Abstract
Laboratory medicine approaches the evaluation of thyroid function mostly through the single determination of the blood level of thyroid stimulating hormone (TSH). Some authors have suggested an upper reference value for TSH of 2.5 mIU/L. This suggestion has not been confirmed by recent clinical studies. These studies have delivered a clinically valid reference range going from 0.3 to 3.5 mIU/L. These values are valid for both for the general population as well as in the setting of fertility and pregnancy.
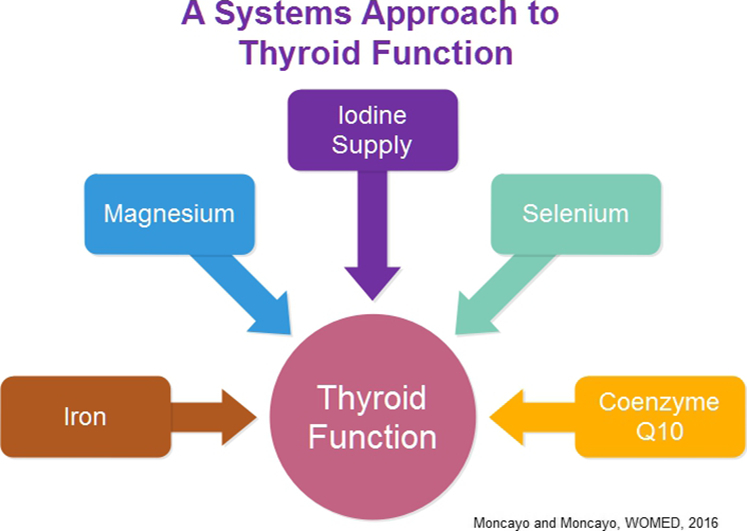
Current biochemical evidence about the elements required to maintain thyroid function shows that these not only include dietary iodine but also magnesium, iron, selenium and coenzyme Q10. Iron is important for the synthesis of thyroid peroxidase; magnesium-ATP contributes to the active process of iodine uptake; iodine has to be sufficiently present in the diet; selenium acts through selenoproteins to protect the thyroid cell during hormone synthesis and in deiodination of thyroxine; coenzyme Q10 influences thyroid vascularity. As a consequence, good clinical practice requires additional biochemical information on the blood levels of magnesium, selenium, coenzyme Q10 as well as iron status.
Since these elements are also important for the maintenance of reproductive function, we postulate that they constitute the connecting link between both endocrine systems.
Graphical abstract
1. Introduction
Clinical practice guides aiming at the improvement of the medical diagnostic and therapeutic process in many medical fields have been published over the past years [1]. Going along with this trend, laboratory medicine recommendations for the interpretation of thyrotropin stimulating hormone (TSH) values appeared in the literature in 2003 and 2007. The first publication came from Baloch and co-workers and was entitled: “Laboratory medicine practice guidelines”, a declaration which implied that clinical data were excluded. The authors made a suggestion about a theoretical upper reference value for TSH [2] by stating: “In the future, it is likely that the upper limit of the serum TSH euthyroid reference range will be reduced to 2.5 mIU/L” (page 34 in [2]). In 2007 Abalovich and co-workers [3] proposed a “desirable TSH level” of 2.5 mIU/L for pregnant women [3]. This report explicitly declared that this recommendation was done on the basis of a poor level of evidence (section 1.1.2. in [3]). With these publications the age of an idealized “likely upper limit” and “desirable TSH level” was started. It has to be stressed that these 2 publications failed to provide any demographic data of the study populations. Consequently the authors could not present any valid statistical evaluations. These 2 serious drawbacks place both publications in the category of low hierarchy level in terms of evidence-based medicine (EBM) [4].
The introduction of a lower upper range value for the evaluation of TSH produced serious alterations in the clinical routine. Clinicians have expressed their concerns arising from the sudden change in diagnostic classification parameters. Fatourechi et al. [5] commented that this measure would lead to an increase of 20% of subjects being “reclassified” from normal to having biochemical hypothyroidism. Such new patients would also require a treatment with thyroid hormones. Following the postulates of Archie Cochrane [6] a treatment should significantly alter the natural history of a disease for the better. To ensure this, the basic diagnostic data have to be reliable in order to avoid errors of falsification [7] and consequently of treatment. One practical way to do this could be to wisely choose a test for any disease following the proposal presented by Evers, Land and Mol [8]. The clue questions in their proposal include: “(1) Is this evidence about a diagnostic test valid? (2) Does the test accurately discriminate between patients who do and patients who do not have a specific disorder? (3) Can the test be applied to this patient who is right now sitting in front of me?”
Keeping in mind current methods of post publication analysis [9], [10] as well as the key postulate of Hilda Bastian on the post-publication culture as to avoid passive consumption of publications [11] we would like to look at the aftermath that has taken place after 2003–2007 in the field of thyroid diseases. This description will be based in part on publications coming from observational studies that originate from our own joint clinical experiences in the fields of reproduction medicine and thyroid diseases. We will consider three topics: 1) studies dealing with reference levels for TSH in the general population and pregnancy, 2) studies on thyroid function and fertility, and 3) a novel systems approach for the description of thyroid function.
