Abstract
Metastasis of esophageal squamous cell carcinoma (ESCC) remains a challenge in clinical practice. In this study, we clarified that integrin β1 (ITGB1) plays critical roles in the metastasis of ESCC. By analyzing the expression of integrin β1 in ESCC specimens, we found that the expression of this integrin was higher in malignant than in normal tissues and that this increase was associated with lymph node metastasis. Moreover, in vitro functional experiments demonstrated that deletion of integrin β1 impaired the motility of ESCC cells, and we also showed that integrin β1 deletion significantly inhibited metastases formation in the lungs and lymph nodes of two murine models. Mechanistically, integrin β1 promoted cellular motility by regulating the FAK-Rac1 signaling pathway. Finally, we found that blocking integrin β1 significantly impaired the resistance of ESCC cells to cisplatin (DDP) treatment based on in vitro and in vivo experiments. Overall, our data suggest that integrin β1 promotes metastasis and confers DDP resistance to ESCC, which provides experimental evidence for targeting this protein to treat ESCC in the future.
Keywords: Integrin β1, metastasis, FAK, chemo-resistance, esophageal cancer
Introduction
Esophageal cancer is the sixth and ninth most common cause of cancer death in males and females, respectively [1]. Two pathological subtypes-squamous cell carcinoma (ESCC) and adenocarcinoma (EAC)-comprise the majority of esophageal cancer cases [2], and more than 90% of patients with esophageal cancer in China are diagnosed with ESCC [2]. Although significant advances have been made in the treatment of ESCC, metastasis remains the leading cause of death of patients. The metastasis of malignant cells consists of consecutive steps: dissemination from the primary lesion, travel through circulatory vessels, and finally, the colonization and formation of overt metastases in distant sites [3]. In this process, cancer cells use multiple molecules to address complex cell-microenvironment interactions.
Among these metastasis-related genes, integrins are believed to mediate cell-cell and cell-matrix crosstalk by relaying outside-inside or inside-outside signals across the cellular membrane. Integrins are heterodimers consisting of 18α and 8β subunits [4]. Specifically, integrin β1 is a member of the β subfamily, which forms dimers with eight different α subunits (α1, α2, α3, α4, α5, α6, α7, and αV) in vertebrates [4]. In the realm of cancer biology, integrin β1 plays critical roles in viability, proliferation, and motility in breast cancer, gastric cancer, non-small lung cancer, laryngeal cancer, liver cancer, and multiple myeloma [5-10]. In the EAC of Barrett’s esophagus, the expression of integrin β1 did not correlate with prognosis [11], but a larger cohort of patients is likely required to confirm this finding. In ESCC, integrins α6β4 and α6β1 are preferentially expressed at the invasive front compared with the normal epithelium, and this change in expression positively correlated with tumor progression [12]. Interestingly, the deletion of integrin β1 sensitized ESCC cells to docetaxel treatment [13], and a similar β1-mediated effect has been identified in several other cancers. However, the function of integrin β1 in esophageal cancer is not fully understood.
In the present report, we used in vitro and in vivo models to test the function of integrin β1 in the invasion and metastasis of ESCC. Blocking integrin β1 expression significantly attenuated the motility of ESCC cells in vitro and inhibited lymph node invasion and pulmonary metastasis formation. Mechanistically, this pro-metastasis function of integrin β1 was mediated by the FAK-Rac1 signaling pathway. Furthermore, we found that integrin β1-null ESCC cells became sensitive to DDP but not paclitaxel, which further supports the use of integrin β1 as a target of ESCC treatment.
Materials and methods
Clinical samples
Clinical samples of patients with ESCC were obtained from the Department of Surgical Oncology of Nanjing First Hospital and the Chinese Academy of Medical Sciences Cancer Hospital. All experiments conducted using human tissues were approved by the ethical committee of Nanjing First Hospital and the ethical committee of the Chinese Academy of Medical Sciences Cancer Hospital.
Cell lines, transfection, and stable cell lines
RPMI 1640 supplemented with 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin was used to culture KYSE30, 30D, KYSE140, KYSE150, KYSE180, KYSE410, KYSE450, KYSE510, TE10 and TE12 ESCC cells. KYSE cells were kindly provided by Dr. Y. Shimada (Kyoto University, Kyoto, Japan) [14]. The normal esophageal epithelial cell line Het-1A was obtained from ATCC (Manassas, VA, USA) and cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin. All cells were maintained at 37°C in a humidified atmosphere (95% air and 5% CO2). These cell lines were authenticated using STR analysis.
Specific siRNAs against integrin β1 or FAK were purchased from Integrated DNA Technologies (Coralville, IA, USA). A pool of three siRNAs was delivered into 30D or TE10 cells using HiperFect reagent (Qiangen, German) according to the manufacturer’s instructions. The final concentration of siRNA was 100 nM.
Lentivirus was prepared as described previously [15]. Briefly, thawed lentivirus was added to 30D or TE10 cells, and puromycin (1 μg/ml) was used to establish stably transduced cell lines. Integrin β1 expression deletion was verified in two clones (shITGB1-3 and shITGB1-7) using qPCR and immunoblot assays. Cells stably transduced with shRNA against GFP (shGFP) were used as the control group. The shRNAs are listed in Table 1.
Table 1.
