Abstract
Currently, there are no Food and Drug Administration (FDA)-approved pharmacotherapies for the treatment of those with traumatic brain injury (TBI). As central mediators of the secondary injury cascade, mitochondria are promising therapeutic targets for prevention of cellular death and dysfunction after TBI. One of the most promising and extensively studied mitochondrial targeted TBI therapies is inhibition of the mitochondrial permeability transition pore (mPTP) by the FDA-approved drug, cyclosporine A (CsA). A number of studies have evaluated the effects of CsA on total brain mitochondria after TBI; however, no study has investigated the effects of CsA on isolated synaptic and non-synaptic mitochondria. Synaptic mitochondria are considered essential for proper neurotransmission and synaptic plasticity, and their dysfunction has been implicated in neurodegeneration. Synaptic and non-synaptic mitochondria have heterogeneous characteristics, but their heterogeneity can be masked in total mitochondrial (synaptic and non-synaptic) preparations. Therefore, it is essential that mitochondria targeted pharmacotherapies, such as CsA, be evaluated in both populations. This is the first study to examine the effects of CsA on isolated synaptic and non-synaptic mitochondria after experimental TBI. We conclude that synaptic mitochondria sustain more damage than non-synaptic mitochondria 24 h after severe controlled cortical impact injury (CCI), and that intraperitoneal administration of CsA (20 mg/kg) 15 min after injury improves synaptic and non-synaptic respiration, with a significant improvement being seen in the more severely impaired synaptic population. As such, CsA remains a promising neuroprotective candidate for the treatment of those with TBI.
Keywords: : cyclosporine A, mitochondria, neuroprotection, synaptic and non-synaptic, traumatic brain injury
Introduction
Traumatic brain injury (TBI) represents a significant health crisis. In the United States, there are more than 5 million persons currently living with a disability resulting from a TBI1 with an associated economic burden of 76.5 billion dollars.2,3 TBI consists of a primary mechanical injury followed by a secondary injury cascade.4 Aspects of this cascade include increases in excitotoxic amino acids such as glutamate, increases in intracellular calcium, mitochondrial dysfunction, production of reactive oxygen and nitrogen species (ROS/RNS), initiation and propagation of lipid peroxidation (LP), formation of LP-derived neurotoxic aldehydes, activation of calcium-dependent proteases such as calpain, cytoskeletal degradation, cell death, and neurologic dysfunction.5–28 The secondary injury cascade should be amenable to therapeutic intervention. Currently, there are no Food and Drug Administration (FDA)-approved pharmacotherapies for the treatment of patients with TBI, however.12
Mitochondria play a central role in the secondary injury cascade. Mitochondria are essential regulators of calcium homeostasis29,30 and buffer increased intracellular calcium after TBI.8 High levels of mitochondrial calcium, however, lead to mitochondrial dysfunction, including increased generation of ROS/RNS, decreased oxidative phosphorylation and adenosine triphosphate (ATP) production, and induction of the mitochondria permeability transition pore (mPTP).7,8,15,28,31–36
The mPTP is a non-selective mega channel located in the inner mitochondrial membrane that is permeable to solutes <1.5 kDa.28 Opening of the mPTP leads to extrusion of calcium back into the cytosol, mitochondrial swelling, and rupture of the outer mitochondrial membrane.28,37 Mitochondrial dysfunction contributes to several aspects of the aforementioned injury cascade, including ROS/RNS induction of LP and formation of the LP-derived neurotoxic aldehydes 4-hydroxynonenal and acrolein, which are capable of covalently binding mitochondrial proteins, further exacerbating mitochondrial dysfunction.7,19,38–42 Additional downstream consequences of mPTP formation include activation of the calcium-dependent protease calpain, cytoskeletal degradation, cell death, and neurologic dysfunction.7,11–13,15,16,28,38,39,43–49 Therefore, as central mediators of the secondary injury cascade, mitochondria are promising therapeutic targets for prevention of cellular death and dysfunction after TBI.
Several therapies targeting mitochondria have been shown to be neuroprotective in experimental models of TBI, including mild uncoupling,50,51 ketogenic diets,52,53 increased antioxidant availability,40,54 and scavenging of neurotoxic aldehydes.19 One of the most promising and extensively studied mitochondrial targeted TBI therapies, however, is inhibition of mPTP by the FDA-approved immunosuppressant, cyclosporine A (CsA). In experimental TBI, CsA or its non-immunosuppressant analog, NIM811, prevent mitochondrial swelling and axonal pathology,20,21 maintain mitochondrial membrane potential and decrease production of reactive oxygen species,33 improve total (synaptic and non-synaptic) mitochondrial respiration,47 prevent oxidative (synaptic and non-synaptic) mitochondrial damage,47,55 improve cortical tissue sparing,56–58 decrease calpain-mediated cytoskeletal degradation and neurodegeneration,48 and improve motor and cognitive function.48,55,59,60
Interestingly, despite the fact that CsA directly targets mitochondria, only a limited number of studies have evaluated the effects of CsA on mitochondria after experimental TBI,33,47,61 with no studies evaluating the effects of CsA on isolated synaptic and non-synaptic mitochondria. Mitochondria are heterogeneous, consisting of both synaptic and non-synaptic populations. Isolated synaptic mitochondria consist of pre-synaptic mitochondria located within the synaptosome, while isolated non-synaptic mitochondria consist of neuronal (axonal, somal, dendritic) and non-neuronal (glial, vascular, etc.) mitochondria.
To our knowledge, there is currently no method available to further separate the non-synaptic mitochondrial population into non-synaptic neuronal and non-synaptic non-neuronal (e.g., glial) populations. Therefore, whereas synaptic mitochondria consist of pure pre-synaptic neuronal mitochondria, non-synaptic mitochondria are isolated from numerous cell types. Synaptic mitochondria are considered essential for proper neurotransmission and synaptic plasticity,62–64 processes that are impaired after TBI.65 Their dysfunction has been implicated in neurodegeneration, as well as degeneration of synapses and neurons absent overt cell death,66–68 and studies reveal synaptic mitochondria to be more susceptible to dysfunction. Importantly, these two mitochondrial populations show different characteristics both in vitro66,69 and in vivo,10,70 including differential responses to pharmacotherapy.70
The heterogeneity of the two populations, however, can be masked in total mitochondrial (synaptic and non-synaptic) preparations, especially because of the high glia to neuron ratio of the cerebral cortex.71 Therefore, it is essential that mitochondria targeted pharmacotherapies, such as CsA, be evaluated in both populations.
This is the first study to examine the effects of CsA on isolated synaptic and non-synaptic mitochondria after experimental TBI. We hypothesized that synaptic mitochondria would sustain more damage than non-synaptic mitochondria 24 h after severe controlled cortical impact (CCI) injury, and that intraperitoneal administration of CsA (20 mg/kg) 15 min after injury would differentially attenuate injury-induced synaptic and non-synaptic respiratory impairment.
Methods
Animals
Young adult male Sprague-Dawley rats (n = 20, Harlan, Indianapolis, IN) weighing 300 to 350 g were used for all studies. Animals were allowed food and water ad libitum and were housed in the Division of Laboratory Animal Resources of the University of Kentucky Medical Center. All animal and husbandry were conducted in accordance with the University of Kentucky Institutional Animal Care and Use Committee. Animals were randomly assigned to experimental groups: sham (n = 6), CCI + vehicle (n = 6), CCI + CsA (n = 8).
CCI TBI
Animals were initially anesthetized with 4% isoflurane and placed in a stereotaxic frame (David Kopf, Tujunga, CA), where they were maintained at 3% isoflurane for the duration of the procedure. A midline incision was made to expose the skull, and a 6 mm craniotomy was made lateral to the sagittal suture midway between lambda and bregma. The exposed brain with intact dura was injured using a computer controlled pneumatic impactor (TBI 03010; Precision Systems and Instrumentation, Fairfax Station, VA) fitted with a 5 mm beveled tip set to impact at ∼3.5 m/sec, 2.2 mm depth, and 500 msec dwell time, as described previously.19 After injury, Surgicel was placed onto the dura, and an 8 mm plastic disk was affixed with tissue adhesive to close the craniotomy site. Body temperature was monitored and maintained at 37°C with a thermo-regulating heating pad. Sham animals underwent all procedures but did not receive an impact injury.
CsA administration
The CsA concentration chosen was based on previously optimized concentrations for CCI.33,72 The CCI + CsA group was administered CsA obtained from the University of Kentucky Medical Center Hospital Pharmacy (Perrigo; Minneapolis, MN; 50 mg/mL) 15 min after injury as a single intraperitoneal dose of 20 mg/kg in saline/650 mg cremophor/33.2% (v/v) ethanol diluted in saline to a final concentration of 10 mg/mL. The injection volume was 0.2 mL/100 g of body weight. CCI + vehicle-treated animals received an equivalent volume of saline/cremophor/ethanol 15 min after injury.
Tissue extraction
Animals were euthanized at 24 h using CO2 anesthetization followed by decapitation, and an 8 mm cortical punch centered over the injury site was collected for analysis of mitochondrial respiration.
Mitochondrial isolation
Mitochondria were isolated as described previously7,19 with modifications to isolate synaptic and non-synaptic populations.10,70 Cortical tissue was homogenized in ice-cold isolation buffer (215 mmol/L mannitol, 75 mmol/L sucrose, 0.1% bovine serum albumin, 20 mmol/L HEPES, 1 mmol/L EGTA, pH 7.2) using Potter-Elvejhem homogenizers. Samples were then centrifuged twice at 1400 × g for 3 min at 4°C. Supernatants were collected and spun at 13,000 × g for 10 min at 4°C.
The crude mitochondrial pellet was resuspended and layered onto a discontinuous 7.5% and 10% Ficoll gradient and centrifuged at 100,000 × g for 30 min at 4°C. The non-synaptic mitochondria pellet was resuspended in isolation buffer without EGTA and centrifuged at 10,000 × g for 10 min at 4°C to remove Ficoll and then resuspended to a final concentration of approximately 10 mg/mL in isolation buffer without EGTA.
The synaptosomal layer was removed from the 7.5–10% Ficoll interface, resuspended in isolation buffer and spun at 13,000 × g for 10 min at 4°C to remove Ficoll. The synaptosome pellet was resuspended in isolation buffer, placed into a nitrogen bomb at 1200 psi for 10 min at 4°C to release synaptic mitochondria,73,74 layered onto a second discontinuous 7.5% and 10% Ficoll gradient, and centrifuged at 100,000 × g for 30 min at 4°C. The synaptic mitochondria pellet was resuspended in isolation buffer without EGTA and centrifuged at 10,000 × g for 10 min at 4°C to remove Ficoll and resuspended in isolation buffer without EGTA. Protein concentrations were determined with a BCA protein assay kit and measured at absorbance 562 nm with a BioTek Synergy HT plate reader (Winooski, VT). Mitochondria were immediately used for respiratory analysis.
Measurement of mitochondrial respiratory function
Mitochondria respiratory rates were measured using a Clark-type electrode in a continuously stirred, sealed, thermostatically controlled chamber (Oxytherm System, Hansatech Instruments, Norfolk, UK) that was maintained at 37°C. Mitochondria (>30 μg) were placed into a chamber containing 250 μL of KCl respiration buffer (125 mmol/L, 2 mmol/L MgCl2, 2.5 mmol/L KH2PO4, 0.1% bovine serum albumin, 20 mmol/L HEPES, pH 7.2). Mitochondria equilibrated for 1 min before complex-I initiation.