2. Clinical studies dealing with reference levels for TSH for adults
In 2005 Kratzsch et al. [12] re-evaluated the topic of reference values for thyroid function investigating 870 subjects. The study included an ultrasound examination in each case. The authors reported a reference range for TSH of 0.3 to 3.63 mIU/L. In 2007 [13] we presented our clinical evaluation of thyroid function tests working with 2570 women in reproductive age and arrived at reference values for TSH of 0.3 to 3.5 mIU/L. These results were validated by the use of a TRH stimulation test in order to define euthyroidism. This publication contains a comparison table where relevant data published by other authors are summarized. The comparison adds to a total number of 69,659 cases. In 2014 Langén et al. [14] carried out a nationwide random sample analysis of the Finnish population with 5709 subjects and came to similar conclusions, i.e. a reference range for TSH between 0.4 and 3.4 mIU/L [14].
Voigtländer and Führer [15] analyzed literature data related to the conflict arising from making a laboratory diagnosis of subclinical hypothyroidism. They recommended that the term subclinical hypothyroidism should not be applied to TSH levels < 4.5 mIU/L.
The suggested reference value of 2.5 mIU/L has also been evaluated in some clinical areas. In relation to coronary heart disease Åsvold et al. [16] (n = 55,412) have observed that lowering the upper TSH reference value did not show any association with events of coronary heart disease. A similar observation has been made by Samuels et al. [17] (n = 132) in relation to health status, cognition and mood. Walsh et al. [18] evaluated whether changing the dose of thyroxine treatment can have an effect on well-being. A series of 52 women were investigated. Using different doses of thyroxine, different TSH levels were achieved. These subgroups showed no significant treatment effects on well-being. They concluded that the target TSH range should not differ from the general laboratory range.
3. Clinical studies dealing with reference levels for TSH in pregnancy
Several authors have evaluated the normal range of thyroid parameters in the setting of pregnancy. Working in an area with sufficient iodine supply, i.e. Innsbruck, Austria, we have evaluated thyroid function parameters in women who had had normal pregnancies [19] (n = 2028). The reference values which we found for TSH were 0.3–3.5 mIU/L. These values did not differ from those obtained in a general female population [13]. The same values also apply to women undergoing in-vitro fertilization (IVF) procedures [20]. In this special setting of IVF, however, caution has to be used when measuring TSH at an early time of pregnancy since blood levels of TSH and fT4 show a unique positive feedback situation with slightly elevated TSH levels. This TSH peculiarity has also been observed by other authors [21]. Parallel to this early elevation of TSH levels we also observed a reciprocal decrease of magnesium levels.
Another issue of interest in the context of pregnancy is the hypothesis that elevated TSH levels could provide a hint as to a compromised cognitive development in children. This concept can be traced back to the investigations done by Elisabeth Man between 1941 and 1972 [22]. In her publications she emphasized the need to treat maternal hypothyroidism sufficiently. This was a difficult task many years ago considering that the first branded L-thyroxine was available only since the 1950s [23]. This situation has improved significantly in the past years, i.e. treatment options are available now worldwide. In 2012 Lazarus et al. [24] showed that treating maternal hypothyroidism did not lead to better cognitive function in children at age 3 years. In 2016 Trumpff et al. [25] demonstrated that thyroid dysfunction of mild degree in the newborn (n = 284), i.e. below a TSH threshold of 5–15 mIU/L, has no connection to impaired psychomotor development in children of preschool age. It has to be noted that studies on thyroid function in children (n = 2194) have to rely on different reference values since they vary with age and sex. In a similar fashion, studies dealing with cognition should observe that this function has multifactorial determinants [26]. One important single element to cognition is iron as it can normalize the cognitive function in young women [27].
4. Thyroid function, thyroid antibodies and fertility
Several publications dealing with thyroid function or thyroid antibodies in the context of fertility have relied on the upper range value for TSH of 2.5 mIU/L. This strategy has resulted in the publication of studies that have presented conflicting results and opinions. One example is the 2013 publication by Bernardi et al. in Fertility Sterility [28]. The authors dealt with the topic of subclinical hypothyroidism and recurrent early pregnancy loss. Their own conclusions dismissed any influence of classifying patients as being subclinical hypothyroid. In 2014 [29] we commented on the clinical weakness this study which came from the use of an arbitrary upper range value for TSH. In 2015 The Practice Committee of the American Society for Reproductive Medicine (ASRM) evaluated the topic extensively and failed to confirm the clinical value of a lowered upper reference value for TSH [30]. Two of their conclusions stated: 1) “There is insufficient evidence that SCH (defined as TSH > 2.5 mIU/L with a normal FT4) is associated with infertility”; 2) “There is fair evidence that SCH, defined as TSH levels > 4 mIU/L, is associated with miscarriage, but insufficient evidence that TSH levels 2.5–4 mIU/L are associated with miscarriage” (both on page 551 in [30]). In 2015 Mintziori et al. working in the setting of in-vitro fertilization (IVF) described the threshold for initiating a therapy with thyroid hormones as a TSH value > 4.0–4.5 mIU/L [31]. The authors had previously shown that there was no relation of pregnancy outcome for TSH values within the normal range [32]. The recent publication by Plowden et al. entitled “Subclinical Hypothyroidism and Thyroid Autoimmunity Are Not Associated With Fecundity, Pregnancy Loss, or Live Birth” [33] can be added to this list of clinical evaluations that convey the same message as we did in 2014.