Primers and shRNAs used in the study
| Names | Sequences |
|---|---|
| ITGB1-F | CAAAGGAACAGCAGAGAAGC |
| ITGB1-R | ATTGAGTAAGACAGGTCCATAAGG |
| GAPDH-F | GGTGAAGGTCGGAGTCAACG |
| GAPDH-R | TGGGTGGAATCATATTGGAACA |
| shITGB1-3-F | ATCCCAGAGGCTCCAAAGATAT |
| shITGB1-3-R | ATATCTTTGGAGCCTCTGGGAT |
| shITGB1-7-F | GCCTTGCATTACTGCTGATAT |
| shITGB1-7-R | ATATCAGCAGTAATGCAAGGC |
GEO data analysis
Two ESCC datasets (GSE23400 and GSE20347) were used to compare the expression of integrin β1 between cancerous tissues and the adjacent normal counterparts.
RT-qPCR and immunoblot analysis
RNAs were extracted using TRIzol (Thermo Fisher Scientific) and reverse-transcribed using the QuantScript RT Kit (TIANGEN BIOTECH, Beijing, China). Quantitative PCR assays were performed using SYBR Premix Ex TaqTM II (TaKaRa, Japan), and GAPDH was used as the internal control. The 2-ΔΔCt method was used to evaluate the relative abundance of target genes. All primers are listed in Table 1.
Cell lysis buffer (10 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, and 0.25% sodium deoxycholate, pH = 7.4) containing protease and phosphatase inhibitors (Roche, Basel, Switzerland) was used to extract protein from cells. The collected protein was then separated using Glycine-SDS-PAGE, and the target proteins were detected with the primary antibodies described in Table 2.
Table 2.
Primary antibodies used in the study
| Names | Cat NO. | Manufacturer |
|---|---|---|
| ITGB1 | 2288-1 | Epitomics |
| ITGB1 | YF102003C | Novus |
| FAK | 13009P | CST |
| p-FAK (Y397) | 3283P | CST |
| p-FAK (Y576/577) | 3281P | CST |
| p-FAK (Y861) | ab81293 | Abcam |
| p-FAK (Y925) | 3284P | CST |
| p130Cas | 13383S | CST |
| p-P130Cas (Y165) | 4015S | CST |
| p-P130Cas (Y249) | 4014S | CST |
| p-P130Cas (Y410) | 4011S | CST |
| Paxillin | 2542s | CST |
| p-Paxillin (Y118) | 2541S | CST |
| Rac1 | 8631S | CST |
| Caspase-3 | 9662S | CST |
| PARP | SC-7150 | Santa Cruz |
| β-Actin | AM2026b | Sigma |
Cell migration and invasion assay
Inserts (6.5 mm in diameter and 8 μm pores, Corning) were used in the migration assay. These inserts were pre-coated with BD Matrigel (26.1 μl/ml) for use in the invasion assay. RPMI 1640 containing 20% FBS acted as the chemo-attractant. TE10 or 30D cells (5×104 cells/well) were incubated in the inserts for 24 h and were fixed using methanol for 15 min at room temperature and stained with crystal violet. Five fields were captured randomly, and the invading cells were counted. The migration and invasion assays were independently performed three times.
The wound healing assay was performed as described previously [15]. Briefly, shGFP and shITGB1-3/7 30D or TE10 cells were plated in 6-well dishes and allowed to reach confluence before wounding the monolayer with a 1-ml pipette tip. After 24 h, images of these scratches were captured using a Leica DM 1L LED inverted microscope. The ratio of the widths at 0 h and 24 h was used to compare the shGFP, shITGB1-3, and shITGB1-7 groups and calculated using the following equation: Ratio of width = (the width at 0 h - The width at 24 h)/the width at 0 hr.
Cellular viability assay
TE10 or 30D cells were plated into 96-well dishes. After the cells were incubated with the indicated concentrations of DDP (0-30 μg/ml) for 24 h, CCK8 was added and the absorbance (OD450) was examined using a microplate reader (BioTek, Vermont, USA).
Flow cytometry assay
Cellular apoptosis was assessed using a FITC Annexin V Apoptosis Kit from BD Biosciences (Franklin Lakes, NJ, USA). The stained cells were analyzed with a BD LSR II (Franklin Lakes, NJ, USA). Each sample was measured in triplicate, and the experiments were independently repeated three times.
Rac1 activation assay
The cells from the shGFP, shITGB1-3, and shITGB1-7 groups were harvested under chemotaxis conditions after 2 h of incubation. Activated Rac1 was detected using an Active GTPase Detection Kit purchased from Cell Signaling Technology (Danvers, MA, USA).
Animal experiments
All immune-deficient mice (Balb/c nude and SCID/Beige) were purchased from Vital River Laboratory Animal Technology (Beijing, China), and all animal experiments were approved by the Beijing Medical Experimental Animal Care Commission.
Two in vivo murine models were used to evaluate the effect of integrin β1 blockage on the metastasis of ESCC cells. In the pulmonary metastasis model, 5×105 30D cells from the shGFP or shITGB1-3/7 group were injected into SCID/Beige mice via the tail vein. After 10 weeks, these mice were sacrificed and their lungs were harvested, fixed with 4% paraformaldehyde, and then stained using 3% picric acid to count metastatic foci. Furthermore, a lymph node metastasis model was generated as described previously [16]. Briefly, 5×105 shGFP or shITGB1-3/7 30D cells were subcutaneously injected into the footpad of Balb/c nude mice. The popliteal lymph nodes were then harvested, stained with H&E, and evaluated.
