Abstract
The Ebola filoviruses are aggressive pathogens that cause severe and often lethal hemorrhagic fever syndromes in humans and nonhuman primates. To date, no effective therapies have been identified. To analyze the entry and fusion properties of Ebola virus, we adapted a human immunodeficiency virus type 1 (HIV-1) virion-based fusion assay by substituting Ebola virus glycoprotein (GP) for the HIV-1 envelope. Fusion was detected by cleavage of the fluorogenic substrate CCF2 by β-lactamase-Vpr incorporated into virions and released as a result of virion fusion. Entry and fusion induced by the Ebola virus GP occurred with much slower kinetics than with vesicular stomatitis virus G protein (VSV-G) and were blocked by depletion of membrane cholesterol and by inhibition of vesicular acidification with bafilomycin A1. These properties confirmed earlier studies and validated the assay for exploring other properties of Ebola virus GP-mediated entry and fusion. Entry and fusion of Ebola virus GP pseudotypes, but not VSV-G or HIV-1 Env pseudotypes, were impaired in the presence of the microtubule-disrupting agent nocodazole but were enhanced in the presence of the microtubule-stabilizing agent paclitaxel (Taxol). Agents that impaired microfilament function, including cytochalasin B, cytochalasin D, latrunculin A, and jasplakinolide, also inhibited Ebola virus GP-mediated entry and fusion. Together, these findings suggest that both microtubules and microfilaments may play a role in the effective trafficking of vesicles containing Ebola virions from the cell surface to the appropriate acidified vesicular compartment where fusion occurs. In terms of Ebola virus GP-mediated entry and fusion to various target cells, primary macrophages proved highly sensitive, while monocytes from the same donors displayed greatly reduced levels of entry and fusion. We further observed that tumor necrosis factor alpha, which is released by Ebola virus-infected monocytes/macrophages, enhanced Ebola virus GP-mediated entry and fusion to human umbilical vein endothelial cells. Thus, Ebola virus infection of one target cell may induce biological changes that facilitate infection of secondary target cells that play a key role in filovirus pathogenesis. Finally, these studies indicate that pseudotyping in the HIV-1 virion-based fusion assay may be a valuable approach to the study of entry and fusion properties mediated through the envelopes of other viral pathogens.
The Ebola and Marburg filoviruses are highly pathogenic viruses that induce hemorrhagic fevers in humans and nonhuman primates (4, 28). Mortality rates range as high as 88% (40, 55, 56). Of the four identified strains of Ebola virus, Zaire, Ivory Coast, Sudan, and Reston, the Zaire strain induces the highest death rates in humans, while the Reston strain has not caused fatal disease in humans (27, 56). The clinical syndrome includes generalized changes in capillary integrity, hypotension, coagulation disorders with variable degrees of hemorrhage, and widespread focal tissue destruction. Mononuclear phagocytic cells form the primary targets for filovirus replication, while endothelial cells serve as secondary targets.
Currently, no effective antiviral therapy is available for Ebola virus infection in humans. Understanding the molecular basis for Ebola virus infection could facilitate the development of new therapeutic approaches. Since viral entry is a very proximal step in the life cycle of the Ebola virus, this process merits further study.
Most studies of viral entry have been performed with either pseudotyped virions in infectivity assays or artificial cell-to-cell fusion assays. Almost without exception, the entry of the pseudotyped virus has been monitored with an enzyme or other biological marker that is expressed when the virus subsequently replicates. Thus, expression of the reporter gene reflects not only the early entry and fusion step but also many postfusion events. As such, these assays are not optimal for the study of virion fusion. Cell-to-cell fusion assays have several potential drawbacks, including pronounced differences in the density of envelope proteins displayed on transfected cells versus virions, differences in the membrane lipid composition of the virions and cells, and the inability of these assays to detect entry that is contingent upon endocytosis of virions.
Recently, a sensitive, specific, and quantitative assay that detects the fusion of human immunodeficiency virus type 1 (HIV-1) virions to target cells was described (7). This assay utilizes HIV virions containing a chimeric β-lactamase-Vpr (BlaM-Vpr) protein. Because Vpr binds to the p6 component of the HIV-1 Gag polyprotein, this protein chimera is efficiently incorporated into newly formed virions. Subsequent fusion of these BlaM-Vpr-containing virions to target cells is detected by the cleavage of the fluorogenic substrate CCF2, which is loaded into the target cells. Endocytosis of HIV virions that do not fuse is not scored in this assay. Cleavage of the β-lactam ring in the CCF2 dye by the β-lactamase component of BlaM-Vpr alters the excited fluorescence emission spectrum of the dye from 520 nm (green) to 447 nm (blue). Fusion can be detected by either epifluorescence microscopy or flow cytometry. Because virtually all cell types are amenable to loading with CCF2, this assay can be used to study HIV-1 fusion in biologically relevant cellular targets, including primary CD4-expressing T lymphocytes and macrophages.
We hypothesized that it might be possible to study the entry and fusion properties of other viral envelopes by constructing pseudotyped HIV-1 virions where the HIV-1 envelope (Env) is replaced with the Env protein from a pathogenic virus of interest. We now describe the production of Ebola virus glycoprotein (GP) pseudotypes of HIV-1, validation of the assay, and the use of these pseudotypes to characterize new features of Ebola virus GP-mediated entry and fusion in biologically relevant target cells.
MATERIALS AND METHODS
Cells, plasmids, and antibodies.
HEK293T cells (23), HeLa cells, and HeLa-CD4 cells (35) were cultured in Dulbecco's modified Eagle's medium (Cellgro, Herndon, Va.) supplemented with 10% fetal bovine serum (FBS; Gemini Bio-Products, Tarzana, Calif.), l-glutamine, and antibiotics. HeLa cells were used to study the entry and fusion of Ebola virus GP- or vesicular stomatitis virus G protein (VSV-G)-pseudotyped virions, while HeLa-CD4 cells were employed in assays comparing the fusion of HIV-1 Env-containing virions.
To prepare monocyte-derived macrophages, buffy coats from healthy volunteer donors were obtained from Stanford Blood Center (Palo Alto, Calif.). Peripheral blood mononuclear cells were isolated by Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation. Monocytes and peripheral blood lymphocytes (PBLs) were separated from peripheral blood mononuclear cells by using CD14 microbeads (Miltenyi Biotech, Sunnyvale, Calif.). Isolated monocytes were >95% CD14+. To promote differentiation into macrophages, monocytes were allowed to adhere to plastic and cultured for 5 days in RPMI 1640 medium (Cellgro) supplemented with 10% human AB-positive serum (Gemini Bio-Products) and 10% FBS. Human umbilical vein endothelial cells (HUVECs) were purchased from Cambrex (Walkersville, Md.) and cultured in endothelial cell basal medium 2 (Cambrex).
An HIV molecular clone lacking the Env gene (pNL4-3 Δenv) was constructed by blunting at the NheI site present in the envelope coding region of HIV-1 NL4-3. The BlaM-Vpr expression vector (pCMV-BlaM-Vpr) was constructed as previously described (7). Briefly, the coding region of BlaM was cloned from the GeneBLAzer β-lactamase vector (Aurora Bioscience, San Diego, Calif.) and linked to the N terminus of Vpr separated by a six-residue glycine spacer.