A commonly addressed topic in reproductive medicine has been a putative influence of thyroid antibodies on the outcome of assisted reproduction procedures. It has to be recalled that the thyroidal target molecules involved, i.e. thyroglobulin and thyroid peroxidase, are exclusively found in the thyroid. The localization of thyroglobulin was described 1977 by Paiement and Leblond [34]. They demonstrated that it follows a strict intracellular distribution within the thyroid follicle (Fig. 6 in [34]). Following iodination of the thyroglobulin molecule its linear structure is changed and so is its antigenicity [35], [36]. An iodine overload is considered as a risk factor for an immune reaction [37]. This risk increases also when selenium deficiency is present [38], [39]. We have previously shown that selenium deficiency is found frequently among patients with thyroid disease [40]. Depending on the functional state of the thyroid the content and structure of thyroglobulin will change [41]. It is conceivable that by this the antigenicity of thyroglobulin could vary resulting in the development of thyroid antibodies. Similar concepts apply to thyroid peroxidase (TPO). TPO is a hemoprotein and it was first localized to thyroid microsomes. TPO catalyzes the oxidation of iodine as well as of guaiacol [42]. Finally, although iodide can be found in the ovarian follicular fluid there is no incorporation of it into organic compounds [43]. In other words, no other organ excepting the thyroid can carry out these specific processes. Unfortunately investigators have disregarded these simple basic notions of thyroid physiology and have dedicated resources to investigate this putative relation in a futile way.
Busnelli et al. [44] have conducted a systematic review looking at the putative relation between thyroid autoimmunity and the outcome of IVF/ICSI procedures. In the introduction they correctly state that the antigens involved are exclusively located in the thyroid. Again, by logical biochemical exclusion, any interaction with different types of tissues in the body cannot be expected. Their main conclusion goes along with this basic line of knowledge, i.e. the serological reaction against thyroid antigens does not have an impact on the outcome of IVF/ICSI. They presented a plea for further research that would look at the process of fertilization aiming at discovering other factors related to it. Tan et al. [45] have looked at the outcome of ICSI in relation to thyroid antibodies and found that serology results did not affect the process of ICSI. This study included a series of 835 women. Similar conclusions about thyroid serology and outcomes of IVF/ICSI have been presented by Unuane et al. [46] in a study that included 2406 women. The reference values for TSH used in the study were 0.27–4.2 mIU/L. They observed that patients who presented thyroid antibodies had higher levels of TSH, i.e. 2.76 ± 4.96 vs. 1.67 ± 0.85 mIU/L, but did not differ in the concentration of fT4 (Table 1, page 146 in [46]). Doing a sub-group analysis on crude cumulative delivery rates according to TSH levels, they found no differences using cut-off values of < 2.5 or < 5.0 mIU/L (Table 3, page 148 in [46]). These authors finished their paper with a positive statement in the sense of taking away the fear of bad outcome due to thyroid serology findings. Negro et al. have addressed the question of treating women with positive thyroid serology with levothyroxine [47]. This study included 3 groups of patients with different laboratory characteristics. The total number of subjects included was 290. They found out that intervention with levothyroxine had no effect on the rate of miscarriage and of preterm delivery. Finally, a large study done with 5076 women by Polyzos has shown that thyroid serology and hypothyroidism are not related to low ovarian reserve [48]. On the other hand, i.e. from the viewpoint of the fertility specialist, it is interesting to note that proposals aiming at the evaluation of ovarian reserve do not mention to include thyroid function [49], [50].
5. Magnesium and iron in a systems approach related to thyroid and ovarian function
The basic functional concept of endocrinology was delivered in June 1905 by Starling at a Croonian Lecture entitled "On the chemical correlation of the functions of the body" [51]. In 2017 we are still working with chemical elements as well as with refined techniques that go into the “OMICS” of body function [52].Consulting any current textbook on endocrinology one will find that the involved chemical messengers of the thyroid function axis include the peripheral thyroid hormones, i.e. fT3 and fT4, and the regulatory pituitary hormone TSH. In addition to this the main ingredient for thyroid hormone synthesis is dietary iodine [53]. For many years these hormones and this chemical element have been the basic elements for the evaluation of thyroid economy and function.
In an observational study we have recently described an acquired condition of magnesium deficiency as the basis of mitochondrial dysfunction that can explain the changes associated with thyroid disease [54]. Further elements that have to be supplemented in case of deficiency are selenium and coenzyme Q10. Adequate correction of this complex deficiency condition can revert the changes in the thyroid [55]. How can this occur? While iodine appears to “simply” be taken up by thyroid tissue [56], the process is an active transport process and by definition it requires energy. In 1968 Tyler [57] showed that iodine uptake depends on the availability of magnesium ATP. Following iodine uptake, thyroid hormone synthesis will be carried out through the interaction of thyroid peroxidase with tyrosine residues on the thyroglobulin molecule [58]. The main regulation of this function occurs through TSH, however it has also been shown that magnesium stimulates peroxidase activity resulting in increased thyroid hormone production [59]. Selenium will protect the thyroid via glutathione peroxidase and coenzyme Q10 will normalize thyroid vascularity [54].
In addition to magnesium, it has also been shown that iron sufficiency is also required for the maintenance of thyroid function. Experimental studies have shown that the activity of thyroid peroxidase, a hemeprotein, decreases under conditions of iron deficiency [60]. Furthermore it has been observed that iron deficiency per se, without anemia, can lead to maternal hypothyroxinemia [61]. In a clinical setting of women in childbearing age in China, Yu et al. [62] described that isolated hypothyroxinemia in pregnant and nonpregnant women can be related to iron deficiency. Already in 2007 Zimmermann, Burgi and Hurrell had described that iron deficiency can be taken as a predictor of maternal thyroid function [63]. This relation is clearly showed in Fig. 1 of their publication where the negative correlation between body iron stores vs. serum TSH levels can be seen. This figure also shows the complementary positive correlation between iron stores and thyroid hormone concentration. Altogether, a better thyroid function is found when iron stores are sufficient. In 2016 Veltri et al. [64] described a similar situation, i.e. increased levels of TSH in conditions of iron deficiency. Besides thyroid function, iron deficiency has been shown to be correlated to a higher risk of small for gestational age babies [65].