Complex-I respiration was initiated with 5 mmol/L pyruvate and 2.5 mmol/L malate, and state II respiration was monitored. Two boluses of 150 μmol/L adenosine diphosphate (ADP) were added to initiate state III respiration. State IV respiration was monitored after 2 μmol/L addition of the ATP synthase inhibitor oligomycin. Maximal state V(I) respiration was initiated by addition of 2 μmol/L of the protonophore FCCP. Complex I was inhibited by addition of 100 nmol/L rotenone. Complex II driven respiration was initiated by addition of 10 mmol/L succinate, and state V(II) was monitored. Respiratory control ratio (RCR) was calculated by dividing the state III respiration rate (second bolus ADP rate) by the state IV respiration rate.7,50
Statistical analysis
Statistical analysis was conducted using Prism version 6.0 (Graph Pad, San Diego, CA). Results are reported as mean ± standard deviation. Initial statistical analysis was performed using a two-way analysis of variance (ANOVA), followed by a Tukey post hoc analysis when appropriate.
Results
The following details the comparative effects of CCI TBI, with and without early CsA treatment, on the respiratory functional status of non-synaptic versus synaptic mitochondria as measured by changes in oxygen utilization during the various respiratory states. Overall, the results show that, in general, synaptic mitochondria are more susceptible to post-traumatic complex I and II-driven dysfunction within the electron transport chain than non-synaptic mitochondria. Nevertheless, early CsA treatment is able to protect respiration that is linked to neuronal ATP production within the more damaged synaptic mitochondria.
State II: Addition of pyruvate + malate to activate mitochondrial complex I
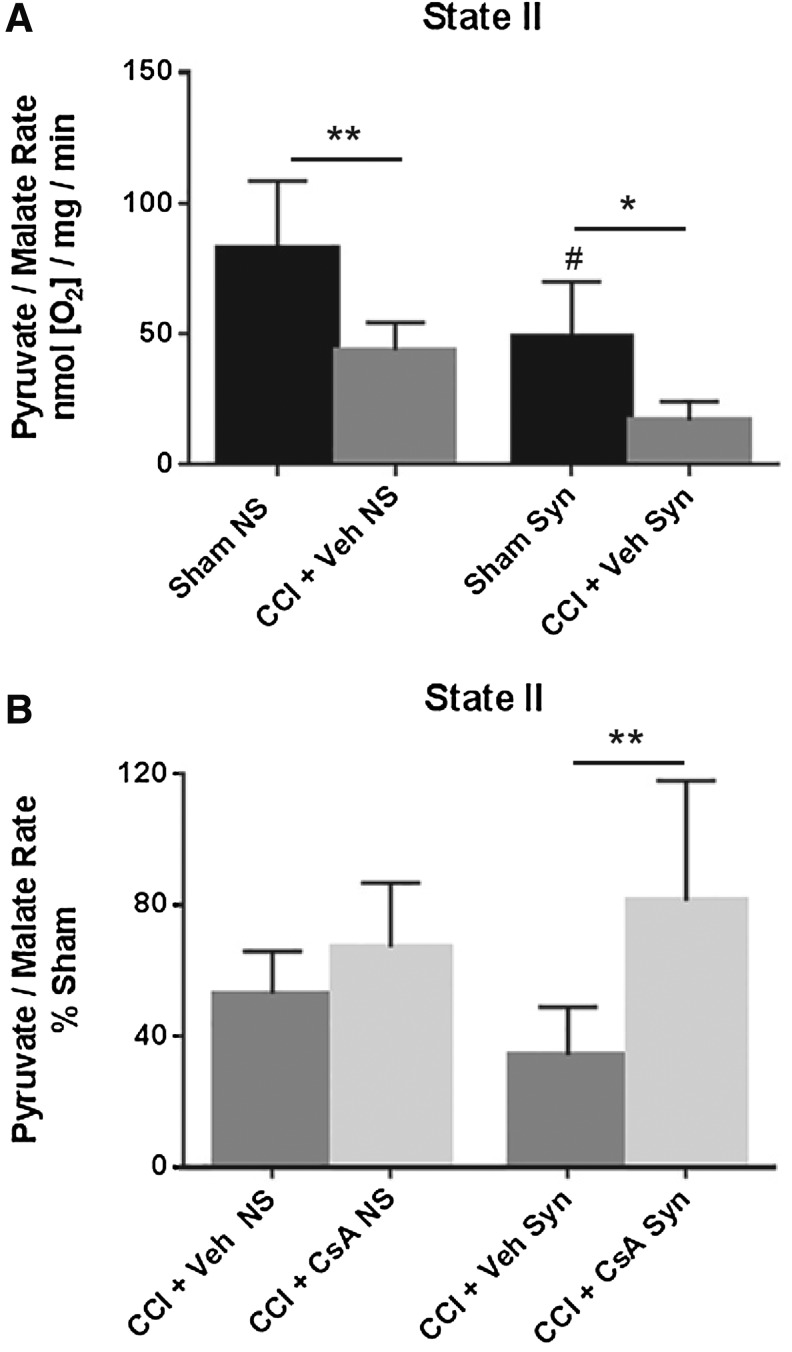
A two-way ANOVA (injury × population) revealed a significant main effect for injury (F [1, 20] = 23.75, p < 0.0001) and population (F [1, 20] = 17.22, p = 0.0005), but not interaction (F [1, 20] = 0.2018, p = 0.6581). Post hoc testing (Tukey) revealed that state II respiration for non-synaptic CCI + vehicle was significantly impaired compared with non-synaptic sham (p < 0.01), synaptic CCI + vehicle was significantly impaired compared with synaptic sham (p < 0.05), and that the state II respiration rate for synaptic sham was significantly decreased compared with non-synaptic sham (p < 0.05) (Fig. 1A).
FIG. 1.
(A) Effect of injury on non-synaptic and synaptic mitochondria for state II respiration (pyruvate/malate) 24 h after severe controlled cortical impact (CCI). (B) Effect of early post-injury (15 min) intraperitoneal administration of cyclosporine A (CsA) (20 mg/kg) on non-synaptic and synaptic mitochondria for state II respiration (pyruvate/malate) 24 h after severe CCI, calculated as % sham. Sham NS, sham non-synaptic (n = 6), Sham Syn, sham synaptic (n = 6), CCI + Veh NS, CCI + vehicle non-synaptic (n = 6), CCI + Veh Syn, CCI + vehicle synaptic (n = 6), CCI + CsA NS, CCI + CsA non-synaptic (n = 8), CCI + CsA Syn, CCI + CsA synaptic (n = 8); values, mean ± standard deviation; two-way analysis of variance followed by Tukey post hoc; *p < 0.05, **p < 0.01, #p < 0.05 compared with non-synaptic sham.
To assess drug effect, state II respiratory rates for CCI + vehicle and CCI + CsA were calculated as a percentage of the sham respiratory rate. A two-way ANOVA (treatment × population) revealed a significant main effect for treatment (F [1, 24] = 11.07, p = 0.0028), but not for population (F [1, 24] = 0.06332, p = 0.8035) or interaction (F [1, 24] = 3.182, p = 0.0871). While CsA improved state II respiration in both injured non-synaptic and injured synaptic mitochondria, post hoc testing (Tukey) revealed that this effect was only significant in the synaptic population (p < 0.01) (Fig. 1B).
State III: Addition of ADP to activate complex V ATP production
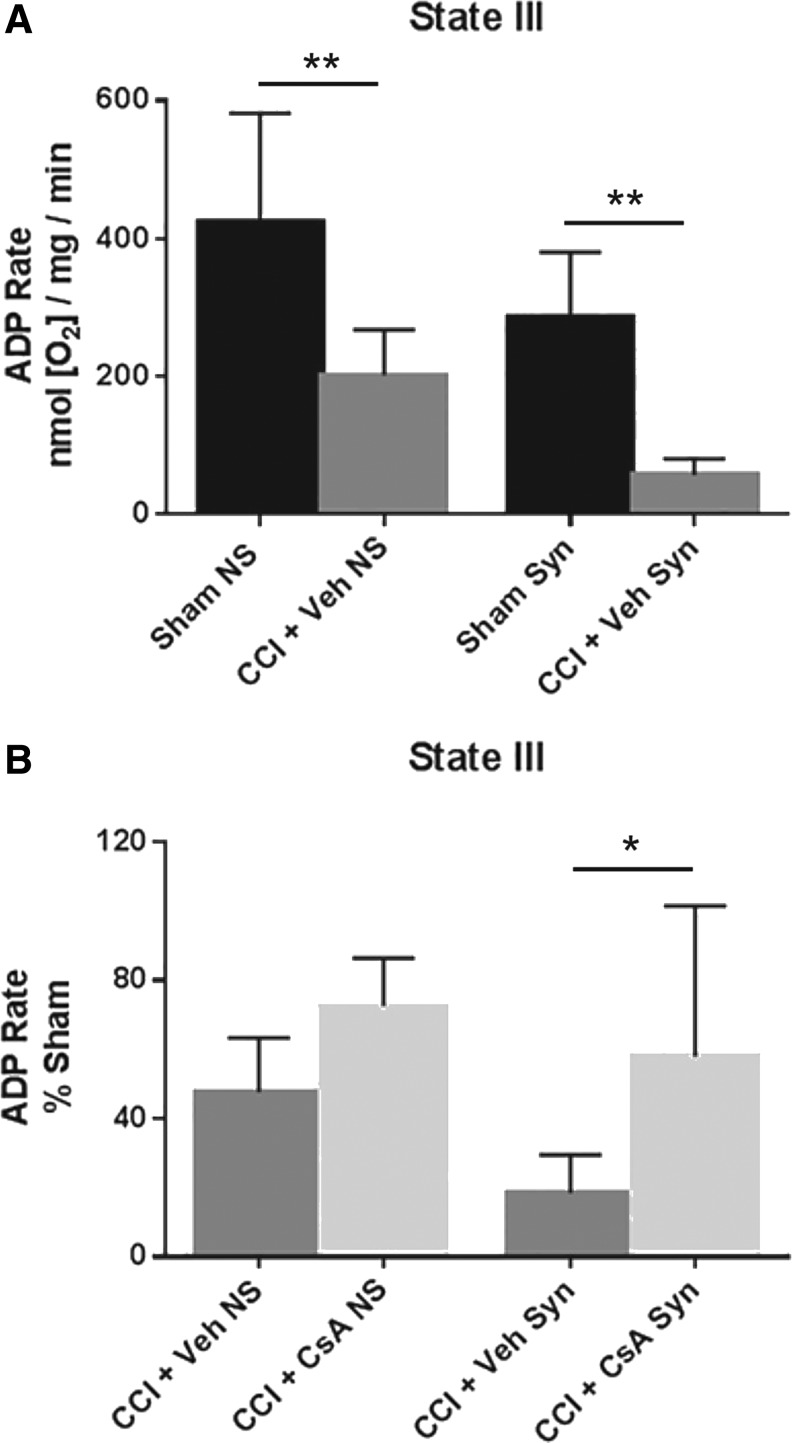
A two-way ANOVA (injury × population) revealed a significant main effect for injury (F [1, 20] = 31.10, p < 0.0001) and population (F [1, 20] = 12.39, p = 0.0022), but not interaction (F [1, 20] = .0075, p = 0.9318). Post hoc testing (Tukey) revealed that state III respiration for non-synaptic CCI + vehicle was significantly impaired compared with non-synaptic sham (p < 0.01), and synaptic CCI + vehicle was significantly impaired compared with synaptic sham (p < 0.01) (Fig. 2A).