The Ebola-Zaire viral envelope GP (Ebola virus GP) expression vector was constructed by cloning cDNA encoding Ebola virus GP (generously provided by A. Sanchez, Centers for Disease Control and Prevention, Atlanta, Ga.) into the mammalian expression pCMVneo expression vector (22) as described elsewhere (18). VSV-G and CXCR4 (X4)-tropic HIV-1HXB2 Env expression vectors were obtained from the NIH AIDS Research and Reference Reagent Program (9, 37). Anti-Ebola virus GP1 monoclonal antibody was kindly provided by M. K. Hart (U.S. Army Medical Research Institute of Infectious Diseases) (53).
Chemical reagents, cytokines, and cell treatment.
Bafilomycin A1, (2-hydroxypropyl)-β-cyclodextrin, nocodazole, and paclitaxel (Taxol) were purchased from Sigma-Aldrich (St. Louis, Mo.). To inhibit endosomal and lysosomal acidification, cells were preincubated with medium containing various concentrations of bafilomycin A1 (5 to 500 nM) for 1 h before the fusion experiments were initiated. To deplete cholesterol in the cell membrane, cells were pretreated with medium containing β-cyclodextrin (1 to 25 mM) for 1 h prior to the initiation of the experiments. Before the addition of the virus, cells were washed extensively with phosphate-buffered saline (PBS) to minimize effects of β-cyclodextrin on the virions. To disrupt or stabilize microtubules, cells were precultured in medium containing nocodazole (0.4 to 10 μM) or paclitaxel (0.8 to 20 μM), respectively, for 30 min prior to experimentation. All of these drugs were maintained in the culture medium throughout the fusion assay. To examine the effects of microfilaments on the viral entry and fusion, cells were cultured with cytochalasin B (CytB) (Sigma-Aldrich) (0.2 to 20 μM), cytochalasin D (CytD) (Sigma-Aldrich) (0.2 to 20 μM), jasplakinolide (Jas) (Molecular Probes, Eugene, Oreg.) (0.04 to 1 μM), or latrunculin A (LatA) (Molecular Probes) (0.04 to 1 μM) for 30 min prior to the virion-based fusion assay. These agents were also maintained in the medium throughout the fusion experiments. To test the effects of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin 1β (IL-1β), which are released by Ebola virus-infected monocytes/macrophages, HUVECs were treated with recombinant human TNF-α (0.1 to 10 ng/ml) (Biosource, Camarillo, Calif.) or IL-1β (10 to 1,000 pg/ml) (eBioscience, San Diego, Calif.) for 24 h prior to analysis in the virion fusion assay.
Pseudotyped virions.
To produce the pseudotyped virions containing either the Ebola virus GP, VSV-G, or HXB2 envelopes, HEK293T cells were cotransfected with expression vectors encoding BlaM-Vpr, NL4-3Δenv, and Ebola virus GP, VSV-G, or HIV-1HXB2 Env. After 48 h, virion-containing supernatants were harvested, centrifuged at low speed to remove cellular debris, and ultracentrifuged at 72,000 × g for 90 min to concentrate the virions. Viral stocks were normalized based on p24-Gag content quantitated by enzyme-linked immunosorbent assay (Perkin-Elmer, Boston, Mass.).
Virion-based fusion assay.
Target cells were incubated with virions containing BlaM-Vpr at 37°C for 3 h, washed in CO2-independent medium (Gibco-Invitrogen, Carlsbad, Calif.), and loaded with CCF2-AM dye at room temperature for 1 h as recommended by the manufacturer (Invitrogen, Carlsbad, Calif.). After two additional washes in medium, the BlaM-CCF2-AM reaction was allowed to develop at room temperature for at least 7 h in medium supplemented with 10% FBS and 2.5 mM probenecid, a nonspecific inhibitor of anion transport (Sigma-Aldrich). No antibiotics were added to the culture medium. Finally, cells were washed twice with PBS and fixed in a 1.2% paraformaldehyde solution prior to analysis. The change in emission fluorescence of CCF2 after cleavage by BlaM-Vpr was monitored by flow cytometry using a three-laser Vantage-SE flow cytometer (Becton Dickinson, San Jose, Calif.).
Immunofluorescence assays.
HeLa cells were cultured to confluence on coverslips in six-well dishes. After exposure to various reagents, the cells were fixed in 3.7% formaldehyde, permeabilized with 0.5% Triton X-100, stained with anti-α-tubulin monoclonal antibody (B-5-1-2 clone; Sigma-Aldrich), washed with 0.1% Tween 20, and stained with fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G (Jackson Immunoresearch Laboratories, West Grove, Pa.). The coverslips were then mounted on slides, and the cells were examined on an immunofluorescent microscope (Eclipse TE300; Nikon, Tokyo, Japan). For staining of actin filaments, cells on the coverslips were fixed, permeabilized with 0.1% Triton X-100, and stained with Texas Red-X phalloidin (Molecular Probes) or anti-β-actin monoclonal antibody (Sigma-Aldrich) followed by staining with Texas Red-conjugated anti-mouse immunoglobulin G (Jackson Immunoresearch Laboratories). The coverslips were then analyzed on an immunofluorescent confocal microscope (Olympus BX60; Olympus, Tokyo, Japan).
RESULTS
Virion-based fusion assay using pseudotyped viruses.
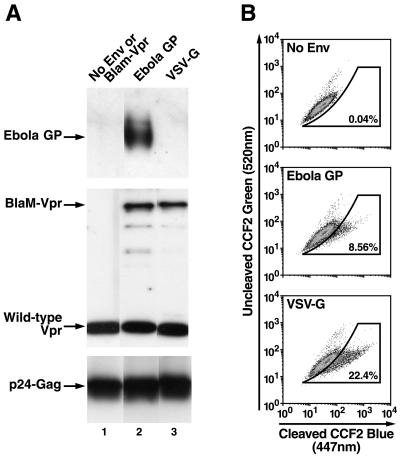
The incorporation of BlaM-Vpr protein into the pseudotyped virions was confirmed by immunoblotting of lysed virions with anti-Vpr polyclonal antibodies (Fig. 1A). Pseudotyped viruses were prepared as described above, ultracentrifuged, and lysed in Laemmli sample buffer containing 5% 2-mercaptoethanol. Wild-type Vpr was detected in all virions, but the BlaM-Vpr chimera was detected only in the virions derived from cells cotransfected with the BlaM-Vpr expression vector DNA. Similarly, Ebola virus GP Env protein was detected in Ebola virus GP-pseudotyped virions by using an anti-Ebola virus GP monoclonal antibody (Fig. 1, lane 2) but not in VSV-G-pseudotyped virions (lane 3) or control (no-Env) virions (lane 1).
FIG. 1.
(A) Incorporation of BlaM-Vpr and Ebola virus GP into pseudotyped HIV virions. Pseudotyped virions were lysed and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting with anti-Vpr polyclonal antibody, anti-p24 Gag monoclonal antibody, or anti-Ebola virus GP mononclonal antibody. (B) Virion-based fusion assay using viral particles pseudotyped with different viral envelopes. HeLa cells were incubated with Ebola GP- or VSV-G-pseudotyped viruses for 3 h at 37°C, and fusion was assayed as described in Materials and Methods. Events occurring within the gate drawn based on data obtained with no-Env virus reflect fusion in this subset of cells. Percent fusion with each viral pseudotype is noted in the insets.