To assess the effect of integrin β1 deletion on the sensitivity to chemotherapy, 2×106 shGFP or shITGB1-3/7 30D cells were subcutaneously injected into Balb/c nude mice. When the tumors reached approximately 30 mm3 in size, DDP (5 mg/kg) was injected every 5 days via the abdomen. After the initial administration of DDP, the tumors were measured once per week. After 5 weeks, the tumors were harvested and analyzed by immunohistochemistry.
Immunohistochemistry
The immunohistochemistry analyses were performed as described previously [15]. Briefly, H&E staining was used to visualize metastatic lesions and assess the pathology of the three murine models. Specimens from patients with ESCC and mice were also stained for specific proteins (such as integrin β1 and cleaved caspase-3). The primary antibodies used for immunohistochemistry are listed in Table 2.
Two investigators independently evaluated the expression of integrin β1 in specimens from patients according to a previously described method [17]. In general, the expression level of integrin β1 was calculated by multiplying its staining intensity by the area of positive staining. The staining intensity was scored as follows: 0 for negative, 1 for light yellow, 2 for yellowish brown, and 3 for brown. The area of positive staining was scored as follows: 0 for 0-5% positive, 1 for 5-25% positive, 2 for 26-50% positive, 3 for 51-75% positive, and 4 for 75%-100% positive. Based on the combination of these scores, the staining was scored as follows: negative (0 points), weak (1-4 points), moderate (5-8 points), and strong (9-12 points).
Statistical analysis
All in vitro cell experiments were independently performed three times, and the results are reported as the mean ± S.D. Student’s t-test was used to analyze these results unless otherwise stated. Paired Student’s t-tests were used to assess the expression of integrin β1 in the two GEO datasets. Animal and immunohistochemistry experiments were performed once. In the bar graphs, *, **, *** and **** indicate P < 0.05, 0.01, 0.001 and 0.0001, respectively.
Results
Clinical properties of integrin β1 expression in human ESCC tissues
We first measured integrin β1 expression in 29 paired ESCC tissues by qPCR, which demonstrated that its mRNA levels were higher in cancerous tissues than in adjacent normal tissue (P < 0.001, Figure 1A). Mining two available expression datasets (GSE23400 and GSE20347) in Gene Expression Omnibus further validated that integrin β1 mRNA expression was elevated in malignant esophageal specimens (Figure 1B and 1C). Furthermore, a comparison of the integrin β1 mRNA level between Het-1A and a panel of ESCC cell lines showed that the expression of this integrin was dramatically increased in malignant cell lines relative to the immortalized epithelial cells (Figure 1D). IHC staining of ESCC specimens (N = 88) showed higher protein levels of integrin β1 in the cancerous epithelium than in normal tissue (Figure 1E). Interestingly, aberrantly increased integrin β1 expression was significantly associated with lymph node metastasis (P = 0.013) and advanced stage ESCC in patients (P < 0.01, Table 3). Taken together, these data imply that integrin β1 overexpression plays important roles in the progression of ESCC.
Figure 1.
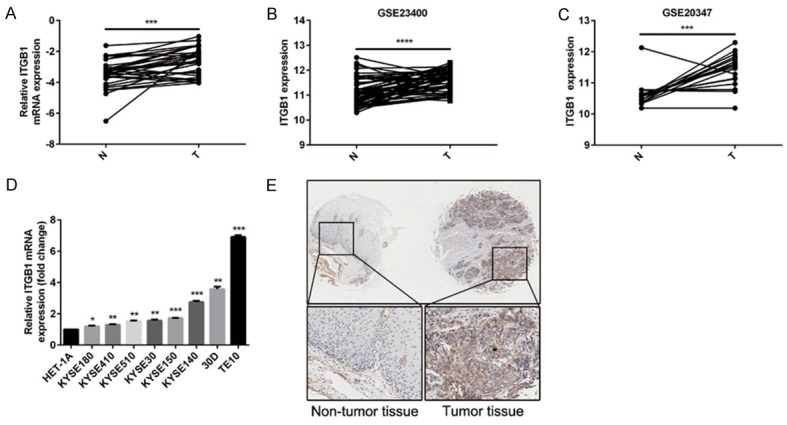
Clinical properties of integrin β1 expression in human ESCC. A: Integrin β1 mRNA expression is higher in cancerous tissues than in adjacent normal tissues (N = 29). GAPDH was used as the internal control. The P-value was determined with the paired t-test. B and C: Mining two ESCC expression datasets (GSE23400 and GSE20347) in GEO showed significantly increased integrin β1 expression in malignant tissues compared with normal tissues. The P-values were determined with the paired t-test. D: The mRNA expression of integrin β1 was significantly lower in an immortalized esophageal epithelial cell line (HET-1A) than in a panel of ESCC cell lines. E: Integrin β1 staining in 88 paired ESCC tissues shows that this integrin was expressed in the membranes of epithelial cells and, importantly, significantly upregulated in cancerous tissues.
Table 3.