Similar to thyroid economy, steroid hormone synthesis is also an active process that requires energy [66]. Usually such biochemical steps are generally taken for granted, and the relation to energy dependency is forgotten. In our model of thyroid disease we consider magnesium to be a central player in energy supply [54]. Magnesium and its cell transport mechanisms, i.e. the transient receptor potential cation channel subfamily M member 7 (TRPM7) [67], [68], have known important relations to reproduction processes. Initiation of embryo development is influenced by TRPM7 channels [69], [70], [71], [72]. On the other hand magnesium deficiency has deleterious effects on fetal outcome [73]. TRPM is functionally expressed in human endometrial cells during the luteal phase [74]. In 1988 Kovács et al. [75] reported the beneficial effects of supplementing magnesium during pregnancy. Using a dose of 15 mmol/d they observed a lower frequency of premature births as well as of low weight at birth.
The Nurses' Health Study II has shown that use of iron supplements, i.e. in the form of non-heme iron, is associated with a lower risk for ovulatory infertility [76]. The relevance of iron in pregnancy has been discussed by Gambling et al. [77]. Recent publications have shown newer associations between elements of iron metabolism and fertility issues. An experimental mouse model has delivered interesting data as to the importance of heme oxygenase-1 in processes such as ovulation, fertilization, implantation and placentation [78]. These results were highlighted in an Editorial by one of the authors as being novel regulators of reproductive processes [79].
6. Conclusions
More than a decade ago the purely theoretical proposals made by Baloch [2] and Abalovich [3] as to an upper reference range for TSH of 2.5 mIU/L for the evaluation of thyroid function appeared in the medical literature. These publications implied that using this reference value, patient evaluation and care would improve.
Table 1.
Reference values for TSH in the literature.
| Author | Year | n | Population | TSH range |
|---|---|---|---|---|
| Baloch [2] | 2003 | Not given | Undefined (adults?) | Single value 2.5 |
| Abalovich [3] | 2007 | Not given | Pregnancy oriented | Single value 2.5 |
| Kratzsch [12] | 2005 | 870 | Healthy blood donors | 0.3–3.63 |
| Moncayo [13] | 2007 | 2570 | Adults | 0.3–3.5 |
| Langén [14] | 2014 | 5709 | General population | 0.4–3.4 |
| Moncayo [19] | 2015 | 2028 | Pregnant women | 0.3–3.5 |
| Kapelari [80] | 2008 | 1209 | Children | Age dependent |
Potential interactions of thyroid function and thyroid serology with pregnancy outcome parameters in the field of reproduction medicine have also not been confirmed.
Besides looking at TSH levels, we propose that clinicians should also determine the iron status and magnesium levels in any situation where thyroid function is being evaluated. This procedure also applies to situations where fertility questions are being considered. A recent recommendation update on the treatment of thyroid diseases during pregnancy made by the American Thyroid Association [81] has still left several clinical areas at an inconclusive situation, even though sufficient data from single studies exists in the medical literature. Furthermore, this sponsored publication contains no references to iron or magnesium. We consider that these omissions could hamper good clinical work.
Altogether the proposals made by Baloch [2] and Abalovich [3] as to an upper TSH reference value of 2.5 mIU/L have to be considered as being flawed. Flawed data belong to a low hierarchy level in terms of evidence-based medicine (EBM) [4]. The situation of flawed publications has been discussed by Casadevall, Grant Steen and Fang [82]. Such publications are to be seen as candidates for retraction. Causes of retraction are mainly laboratory errors, analytical errors and irreproducible results. If a publication turns out to be invalid then even damage might ensue [83].
Current biochemical evidence about the elements required to maintain thyroid function show that these not only include dietary iodine but also magnesium, iron, selenium and coenzyme Q10. Iron is important for the synthesis of thyroid peroxidase; magnesium-ATP contributes to the active process of iodine uptake; iodine has to be sufficiently present in the diet; selenium acts through selenoproteins to protect the thyroid cell during hormone synthesis and in deiodination of thyroxine; coenzyme Q10 influences thyroid vascularity. As a consequence, good clinical practice requires additional biochemical information on the blood levels of magnesium, selenium, coenzyme Q10 as well as iron status.
Since these elements are also important for the maintenance of reproductive function, we postulate that they constitute the connecting link between both endocrine systems.
Fig. 1.