Next, we tested the ability of HIV-1 virions pseudotyped with Ebola virus GP or VSV-G to enter and fuse to HeLa cells. HeLa cells were incubated with the virions for 3 h at 37°C, washed twice, loaded with CCF2-AM, and analyzed by flow cytometry. Although we observed no significant increase in blue fluorescence in the no-Env virus-infected control, ∼8 to 9% of cells incubated with Ebola virus GP-pseudotyped virions and ∼22% of cells incubated with VSV-G-pseudotyped virions displayed increased blue fluorescence (447 nm) (Fig. 1B). These results suggest that the BlaM-Vpr protein chimera present in the pseudotyped virions can be used to detect entry and fusion of HIV-1 virions pseudotyped with either the Ebola virus GP or VSV-G proteins. However, additional studies of the properties of Ebola virus GP-mediated virion entry and fusion were required to validate that the entry pathway utilized was identical to that described for this filovirus.
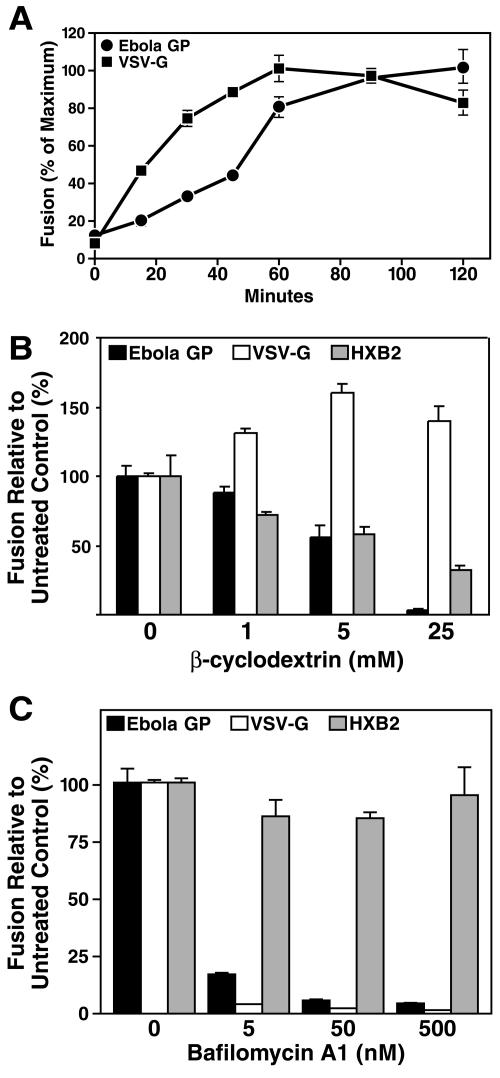
Comparison of the time course of Ebola virus GP-pseudotyped virion entry versus that of VSV-G-pseudotyped virions.
We next compared the time required for virion entry and fusion mediated by Ebola virus GP and VSV-G. A previous study demonstrated that Ebola virus GP- or Marburg virus GP-mediated entry occurs much more slowly (time required to reach 50% of the maximum entry and fusion events [T1/2], 3 to 4 h) than VSV-G (T1/2 of 1 to 1.5 h) (11). Cells were incubated with Ebola virus GP- or VSV-G-pseudotyped virions at 4°C for 1 h and washed to remove unbound viral particles. Postadsorptive events required for entry and fusion were initiated by replacing the medium with prewarmed (37°C) medium and shifting the cells to 37°C. At the conclusion of each incubation, cells were washed with ice-cold PBS and treated with trypsin to remove surface bound virions. All the samples were loaded with CCF2-AM at the same time. Inspection of the time course for entry and fusion of Ebola virus GP-pseudotyped virions revealed a T1/2 of ∼50 min, while the VSV-G-pseudotyped virions displayed a T1/2 of ∼20 min (Fig. 2A). Although Ebola virus GP-mediated kinetics of virion entry were slower than those of VSV-G, in agreement with the previous study (11), the T1/2 required for entry and fusion of both types of the virions was shorter. It is possible that these differences stem from the use of different target cells (HeLa in our studies and HEK293T in reference 11). Nevertheless, Ebola virus GP-pseudotyped-virion entry and fusion were approximately 2 to 2.5 times slower than VSV-G-pseudotyped-virion entry and fusion in both studies.
FIG. 2.
(A) Entry and fusion kinetics. The time courses of Ebola virus GP- and VSV-G-pseudotyped virion entry and fusion were studied. Ebola virus GP- or VSV-G-pseudotyped virions were prebound to cells at 4°C for 1 h. After thorough washing to remove unbound viral particles, entry and fusion were initiated by shifting the cells to 37°C. At various times, cells were washed with ice-cold PBS and treated with trypsin to terminate the fusion reaction. Values represent the extent of fusion relative to the maximum level of fusion obtained. (B) Effect of the cholesterol-sequestering agent β-cyclodextrin on Ebola virus GP-, VSV-G-, or HIV-1HXB2 Env-mediated entry and fusion. Cholesterol was depleted with the cholesterol-binding resin β-cyclodextrin. HeLa cells were treated with graded doses of cyclodextrin for 30 min at 37°C, washed three times to deplete the reagent, and incubated with Ebola virus GP-, VSV-G-, or HIV-1HXB2 Env-pseudotyped virions, and entry and fusion were measured. Cells were pretreated with various concentrations of β-cyclodextrin for 30 min at 37°C and thoroughly washed three times with PBS. The cells were then incubated with pseudotyped virions for 3 h at 37°C, and fusion was measured. (C) Effects of bafilomycin A1 on Ebola virus GP-, VSV-G-, and HIV-1HXB2 Env-pseudotyped virus entry and fusion. Cells were pretreated with various concentrations of bafilomycin A1 at 37°C for 1 h and incubated with each of the indicated pseudotyped virions at 37°C for 3 h. Bafilomycin A1 was maintained in the cultures throughout the experiment. Values are levels of fusion relative to that in untreated controls.
Cholesterol depletion from membranes impairs Ebola virus GP-pseudotyped-virion entry and fusion.
Prior studies have implicated the involvement of lipid rafts in both filovirus budding and entry (3, 11). In agreement with these prior studies, we found that disruption of lipid rafts by depletion of cholesterol with β-cyclodextrin produced dose-related decreases in the entry and fusion of Ebola virus GP-pseudotyped virions (Fig. 2B). As a positive control, such treatment of β-cyclodextrin inhibited HIV-1HXB2 Env-mediated entry and fusion (34, 41). Conversely, the entry and fusion of VSV-G-pseudotyped virions, which are known to utilize the clathrin-mediated endocytotic pathway (49), were not impaired and in fact were slightly enhanced by β-cyclodextrin. These studies further suggest that the fusion events measured with the Ebola virus GP-pseudotyped virions reflect utilization of the expected postadsorptive pathway. Whether the pathway accessed by Ebola virus GP involves caveolae remains controversial (11, 44).
Ebola virus entry and fusion are pH dependent and require vesicular acidification.