FIG. 2.
(A) Effect of injury on non-synaptic and synaptic mitochondria for state III respiration (pyruvate/malate/adenosine diphosphate [ADP]) 24 h after severe controlled cortical impact (CCI). (B) Effect of early post-injury (15 min) intraperitoneal administration of cyclosporine A (CsA) (20 mg/kg) on non-synaptic and synaptic mitochondria for state III respiration (pyruvate/malate/ADP) 24 h after severe CCI, calculated as % sham. Sham NS, sham non-synaptic (n = 6), Sham Syn, sham synaptic (n = 6); CCI + Veh NS, CCI + vehicle non-synaptic (n = 6); CCI + Veh Syn, CCI + vehicle synaptic (n = 6); CCI + CsA NS, CCI + CsA non-synaptic (n = 8); CCI + CsA Syn, CCI + CsA synaptic (n = 8); values = mean ± standard deviation; two-way analysis of variance followed by Tukey post hoc; *p < 0.05, **p < 0.01.
To assess drug effect, state III respiratory rates for CCI + vehicle and CCI + CsA were calculated as a percentage of the sham respiratory rate. A two-way ANOVA revealed a significant main effect for treatment (F [1, 24] = 10.45, p = 0.0036), and population (F [1, 24] = 4.847, p = 0.0375), but not interaction (F [1, 24] = 0.5409, p = 0.4692). While CsA improved state III respiration in both injured non-synaptic and injured synaptic mitochondria, post hoc testing (Tukey) revealed this effect was only significant in the synaptic population (p < 0.05) (Fig. 2B).
State IV: Addition of oligomycin to inhibit complex V ATP production
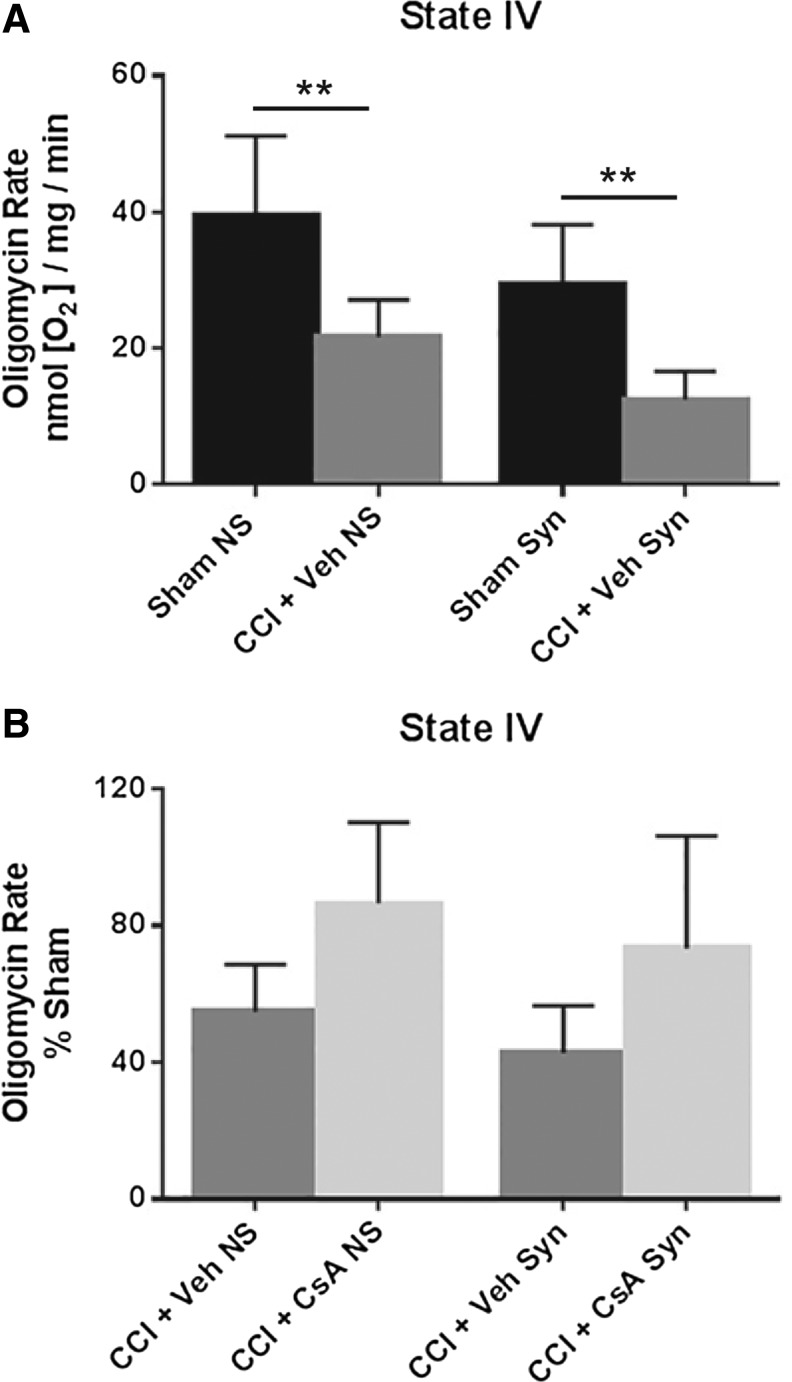
A two-way ANOVA (injury × population) revealed a significant main effect for injury (F [1, 20] = 27.77, p < 0.0001) and population (F [1, 20] = 8.680, p = .0080), but not interaction (F [1, 20] = .0276, p = 0.8698). Post hoc testing (Tukey) revealed that state IV respiration for non-synaptic CCI + vehicle was significantly impaired compared with non-synaptic sham (p < 0.01), and synaptic CCI + vehicle was significantly impaired compared with synaptic sham (p < 0.01) (Fig. 3A).
FIG. 3.
(A) Effect of injury on non-synaptic and synaptic mitochondria for state IV respiration (oligomycin) 24 h after severe controlled cortical impact (CCI). (B) Effect of early post-injury (15 min) intraperitoneal administration of cyclosporine A (CsA) (20 mg/kg), on non-synaptic and synaptic mitochondria for state IV respiration (oligomycin) 24 h after severe CCI, calculated as % sham. Sham NS, sham non-synaptic (n = 6); Sham Syn, sham synaptic (n = 6); CCI + Veh NS, CCI + vehicle non-synaptic (n = 6); CCI + Veh Syn, CCI + vehicle synaptic (n = 6); CCI + CsA NS, CCI + CsA non-synaptic (n = 8); CCI + CsA Syn, CCI + CsA synaptic (n = 8); values = mean ± standard deviation; two-way analysis of variance followed by Tukey post hoc; **p < 0.01.
To assess drug effect, state IV respiratory rates for CCI + vehicle and CCI + CsA were calculated as a percentage of the sham respiratory rate. A two-way ANOVA revealed a significant main effect for treatment (F [1, 24] = 11.91, p = 0.0021), but not population (F [1, 24] = 1.964, p = 0.1738) or interaction (F [1, 24] = 0.0046, p = 0.9465). While CsA improved state IV respiration in both injured non-synaptic and injured synaptic mitochondria, post hoc testing (Tukey) revealed these effects were not significant (Fig. 3B).
RCR (state III/state IV): Difference in oxygen utilization between activation and inhibition of ATP production
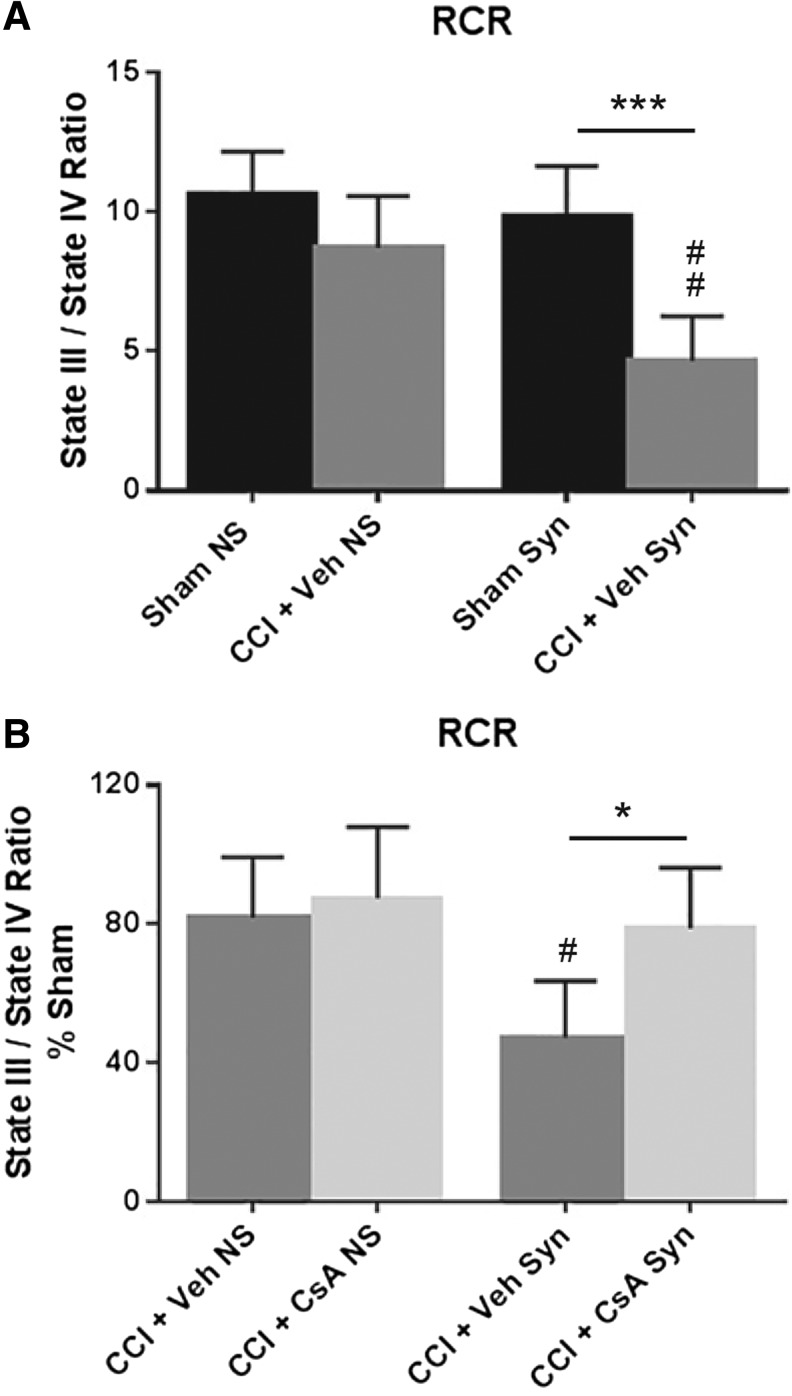
A two-way ANOVA (injury × population) revealed a significant main effect for injury (F [1, 20] = 26.02, p < 0.0001), population (F [1, 20] = 12.23, p = 0.0023), and interaction (F [1, 20] = 5.418, p = 0.0305). Post hoc testing (Tukey) revealed that RCR for synaptic CCI + vehicle was significantly impaired compared with synaptic sham (p < 0.001) and that synaptic CCI + vehicle was significantly impaired compared with non-synaptic CCI + vehicle (p < 0.01) (Fig. 4A]. To assess drug effect, RCR for CCI + vehicle and CCI + CsA was calculated as a percentage of sham RCR. A two-way ANOVA revealed a significant main effect for treatment (F [1, 24) = 7.092, p = 0.0136) and population (F [1, 24] = 9.680, p = 0.0048), but not interaction (F [1, 24] = 3.437, p = 0.0761). Post hoc testing (Tukey) revealed that CsA treatment significantly improved RCR in the injured synaptic population (p < 0.05) and that the RCR for synaptic CCI + vehicle is significantly impaired compared with the RCR for non-synaptic CCI + vehicle (p < 0.05) (Fig. 4B).