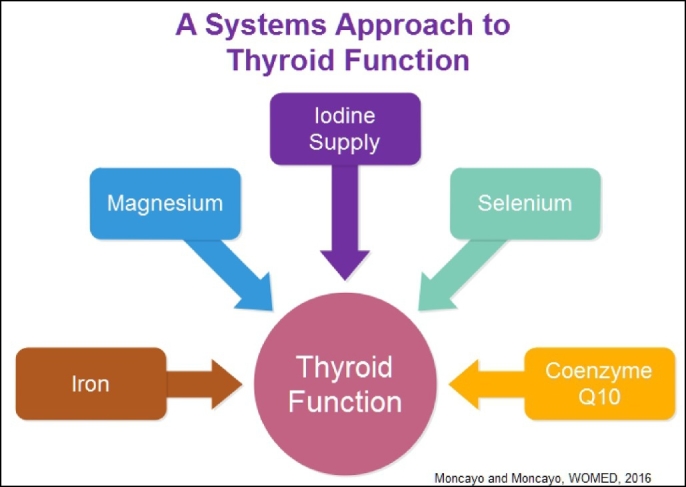
The elements involved in thyroid function.
Transparency document
Transparency document
Acknowledgements
This paper reflects our daily clinical work with patients being treated at our Institution with questions about fertility and thyroid function. We are grateful to them for their time and compliance. Inspiration for this writing was taken from Mark Twain [84]: “Supposing is good, but finding out is better”.
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- 1.Upshur R.E. Do clinical guidelines still make sense? No. Ann. Fam. Med. 2014;12:202–203. doi: 10.1370/afm.1654. (PM:24821890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baloch Z. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3–126. doi: 10.1089/105072503321086962. (PM:12625976) [DOI] [PubMed] [Google Scholar]
- 3.Abalovich M. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2007;92(Suppl. 8):s1–s47. doi: 10.1210/jc.2007-0141. (PM:17948378) [DOI] [PubMed] [Google Scholar]
- 4.Guyatt G. Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA. 1992;268:2420–2425. doi: 10.1001/jama.1992.03490170092032. (PM:1404801) [DOI] [PubMed] [Google Scholar]
- 5.Fatourechi V. Effects of reducing the upper limit of normal TSH values. JAMA. 2003;290:3195–3196. doi: 10.1001/jama.290.24.3195-b. (PM:14693871) [DOI] [PubMed] [Google Scholar]
- 6.Cochrane A.L. Nuffield Provincial Hospitals Trust; Nuffield: 1972. Effectiveness and Efficiency: Random Reflections on Health Services. [Google Scholar]
- 7.Popper K.R. J.C.B. Mohr; Tübingen: 1984. Logik Der Forschung. [Google Scholar]
- 8.Evers J.L. Evidence-based medicine for diagnostic questions. Semin. Reprod. Med. 2003;21:9–15. doi: 10.1055/s-2003-39990. (PM:12806555) [DOI] [PubMed] [Google Scholar]
- 9.Ioannidis J.P. Meta-research: the art of getting it wrong. Res. Synth. Methods. 2010;1:169–184. doi: 10.1002/jrsm.19. (PM:26061464) [DOI] [PubMed] [Google Scholar]
- 10.Kriegeskorte N. Open evaluation: a vision for entirely transparent post-publication peer review and rating for science. Front. Comput. Neurosci. 2012;6:79. doi: 10.3389/fncom.2012.00079. (PM:23087639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bastian H. A stronger post-publication culture is needed for better science. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001772. (PM:25548904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kratzsch J. New reference intervals for thyrotropin and thyroid hormones based on National Academy of Clinical Biochemistry criteria and regular ultrasonography of the thyroid. Clin. Chem. 2005;51:1480–1486. doi: 10.1373/clinchem.2004.047399. (PM:15961550) [DOI] [PubMed] [Google Scholar]
- 13.Moncayo H. Diagnostic accuracy of basal TSH determinations based on the intravenous TRH stimulation test: an evaluation of 2570 tests and comparison with the literature. BMC Endocr. Disord. 2007;7:5. doi: 10.1186/1472-6823-7-5. (PM:17678551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langén V.L. Thyroid-stimulating hormone reference range and factors affecting it in a nationwide random sample. Clin. Chem. Lab. Med. 2014;52:1807–1813. doi: 10.1515/cclm-2014-0287. (PM:24950512) [DOI] [PubMed] [Google Scholar]
- 15.Voigtländer R., Führer D. Latente Hypothyreose - Laborkonstellation oder Krankheit? Dtsch. Med. Wochenschr. 2016;141:1134–1136. doi: 10.1055/s-0042-107439. (PM:27509338) [DOI] [PubMed] [Google Scholar]
- 16.Åsvold B.O. Thyroid function within the normal range and risk of coronary heart disease: an individual participant data analysis of 14 cohorts. JAMA Intern. Med. 2015;175:1037–1047. doi: 10.1001/jamainternmed.2015.0930. (PM:25893284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuels M.H. Effect of thyroid function variations within the laboratory reference range on health status, mood, and cognition in levothyroxine-treated subjects. Thyroid. 2016;26:1173–1184. doi: 10.1089/thy.2016.0141. (PM:27338133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh J.P. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J. Clin. Endocrinol. Metab. 2006;91:2624–2630. doi: 10.1210/jc.2006-0099. (PM:16670161) [DOI] [PubMed] [Google Scholar]
- 19.Moncayo R. Thyroid function parameters in normal pregnancies in an iodine sufficient population. BBA Clin. 2015;3:90–95. doi: 10.1016/j.bbacli.2014.12.006. (PM:26674060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stuefer S. The role of magnesium and thyroid function in early pregnancy after in-vitro fertilization (IVF): new aspects in endocrine physiology. BBA Clin. 2015;3:196–204. doi: 10.1016/j.bbacli.2015.02.006. (PM:26675754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinblatt S. Thyroid stimulating hormone levels rise after assisted reproductive technology. J. Assist. Reprod. Genet. 2013;30:1347–1352. doi: 10.1007/s10815-013-0081-3. (PM:23955685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Man E.B. Thyroid function in pregnancy and infancy. Maternal hypothyroxinemia and retardation of progeny. CRC Crit. Rev. Clin. Lab. Sci. 1972;3:203–225. doi: 10.3109/10408367209151327. (PM:4115124) [DOI] [PubMed] [Google Scholar]