Prior studies have suggested that Ebola virus GP-mediated entry and fusion require acidification within an internal vesicle (8, 50, 54). To test whether acidification is required for detection of entry and fusion with Ebola virus GP-pseudotyped virions, target cells were pretreated with the vacuolar ATPase inhibitor bafilomycin A1 for 1 h at 37°C, followed by incubation with virions pseudotyped with Ebola virus GP, VSV-G, or HIV-1HXB2 Env. Bafilomycin A1 treatment almost completely blocked the detection of entry and fusion mediated by Ebola virus GP and VSV-G but had little effect on fusion induced by HIV-1HXB2 Env in these HeLa-CD4 cells (Fig. 2C). Of note, bafilomycin A1 treatment has been shown to enhance HIV fusion or productive entry in other cell types, including primary CD4 T lymphocytes (43) and HeLa Magi cells (14). These data confirm that acidification is required for the detection of Ebola virus GP-pseudotyped-virion entry and fusion with the β-lactamase-Vpr reporter system. These kinetics, plus the dependence on cholesterol and vesicle acidification, support the notion that the Ebola virus GP-pseudotyped virions access the expected entry-and-fusion pathway utilized by filoviruses.
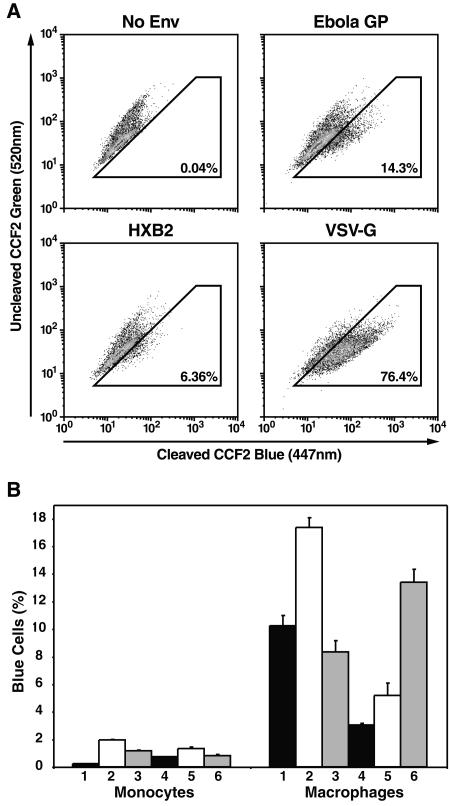
Comparison of Ebola virus GP-pseudotyped-virion fusion to monocytes and macrophages.
Having validated the use of Ebola virus GP pseudotypes in the virion-based fusion assay, we next investigated entry and fusion of these pseudotyped virions in physiologically relevant target cells. Specifically, we incubated either primary monocytes or monocyte-derived macrophages, key targets of Ebola virus infection in humans, with virions pseudotyped with Ebola virus GP, HIV-1HXB2 Env, or VSV-G for 3 h at 37°C. Entry and fusion of Ebola virus GP-pseudotyped virions were detected in ∼14% of the macrophages, while HIV-1HXB2 Env- and VSV-G-mediated entry and fusion were detected in ∼6 and ∼76% of the macrophages, respectively (Fig. 3A). Conversely, and consistent with prior results (17, 19, 42, 58), Ebola virus GP pseudotypes did not detectably fuse to either unstimulated PBLs or phytohemagglutinin-activated PBLs (<0.05%; data not shown). It has been reported that both macrophages and monocytes from humans and nonhuman primates are susceptible to Ebola virus infection (13, 17, 19, 21, 24, 45, 46). Stroher and colleagues have in fact reported similar replication of filoviruses in monocytes and macrophages in vitro by utilizing an immunoplaque assay (46). Using matched monocytes and macrophages from six donors, we observed much lower levels of Ebola virus GP-mediated entry and fusion with monocytes than macrophages (Fig. 3B). These findings raise the possibility that key receptors required for efficient Ebola virus entry and fusion, while present on monocytes, may be upregulated as a consequence of the differentiation of monocytes into macrophages. Other postadsorption differences in the handling of the Ebola virus GP-pseudotyped virions could also contribute to these differences. Whether macrophages display a postfusion impairment in Ebola virus replication that could explain the differences in our results with those of Stroher et al. is unknown. Nevertheless, our findings reveal that monocytes display decreased susceptibility to Ebola virus GP-mediated entry and fusion compared with macrophages.
FIG. 3.
Ebola virus GP-mediated entry and fusion to human monocyte-derived macrophages. (A) Monocyte-derived macrophages were incubated for 3 h with BlaM-Vpr-containing virions lacking Env or pseudotyped with Ebola virus GP, HIV-1HXB2, or VSV-G. (B) Efficiency of Ebola virus GP-mediated entry and fusion in human monocytes versus macrophages. Human monocytes and macrophages (2 × 106) derived from six donors were incubated with the same concentration of Ebola virus GP pseudotypes for 3 h. Percentages of blue cells represent cells supporting virion entry and fusion. Error bars indicate standard deviations derived from triplicate samples.
Cytoskeletal proteins play a key role in Ebola virus GP-mediated entry and fusion.
Ebola virus enters target cells by an endocytic pathway (18). Subsequent trafficking of internalized vesicles within the cell often depends on various components of the cytoskeleton. For example, the transfer of internalized cargoes from early endosomes to late endosomes is dependent on intact microtubules (36). To assess whether microtubules are required for effective Ebola virus GP-mediated entry and fusion, we tested the effects of reagents that either inhibit or enhance microtubule assembly. Treatment of HeLa cells with nocodazole, which effectively disrupted microtubules based on staining with α-tubulin (Fig. 4A), inhibited Ebola GP-mediated entry and fusion in a dose-dependent manner (Fig. 4B). In contrast, entry and fusion of VSV-G and HIV-1HXB2 Env pseudotypes were not affected by nocodazole. Similar differential effects on virion entry and fusion were obtained with colchicine, a second microtubule-disrupting agent (data not shown). In contrast, when cells were treated with taxol, which stabilizes microtubules by enhancing bundle formation (Fig. 4A), a marked enhancement of Ebola virus GP-mediated entry and fusion was observed (Fig. 4C). Consistent with the microtubule disrupting experiments, entry and fusion of VSV-G and HIV-1HXB2 Env pseudotypes were not altered by pretreatment of the cells with taxol.
FIG. 4.
Effects of the microtubule-disrupting agent nocodazole and the microtubule-stabilizing agent taxol on Ebola virus GP-pseudotyped entry and fusion. (A) Effects of nocodazole and taxol on the microtubule network present in HeLa cells. Cells were treated with nocodazole (10 μM) or taxol (20 μM) for 1 h at 37°C followed by immunostaining with anti-α-tubulin antibody. (B and C) HeLa or HeLa-CD4 cells were pretreated with the indicated concentrations of nocodazole (B) or taxol (C) at 37°C for 30 min and incubated with each of the pseudotyped virions in the continued presence of each drug at 37°C for 3 h. Values in panels B and C are the levels of fusion relative to that in untreated controls. (D) Effect of taxol (Tx) on entry and fusion kinetics. Cells were pretreated with 10 μM taxol or a dimethyl sulfoxide control for 1 h at 4°C prior to assessment of entry and fusion kinetics with the indicated virion pseudotypes. Values represent the extent of fusion relative to the maximum level of fusion obtained.