FIG. 4.
(A) Effect of injury on non-synaptic and synaptic mitochondria for respiratory control ratio (RCR) (state III/state IV) 24 h after severe controlled cortical impact (CCI). (B) Effect of early post-injury (15 min) intraperitoneal administration of cyclosporine A (CsA) (20 mg/kg), on non-synaptic and synaptic mitochondria for RCR (state III/state IV) 24 h after severe CCI, calculated as % sham. Sham NS, sham non-synaptic (n = 6); Sham Syn, sham synaptic (n = 6); CCI + Veh NS, CCI + vehicle non-synaptic (n = 6); CCI + Veh Syn, CCI + vehicle synaptic (n = 6); CCI + CsA NS, CCI + CsA non-synaptic (n = 8); CCI + CsA Syn, CCI + CsA synaptic (n = 8); values = mean ± standard deviation; two-way analysis of variance followed by Tukey post hoc; *p < 0.05, **p < 0.001, #p < 0.05 vs non-synaptic vehicle, ##p < 0.01 vs. non-synaptic vehicle.
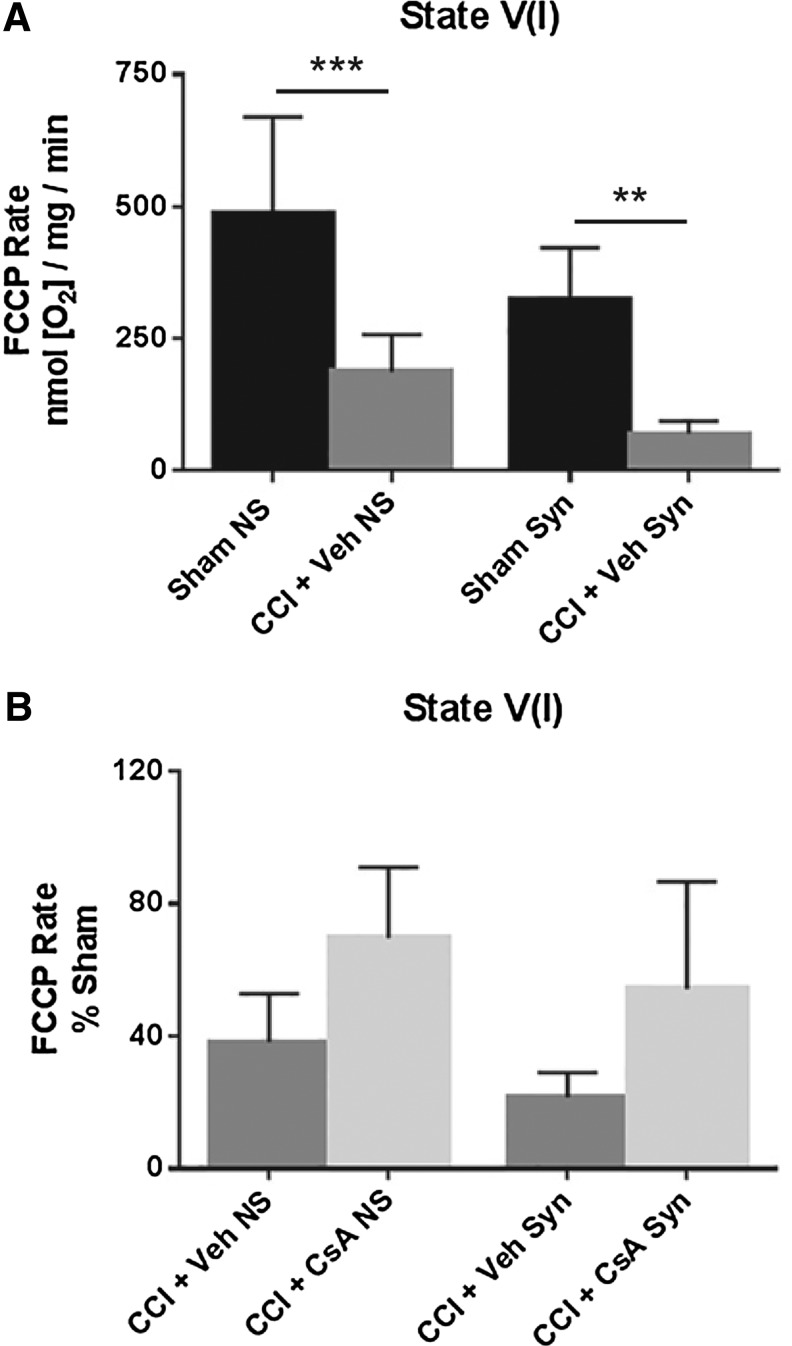
State V(I): Maximal complex I-driven respiration after addition of the protonophore FCCP
A two-way ANOVA (injury × population) revealed a significant main effect for injury (F [1, 20] = 37.82, p < 0.0001) and population (F [1, 20] = 9.556, p = 0.0058), but not interaction (F [1, 20] = 0.2492, p = 0.6231]. Post hoc testing (Tukey) revealed that state V(I) respiration for non-synaptic CCI + vehicle was significantly impaired compared with non-synaptic sham (p < 0.001), and synaptic CCI + vehicle was significantly impaired compared with synaptic sham (p < 0.01) (Fig. 5A).
FIG. 5.
(A) Effect of injury on non-synaptic and synaptic mitochondria for state V(I) respiration (FCCP) 24 h after severe controlled cortical impact (CCI). (B) Effect of early post-injury (15 min) intraperitoneal administration of cyclosporine A (CsA) (20 mg/kg) on non-synaptic and synaptic mitochondria for state V(I) respiration (FCCP) 24 h after severe CCI, calculated as % sham. Sham NS, sham non-synaptic (n = 6); Sham Syn, sham synaptic (n = 6); CCI + Veh NS, CCI + vehicle non-synaptic (n = 6); CCI + Veh Syn, CCI + vehicle synaptic (n = 6); CCI + CsA NS, CCI + CsA non-synaptic (n = 8); CCI + CsA Syn, CCI + CsA synaptic (n = 8); values = mean ± standard deviation; two-way analysis of variance followed by Tukey post hoc; **p < 0.01, ***p < 0.001.
To assess drug effect, state V(I) respiratory rates for CCI + vehicle and CCI + CsA were calculated as a percentage of the sham respiratory rate. A two-way ANOVA revealed a significant main effect for treatment (F [1, 24] = 14.25, p = 0.0009), but not population (F [1, 24] = 3.580, p = 0.0706) or interaction (F [1, 24] = 0.0084, p = 0.9276). While CsA improved state V(I) respiration in both injured non-synaptic and injured synaptic mitochondria, post hoc testing (Tukey) revealed neither effect was statistically significant (Fig. 5B).
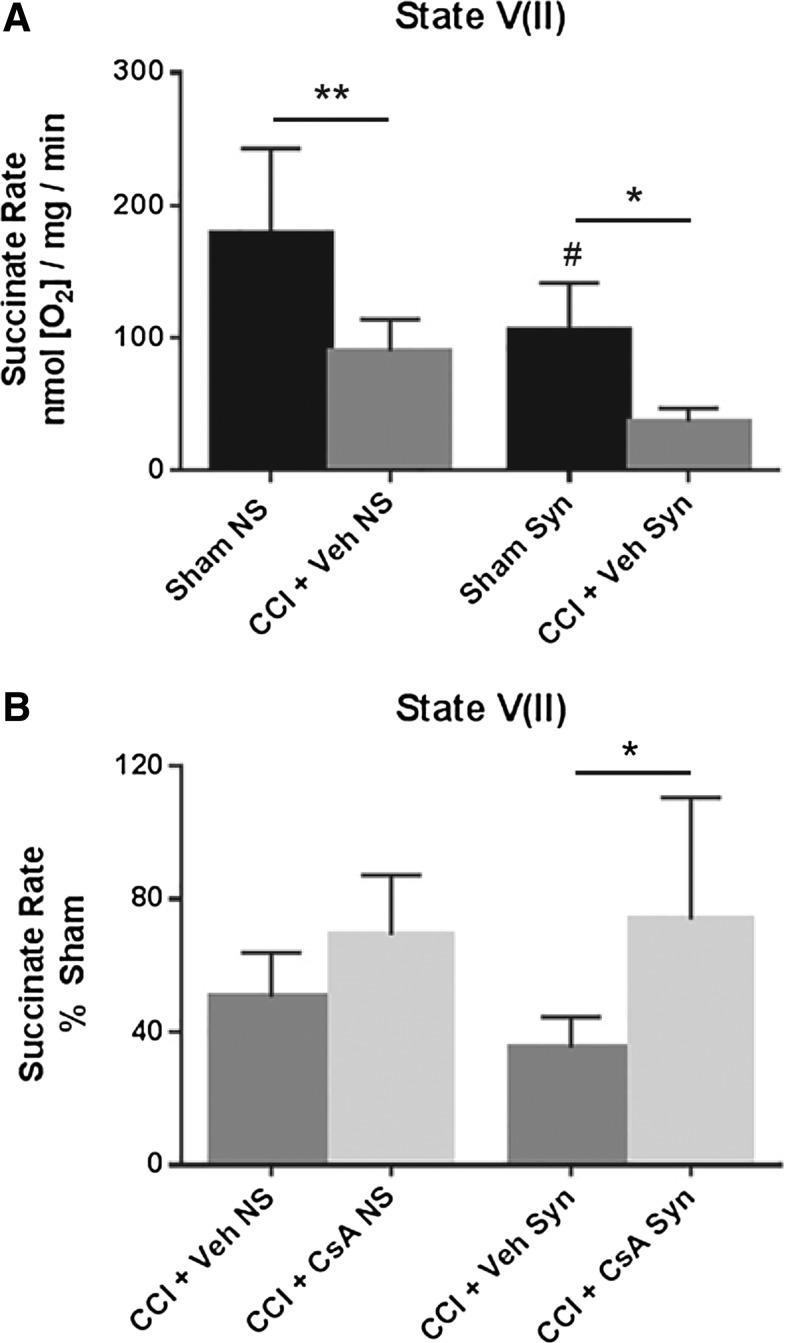
State V(II): Succinate-sctivated complex II-driven respiration after inhibition of complex I with rotenone
A two-way ANOVA (injury × population) revealed a significant main effect for injury (F [1, 20] = 23.97, p < 0.0001) and population (F [1, 20] = 15.37 = 0.0008), but not for interaction (F [1, 20] = 0.3787, p = 0.5452). Post hoc testing (Tukey) revealed that state V(II) respiration for non-synaptic CCI + vehicle was significantly impaired compared with non-synaptic sham (p < 0.01), synaptic CCI + vehicle was significantly impaired compared with synaptic sham (p < 0.05), and that the state V(II) respiration rate for synaptic sham was significantly decreased compared with non-synaptic sham (p < 0.05) (Fig. 6A).