- 23.McAninch E.A., Bianco A.C. The history and future of treatment of hypothyroidism. Ann. Intern. Med. 2016;164:50–56. doi: 10.7326/M15-1799. (PM:26747302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazarus J.H. Antenatal thyroid screening and childhood cognitive function. N. Engl. J. Med. 2012;366:493–501. doi: 10.1056/NEJMoa1106104. (PM:22316443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trumpff C. Neonatal thyroid-stimulating hormone concentration and psychomotor development at preschool age. Arch. Dis. Child. 2016;101:1100–1106. doi: 10.1136/archdischild-2015-310006. (PM:27402733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moncayo R., Ortner K. Multifactorial determinants of cognition – thyroid function is not the only one. BBA Clin. 2015;3:289–298. doi: 10.1016/j.bbacli.2015.04.002. (PM:26672993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray-Kolb L.E., Beard J.L. Iron treatment normalizes cognitive functioning in young women. Am. J. Clin. Nutr. 2007;85:778–787. doi: 10.1093/ajcn/85.3.778. (PM:17344500) [DOI] [PubMed] [Google Scholar]
- 28.Bernardi L.A. Impact of subclinical hypothyroidism in women with recurrent early pregnancy loss. Fertil. Steril. 2013;100:1326–1331. doi: 10.1016/j.fertnstert.2013.07.1975. (PM:23954357) [DOI] [PubMed] [Google Scholar]
- 29.Moncayo H., Moncayo R. The lack of clinical congruence in diagnosis and research in relation to subclinical hypothyroidism. Fertil. Steril. 2014;101 doi: 10.1016/j.fertnstert.2014.01.010. (PM:24534287) [DOI] [PubMed] [Google Scholar]
- 30.Practice Committee of the American Society for Reproductive Medicine Subclinical hypothyroidism in the infertile female population: a guideline. Fertil. Steril. 2015;104:545–553. doi: 10.1016/j.fertnstert.2015.05.028. (PM:26239023) [DOI] [PubMed] [Google Scholar]
- 31.Mintziori G. Thyroid function and IVF outcome: when to investigate and when to intervene? Curr. Opin. Obstet. Gynecol. 2016;28:191–197. doi: 10.1097/GCO.0000000000000263. (PM:26967594) [DOI] [PubMed] [Google Scholar]
- 32.Mintziori G. Association of TSH concentrations and thyroid autoimmunity with IVF outcome in women with TSH concentrations within normal adult range. Gynecol. Obstet. Investig. 2014;77:84–88. doi: 10.1159/000357193. (PM:24356283) [DOI] [PubMed] [Google Scholar]
- 33.Plowden T.C. Subclinical hypothyroidism and thyroid autoimmunity are not associated with fecundity, pregnancy loss, or live birth. J. Clin. Endocrinol. Metab. 2016;101:2358–2365. doi: 10.1210/jc.2016-1049. (PM:27023447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paiement J., Leblond C.P. Localization of thyroiglobulin antigenicity in rat thyroid sections using antibodies labeled with peroxidase or 125I-radioiodine. J. Cell Biol. 1977;74:992–1015. doi: 10.1083/jcb.74.3.992. (PM:71304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saboori A.M. Iodination of human thyroglobulin (Tg) alters its immunoreactivity. I. Iodination alters multiple epitopes of human Tg. Clin. Exp. Immunol. 1998;113:297–302. doi: 10.1046/j.1365-2249.1998.00643.x. (PM:9717981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakata S. Synthesis and examination of antigenicity of four hormonogenic sites and two non-hormonogenic sites of human thyroglobulin. Mol. Cell. Endocrinol. 1991;79:93–98. doi: 10.1016/0303-7207(91)90099-e. (PM:1936549) [DOI] [PubMed] [Google Scholar]
- 37.Luo Y. Iodine excess as an environmental risk factor for autoimmune thyroid disease. Int. J. Mol. Sci. 2014;15:12895–12912. doi: 10.3390/ijms150712895. (PM:25050783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Contempre B. Selenium deficiency aggravates the necrotizing effects of a high iodide dose in iodine deficient rats. Endocrinology. 1993;132:1866–1868. doi: 10.1210/endo.132.4.8462484. (PM:8462484) [DOI] [PubMed] [Google Scholar]
- 39.Contempre B. Effects of selenium deficiency on thyroid necrosis, fibrosis and proliferation: a possible role in myxoedematous cretinism. Eur. J. Endocrinol. 1995;133:99–109. doi: 10.1530/eje.0.1330099. (PM:7627345) [DOI] [PubMed] [Google Scholar]
- 40.Moncayo R. The role of selenium, vitamin C, and zinc in benign thyroid diseases and of Se in malignant thyroid diseases: low selenium levels are found in subacute and silent thyroiditis and in papillary and follicular carcinoma. BMC Endocr. Disord. 2008;8:2. doi: 10.1186/1472-6823-8-2. (PM:18221503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gérard A.C. Evidence for processing of compact insoluble thyroglobulin globules in relation with follicular cell functional activity in the human and the mouse thyroid. Eur. J. Endocrinol. 2004;150:73–80. doi: 10.1530/eje.0.1500073. (PM:14713282) [DOI] [PubMed] [Google Scholar]