Next, we assessed whether paclitaxel altered the kinetics of entry and fusion mediated through Ebola virus GP. As described above, viruses and target cells were mixed and allowed to bind at 4°C. Such treatment at 4°C induces microtubule depolymerization (51). We independently confirmed that cooling of HeLa cells to 4°C resulted in depolymerization of microtubules, based on differences in the immunostaining pattern obtained with anti-α-tubulin antibodies (data not shown). Ebola virus GP-pseudotyped virions entered more rapidly in the presence of taxol than in the absence of taxol (Fig. 4D). Conversely, taxol exerted no effects on the kinetics of entry and fusion observed with the VSV-G-pseudotyped virions. These findings further support the conclusion that intact microtubules play an important role in Ebola virus entry and fusion, perhaps due to microtubule-dependent trafficking of virus-containing vesicles within the cell to the site of fusion.
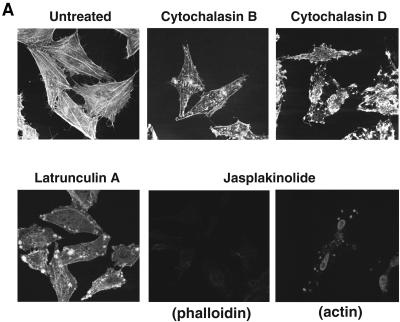
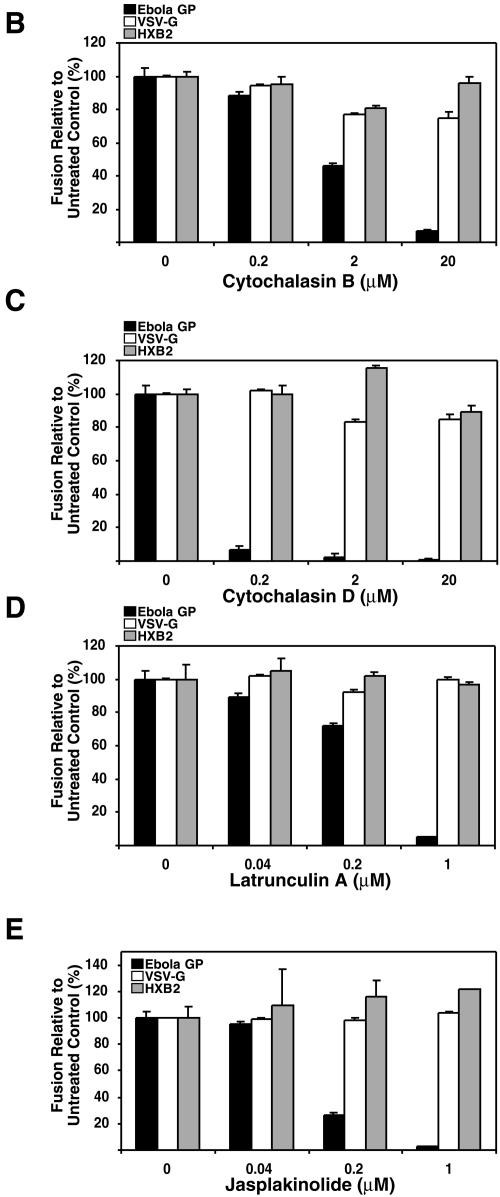
Next, we examined the effects of chemical agents that affect the integrity and/or function of actin filaments on the entry and fusion of Ebola virus GP pseudotypes. When HeLa cells were treated with CytB or CytD, we observed that actin filaments were disrupted based on staining with phalloidin (Fig. 5A) and that the entry and fusion of Ebola virus GP pseudotypes were inhibited in a dose-dependent manner (Fig. 5B and C). VSV-G-mediated entry and fusion were slightly attenuated. Both positive and negative effects of the cytochalasins have been previously described for VSV infection (20, 30, 48). Consistent with a prior report (6), HIV-1HXB2 Env-mediated fusion was not affected by the cytochalasins. Treatment with LatA, which sequesters actin monomers (Fig. 5A), also inhibited the entry and fusion of Ebola virus GP pseudotypes while not affecting either VSV-G or HIV-1HXB2 Env-mediated entry and fusion (Fig. 5D). We similarly tested Jas, which binds to and stabilizes actin filaments. No detectable labeling was observed by using phalloidin staining, consistent with the fact that Jas and phalloidin compete for the same binding on actin (5). This result indicates that the phalloidin binding sites were occupied by Jas in these cells. Using antiactin antibodies for staining, we observed aggregation of actin filaments by Jas (Fig. 5A), consistent with a recent report (6). When the cells were treated with Jas, Ebola virus GP-mediated entry and fusion were inhibited in a dose-dependent manner (Fig. 5E). In contrast, neither VSV-G nor HIV-1HXB2 Env-mediated entry and fusion were affected. Together, these findings suggest that actin filaments also play a key role in the entry and/or fusion of Ebola virus GP pseudotypes.
FIG. 5.
Effects of actin-disrupting or -stabilizing agents on Ebola virus GP-mediated entry and fusion. (A) Fluorescence labeling of actin filaments in HeLa cells treated with CytB (20 μM), CytD (20 μM), LatA (1 μM), or Jas (1 μM) for 30 min at 37 °C followed by labeling with Texas Red-X phalloidin (CyB, CyD, LatA, and Jas) or anti-β-actin antibody (Jas). (B to E) HeLa and HeLa-CD4 cells were pretreated with the indicated concentrations of CytB (B), CytD (C), LatA (D), or Jas (E) at 37°C for 30 min and incubated with each of the pseudotyped virions in the continued presence of each drug at 37°C for 3 h. Values represent the level of entry and fusion relative to that in untreated controls.
TNF-α enhances Ebola virus entry and fusion.
Several inflammatory cytokines, including TNF-α, alpha interferon (IFN-α), IFN-γ, and IL-1β, are present in increased amounts in the plasma of patients infected with Ebola virus (1, 2, 33, 52). Increases in plasma TNF-α and IFN-γ levels are associated with fatal infection, whereas elevated IL-1β and IL-6 during the symptomatic phase are often associated with nonfatal infection (1, 52). These cytokines are also secreted by filovirus-infected monocytes and macrophages in vitro. However, the precise function of these cytokines in Ebola virus-induced pathogenesis remains controversial. Using the pseudotyped virion entry and fusion assay, we investigated whether these cytokines altered Ebola virus GP-mediated entry and fusion. Ebola virus GP-mediated entry and fusion were detectable in HUVECs; endothelial cells form important secondary cellular targets for Ebola virus infection in vivo, and their infection plays a key role in filovirus disease progression. When HUVECs were treated with TNF-α for 24 h and then cultured with Ebola virus GP or VSV-G pseudotypes, we observed enhanced entry and fusion of the Ebola pseudotypes but only slight inhibition of the entry and fusion of the VSV-G-pseudotyped virions (Fig. 6A). In contrast, treatment with IL-1β for 24 h did not similarly enhance entry and fusion of Ebola virus GP-pseudotyped virions with HUVECs (Fig. 6B). These enhancing effects of TNF-α did not appear to involve more extensive microtubule polymerization in the HUVECs, based on α-tubulin staining patterns (data not shown). These findings raise the possibility that TNF-α, produced as a consequence of Ebola virus infection of macrophages, may increase the susceptibility of endothelial cells to infection with this viral pathogen. Infection of these endothelial cells likely plays a key role in the loss of capillary integrity observed in filoviral infections.