Clinicopathologic properties of Integrin β1 expression in ESCC specimens
| Characteristics | Number of cases | ITGB1 expression | P value | |
|---|---|---|---|---|
|
| ||||
| Low (n = 59) | High (n = 29) | |||
| Age group | 0.934 | |||
| ≤60 | 40 | 27 | 13 | |
| >60 | 48 | 32 | 16 | |
| Sex, N | 0.819 | |||
| Male | 71 | 48 | 23 | |
| Famale | 17 | 11 | 6 | |
| Histological type | 0.741 | |||
| High | 9 | 7 | 2 | |
| Middle | 57 | 37 | 20 | |
| Low | 22 | 15 | 7 | |
| T stage, N | 0.002 | |||
| T1/T2 | 20 | 19 | 1 | |
| T3/T4 | 68 | 40 | 28 | |
| Lymph node metastasis | 0.013 | |||
| Yes | 41 | 22 | 19 | |
| No | 47 | 37 | 10 | |
| TNM stage | 0.003 | |||
| I/II | 50 | 40 | 10 | |
| III/IV | 38 | 19 | 19 | |
Integrin β1 deficiency suppresses the motility of ESCC cells in vitro
Because metastasis is the primary cause of death in ESCC, we focused on the possible role of integrin β1 in the motility of ESCC cells. To this end, we first measured integrin β1 expression in a panel of ESCC cell lines by immunoblotting (Figure 2A). siRNAs dramatically reduced integrin β1 expression in 30D cells, and these cells were used for functional analysis (Figure 2B). Specifically, Transwell assays demonstrated that reduced integrin β1 expression significantly inhibited the migration and invasion of 30D cells in vitro (P < 0.01, Figure 2C). Integrin β1 expression was then stably deleted in 30D or TE10 cells using shRNAs (Figure 2D). Similarly, cellular motility was also inhibited when integrin β1 expression was knocked down in these cells (shITGB1 30D or TE10) compared with the respective control cells (shGFP 30D or TE10) (P < 0.05, Figure 2E and 2F).
Figure 2.
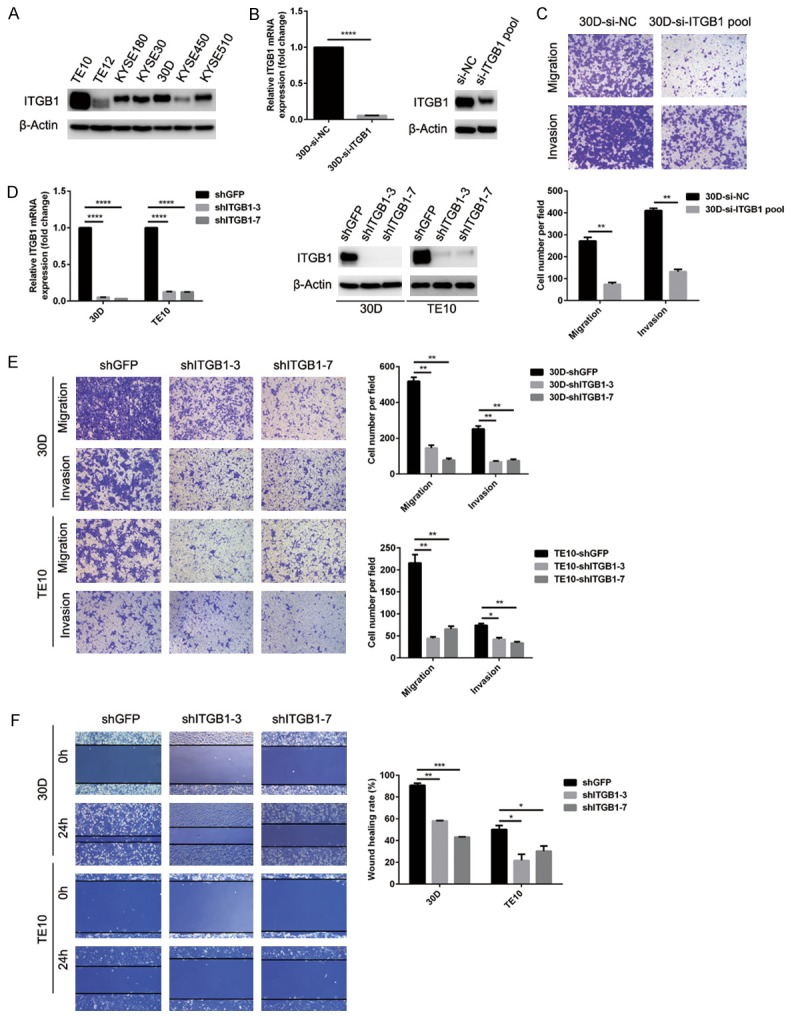
Knockdown of integrin β1 expression attenuates the motility of ESCC cells in vitro. (A) The protein level of integrin β1 was measured in a panel of ESCC cells using immunoblots. TE10 and 30D expressed higher levels of integrin β1 than the remaining tested cell lines. (B and C) siRNA knockdown of integrin β1 expression dramatically inhibited the migration and invasion of 30D cells in vitro. (D-F) The downregulation of integrin β1 expression in 30D or TE10 cells (shGFP and shITGB1-3/7 group) was verified using RT-qPCR and immunoblots (D). Stable knockdown of integrin β1 expression in 30D and TE10 cells significantly impeded cell motility compared with control cells based on Transwell assays (E) and wound healing assays (F). In all Transwell assays, five fields were randomly selected and the invading cells were counted. All scale bars in these images indicate 100 μm.