- 42.Hosoya T., Morrison M. A study of the hemoproteins of thyroid microsomes with emphasis on the thyroid peroxidase. Biochemistry. 1967;6:1021–1026. doi: 10.1021/bi00856a010. (PM:4382244) [DOI] [PubMed] [Google Scholar]
- 43.Bengtsson G. Distribution and fate of 131-I in the mammalian ovary. Acta Endocrinol. 1963;42:122–128. doi: 10.1530/acta.0.0420122. (PM:13967437) [DOI] [PubMed] [Google Scholar]
- 44.Busnelli A. The impact of thyroid autoimmunity on IVF/ICSI outcome: a systematic review and meta-analysis. Hum. Reprod. Update. 2016;22:775–790. doi: 10.1093/humupd/dmw019. (PM:27323769) [DOI] [PubMed] [Google Scholar]
- 45.Tan S. Thyroid autoantibodies per se do not impair intracytoplasmic sperm injection outcome in euthyroid healthy women. Eur. J. Endocrinol. 2014;170:495–500. doi: 10.1530/EJE-13-0790. (PM:24394727) [DOI] [PubMed] [Google Scholar]
- 46.Unuane D. Impact of thyroid autoimmunity on cumulative delivery rates in in vitro fertilization/intracytoplasmic sperm injection patients. Fertil. Steril. 2016;106:144–150. doi: 10.1016/j.fertnstert.2016.03.011. (PM:27036234) [DOI] [PubMed] [Google Scholar]
- 47.Negro R. Impact of levothyroxine in miscarriage and preterm delivery rates in first trimester thyroid antibody-positive women with TSH less than 2.5 mIU/L. J. Clin. Endocrinol. Metab. 2016;101:3685–3690. doi: 10.1210/jc.2016-1803. (PM:27459527) [DOI] [PubMed] [Google Scholar]
- 48.Polyzos N.P. Thyroid autoimmunity, hypothyroidism and ovarian reserve: a cross-sectional study of 5000 women based on age-specific AMH values. Hum. Reprod. 2015;30:1690–1696. doi: 10.1093/humrep/dev089. (PM:25948573) [DOI] [PubMed] [Google Scholar]
- 49.Ferraretti A.P. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum. Reprod. 2011;26:1616–1624. doi: 10.1093/humrep/der092. (PM:21505041) [DOI] [PubMed] [Google Scholar]
- 50.Poseidon Group A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil. Steril. 2016;105:1452–1453. doi: 10.1016/j.fertnstert.2016.02.005. (PM:26921622) [DOI] [PubMed] [Google Scholar]
- 51.Starling E.H. Croonian lecture: on the chemical correlation of the functions of the body I. Lancet. 1905;166:339–341. [Google Scholar]
- 52.Silvestri E. Studies of complex biological systems with applications to molecular medicine: the need to integrate transcriptomic and proteomic approaches. J. Biomed. Biotechnol. 2011;2011:810242. doi: 10.1155/2011/810242. (PM:20981256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mondal S. Chemistry and biology in the biosynthesis and action of thyroid hormones. Angew. Chem. Int. Ed. Eng. 2016;55:7606–7630. doi: 10.1002/anie.201601116. (PM:27226395) [DOI] [PubMed] [Google Scholar]
- 54.Moncayo R., Moncayo H. The WOMED model of benign thyroid disease: acquired magnesium deficiency due to physical and psychological stressors relates to dysfunction of oxidative phosphorylation. BBA Clin. 2015;3:44–64. doi: 10.1016/j.bbacli.2014.11.002. (PM:26675817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moncayo R., Moncayo H. Proof of concept of the WOMED model of benign thyroid disease: restitution of thyroid morphology after correction of physical and psychological stressors and magnesium supplementation. BBA Clin. 2015;3:113–122. doi: 10.1016/j.bbacli.2014.12.005. (PM:26672672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pitt-Rivers R. Localization of protein-bound radioactive iodine in rat thyroid glands labelled with 125-I or 131-I. Biochem. J. 1964;90:205–208. doi: 10.1042/bj0900205. (PM:5832293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tyler D.D. Influence of mitochondrial inhibitors on the respiration and energy-dependent uptake of iodide by thyroid slices. Biochem. J. 1968;106:123–133. doi: 10.1042/bj1060123. (PM:4238489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hosoya T. Localization of peroxidase and other microsomal enzymes in thyroid cells. Biochemistry. 1971;10:3086–3093. doi: 10.1021/bi00792a016. (PM:4399555) [DOI] [PubMed] [Google Scholar]
- 59.Chandra A.K. Effects of magnesium on cytomorphology and enzyme activities in thyroid of rats. Indian J. Exp. Biol. 2014;52:787–792. (PM:25141541) [PubMed] [Google Scholar]
- 60.Hess S.Y. Iron deficiency anemia reduces thyroid peroxidase activity in rats. J. Nutr. 2002;132:1951–1955. doi: 10.1093/jn/132.7.1951. (PM:12097675) [DOI] [PubMed] [Google Scholar]
- 61.Hu X. Iron deficiency without anemia causes maternal hypothyroxinemia in pregnant rats. Nutr. Res. 2014;34:604–612. doi: 10.1016/j.nutres.2014.06.007. (PM:25150119) [DOI] [PubMed] [Google Scholar]