FIG. 6.
(A) Effect of injury on non-synaptic and synaptic mitochondria for state V(II) respiration (rotenone/succinate) 24 h after severe controlled cortical impact (CCI). (B) Effect of early post-injury (15 min) intraperitoneal administration of CsA (20 mg/kg) on non-synaptic and synaptic mitochondria for state V(II) respiration (rotenone/succinate) 24 h after severe CCI, calculated as % sham. Sham NS, sham non-synaptic (n = 6); Sham Syn, sham synaptic (n = 6); CCI + Veh NS, CCI + vehicle non-synaptic (n = 6); CCI + Veh Syn, CCI + vehicle synaptic (n = 6); CCI + CsA NS, CCI + CsA non-synaptic (n = 8); CCI + CsA Syn, CCI + CsA synaptic (n = 8); values = mean ± standard deviation; two-way analysis of variance followed by Tukey post hoc; *p < 0.05, **p < 0.01, #p < 0.05 vs. non-synaptic sham.
To assess drug effect, state V(II) respiratory rates for CCI + vehicle and CCI + CsA were calculated as a percentage of the sham respiratory rate. A two-way ANOVA revealed a significant main effect for treatment (F [1, 24] = 10.45, p = 0.0035), but not for population (F [1, 24] = 0.3533, p = 0.5578) or interaction (F [1, 24] = 1.272, p = 0.2706). While CsA improved state V(II) respiration in both injured non-synaptic and injured synaptic mitochondria, post hoc testing (Tukey) revealed this effect was only significant in the synaptic population (p < 0.05) (Fig. 6B).
Discussion
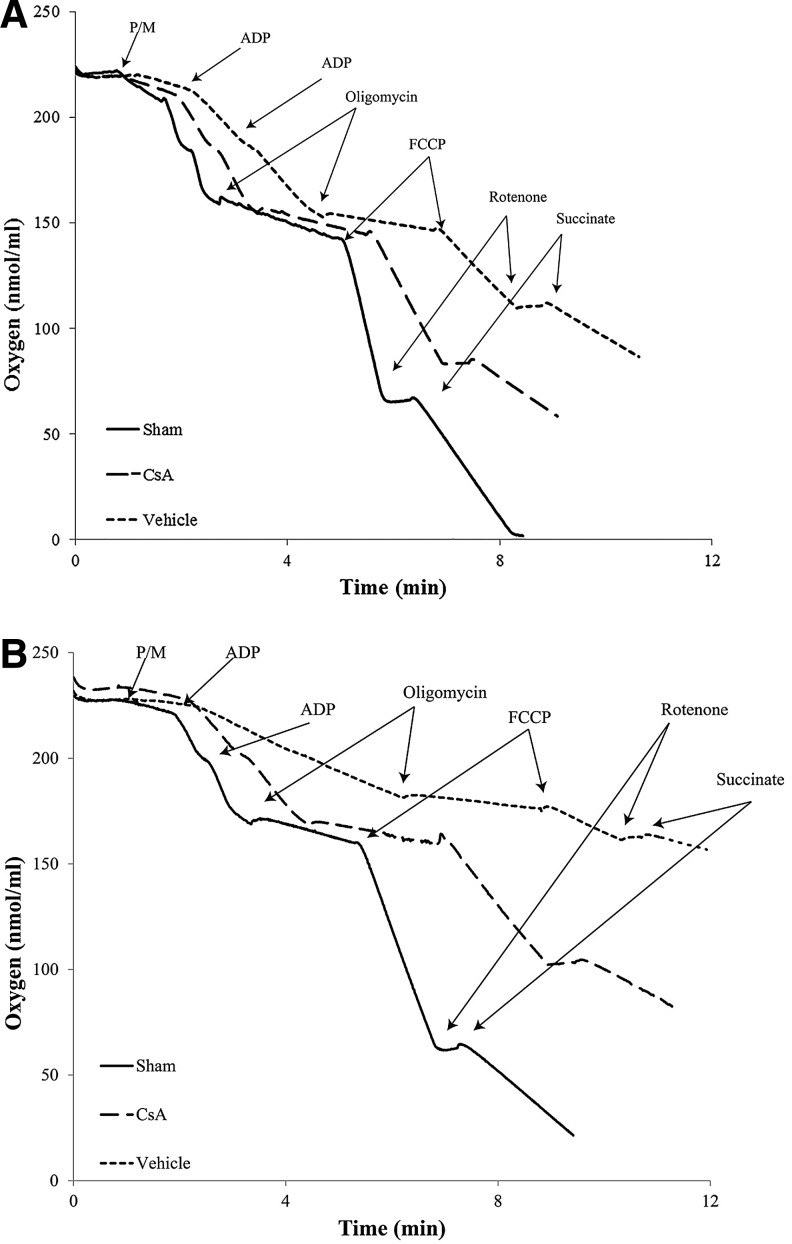
These results indicate that synaptic mitochondria sustain more damage than non-synaptic mitochondria 24 h after severe CCI and that intraperitoneal administration of CsA (20 mg/kg) 15 min after injury improves synaptic and non-synaptic respiration (Fig. 7), with a significant improvement being seen in the more severely impaired synaptic population.
FIG. 7.
Representative oxymetric traces indicating rates of oxygen consumption of sham, vehicle, and cyclosporine A (CsA) (20 mg/kg, intraperitoneally, 15 min post-injury) for (A) non-synaptic and (B) synaptic mitochondria isolated from ipsilateral cortex 24 h after severe controlled cortical impact (CCI). Purified mitochondrial protein (>30 μg) was suspended in respiration buffer (125 mmol/L KCl, 2 mmol/L MgCl2, 2.5 mmol/L KH2PO4, 0.1% BSA, 20 mmol/L HEPES, pH 7.2) in a final volume of 250 μl, and oxygen consumption rates were measured using a Clark-type oxygen electrode in the presence of 5 mmol/L pyruvate and 2.5 mmol/L malate (state II), two boluses of 150 μmol/L ATP (state III), 2 μmol/L oligomycin (state IV), 2 μmol/L FCCP (state VI), and 100 nmol/L rotenone and 10 mmol/L succinate (state VII). Sham, sham; CsA, CCI + CsA; vehicle, CCI + vehicle; ADP, adenosine diphosphate.
CsA is an FDA-approved immunosuppressant, used clinically to prevent organ rejection after transplant through a mechanism that involves inhibition of calcineurin and T-cell activation.75 CsA, however, also has the ability to bind the mitochondrial matrix protein cyclophilin D,76,77 which inhibits its interaction with the adenine nucleotide transporter, an inner mitochondrial membrane protein, thus preventing formation of mPTP.78 It is through inhibition of mPTP that CsA exerts its neuroprotective effects after TBI.
NIM811, a non-immunosuppressive analog of CsA unable to bind calcinuerin, but capable of binding cyclophilin D and inhibiting mPTP,79 maintains the neuroprotective effects of CsA, improving mitochondrial respiration, decreasing oxidative damage, decreasing calpain-mediated cytoskeletal degradation and neurodegeneration, and improving motor and cognitive function after experimental TBI.47,48,55
Under normal physiologic conditions CsA is minimally blood–brain barrier penetrable.80 After TBI, however, CsA is able to enter the CNS because of blood–brain barrier breakdown,81 and although concerns have been raised regarding CsA and neurotoxicity,82–89 two phase II clinical trials show CsA to be safe for use in patients with severe TBI.90,91
Despite the fact that the neuroprotective effects of CsA rely on direct protection of mitochondria,28,47,48,58 many experimental TBI studies using CsA have focused on outcome measures downstream of mitochondrial dysfunction, such as cortical lesion volume,56–58,72 axonal pathology,20,21,48,92,93 behavior,48,59 and synaptic plasticity.94 Although important work does confirm the ability of CsA to protect mitochondria after injury, those studies focused on acute injury,33 microscopic structure analysis,21 or more indirect measures of mitochondrial function such as brain oxygen consumption59 and whole brain ATP levels.95 Only two studies have assessed mitochondrial bioenergetics via oxygen consumption rates, one of which did not extend beyond 12 h and was conducted in mice,47 and one of which used juvenile animals.61 Importantly, none of these mitochondria-focused studies evaluated isolated synaptic and non-synaptic populations.
While it should be noted that Sullivan and coworkers33 showed that CsA maintains mitochondrial membrane potential in isolated non-synaptic mitochondria and intact synaptosomes acutely (30–60 min) after CCI, this is the first study to examine the effects of CsA on isolated and purified synaptic and non-synaptic mitochondria after experimental TBI. As such, CsA was administered 15 min post-injury. Early administration allows for the greatest chance of neuroprotection and allows for contextualization of future therapeutic window studies. The 20 mg/kg intraperitoneal dosage used is considered optimal and has been used extensively in previous experimental TBI studies.33,47,48,57,58,61,72,93,94
The CsA used in this study is solubilized in a vehicle containing cremophor. Toxicity concerns have been raised in regard to cremophor, and reported side effects include hypersensitivity reactions, hyperlipidemia, erythrocyte aggregation, and peripheral neuropathy, but are usually associated with intravenous infusions or high cremophor concentrations.96 Several studies have shown that cremophor has the ability to impair mitochondrial function. These studies, however, were performed in mitochondria isolated from heart muscle, skeletal muscle, and kidney tissue, and cremophor was either administered chronically or in vitro.97–99
Interestingly, chronic administration of cremophor or CsA + cremophor impairs heart and skeletal muscle mitochondrial similarly, suggesting that any cremophor toxicity is still observable when administered along with CsA. The safety of low doses of CsA + cremophor, such as the dose used in this study (20 mg/kg intraperitoneally), is well supported in the literature, however. For example, administration of CsA + cremophor to sham animals has no effect on long-term potentiation,94 motor or cognitive function,60 or brain metabolism,95 although chronic administration can result in weight loss.60
Although it is feasible that administration of vehicle or CsA to sham animals could alter basal mitochondrial respiration, evaluating the protective effect of CsA after injury in comparison with sham animals not receiving vehicle or drug is the most clinically appropriate assessment. Therefore, neither vehicle nor CsA was administered to sham animals for this study.
The respiratory function of isolated synaptic and non-synaptic mitochondria was assessed 24 h after injury. Total (synaptic and non-synaptic) mitochondrial respiratory dysfunction peaks between 12 and 24 h after severe CCI in the rat, 8 with several studies confirming mitochondrial respiratory dysfunction 24 h after injury.8,50,100
In sham animals, synaptic mitochondria showed decreased respiratory rates compared with non-synaptic mitochondria, decreases that were only significant in state II and state V(II). Previous studies have also shown that healthy synaptic mitochondria have decreased respiration rates compared with healthy non-synaptic mitochondria,10,101 as well as decreased pyruvate dehydrogenase activity,102 the enzyme responsible for generating the nicotinamide adenine dinucleotide that feeds into complex I of the electron transport chain. Because non-synaptic mitochondria contain mitochondria from both neurons and glia, it is possible that the higher respiration rates found in non-synaptic mitochondria are because of astrocytic mitochondria; mitochondria isolated from cultured astrocytes have higher respiration rates than mitochondria isolated from cultured neurons as well as higher levels of electron transport chain subunits.74
RCR (state III/state IV) is considered one of the best general measures of mitochondrial function and health and represents the ability of mitochondria to couple oxidation of substrates with generation of ATP via ADP phosphorylation under minimal proton leak. Therefore, healthy mitochondria will have a high RCR, while damaged mitochondria will have a low RCR.103 In this study, the RCR for synaptic mitochondria was significantly reduced 24 h after injury compared with sham, while the RCR for non-synaptic mitochondria was not. The RCR for injured synaptic mitochondria was also significantly reduced compared with the RCR for injured non-synaptic mitochondria. Interestingly, statistical analysis revealed a significant interaction between injury and population for this measure, indicating that synaptic mitochondria are indeed more susceptible to injury.