FIG. 6.
Effects of TNF-α and IL-1β on Ebola virus GP-mediated virion entry and fusion in HUVECs. HUVECs were treated with various concentrations of TNF-α (A) or IL-1β (B) for 24 h, and levels of entry and fusion obtained with Ebola virus GP and VSV-G pseudotypes were assessed. Values are levels of fusion relative to that in untreated controls.
DISCUSSION
Insights into Ebola virus pathogenesis have been complicated by the highly pathogenic nature of this virus and the requirement for biosafety level 4 containment procedures for study of the infectious virus. We now describe studies of Ebola virus GP-mediated entry and fusion employing HIV-1 virions pseudotyped with Ebola virus GP and containing a BlaM-Vpr protein chimera that permits detection of the fusion of these virions. We confirmed what Ebola virus GP is effectively incorporated into BlaM-Vpr-containing HIV virions and that the entry and fusion of Ebola pseudotypes are detectable by flow cytometry in HeLa cells. Consistent with prior results, the entry and fusion of Ebola virus GP-pseudotyped virions occurred with slow kinetics, were blocked by depletion of membrane cholesterol, and were impaired by inhibition of vesicular acidification with bafilomycin A1. These properties validated the assay, permitting assessment of other properties of Ebola virus GP-mediated entry and fusion.
We found that both human monocytes and macrophages support entry and fusion of Ebola virus GP-pseudotyped virions. However, levels of entry and fusion were markedly higher in macrophages than in monocytes despite use of the same number of cells and the same multiplicity of infection. These results are somewhat at odds with a prior report indicating that the replication of filoviruses in monocytes was similar to that which occurs in macrophages (46). It is possible that postfusion events in macrophages may slow productive infection in these cells, giving rise to comparable levels of infection in macrophages and monocytes. Our findings suggest that the changes in gene expression that accompany the differentiation of monocytes into macrophages are associated with the acquisition of increased receptivity to Ebola virus GP-mediated entry and fusion. In terms of the susceptibility of other primary cells to Ebola virus GP-mediated entry and fusion, we observed that neither resting PBLs nor mitogen-activated PBLs support this response. The latter findings are consistent with prior infection results obtained in vivo (17, 19, 42, 58).
The endocytic pathway has many important cellular functions, including the uptake of extracellular nutrients, regulation of cell surface receptor expression, and antigen presentation. This pathway is also utilized by viruses, toxins, and microorganisms to gain entry into cells (36). The cytoskeleton plays an important role in endocytosis, including the transfer of cargo from early to late endosomes and the transport of endocytosed proteins from one plasma membrane to another by transcytosis (36). Microtubule-depolymerizing agents like nocodazole inhibit the uptake of bacteria, such as Listeria monocytogenes (31), and transferrin (25), which are transported from early endosomes to late endosomes. Entry of enveloped viruses, such as ecotropic murine leukemia virus, human enterovirus, simian virus 40, influenza virus, and vaccinia virus, similarly requires the presence of an intact microtubule network (16, 29, 32, 38, 47).
To explore the potential requirement of microtubule-dependent virion transport prior to fusion, we investigated the effects of the microtubule-disrupting agent nocodazole and the microtubule-stabilizing agent taxol on the entry and fusion of Ebola virus GP, VSV-G, and HIV-1 Env pseudotypes. Ebola virus GP-mediated virion entry and fusion were markedly attenuated by nocodazole and enhanced by taxol. Conversely, these drugs exerted no significant effects on VSV-G-mediated entry and fusion, which occur in early endosomes. These findings are consistent with the possibility that, after Ebola virions are internalized, they traffic to a more distal acidified compartment, possibly late endosomes, and that this trafficking requires intact microtubules. This requirement for an additional transport step, compared to VSV-G-pseudotyped virions, also could explain the slower kinetics of entry and fusion observed with Ebola virus GP-pseudotyped virions. Alternatively, the Ebola virus and VSV may utilize distinct cellular entry pathways that display a different dependence on microtubules. Finally, it is possible that disruption of microtubules could differentially alter the membrane distribution of key receptors required for Ebola virus GP- versus VSV-G-mediated entry and fusion.
Actin filaments are also involved in endocytosis or internalization of several ligands and pathogens (10, 12, 15). In terms of viruses, the entry of simian virus 40 utilizes an endocytic pathway that requires the presence of actin filaments (39). However, the role of actin filaments in viral entry into mammalian cells remains rather controversial. To assess the role of actin microfilaments in Ebola virus GP-mediated entry and fusion, we tested CytB and CytD, which cap actin filaments, LatA, which sequesters actin monomers by binding to G-actin, and Jas, which stabilizes and promotes actin assembly on the entry and fusion of Ebola virus GP, VSV-G, and HIV-1 Env pseudotypes. Ebola virus GP-mediated entry and fusion were inhibited by treatment with each of these microfilament agents. Conversely, VSV-G- and HIV-1 Env-mediated entry and fusion were not significantly affected. These findings suggest that, in addition to microtubules, actin filaments play an important role in the events controlling Ebola virus GP-mediated entry and fusion.
The primary targets of Ebola virus infection are monocytes/macrophages, which respond by producing and releasing large quantities of several chemokines and proinflammatory cytokines, including TNF-α, IFN-γ, and IL-1β (13, 21, 24, 26, 46, 52). Earlier studies using samples from the infected patients revealed that elevated levels of TNF-α (236 ± 265 pg/ml) in plasma are often present in patients dying of Ebola hemorrhagic fever. Conversely, TNF-α levels are lower in patients experiencing nonfatal forms of infection (113 ± 53 pg/ml) (52). These results contrast sharply with the elevations of plasma IL-1β that occur in nonfatal Ebola virus infection (∼15.2 to 28.3 pg/ml) versus the undetectable levels of IL-1β (<10 pg/ml) in patients dying of this infection (1). TNF-α released from infected monocytes/macrophages can produce sharp changes in vascular permeability through its effects on human endothelial cells (13). We observed that levels of Ebola virus GP-mediated entry and fusion occurring in HUVECs were increased in a dose-related manner after treatment of these cells with TNF-α. This finding raises the possibility that changes in the level of filoviral entry and fusion into endothelial cells contributes to Ebola virus disease progression. Conversely, IL-1β exerts little or no effect on these entry and fusion events in endothelial cells. TNF-α did not appear to act by stabilizing microtubule formation in HUVECs, as shown in α-tubulin staining studies. Overall, these findings suggest that TNF-α production by macrophages infected with Ebola virus may in turn promote increased entry and fusion of the virus into key secondary cellular targets, namely, endothelial cells, thereby contributing to the profound compromise in vascular integrity observed in Ebola virus-induced disease.
On a more general note, our studies demonstrate that it is possible to adapt the HIV-1 virion-based fusion assay to study the entry properties conferred by other viral envelopes. Our studies have focused chiefly on Ebola virus GP-mediated entry, although VSV-G pseudotypes have also been successfully employed in our studies and those of Wyma et al. (57). The availability of this rapid, sensitive, and specific virion fusion assay could thus facilitate further study of the envelopes of many pathogenic viruses.