- 62.Yu X. Iron deficiency, an independent risk factor for isolated hypothyroxinemia in pregnant and nonpregnant women of childbearing age in China. J. Clin. Endocrinol. Metab. 2015;100:1594–1601. doi: 10.1210/jc.2014-3887. (PM:25599388) [DOI] [PubMed] [Google Scholar]
- 63.Zimmermann M.B. Iron deficiency predicts poor maternal thyroid status during pregnancy. J. Clin. Endocrinol. Metab. 2007;92:3436–3440. doi: 10.1210/jc.2007-1082. (PM:17566085) [DOI] [PubMed] [Google Scholar]
- 64.Veltri F. Prevalence of thyroid autoimmunity and dysfunction in women with iron deficiency during early pregnancy: is it altered? Eur. J. Endocrinol. 2016;175:191–199. doi: 10.1530/EJE-16-0288. (PM:27450694) [DOI] [PubMed] [Google Scholar]
- 65.Alwan N.A. Maternal iron status in early pregnancy and birth outcomes: insights from the Baby's Vascular health and Iron in Pregnancy study. Br. J. Nutr. 2015;113:1985–1992. doi: 10.1017/S0007114515001166. (PM:25946517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stocco D.M. Intramitochondrial cholesterol transfer. Biochim. Biophys. Acta. 2000;1486:184–197. doi: 10.1016/s1388-1981(00)00056-1. (PM:10856721) [DOI] [PubMed] [Google Scholar]
- 67.Schlingmann K.P. TRPM6 and TRPM7—gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta. 2007;1772:813–821. doi: 10.1016/j.bbadis.2007.03.009. (PM:17481860) [DOI] [PubMed] [Google Scholar]
- 68.Paravicini T.M. TRPM7: a unique channel involved in magnesium homeostasis. Int. J. Biochem. Cell Biol. 2012;44:1381–1384. doi: 10.1016/j.biocel.2012.05.010. (PM:22634382) [DOI] [PubMed] [Google Scholar]
- 69.Liu W. TRPM7 regulates gastrulation during vertebrate embryogenesis. Dev. Biol. 2011;350:348–357. doi: 10.1016/j.ydbio.2010.11.034. (PM:21145885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Komiya Y. Magnesium and embryonic development. Magnes. Res. 2014;27:1–8. doi: 10.1684/mrh.2014.0356. (PM:24721994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Komiya Y., Runnels L.W. TRPM channels and magnesium in early embryonic development. Int. J. Dev. Biol. 2015;59:281–288. doi: 10.1387/ijdb.150196lr. (PM:26679946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carvacho I. TRPM7-like channels are functionally expressed in oocytes and modulate post-fertilization embryo development in mouse. Sci. Rep. 2016;6:34236. doi: 10.1038/srep34236. (PM:27681336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Almonte R.A. Gestational magnesium deficiency is deleterious to fetal outcome. Biol. Neonate. 1999;76:26–32. doi: 10.1159/000014128. (PM:10364636) [DOI] [PubMed] [Google Scholar]
- 74.De Clercq K. Functional expression of transient receptor potential channels in human endometrial stromal cells during the luteal phase of the menstrual cycle. Hum. Reprod. 2015;30:1421–1436. doi: 10.1093/humrep/dev068. (PM:25820697) [DOI] [PubMed] [Google Scholar]
- 75.Kovács L. Magnesium substitution in pregnancy. A prospective, randomized double-blind study. Geburtshilfe Frauenheilkd. 1988;48:595–600. (PM:3063587) [PubMed] [Google Scholar]
- 76.Chavarro J.E. Iron intake and risk of ovulatory infertility. Obstet. Gynecol. 2006;108:1145–1152. doi: 10.1097/01.AOG.0000238333.37423.ab. (PM:17077236) [DOI] [PubMed] [Google Scholar]
- 77.Gambling L. Fetal regulation of iron transport during pregnancy. Am. J. Clin. Nutr. 2011;94:1903S–1907S. doi: 10.3945/ajcn.110.000885. (PM:21543532) [DOI] [PubMed] [Google Scholar]
- 78.Zenclussen M.L. Heme oxygenase-1 is critically involved in placentation, spiral artery remodeling, and blood pressure regulation during murine pregnancy. Front. Pharmacol. 2014;5:291. doi: 10.3389/fphar.2014.00291. (PM:25628565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wong R.J., Zenclussen A.C. Editorial: heme oxygenases: novel regulators of reproductive processes. Front. Pharmacol. 2015;6:282. doi: 10.3389/fphar.2015.00282. (PM:26640438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kapelari K. Pediatric reference intervals for thyroid hormone levels from birth to adulthood: a retrospective study. BMC Endocr. Disord. 2008;8:15. doi: 10.1186/1472-6823-8-15. (PM:19036169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alexander E.K. 2016 guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017 (PM:28056690) [Google Scholar]
- 82.Casadevall A. Sources of error in the retracted scientific literature. FASEB J. 2014;28:3847–3855. doi: 10.1096/fj.14-256735. (PM:24928194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Montgomery K., Oliver A.L. Conceptualizing fraudulent studies as viruses: new models for handling retractions. Minerva. 2016 [Google Scholar]
- 84.Twain M. Supposing is good, but finding out is better. In: De Voto B., editor. Mark Twain in Eruption: Hitherto Unpublished Pages About Men and Events. Harper; 1940. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document