Knockdown of integrin β1 impedes metastasis of ESCC cells in vivo
To further investigate the role of integrin β1 in ESCC metastasis, we used experimental murine lung and lymph node metastasis models. Depletion of integrin β1 in 30D cells suppressed the formation of overt pulmonary metastases (P < 0.001, Figure 3A), and H&E staining revealed that more metastatic foci formed in mice from the shGFP group (Figure 3B). As shown in Table 3, integrin β1 was closely associated with the lymph node metastasis of ESCC. Thus, we inoculated the footpads of mice with integrin β1-depleted or control 30D cells. The popliteal lymph nodes were harvested 60 days later, which showed that the lymph nodes from the two shITGB1 groups were lighter than those from the shGFP group (P < 0.001, Figure 3C and 3D). Accordingly, H&E staining showed that far less integrin β1-depleted 30D cells invaded the popliteal lymph nodes (Figure 3E). Taken together, these results demonstrate that integrin β1 promotes the motility and metastasis of ESCC cells.
Figure 3.
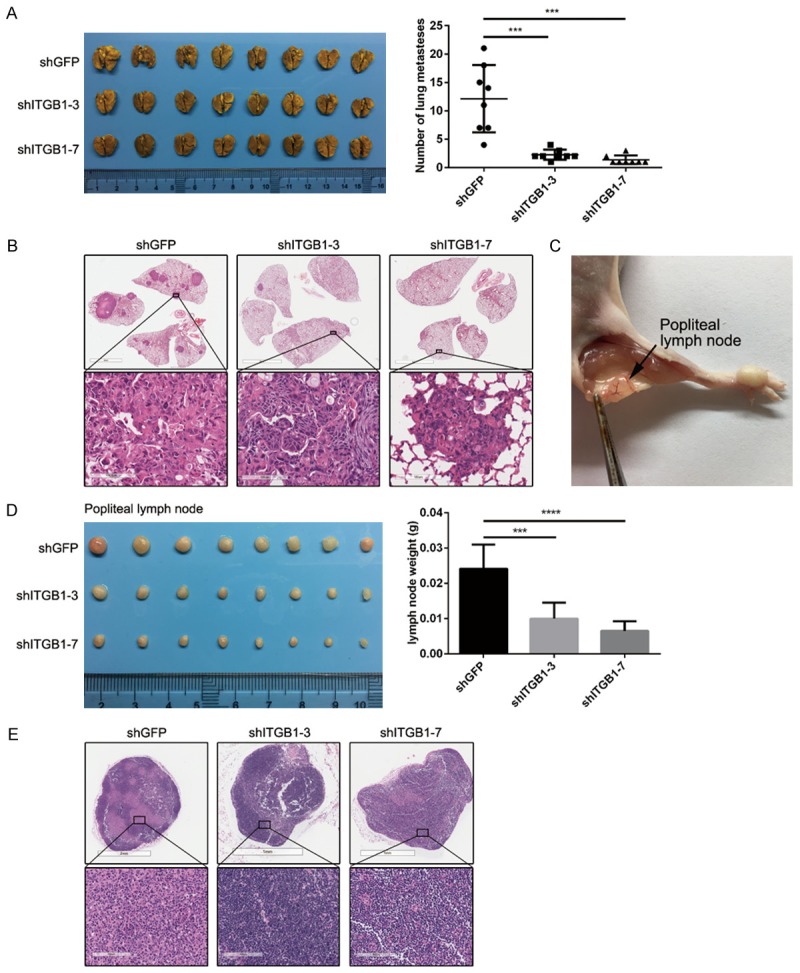
Blocking integrin β1 expression inhibits the metastasis of ESCC cells in vivo. (A and B) In the pulmonary metastasis model, β1-null 30D cells (shITGB1-3/7) formed far fewer metastases than control cells (shGFP) in the lungs of SCID/Beige mice (N = 8 for each group). The lungs were stained using 3% picric acid (A). H&E staining of fixed sections of these lungs shows the metastatic foci (B). The scale bars in the top three images indicate 4 mm, whereas those in the bottom three images indicate 100 μm (B). (C) Representative image showing the method used to harvest popliteal lymph nodes. (D and E) In the lymph node metastasis model, the popliteal lymph nodes of Balb/c nude mice in the shITGB1-3/7 group were smaller than those of the shGFP group (N = 8 for each group) (D). Representative images of the H&E-stained lymph nodes showing the metastatic lesions (E). The scale bars in the top three images indicate 1 mm or 2 mm, whereas those in the bottom three images indicate 100 μm (E).
Knockdown of integrin β1 inhibits the motility of ESCC cells by deactivating the FAK-Rac1 pathway
FAK is a critical signaling node in the regulation of cellular motility, and silencing FAK significantly impeded the migration and invasion of both 30D and TE10 cells in vitro (P < 0.05, Figure 4A and 4B). Therefore, we analyzed the interaction between integrin β1 and FAK signaling. Under the adherent or chemotaxis condition (2 h), depletion of integrin β1 elicited the de-phosphorylation of FAK, P130/Cas, and Paxillin and reduced the levels of activated Rac1 in 30D cells (Figure 4C and 4D). The mechanism of integrin β1 in TE10 cells was analogous to that in 30D cells: blocking the expression of this integrin also de-activated FAK, P130/Cas, and Paxillin under chemotaxis conditions (2 h, Figure 4E). These results demonstrate that integrin β1 promotes the motility of ESCC cells, at least in part, via the FAK-Rac1 pathway.
