, and H. Cao. 2003. Evaluation of antigen-specific responses using in vitro enriched T cells. J. Immunol. Methods 274:139-147. [DOI] [PubMed] [Google Scholar]
- 21.Jost, S., M. C. Bernard, L. Kaiser, S. Yerly, B. Hirschel, A. Samri, B. Autran, L. E. Goh, and L. Perrin. 2002. A patient with HIV-1 superinfection. N. Engl. J. Med. 347:731-736. [DOI] [PubMed] [Google Scholar]
- 22.Klenerman, P., and R. M. Zinkernagel. 1998. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature 394:482-485. [DOI] [PubMed] [Google Scholar]
- 23.Koelsch, K. K., D. M. Smith, S. J. Little, C. C. Ignacio, T. R. Macaranas, A. J. Leigh Brown, C. J. Petropoulos, D. D. Richman, and J. K. Wong. 2003. Clade B HIV-1 superinfection with wild-type virus after primary infection with drug-resistant clade B virus. AIDS 17:F11-F16. [DOI] [PubMed] [Google Scholar]
- 24.Koenig, S., A. J. Conley, Y. A. Brewah, G. M. Jones, S. Leath, L. J. Boots, V. Davey, G. Pantaleo, J. F. Demarest, C. Carter, et al. 1995. Transfer of HIV-1 specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nat. Med. 1:330-336. [DOI] [PubMed] [Google Scholar]
- 25.Kuiken, C., B. Foley, B. Hahn, B. Korber, P. A. Marx, F. McCutchan, J. W. Mellors, J. I. Mullins, and S. Wolinsky. 2002. HIV sequence compendium 2001. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 26.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Arizona State University, Tempe, Ariz. [DOI] [PubMed]
- 27.Kusumi, K., B. Conway, S. Cunningham, A. Berson, C. Evans, A. K. Iversen, D. Colvin, M. V. Gallo, S. Coutre, E. G. Shaper, D. V. Faulkner, A. DeRonde, S. Volkman, C. Williams, M. S. Hirsch, and J. I. Mullins. 1992. Human immunodeficiency virus type 1 envelope gene structure and diversity in vivo and after cocultivation in vitro. J. Virol. 66:875-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Learn, G. H., D. Muthui, S. J. Brodie, T. Zhu, K. Diem, J. I. Mullins, and L. Corey. 2002. Virus population homogenization following acute human immunodeficiency virus type 1 infection. J. Virol. 76:11953-11959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leigh Brown, A. J., S. D. W. Frost, B. Good, E. S. Daar, V. Simon, M. Markowitz, A. C. Collier, E. Connick, B. Conway, J. B. Margolick, J.-P. Routy, J. Corbeil, N. Hellmann, D. D. Richman, and S. J. Little. 2004. Genetic basis of hypersusceptibility to protease inhibitors and low replicative capacity of human immunodeficiency virus type 1 strains in primary infection. J. Virol. 78:2242-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mellquist, J. L., G. Becker-Pergola, J. Gu, L. Guay, L. Himes, P. Kataaha, F. Mmiro, C. Ndugwa, J. B. Jackson, and S. H. Eshleman. 1999. Dual transmission of subtype A and D HIV type 1 viruses from a Ugandan woman to her infant. AIDS Res. Hum. Retrovir. 15:217-221. [DOI] [PubMed] [Google Scholar]
- 31.Meyerhans, A., R. Cheynier, J. Albert, M. Seth, S. Kwok, J. Sninsky, L. Morfeldt Manson, B. Asjo, and S. Wain-Hobson. 1989. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell 58:901-910. [DOI] [PubMed] [Google Scholar]
- 32.Oxenius, A., D. A. Price, A. Trkola, C. Edwards, E. Gostick, H. T. Zhang, P. J. Easterbrook, T. Tun, A. Johnson, A. Waters, E. C. Holmes, and R. E. Phillips. 2004. Loss of viral control in early HIV-1 infection is temporally associated with sequential escape from CD8+ T cell responses and decrease in HIV-1-specific CD4+ and CD8+ T cell frequencies. J. Infect. Dis. 190:713-721. [DOI] [PubMed] [Google Scholar]
- 33.Pedroza Martins, L., N. Chenciner, B. Åsjö, A. Meyerhans, and S. Wain-Hobson. 1991. Independent fluctuation of human immunodeficiency virus type 1 rev and gp41 quasispecies in vivo. J. Virol. 65:4502-4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedroza Martins, L., N. Chenciner, and S. Wain-Hobson. 1992. Complex intrapatient sequence variation in the V1 and V2 hypervariable regions of the HIV-1 gp 120 envelope sequence. Virology 191:837-845. [DOI] [PubMed] [Google Scholar]
- 35.Peeters, M. 2000. Recombinant HIV sequences: their role in the global epidemic, p. I-39-I-54. In C. L. Kuiken, B. Foley, B. Hahn, B. Korber, F. McCutchan, P. A. Marx, J. W. Mellors, J. I. Mullins, J. Sodroski, and S. Wolinsky (ed.), HIV sequence compendium 2000. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.Mex.