Importantly, the decrease in synaptic RCR was significantly attenuated by CsA administration. While RCR represents an overall measure of mitochondrial function, its value is affected by multiple aspects of oxidative phosphorylation.103 Therefore, in addition to RCR we assessed individual states of respiration to identify specific aspects of bioenergetic impairment.
State II respiration is measured after addition of the complex-I substrates pyruvate and malate, but before ADP addition, and represents a slow state of respiration. Severe CCI significantly reduced state II respiration for both synaptic and non-synaptic mitochondria 24 h after injury. Decreases in state II respiration are consistent with the fact that pyruvate dehydrogenase, the enzyme linking glycolysis with the citric acid cycle, is known to have decreased activity after TBI.104,105 While CsA was able to improve injury induced decreases in state II respiration in both populations, the improvements only reached statistical significance in the synaptic population.
State III respiration is measured after addition of ADP, allowing coupling of oxidative phosphorylation, and is considered one of the most important states of respiration to measure after injury, because decreases in state III respiration are indicative of defects in complex I-driven substrate oxidation and/or ATP turnover.103 Severe CCI significantly reduced state III respiration for both synaptic and non-synaptic mitochondria 24 h after injury. While CsA was able to improve injury-induced decreases in state III respiration in both populations, the improvements only reached statistical significance in the synaptic population. Importantly, the ability of CsA to significantly improve state III synaptic respiration is a major contributory factor to the ability of CsA to also significantly improve synaptic RCR (state III/IV) after injury.
State IV is measured after addition of the ATP synthase inhibitor oligomycin, which returns the mitochondria to a basal state of respiration. Severe CCI significantly reduced state IV respiration for both synaptic and non-synaptic mitochondria 24 h after injury. While CsA was able to improve injury-induced decreases in state IV respiration in both populations, the improvements were not statistically significant.
State V(I) respiration is measured after addition of the protonophore FCCP, which uncouples substrate oxidation from ATP production, and is used to assess maximal respiration, with decreases in state V(I) respiration being indicative of defects in complex-I driven substrate oxidation.103 Severe CCI significantly reduced state V(I) respiration for both synaptic and non-synaptic mitochondria 24 h after injury. While CsA was able to improve injury-induced decreases in state V(I) respiration in both populations, the improvements were not statistically significant.
State V(II) respiration is assessed after addition of the complex-I inhibitor rotenone and the complex-II substrate succinate. Therefore, decreases in state V(II) respiration are indicative of defects in complex-II driven respiration. Severe CCI significantly reduced state V(II) respiration for both synaptic and non-synaptic mitochondria 24 h after injury. While, CsA improved injury-induced decreases in complex-II driven respiration in both populations, the improvements only reached statistical significance in the synaptic population.
In summary, synaptic mitochondria sustain more damage 24 h after severe CCI than non-synaptic mitochondria, as best evidenced by the changes in RCR. This is in agreement with previous work indicating that synaptic mitochondria are more susceptible to dysfunction. For example, synaptic mitochondria are more susceptible to damage 3 h after moderate CCI compared with non-synaptic mitochondria, an effect that is compounded by aging.10 In addition, in vitro, healthy synaptic mitochondria have a decreased ability to buffer calcium before undergoing permeability transition compared with non-synaptic mitochondria.66
Several hypotheses have been offered to explain the increased vulnerability of synaptic mitochondria compared with non-synaptic mitochondria, including high concentrations of cyclophilin D, leading to increased susceptibility toward calcium-induced permeability transition,69 as well as increased exposure of synaptic mitochondria to oxidative damage.66,69 Because non-synaptic mitochondria contain both neuronal and non-neuronal cell types, however, and mitochondria isolated from neurons and glia have differing properties,69,74 part of the increased susceptibility of synaptic mitochondria to injury may be because synaptic mitochondria represent of a more purely neuronal population,66,69,106 rather than their specific subcellular localization to the pre-synaptic terminal. It is likely, however, that both cell type and subcellular localization contribute to synaptic mitochondria vulnerability.
These results also indicate that intraperitoneal administration of CsA (20 mg/kg) 15 min after injury attenuates respiratory dysfunction in both populations 24 h after severe CCI, with a statistically significant improvement being seen in the synaptic population. Although, there was a significant decrease in each respiration state after injury for the non-synaptic mitochondria, the non-synaptic RCR was unaffected by injury, indicating no overall bioenergetics dysfunction.103 It is therefore likely that the improvements CsA had on the non-synaptic respiration states were only non-significant because of a lack of robust injury effect in this population, and that CsA would offer significant improvements at time points later than 24 h when non-synaptic mitochondria are more significantly impaired.
The fact that CsA is able to significantly protect synaptic mitochondria after injury is impressive, because synaptic mitochondria do not always respond as favorably to pharmaceutical intervention as the non-synaptic population. For example, following spinal cord injury, the catalytic peroxynitrite inhibitor, tempol, is only effective in non-synaptic mitochondria, while the mitochondrial uncoupler 2–4 DNP works in both populations, but has a shorter therapeutic window in the synaptic mitochondria.70
Future studies will investigate whether there is, indeed, a difference in the therapeutic window for CsA between synaptic and non-synaptic mitochondria. If so, studies may explain the fact that while CsA is able to improve cortical tissue sparing when administration is delayed up to 8 h, it is significantly more protective when administered within 3 h of injury.56 Further, the ability of CsA to protect synaptic neuronal mitochondrial respiration is made more impressive by the knowledge that there are higher concentrations of the CsA target protein cyclophilin D in synaptic mitochondria,66 gamma-aminobutyric acid-ergic interneurons,106 and cultured neurons when compared with cultured astrocytes.69
In fact, in vitro healthy synaptic mitochondria require higher concentrations of CsA to prevent calcium-induced permeability transition.69 Future studies will therefore evaluate the ability of CsA to attenuate calcium-induced permeability transition in both populations after injury, as well as the ability of CsA to retain its synaptic neuroprotective effect at time points beyond 24 h.
Conclusion
This study confirms that synaptic mitochondria are more vulnerable than non-synaptic mitochondria after experimental TBI, and therefore emphasizes the need for further characterization of synaptic and non-synaptic mitochondria after experimental TBI, including the contribution each population makes to TBI pathology, as well as the response each population has to mitochondrial-directed pharmacotherapies.
While the pathology of TBI is complex and factors other than mitochondrial dysfunction contribute to downstream processes such as cytoskeletal degradation, neurodegeneration, and neurologic impairment, it is likely that successful protection of the more vulnerable synaptic population greatly contributes to inhibition of these downstream processes. Because this study confirms the ability of CsA to significantly improve synaptic respiration after injury, CsA remains a promising neuroprotective candidate for the treatment of those with TBI.
Acknowledgments
This work was supported by 5R01 NS083405, 5R01 NS084857, 5P30 NS051220, 1F30 NS096876, and funding from the Kentucky Spinal Cord & Head Injury Research Trust (KSCHIRT).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Thurman D.J., Alverson C., Dunn K.A., Guerrero J., and Sniezek J.E. (1999). Traumatic brain injury in the United States: a public health perspective. J. Head Trauma Rehabil. 14, 602–615 [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein E.A, Corso P.S., and Miller T.R. (2006). The Incidence and Economic Burden of Injuries in the United States. Oxford University Press: New York [Google Scholar]
- 3.Coronado V.G., McGuire L.C., Faul M.F., Sugerman D.E., and Pearson W.S. (2012). Traumatic brain injury epidemiology and public health issues. in: Brain Injury Medicine: Principles and Practice. Zasler N.D., Katz D.I., and Zafonte R.D. (eds). Demos Medical Publishing: New York, pps. 84–100 [Google Scholar]
- 4.Maas A.I., Stocchetti N., and Bullock R. (2008). Moderate and severe traumatic brain injury in adults. Lancet Neurol. 7, 728–741 [DOI] [PubMed] [Google Scholar]
- 5.Faden A.I., Demediuk P., Panter S.S., and Vink R. (1989). The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 244, 798–800 [DOI] [PubMed] [Google Scholar]
- 6.Weber J.T. (2012). Altered calcium signaling following traumatic brain injury. Front. Pharmacol. 3, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh I.N., Sullivan P.G., Deng Y., Mbye L.H., and Hall E.D. (2006). Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J. Cereb. Blood Flow Metab. 26, 1407–1418 [DOI] [PubMed] [Google Scholar]
- 8.Xiong Y., Gu Q., Peterson P.L., Muizelaar J.P., and Lee C.P. (1997). Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J. Neurotrauma 14, 23–34 [DOI] [PubMed] [Google Scholar]
- 9.Gilmer L.K., Roberts K.N., Joy K., Sullivan P.G., and Scheff S.W. (2009). Early mitochondrial dysfunction after cortical contusion injury. J. Neurotrauma 26, 1271–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilmer L.K., Ansari M.A., Roberts K.N., and Scheff S.W. (2010). Age-related mitochondrial changes after traumatic brain injury. J. Neurotrauma 27, 939–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall E.D., Wang J.A., Bosken J.M., and Singh I.N. (2016). Lipid peroxidation in brain or spinal cord mitochondria after injury. J. Bioenerg. Biomembr. 48, 169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall E.D., Vaishnav R.A., and Mustafa A.G. (2010). Antioxidant therapies for traumatic brain injury. Neurotherapeutics 7, 51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bains M., and Hall E.D. (2012). Antioxidant therapies in traumatic brain and spinal cord injury. Biochim. Biophys. Acta 1822, 675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall E.D., Andrus P.K., Yonkers P.A., Smith S.L., Zhang J.R., Taylor B.M., and Sun F.F. (1994). Generation and detection of hydroxyl radical following experimental head injury. Ann. N. Y. Acad. Sci. 738, 15–24 [DOI] [PubMed] [Google Scholar]