Figure 4.
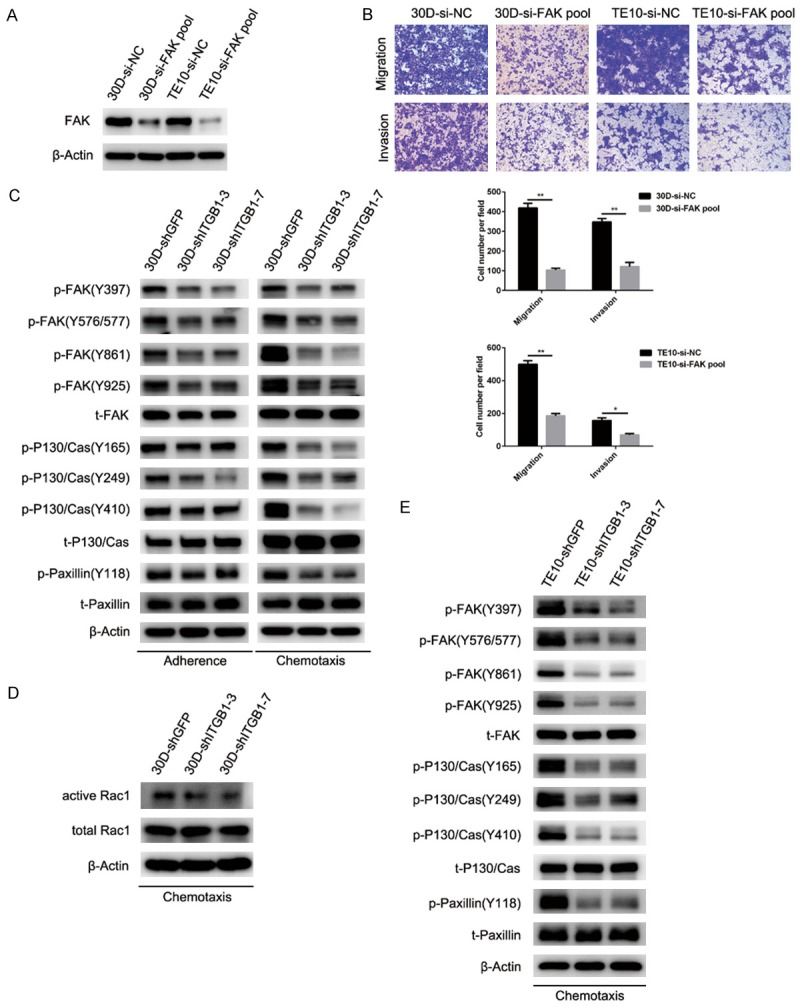
Deletion of integrin β1 expression inhibits the motility of ESCC cells by suppressing FAK-Rac1 signaling. A: siRNA knockdown of FAK expression significantly reduced the FAK level in 30D cells or TE10 cells. B: Deletion of FAK in 30D or TE10 cells impeded their motility in vitro relative to the control cells. In the Transwell assays, five fields were randomly selected and the invading cells were counted. All scale bars in these images indicate 100 μm. C: In the stably β1-deleted 30D cells (30D-shITGB1-3/7), the levels of phosphorylated FAK (Y397/576/577/861/925), P130/Cas (Y16/249/410), and paxillin (Y118) were reduced compared with control cells (30D-shGFP) under adherent or chemotaxis conditions (2 h). D: Under chemotaxis conditions (2 h), 30D cells from the shITGB1-3/7 group expressed lower levels of Rac1-GTP than shGFP cells. E: Stable deletion of integrin β1 in TE10 cells (TE10-shITGB1-3/7) also reduced the level of phosphorylated FAK (Y397/576/577/861/925), P130/Cas (Y16/249/410), and paxillin (Y118) relative to the control ones (TE10-shGFP) under chemotaxis conditions for 2 h.
Integrin β1 deficiency sensitizes ESCC cells to DDP
Pro-metastasis genes usually endow malignant cells with stronger resistance to chemotherapy; thus, we assessed the role of integrin β1 in chemotherapy resistance. DDP is a widely used first-line drug for the treatment of ESCC, and we found that stably knocking down integrin β1 sensitized 30D and TE10 cells to a wide range of concentrations of DDP (2.5-30 μg/ml, Figure 5A). This sensitizing effect depended largely on the dramatic increase in apoptosis caused by integrin β1 deficiency (Figure 5B and 5C). We then subcutaneously inoculated Balb/c nude mice with shGFP or shITGB1-3/7 30D cells and treated the mice with injections of normal saline (NS) or DDP. The growth of malignant cells in vivo significantly differed by group: tumors in the shITGB1 group grew more slowly than those from the shGFP group during one month of DDP treatment (Figure 5D). Moreover, IHC staining showed a higher level of cleaved caspase-3 in integrin β1-depleted cells than in control cells (Figure 5E). Interestingly, the knockdown of integrin β1 did not sensitize 30D and TE10 cells to paclitaxel treatment (Figure 5F). Taken together, these data indicate that depletion of integrin β1 enhanced the cytotoxicity of some chemotherapy drugs (such as DDP).