- 36.Petropoulos, C. J., N. T. Parkin, K. L. Limoli, Y. S. Lie, T. Wrin, W. Huang, H. Tian, D. A. Smith, G. A. Winslow, D. J. Capon, and J. M. Whitcomb. 2000. A novel phenotypic drug susceptibility assay for HIV-1. Antimicrob. Agents Chemother. 44:920-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramos, A., D. J. Hu, L. Nguyen, K. O. Phan, S. Vanichseni, N. Promadej, K. Choopanya, M. Callahan, N. L. Young, J. McNicholl, T. D. Mastro, T. M. Folks, and S. Subbarao. 2002. Intersubtype human immunodeficiency virus type 1 superinfection following seroconversion to primary infection in two injection drug users. J. Virol. 76:7444-7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramos, A., A. Tanuri, M. Schechter, M. A. Rayfield, D. J. Hu, M. C. Cabral, C. I. Bandea, J. Baggs, and D. Pieniazek. 1999. Dual and recombinant infections: an integral part of the HIV-1 epidemic in Brazil. Emerg. Infect. Dis. 5:65-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodrigo, A. G., E. G. Shpaer, E. L. Delwart, A. K. Iversen, M. V. Gallo, J. Brojatsch, M. S. Hirsch, B. D. Walker, and J. I. Mullins. 1999. Coalescent estimates of HIV-1 generation time in vivo. Proc. Natl. Acad. Sci. USA 96:2187-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sala, M., E. Pelletier, and S. Wain-Hobson. 1995. HIV-1 gp120 sequences from a doubly infected drug user. AIDS Res. Hum. Retrovir. 11:653-655. [DOI] [PubMed] [Google Scholar]
- 41.Sala, M., G. Zambruno, J. P. Vartanian, A. Marconi, U. Bertazzoni, and S. Wain-Hobson. 1994. Spatial discontinuities in human immunodeficiency virus type 1 quasispecies derived from epidermal Langerhans cells of a patient with AIDS and evidence for double infection. J. Virol. 68:5280-5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shankarappa, R., J. B. Margolick, S. J. Gange, A. G. Rodrigo, D. Upchurch, H. Farzadegan, P. Gupta, C. R. Rinaldo, G. H. Learn, X. He, X. L. Huang, and J. I. Mullins. 1999. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J. Virol. 73:10489-10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simmonds, P., P. Balfe, C. A. Ludlam, J. O. Bishop, and A. J. Leigh Brown. 1990. Analysis of sequence diversity in hypervariable regions of the external glycoprotein of human immunodeficiency virus type 1. J. Virol. 64:5840-5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simmonds, P., L. Q. Zhang, F. McOmish, P. Balfe, C. A. Ludlam, and A. J. Leigh Brown. 1991. Discontinuous sequence change of human immunodeficiency virus (HIV) type 1 env sequences in plasma viral and lymphocyte-associated proviral populations in vivo: implications for models of HIV pathogenesis. J. Virol. 65:6266-6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vartanian, J. P., A. Meyerhans, M. Henry, and S. Wain-Hobson. 1992. High-resolution structure of an HIV-1 quasispecies: identification of novel coding sequences. AIDS 6:1095-1098. [DOI] [PubMed] [Google Scholar]
- 46.Wolfs, T. F., J. J. de Jong, H. Van den Berg, J. M. Tijnagel, W. J. Krone, and J. Goudsmit. 1990. Evolution of sequences encoding the principal neutralization epitope of human immunodeficiency virus 1 is host dependent, rapid, and continuous. Proc. Natl. Acad. Sci. USA 87:9938-9942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolfs, T. F., G. Zwart, M. Bakker, M. Valk, C. L. Kuiken, and J. Goudsmit. 1991. Naturally occurring mutations within HIV-1 V3 genomic RNA lead to antigenic variation dependent on a single amino acid substitution. Virology 185:195-205. [DOI] [PubMed] [Google Scholar]
- 48.Wolfs, T. F. W., G. Zwart, M. Bakker, and J. Goudsmit. 1992. HIV-1 genomic RNA diversification following sexual and parenteral virus transmission. Virology 189:103-110. [DOI] [PubMed] [Google Scholar]
- 49.Yang, O. O., W. J. Boscardin, J. Matud, M. A. Hausner, L. E. Hultin, P. M. Hultin, R. Shih, J. Ferbas, F. P. Siegal, M. Shodell, G. M. Shearer, E. Grene, M. Carrington, S. O'Brien, C. B. Price, R. Detels, B. D. Jamieson, and J. V. Giorgi. 2002. Immunologic profile of highly exposed yet HIV type 1-seronegative men. AIDS Res. Hum. Retrovir. 18:1051-1065. [DOI] [PubMed] [Google Scholar]
- 50.Yang, O. O., S. A. Kalams, A. Trocha, H. Cao, A. Luster, R. P. Johnson, and B. D. Walker. 1997. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 71:3120-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu, T., N. Wang, R. Carr, S. M. Wolinsky, and D. D. Ho. 1995. Evidence for coinfection by multiple strains of human immunodeficiency virus type 1 subtype B in an acute seroconvertor. J. Virol. 69:1324-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]