- 15.Deng Y., Thompson B.M., Gao X., and Hall E.D. (2007). Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Exp. Neurol. 205, 154–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh I.N., Sullivan P.G., and Hall E.D. (2007). Peroxynitrite-mediated oxidative damage to brain mitochondria: protective effects of peroxynitrite scavengers. J. Neurosci. Res. 85, 2216–2223 [DOI] [PubMed] [Google Scholar]
- 17.Deng-Bryant Y., Singh I.N., Carrico K.M., and Hall E.D. (2008). Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J. Cereb. Blood Flow Metab. 28, 1114–1126 [DOI] [PubMed] [Google Scholar]
- 18.Smith S.L., Andrus P.K., Zhang J.R., and Hall E.D. (1994). Direct measurement of hydroxyl radicals, lipid peroxidation, and blood-brain barrier disruption following unilateral cortical impact head injury in the rat. J. Neurotrauma 11, 393–404 [DOI] [PubMed] [Google Scholar]
- 19.Singh I.N., Gilmer L.K., Miller D.M., Cebak J.E., Wang J.A., and Hall E.D. (2013). Phenelzine mitochondrial functional preservation and neuroprotection after traumatic brain injury related to scavenging of the lipid peroxidation-derived aldehyde 4-hydroxy-2-nonenal. J. Cereb. Blood Flow Metab. 33, 593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okonkwo D.O., Buki A., Siman R. and Povlishock J.T. (1999). Cyclosporin A limits calcium-induced axonal damage following traumatic brain injury. Neuroreport 10, 353–358 [DOI] [PubMed] [Google Scholar]
- 21.Okonkwo D.O., and Povlishock J.T. (1999). An intrathecal bolus of cyclosporin A before injury preserves mitochondrial integrity and attenuates axonal disruption in traumatic brain injury. J. Cereb. Blood Flow Metab. 19, 443–451 [DOI] [PubMed] [Google Scholar]
- 22.Buki A., Siman R., Trojanowski J.Q., and Povlishock J.T. (1999). The role of calpain-mediated spectrin proteolysis in traumatically induced axonal injury. J. Neuropathol. Exp. Neurol. 58, 365–375 [DOI] [PubMed] [Google Scholar]
- 23.Saatman K.E., Bozyczko-Coyne D., Marcy V., Siman R., and McIntosh T.K. (1996). Prolonged calpain-mediated spectrin breakdown occurs regionally following experimental brain injury in the rat. J. Neuropathol. Exp. Neurol. 55, 850–860 [DOI] [PubMed] [Google Scholar]
- 24.Springer J.E. (2002). Apoptotic cell death following traumatic injury to the central nervous system. J. Biochem. Mol. Biol. 35, 94–105 [DOI] [PubMed] [Google Scholar]
- 25.Lifshitz J., Friberg H., Neumar R.W., Raghupathi R., Welsh F.A., Janmey P., Saatman K.E., Wieloch T., Grady M.S., and McIntosh T.K. (2003). Structural and functional damage sustained by mitochondria after traumatic brain injury in the rat: evidence for differentially sensitive populations in the cortex and hippocampus. J. Cereb. Blood Flow Metab. 23, 219–231 [DOI] [PubMed] [Google Scholar]
- 26.Lifshitz J., Sullivan P.G., Hovda D.A., Wieloch T., and McIntosh T.K. (2004). Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion 4, 705–713 [DOI] [PubMed] [Google Scholar]
- 27.Raghupathi R. (2004). Cell death mechanisms following traumatic brain injury. Brain Pathol. 14, 215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan P.G., Rabchevsky A.G., Waldmeier P.C., and Springer J.E. (2005). Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J. Neurosci. Res. 79, 231–239 [DOI] [PubMed] [Google Scholar]
- 29.Rizzuto R., Pinton P., Brini M., Chiesa A., Filippin L., and Pozzan T. (1999). Mitochondria as biosensors of calcium microdomains. Cell Calcium 26, 193–199 [DOI] [PubMed] [Google Scholar]
- 30.Rizzuto R., Bernardi P., and Pozzan T. (2000). Mitochondria as all-round players of the calcium game. J. Physiol. 529, 37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghafourifar P., and Richter C. (2000). Peroxynitrite formed by mitochondrial NO synthase promotes mitochondrial Ca2+ release. Free Radic. Biol. Med. 29, 343–348 [DOI] [PubMed] [Google Scholar]
- 32.Hansson M.J., Mansson R., Morota S., Uchino H., Kallur T., Sumi T., Ishii N., Shimazu M., Keep M.F., Jegorov A., and Elmer E. (2008). Calcium-induced generation of reactive oxygen species in brain mitochondria is mediated by permeability transition. Free Radic. Biol. Med. 45, 284–294 [DOI] [PubMed] [Google Scholar]
- 33.Sullivan P.G., Thompson M.B., and Scheff S.W. (1999). Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp. Neurol. 160, 226–234 [DOI] [PubMed] [Google Scholar]
- 34.Xiong Y., Shie F.S., Zhang J., Lee C.P., and Ho Y.S. (2005). Prevention of mitochondrial dysfunction in post-traumatic mouse brain by superoxide dismutase. J. Neurochem. 95, 732–744 [DOI] [PubMed] [Google Scholar]
- 35.Xiong Y., Peterson P.L., Verweij B.H., Vinas F.C., Muizelaar J.P., and Lee C.P. (1998). Mitochondrial dysfunction after experimental traumatic brain injury: combined efficacy of SNX-111 and U-101033E. J. Neurotrauma 15, 531–544 [DOI] [PubMed] [Google Scholar]
- 36.Bernardi P. (1996). The permeability transition pore. Control points of a cyclosporin A-sensitive mitochondrial channel involved in cell death. Biochim. Biophys. Acta 1275, 5–9 [DOI] [PubMed] [Google Scholar]
- 37.Galluzzi L., Blomgren K., and Kroemer G. (2009). Mitochondrial membrane permeabilization in neuronal injury. Nat. Rev. Neurosci. 10, 481–494 [DOI] [PubMed] [Google Scholar]
- 38.Vaishnav R.A., Singh I.N., Miller D.M., and Hall E.D. (2010). Lipid peroxidation-derived reactive aldehydes directly and differentially impair spinal cord and brain mitochondrial function. J. Neurotrauma 27, 1311–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mustafa A.G., Singh I.N., Wang J., Carrico K.M., and Hall E.D. (2010). Mitochondrial protection after traumatic brain injury by scavenging lipid peroxyl radicals. J. Neurochem. 114, 271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller D.M., Singh I.N., Wang J.A., and Hall E.D. (2014). Nrf2-ARE activator carnosic acid decreases mitochondrial dysfunction, oxidative damage and neuronal cytoskeletal degradation following traumatic brain injury in mice. Exp. Neurol. 264, 103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo J., and Shi R. (2005). Acrolein induces oxidative stress in brain mitochondria. Neurochem. Int. 46, 243–252 [DOI] [PubMed] [Google Scholar]
- 42.Shi R., Rickett T., and Sun W. (2011). Acrolein-mediated injury in nervous system trauma and diseases. Mol. Nutr. Food Res. 55, 1320–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pocernich C.B., and Butterfield D.A. (2003). Acrolein inhibits NADH-linked mitochondrial enzyme activity: implications for Alzheimer's disease. Neurotox. Res. 5, 515–520 [DOI] [PubMed] [Google Scholar]
- 44.Picklo M.J., and Montine T.J. (2001). Acrolein inhibits respiration in isolated brain mitochondria. Biochim. Biophys. Acta 1535, 145–152 [DOI] [PubMed] [Google Scholar]
- 45.Picklo M.J., Amarnath V., McIntyre J.O., Graham D.G., and Montine T.J. (1999). 4-Hydroxy-2(E)-nonenal inhibits CNS mitochondrial respiration at multiple sites. J. Neurochem. 72, 1617–1624 [DOI] [PubMed] [Google Scholar]
- 46.Keller J.N., Mark R.J., Bruce A.J., Blanc E., Rothstein J.D., Uchida K., Waeg G., and Mattson M.P. (1997). 4-Hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neuroscience 80, 685–696 [DOI] [PubMed] [Google Scholar]
- 47.Mbye L.H., Singh I.N., Sullivan P.G., Springer J.E., and Hall E.D. (2008). Attenuation of acute mitochondrial dysfunction after traumatic brain injury in mice by NIM811, a non-immunosuppressive cyclosporin A analog. Exp. Neurol. 209, 243–253 [DOI] [PubMed] [Google Scholar]
- 48.Mbye L.H., Singh I.N., Carrico K.M., Saatman K.E., and Hall E.D. (2009). Comparative neuroprotective effects of cyclosporin A and NIM811, a nonimmunosuppressive cyclosporin A analog, following traumatic brain injury. J. Cereb. Blood Flow Metab. 29, 87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mustafa A.G., Wang J.A., Carrico K.M., and Hall E.D. (2011). Pharmacological inhibition of lipid peroxidation attenuates calpain-mediated cytoskeletal degradation after traumatic brain injury. J. Neurochem. 117, 579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pandya J.D., Pauly J.R., and Sullivan P.G. (2009). The optimal dosage and window of opportunity to maintain mitochondrial homeostasis following traumatic brain injury using the uncoupler FCCP. Exp. Neurol. 218, 381–389 [DOI] [PubMed] [Google Scholar]
- 51.Pandya J.D., Pauly J.R., Nukala V.N., Sebastian A.H., Day K.M., Korde A.S., Maragos W.F., Hall E.D., and Sullivan P.G. (2007). Post-injury administration of mitochondrial uncouplers increases tissue sparing and improves behavioral outcome following traumatic brain injury in rodents. J. Neurotrauma 24, 798–811 [DOI] [PubMed] [Google Scholar]
- 52.Prins M.L., Fujima L.S., and Hovda D.A. (2005). Age-dependent reduction of cortical contusion volume by ketones after traumatic brain injury. J. Neurosci. Res. 82, 413–420 [DOI] [PubMed] [Google Scholar]
- 53.Greco T., Glenn T.C., Hovda D.A., and Prins M.L. (2015). Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J. Cereb. Blood Flow Metab. 36, 1603–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pandya J.D., Readnower R.D., Patel S.P., Yonutas H.M., Pauly J.R., Goldstein G.A., Rabchevsky A.G., and Sullivan P.G. (2014). N-acetylcysteine amide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Exp. Neurol. 257, 106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Readnower R.D., Pandya J.D., McEwen M.L., Pauly J.R., Springer J.E., and Sullivan P.G. (2011). Post-injury administration of the mitochondrial permeability transition pore inhibitor, NIM811, is neuroprotective and improves cognition after traumatic brain injury in rats. J. Neurotrauma 28, 1845–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sullivan P.G., Sebastian A.H., and Hall E.D. (2011). Therapeutic window analysis of the neuroprotective effects of cyclosporine A after traumatic brain injury. J. Neurotrauma 28, 311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sullivan P.G., Thompson M., and Scheff S.W. (2000). Continuous infusion of cyclosporin A postinjury significantly ameliorates cortical damage following traumatic brain injury. Exp. Neurol. 161, 631–637 [DOI] [PubMed] [Google Scholar]
- 58.Scheff S.W., and Sullivan P.G. (1999). Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J. Neurotrauma 16, 783–792 [DOI] [PubMed] [Google Scholar]
- 59.Alessandri B., Rice A.C., Levasseur J., DeFord M., Hamm R.J., and Bullock M.R. (2002). Cyclosporin A improves brain tissue oxygen consumption and learning/memory performance after lateral fluid percussion injury in rats. J. Neurotrauma 19, 829–841 [DOI] [PubMed] [Google Scholar]