Figure 5.
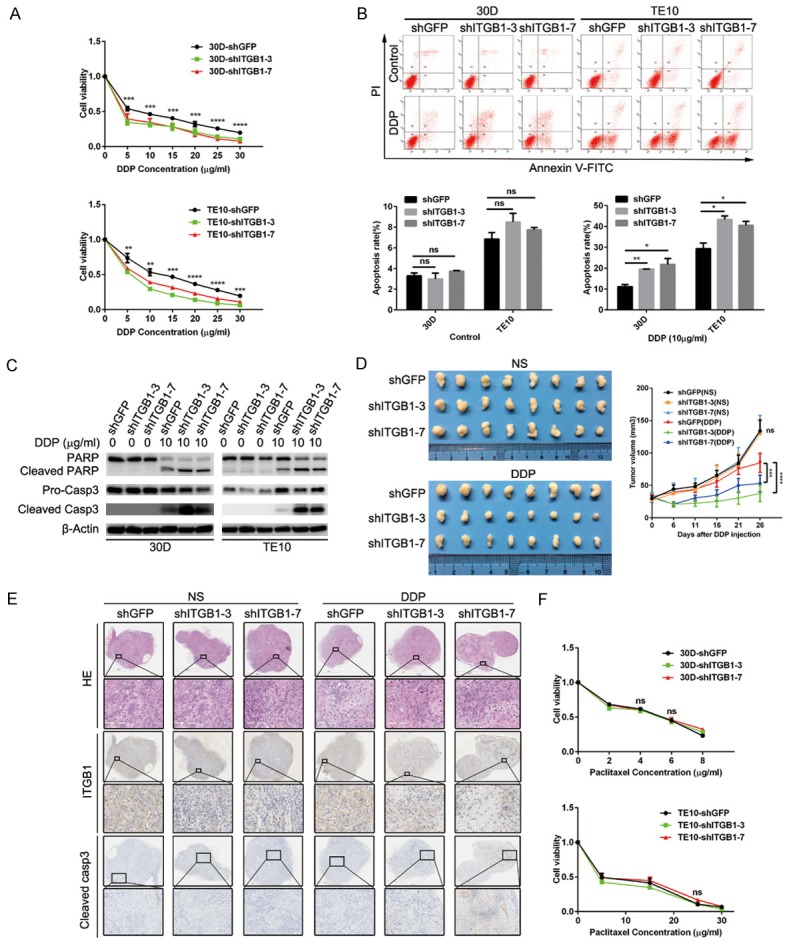
Reduced integrin β1 expression sensitizes ESCC cells to DDP administration. A: Integrin β1 deletion in 30D or TE10 cells impaired cellular viability when exposed to the indicated concentrations of DDP. B: The apoptosis rates were analyzed using flow cytometry, which showed that integrin β1 deletion promoted DDP-mediated apoptosis (10 μg/ml) in 30D or TE10 cells. C: Immunoblotting assays confirmed that DDP (10 μg/ml) treatment promoted apoptosis in integrin β1-null 30D or TE10 cells, as demonstrated by the dramatic increases in PARP and caspase-3. D: In vivo, the growth of xenografts formed by subcutaneously injected 30D cells was inhibited by 26 days of DDP treatment. Xenografts in the shITGB1-3/7 group were smaller than those in the shGFP group. E: Immunohistochemistry staining showed decreases in integrin β1 expression and increases in cleaved caspase-3 expression in the harvested xenografts. The scale bars indicate 1 mm or 2 mm in the original images and 100 μm or 200 μm in the magnified images. F: The deletion of integrin β1 did not affect the viability of 30D or TE10 cells when they were exposed to the indicated concentrations of paclitaxel.
Discussion
Metastasis is a major health problem for patients with cancer that urgently requires effective treatments in clinical practice. To this end, identifying metastasis-related genes and clarifying their mechanisms of action are vital steps to develop new treatment strategies. In ESCC, several proteins and non-coding RNAs have been reported to promote or inhibit the invasion and metastasis of malignant cells [15,18]. Here, we delineate the pro-metastasis role of integrin β1 in ESCC for the first time based on two murine models. In the pulmonary metastasis model, shITGB1-3/7 cells formed fewer and smaller metastases than shGFP cells. Accordingly, integrins α3β1 and α6β1 have been shown to facilitate the extravasation of cancer cells, as β1-ablated cells failed to form functional protrusions during the transmigration of the endothelial barrier [19]. ESCC cells likely exit blood vessels via similar mechanisms, which may explain why shITGB1 cells formed far fewer metastatic foci.
In addition to blood vessels, lymph nodes constitute another major metastatic route. Thus, lymph node metastasis serves as an important index to assess cancer progression and usually indicates poor prognosis in a wide range of cancers [20]. Because higher integrin β1 expression positively correlates with lymph node metastasis in ESCC specimens (Table 3), we used a murine model to analyze the ability of integrin β1 deletion to attenuate lymph node invasion. Popliteal lymph nodes from the shITGB1-3/7 group were significantly smaller than those from the shGFP group, and the H&E staining of lymph node sections showed fewer metastases. Interestingly, larger metastases were observed in several shGFP lymph nodes, which is analogous to the observations made in the pulmonary metastasis model. However, the ability of integrin β1 to stimulate the proliferation of these metastatic cells in lymph nodes is unknown. In this case, the similarity of the pro-survival and pro-proliferation mechanisms of integrin β1 in the lungs and lymph nodes warrants further investigation.