Acknowledgments
We thank the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, for HXB2-env donated by Kathleen Page and Dan Littman and pHEF-VSVG donated by Lung-Ji Chang. We also thank Anthony Sanchez (Centers for Disease Control and Prevention) and Stephen Y. Chan for generously providing cDNA encoding Ebola virus GP and an Ebola virus GP expression vector, respectively, and Mary Kate Hart (U.S. Army Medical Research Institute of Infectious Diseases) for the gift of anti-Ebola virus GP monoclonal antibodies. We thank Marty Bigos, Dax Arguello, and Valerie Stepps for expertise in flow cytometry, Stephen Ordway and Gary Howard for editorial assistance, John C. W. Carroll for graphics assistance, and Robin Givens for manuscript assistance.
This work was supported in part by grants from Universitywide AIDS Research Program CC02-SF-002 and NIH P01 HD40543-03 to W.C.G.
REFERENCES
- 1.Baize, S., E. M. Leroy, A. J. Georges, M. C. Georges-Courbot, M. Capron, I. Bedjabaga, J. Lansoud-Soukate, and E. Mavoungou. 2002. Inflammatory responses in Ebola virus-infected patients. Clin. Exp. Immunol. 128:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baize, S., E. M. Leroy, M. C. Georges-Courbot, M. Capron, J. Lansoud-Soukate, P. Debre, S. P. Fisher-Hoch, J. B. McCormick, and A. J. Georges. 1999. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat. Med. 5:423-426. [DOI] [PubMed] [Google Scholar]
- 3.Bavari, S., C. M. Bosio, E. Wiegand, G. Ruthel, A. B. Will, T. W. Geisbert, M. Hevey, C. Schmaljohn, A. Schmaljohn, and M. J. Aman. 2002. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J. Exp. Med. 195:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowen, E. T., G. Lloyd, W. J. Harris, G. S. Platt, A. Baskerville, and E. E. Vella. 1977. Viral haemorrhagic fever in southern Sudan and northern Zaire. Preliminary studies on the aetiological agent. Lancet i:571-573. [DOI] [PubMed] [Google Scholar]
- 5.Bubb, M. R., A. M. Senderowicz, E. A. Sausville, K. L. Duncan, and E. D. Korn. 1994. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem. 269:14869-14871. [PubMed] [Google Scholar]
- 6.Campbell, E. M., R. Nunez, and T. J. Hope. 2004. Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity. J. Virol. 78:5745-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavrois, M., C. De Noronha, and W. C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 20:1151-1154. [DOI] [PubMed] [Google Scholar]
- 8.Chan, S. Y., R. F. Speck, M. C. Ma, and M. A. Goldsmith. 2000. Distinct mechanisms of entry by envelope glycoproteins of Marburg and Ebola (Zaire) viruses. J. Virol. 74:4933-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, L. J., V. Urlacher, T. Iwakuma, Y. Cui, and J. Zucali. 1999. Efficacy and safety analyses of a recombinant human immunodeficiency virus type 1 derived vector system. Gene Ther. 6:715-728. [DOI] [PubMed] [Google Scholar]
- 10.Dramsi, S., and P. Cossart. 1998. Intracellular pathogens and the actin cytoskeleton. Annu. Rev. Cell. Dev. Biol. 14:137-166. [DOI] [PubMed] [Google Scholar]
- 11.Empig, C. J., and M. A. Goldsmith. 2002. Association of the caveola vesicular system with cellular entry by filoviruses. J. Virol. 76:5266-5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engqvist-Goldstein, A. E., and D. G. Drubin. 2003. Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell. Dev. Biol. 19:287-332. [DOI] [PubMed] [Google Scholar]
- 13.Feldmann, H., H. Bugany, F. Mahner, H. D. Klenk, D. Drenckhahn, and H. J. Schnittler. 1996. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J. Virol. 70:2208-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredericksen, B. L., B. L. Wei, J. Yao, T. Luo, and J. V. Garcia. 2002. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J. Virol. 76:11440-11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujimoto, L. M., R. Roth, J. E. Heuser, and S. L. Schmid. 2000. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic 1:161-171. [DOI] [PubMed] [Google Scholar]
- 16.Geada, M. M., I. Galindo, M. M. Lorenzo, B. Perdiguero, and R. Blasco. 2001. Movements of vaccinia virus intracellular enveloped virions with GFP tagged to the F13L envelope protein. J. Gen. Virol. 82:2747-2760. [DOI] [PubMed] [Google Scholar]
- 17.Geisbert, T. W., L. E. Hensley, T. R. Gibb, K. E. Steele, N. K. Jaax, and P. B. Jahrling. 2000. Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Lab. Investig. 80:171-186. [DOI] [PubMed] [Google Scholar]
- 18.Geisbert, T. W., and P. B. Jahrling. 1995. Differentiation of filoviruses by electron microscopy. Virus Res. 39:129-150. [DOI] [PubMed] [Google Scholar]
- 19.Geisbert, T. W., P. B. Jahrling, M. A. Hanes, and P. M. Zack. 1992. Association of Ebola-related Reston virus particles and antigen with tissue lesions of monkeys imported to the United States. J. Comp Pathol. 106:137-152. [DOI] [PubMed] [Google Scholar]
- 20.Genty, N., and F. Bussereau. 1980. Is cytoskeleton involved in vesicular stomatitis virus reproduction? J. Virol. 34:777-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibb, T. R., D. A. Norwood, Jr., N. Woollen, and E. A. Henchal. 2002. Viral replication and host gene expression in alveolar macrophages infected with Ebola virus (Zaire strain). Clin. Diagn. Lab. Immunol. 9:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldsmith, M. A., W. Xu, M. C. Amaral, E. S. Kuczek, and W. C. Greene. 1994. The cytoplasmic domain of the interleukin-2 receptor beta chain contains both unique and functionally redundant signal transduction elements. J. Biol. Chem. 269:14698-14704. [PubMed] [Google Scholar]
- 23.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-74. [DOI] [PubMed] [Google Scholar]
- 24.Gupta, M., S. Mahanty, R. Ahmed, and P. E. Rollin. 2001. Monocyte-derived human macrophages and peripheral blood mononuclear cells infected with Ebola virus secrete MIP-1 α and TNF-α and inhibit poly-IC-induced IFN-α in vitro. Virology 284:20-25. [DOI] [PubMed] [Google Scholar]
- 25.Hamm-Alvarez, S. F., M. Sonee, K. Loran-Goss, and W. C. Shen. 1996. Paclitaxel and nocodazole differentially alter endocytosis in cultured cells. Pharm. Res. 13:1647-1656. [DOI] [PubMed] [Google Scholar]
- 26.Hensley, L. E., H. A. Young, P. B. Jahrling, and T. W. Geisbert. 2002. Proinflammatory response during Ebola virus infection of primate models: possible involvement of the tumor necrosis factor receptor superfamily. Immunol. Lett. 80:169-179. [DOI] [PubMed] [Google Scholar]