- 60.Riess P., Bareyre F.M., Saatman K.E., Cheney J.A., Lifshitz J., Raghupathi R., Grady M.S., Neugebauer E., and McIntosh T.K. (2001). Effects of chronic, post-injury Cyclosporin A administration on motor and sensorimotor function following severe, experimental traumatic brain injury. Restor. Neurol. Neurosci. 18, 1–8 [PubMed] [Google Scholar]
- 61.Kilbaugh T.J., Bhandare S., Lorom D.H., Saraswati M., Robertson C.L., and Margulies S.S. (2011). Cyclosporin A preserves mitochondrial function after traumatic brain injury in the immature rat and piglet. J. Neurotrauma 28, 763–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sheng Z.H., and Cai Q. (2012). Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat. Rev. Neurosci. 13, 77–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng A., Hou Y., and Mattson M.P. (2010). Mitochondria and neuroplasticity. ASN Neuro. 2, e00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MacAskill A.F., Atkin T.A., and Kittler J.T. (2010). Mitochondrial trafficking and the provision of energy and calcium buffering at excitatory synapses. Eur. J. Neurosci. 32, 231–240 [DOI] [PubMed] [Google Scholar]
- 65.Sullivan P.G., Keller J.N., Mattson M.P., and Scheff S.W. (1998). Traumatic brain injury alters synaptic homeostasis: implications for impaired mitochondrial and transport function. J. Neurotrauma 15, 789–798 [DOI] [PubMed] [Google Scholar]
- 66.Brown M.R., Sullivan P.G., and Geddes J.W. (2006). Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J. Biol. Chem. 281, 11658–11668 [DOI] [PubMed] [Google Scholar]
- 67.Mattson M.P., Keller J.N., and Begley J.G. (1998). Evidence for synaptic apoptosis. Exp. Neurol. 153, 35–48 [DOI] [PubMed] [Google Scholar]
- 68.Ikegami K., and Koike T. (2003). Non-apoptotic neurite degeneration in apoptotic neuronal death: pivotal role of mitochondrial function in neurites. Neuroscience 122, 617–-626 [DOI] [PubMed] [Google Scholar]
- 69.Naga K.K., Sullivan P.G., and Geddes J.W. (2007). High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition. J. Neurosci. 27, 7469–7475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patel S.P., Sullivan P.G., Pandya J.D., and Rabchevsky A.G. (2009). Differential effects of the mitochondrial uncoupling agent, 2,4-dinitrophenol, or the nitroxide antioxidant, Tempol, on synaptic or nonsynaptic mitochondria after spinal cord injury. J. Neurosci. Res. 87, 130–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Azevedo F.A., Carvalho L.R., Grinberg L.T., Farfel J.M., Ferretti R.E., Leite R.E., Jacob Filho W., Lent R., and Herculano-Houzel S. (2009). Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 513, 532–541 [DOI] [PubMed] [Google Scholar]
- 72.Sullivan P.G., Rabchevsky A.G., Hicks R.R., Gibson T.R., Fletcher-Turner A., and Scheff S.W. (2000). Dose-response curve and optimal dosing regimen of cyclosporin A after traumatic brain injury in rats. Neuroscience 101, 289–295 [DOI] [PubMed] [Google Scholar]
- 73.Brown M.R., Sullivan P.G., Dorenbos K.A., Modafferi E.A., Geddes J.W., and Steward O. (2004). Nitrogen disruption of synaptoneurosomes: an alternative method to isolate brain mitochondria. J. Neurosci. Methods 137, 299–303 [DOI] [PubMed] [Google Scholar]
- 74.Kristian T., Hopkins I.B., McKenna M.C., and Fiskum G. (2006). Isolation of mitochondria with high respiratory control from primary cultures of neurons and astrocytes using nitrogen cavitation. J. Neurosci. Methods 152, 136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y., and Chen X.J. (2013). Adenine nucleotide translocase, mitochondrial stress, and degenerative cell death. Oxid. Med. Cell Longev. 2013, 146860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nicolli A., Basso E., Petronilli V., Wenger R.M., and Bernardi P. (1996). Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel. J Biol. Chem. 271, 2185–2192 [DOI] [PubMed] [Google Scholar]
- 77.Baines C.P., Kaiser R.A., Purcell N.H., Blair N.S., Osinska H., Hambleton M.A., Brunskill E.W., Sayen M.R., Gottlieb R.A., Dorn G.W., Robbins J., and Molkentin J.D. (2005). Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434, 658–662 [DOI] [PubMed] [Google Scholar]
- 78.Halestrap A.P., and Davidson A.M. (1990). Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem. J. 268, 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Waldmeier P.C., Feldtrauer J.J., Qian T., and Lemasters J.J. (2002). Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol. Pharmacol. 62, 22–29 [DOI] [PubMed] [Google Scholar]
- 80.Begley D.J., Squires L.K., Zlokovic B.V., Mitrovic D.M., Hughes C.C., Revest P.A., and Greenwood J. (1990). Permeability of the blood-brain barrier to the immunosuppressive cyclic peptide cyclosporin A. J. Neurochem. 55, 1222–1230 [DOI] [PubMed] [Google Scholar]
- 81.Baldwin S.A., Fugaccia I., Brown D.R., Brown L.V., and Scheff S.W. (1996). Blood-brain barrier breach following cortical contusion in the rat. J. Neurosurg. 85, 476–481 [DOI] [PubMed] [Google Scholar]
- 82.Famiglio L., Racusen L., Fivush B., Solez K., and Fisher R. (1989). Central nervous system toxicity of cyclosporine in a rat model. Transplantation 48, 316–321 [DOI] [PubMed] [Google Scholar]
- 83.Walker R.W., and Brochstein J.A. (1988). Neurologic complications of immunosuppressive agents. Neurol. Clin. 6, 261–278 [PubMed] [Google Scholar]
- 84.Wijdicks E.F., Wiesner R.H., and Krom R.A. (1995). Neurotoxicity in liver transplant recipients with cyclosporine immunosuppression. Neurology 45, 1962–1964 [DOI] [PubMed] [Google Scholar]
- 85.de Groen P.C., Aksamit A.J., Rakela J., Forbes G.S., and Krom R.A. (1987). Central nervous system toxicity after liver transplantation. The role of cyclosporine and cholesterol. N. Engl. J. Med. 317, 861–866 [DOI] [PubMed] [Google Scholar]
- 86.Dixon C.E., Bramlett H.M., Dietrich W.D., Shear D.A., Yan H.Q., Deng-Bryant Y., Mondello S., Wang K.K., Hayes R.L., Empey P., Povlishock J., Tortella F.C., and Kochanek P.M. (2016). Cyclosporine treatment in traumatic brain injury: operation brain trauma therapy. J. Neurotrauma 33, 553–566 [DOI] [PubMed] [Google Scholar]
- 87.Berden J.H., Hoitsma A.J., Merx J.L., and Keyser A. (1985). Severe central-nervous-system toxicity associated with cyclosporin. Lancet 1, 219–220 [DOI] [PubMed] [Google Scholar]
- 88.Hughes R.L. (1990). Cyclosporine-related central nervous system toxicity in cardiac transplantation. N. Engl. J. Med. 323, 420–421 [DOI] [PubMed] [Google Scholar]
- 89.Reece D.E., Frei-Lahr D.A., Shepherd J.D., Dorovini-Zis K., Gascoyne R.D., Graeb D.A., Spinelli J.J., Barnett M.J., Klingemann H.G., Herzig G.P., and et al. (1991). Neurologic complications in allogeneic bone marrow transplant patients receiving cyclosporin. Bone Marrow Transplant. 8, 393–401 [PubMed] [Google Scholar]
- 90.Mazzeo A.T., Brophy G.M., Gilman C.B., Alves O.L., Robles J.R., Hayes R.L., Povlishock J.T., and Bullock M.R. (2009). Safety and tolerability of cyclosporin a in severe traumatic brain injury patients: results from a prospective randomized trial. J. Neurotrauma 26, 2195–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hatton J., Rosbolt B., Empey P., Kryscio R., and Young B. (2008). Dosing and safety of cyclosporine in patients with severe brain injury. J. Neurosurg. 109, 699–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okonkwo D.O., Melon D.E., Pellicane A.J., Mutlu L.K., Rubin D.G., Stone J.R., and Helm G.A. (2003). Dose-response of cyclosporin A in attenuating traumatic axonal injury in rat. Neuroreport 14, 463–466 [DOI] [PubMed] [Google Scholar]
- 93.Colley B.S., Phillips L.L., and Reeves T.M. (2010). The effects of cyclosporin-A on axonal conduction deficits following traumatic brain injury in adult rats. Exp. Neurol. 224, 241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Albensi B.C., Sullivan P.G., Thompson M.B., Scheff S.W., and Mattson M.P. (2000). Cyclosporin ameliorates traumatic brain-injury-induced alterations of hippocampal synaptic plasticity. Exp. Neurol. 162, 385–389 [DOI] [PubMed] [Google Scholar]
- 95.Signoretti S., Marmarou A., Tavazzi B., Dunbar J., Amorini A.M., Lazzarino G., and Vagnozzi R. (2004). The protective effect of cyclosporin A upon N-acetylaspartate and mitochondrial dysfunction following experimental diffuse traumatic brain injury. J. Neurotrauma 21, 1154–1167 [DOI] [PubMed] [Google Scholar]
- 96.Gelderblom H., Verweij J., Nooter K., and Sparreboom A. (2001). Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 37, 1590–1598 [DOI] [PubMed] [Google Scholar]
- 97.Sanchez H., Bigard X., Veksler V., Mettauer B., Lampert E., Lonsdorfer J. and Ventura-Clapier R. (2000). Immunosuppressive treatment affects cardiac and skeletal muscle mitochondria by the toxic effect of vehicle. J Mol Cell Cardiol 32, 323–331 [DOI] [PubMed] [Google Scholar]
- 98.Sanchez H., Zoll J., Bigard X., Veksler V., Mettauer B., Lampert E., Lonsdorfer J. and Ventura-Clapier R. (2001). Effect of cyclosporin A and its vehicle on cardiac and skeletal muscle mitochondria: relationship to efficacy of the respiratory chain. Br J Pharmacol 133, 781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nassberger L. (1990). Effect of cyclosporin A and different vehicles on ATP production in mitochondria isolated from the rat kidney cortex. Pharmacol Toxicol 67, 147–150 [DOI] [PubMed] [Google Scholar]
- 100.Sauerbeck A., Gao J., Readnower R., Liu M., Pauly J.R., Bing G., and Sullivan P.G. (2011). Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp. Neurol. 227, 128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gilmer L.K., Ansari M.A., Roberts K.N., and Scheff S.W. (2010). Age-related changes in mitochondrial respiration and oxidative damage in the cerebral cortex of the Fischer 344 rat. Mech. Ageing Dev. 131, 133–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lai J.C., and Clark J.B. (1976). Preparation and properties of mitochondria derived from synaptosomes. Biochem. J. 154, 423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brand M.D., and Nicholls D.G. (2011). Assessing mitochondrial dysfunction in cells. Biochem. J. 435, 297–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Opii W.O., Nukala V.N., Sultana R., Pandya J.D., Day K.M., Merchant M.L., Klein J.B., Sullivan P.G., and Butterfield D.A. (2007). Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J. Neurotrauma 24, 772–789 [DOI] [PubMed] [Google Scholar]
- 105.Robertson C.L., Saraswati M., and Fiskum G. (2007). Mitochondrial dysfunction early after traumatic brain injury in immature rats. J. Neurochem. 101, 1248–1257 [DOI] [PubMed] [Google Scholar]
- 106.Hazelton J.L., Petrasheuskaya M., Fiskum G., and Kristian T. (2009). Cyclophilin D is expressed predominantly in mitochondria of gamma-aminobutyric acidergic interneurons. J. Neurosci. Res. 87, 1250–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]