- 27.Jahrling, P. B., T. W. Geisbert, D. W. Dalgard, E. D. Johnson, T. G. Ksiazek, W. C. Hall, and C. J. Peters. 1990. Preliminary report: isolation of Ebola virus from monkeys imported to USA. Lancet 335:502-505. [DOI] [PubMed] [Google Scholar]
- 28.Johnson, K. M., J. V. Lange, P. A. Webb, and F. A. Murphy. 1977. Isolation and partial characterisation of a new virus causing acute haemorrhagic fever in Zaire. Lancet i:569-571. [DOI] [PubMed] [Google Scholar]
- 29.Kizhatil, K., and L. M. Albritton. 1997. Requirements for different components of the host cell cytoskeleton distinguish ecotropic murine leukemia virus entry via endocytosis from entry via surface fusion. J. Virol. 71:7145-7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koch, G., and F. Koch. 1978. The use of cytochalasins in studies on the molecular biology of virus-host cell interactions. Front. Biol. 46:475-498. [PubMed] [Google Scholar]
- 31.Kuhn, M. 1998. The microtubule depolymerizing drugs nocodazole and colchicine inhibit the uptake of Listeria monocytogenes by P388D1 macrophages. FEMS Microbiol. Lett. 160:87-90. [DOI] [PubMed] [Google Scholar]
- 32.Lakadamyali, M., M. J. Rust, H. P. Babcock, and X. Zhuang. 2003. Visualizing infection of individual influenza viruses. Proc. Natl. Acad. Sci. USA 100:9280-9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leroy, E. M., S. Baize, V. E. Volchkov, S. P. Fisher-Hoch, M. C. Georges- Courbot, J. Lansoud-Soukate, M. Capron, P. Debre, J. B. McCormick, and A. J. Georges. 2000. Human asymptomatic Ebola infection and strong inflammatory response. Lancet 355:2210-2215. [DOI] [PubMed] [Google Scholar]
- 34.Liao, Z., L. M. Cimakasky, R. Hampton, D. H. Nguyen, and J. E. Hildreth. 2001. Lipid rafts and HIV pathogenesis: host membrane cholesterol is required for infection by HIV type 1. AIDS Res. Hum. Retrovir. 17:1009-1019. [DOI] [PubMed] [Google Scholar]
- 35.Maddon, P. J., A. G. Dalgleish, J. S. McDougal, P. R. Clapham, R. A. Weiss, and R. Axel. 1986. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell 47:333-348. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee, S., R. N. Ghosh, and F. R. Maxfield. 1997. Endocytosis. Physiol. Rev. 77:759-803. [DOI] [PubMed] [Google Scholar]
- 37.Page, K. A., N. R. Landau, and D. R. Littman. 1990. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J. Virol. 64:5270-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 39.Pelkmans, L., D. Puntener, and A. Helenius. 2002. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296:535-539. [DOI] [PubMed] [Google Scholar]
- 40.Peters, C. J., and A. S. Khan. 1999. Filovirus diseases. Curr. Top. Microbiol. Immunol. 235:85-95. [DOI] [PubMed] [Google Scholar]
- 41.Popik, W., T. M. Alce, and W. C. Au. 2002. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4(+) T cells. J. Virol. 76:4709-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryabchikova, E. I., L. V. Kolesnikova, and S. V. Luchko. 1999. An analysis of features of pathogenesis in two animal models of Ebola virus infection. J. Infect. Dis. 179(Suppl. 1):S199-S202. [DOI] [PubMed] [Google Scholar]
- 43.Schaeffer, E., V. B. Soros, and W. C. Greene. 2004. Compensatory link between fusion and endocytosis of human immunodeficiency virus type 1 in human CD4 T lymphocytes. J. Virol. 78:1375-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simmons, G., A. J. Rennekamp, N. Chai, L. H. Vandenberghe, J. L. Riley, and P. Bates. 2003. Folate receptor alpha and caveolae are not required for Ebola virus glycoprotein-mediated viral infection. J. Virol. 77:13433-13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steele, K., B. Crise, A. Kuehne, and W. Kell. 2001. Ebola virus glycoprotein demonstrates differential cellular localization in infected cell types of nonhuman primates and guinea pigs. Arch. Pathol. Lab. Med. 125:625-630. [DOI] [PubMed] [Google Scholar]
- 46.Stroher, U., E. West, H. Bugany, H. D. Klenk, H. J. Schnittler, and H. Feldmann. 2001. Infection and activation of monocytes by Marburg and Ebola viruses. J. Virol. 75:11025-11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stuart, A. D., H. E. Eustace, T. A. McKee, and T. D. Brown. 2002. A novel cell entry pathway for a DAF-using human enterovirus is dependent on lipid rafts. J. Virol. 76:9307-9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Superti, F., L. Seganti, and N. Orsi. 1988. Effect of cellular inhibitors on the infection of various susceptible cells with vesicular stomatitis virus. Acta Virol. 32:487-493. [PubMed] [Google Scholar]
- 49.Superti, F., L. Seganti, F. M. Ruggeri, A. Tinari, G. Donelli, and N. Orsi. 1987. Entry pathway of vesicular stomatitis virus into different host cells. J. Gen. Virol. 68(Pt. 2):387-399. [DOI] [PubMed] [Google Scholar]
- 50.Takada, A., C. Robison, H. Goto, A. Sanchez, K. G. Murti, M. A. Whitt, and Y. Kawaoka. 1997. A system for functional analysis of Ebola virus glycoprotein. Proc. Natl. Acad. Sci. USA 94:14764-14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terasaki, M., L. B. Chen, and K. Fujiwara. 1986. Microtubules and the endoplasmic reticulum are highly interdependent structures. J Cell Biol. 103:1557-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villinger, F., P. E. Rollin, S. S. Brar, N. F. Chikkala, J. Winter, J. B. Sundstrom, S. R. Zaki, R. Swanepoel, A. A. Ansari, and C. J. Peters. 1999. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J. Infect. Dis. 179(Suppl. 1):S188-S191. [DOI] [PubMed] [Google Scholar]
- 53.Wilson, J. A., M. Hevey, R. Bakken, S. Guest, M. Bray, A. L. Schmaljohn, and M. K. Hart. 2000. Epitopes involved in antibody-mediated protection from Ebola virus. Science 287:1664-1666. [DOI] [PubMed] [Google Scholar]
- 54.Wool-Lewis, R. J., and P. Bates. 1998. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J. Virol. 72:3155-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.World Health Organization. 1978. Ebola haemorrhagic fever in Sudan, 1976. Report of a WHO/International Study Team. Bull. W. H. O. 56:247-270. [PMC free article] [PubMed] [Google Scholar]
- 56.World Health Organization. 1978. Ebola haemorrhagic fever in Zaire, 1976. Bull. W. H. O. 56:271-293. [PMC free article] [PubMed] [Google Scholar]
- 57.Wyma, D. J., J. Jiang, J. Shi, J. Zhou, J. E. Lineberger, M. D. Miller, and C. Aiken. 2004. Coupling of human immunodeficiency virus type 1 fusion to virion maturation: a novel role of the gp41 cytoplasmic tail. J. Virol. 78:3429-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaki, S. R., and C. S. Goldsmith. 1999. Pathologic features of filovirus infections in humans. Curr. Top. Microbiol. Immunol. 235:97-116. [DOI] [PubMed] [Google Scholar]