Because integrin β1 regulates viability and proliferation, several groups reported that β1-null cells from various cancer types became sensitive to chemotherapy and radiotherapy [21-23]. In this study, downregulation of integrin β1 sensitized ESCC cells to DDP treatment by promoting apoptosis. However, paclitaxel did not significantly inhibit the growth of β1-null cells compared with control cells. Nevertheless, a previous report showed that blocking integrin β1 with siRNA restored the sensitivity of ESCC cells to docetaxel administration [13]. Because both paclitaxel and docetaxel target microtubules, this differential response is likely due to distinct cellular contexts. Therefore, the lack of response in our system likely represents the insensitivity of ESCC to paclitaxel treatment.
In conclusion, we demonstrated the pro-metastasis function of integrin β1 in ESCC cells based on in vitro and in vivo models. These results provide preliminary data to support the targeting of integrin β1 to treat ESCC metastasis. For example, the macrolide compound F806 has been shown to bind integrin β1 (R610) in experimental studies to induce anoikis and inhibit the growth of ESCC cells [24]. However, current experimental therapies primarily focus on the growth of ESCC cells or subcutaneous xenografts because metastasis models fail to accurately represent clinical disease progression. Thus, novel cellular or animal models should be developed to assess the utility of targeting integrin β1 to treat metastasis.
Acknowledgements
This work was supported by the Beijing Training Project for the Leading Talents in S & T (Z151100000315009), the National Natural Science Foundation of China (NSFC) (No. 81372656, No. 81672423, No. 81602153), the Jiangsu Provincial Conditional Construction and the People’s Livelihood Scientific and Technological Special Project for “Clinical Science and Technology” (BL2014011), the 333 Project Foundation of Jiangsu Province (No. BRA2014355), and the Six Talent Peaks Project of Jiangsu Province (WSN-084).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499–2509. doi: 10.1056/NEJMra1314530. [DOI] [PubMed] [Google Scholar]
- 3.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 5.Itou J, Tanaka S, Li W, Iida A, Sehara-Fujisawa A, Sato F, Toi M. The Sal-like 4-integrin alpha6beta1 network promotes cell migration for metastasis via activation of focal adhesion dynamics in basal-like breast cancer cells. Biochim Biophys Acta. 2017;1864:76–88. doi: 10.1016/j.bbamcr.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Hu C, Ni Z, Li BS, Yong X, Yang X, Zhang JW, Zhang D, Qin Y, Jie MM, Dong H, Li S, He F, Yang SM. hTERT promotes the invasion of gastric cancer cells by enhancing FOXO3a ubiquitination and subsequent ITGB1 upregulation. Gut. 2017;66:31–42. doi: 10.1136/gutjnl-2015-309322. [DOI] [PubMed] [Google Scholar]
- 7.Navab R, Strumpf D, To C, Pasko E, Kim KS, Park CJ, Hai J, Liu J, Jonkman J, Barczyk M, Bandarchi B, Wang YH, Venkat K, Ibrahimov E, Pham NA, Ng C, Radulovich N, Zhu CQ, Pintilie M, Wang D, Lu A, Jurisica I, Walker GC, Gullberg D, Tsao MS. Integrin alpha11beta1 regulates cancer stromal stiffness and promotes tumorigenicity and metastasis in non-small cell lung cancer. Oncogene. 2016;35:1899–1908. doi: 10.1038/onc.2015.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klobucar M, Sedic M, Gehrig P, Grossmann J, Bilic M, Kovac-Bilic L, Pavelic K, Kraljevic Pavelic S. Basement membrane protein ladinin-1 and the MIF-CD44-beta1 integrin signaling axis are implicated in laryngeal cancer metastasis. Biochim Biophys Acta. 2016;1862:1938–1954. doi: 10.1016/j.bbadis.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Wong KF, Liu AM, Hong W, Xu Z, Luk JM. Integrin alpha2beta1 inhibits MST1 kinase phosphorylation and activates Yes-associated protein oncogenic signaling in hepatocellular carcinoma. Oncotarget. 2016;7:77683–77695. doi: 10.18632/oncotarget.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shain KH, Yarde DN, Meads MB, Huang M, Jove R, Hazlehurst LA, Dalton WS. Beta1 integrin adhesion enhances IL-6-mediated STAT3 signaling in myeloma cells: implications for microenvironment influence on tumor survival and proliferation. Cancer Res. 2009;69:1009–1015. doi: 10.1158/0008-5472.CAN-08-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottger TC, Youssef V, Dutkowski P, Seifert J, Maschek H, Brenner W, Junginger T. Beta1 integrin expression in adenocarcinoma of Barrett’s esophagus. Hepatogastroenterology. 1999;46:938–943. [PubMed] [Google Scholar]
- 12.Vay C, Hosch SB, Stoecklein NH, Klein CA, Vallbohmer D, Link BC, Yekebas EF, Izbicki JR, Knoefel WT, Scheunemann P. Integrin expression in esophageal squamous cell carcinoma: loss of the physiological integrin expression pattern correlates with disease progression. PLoS One. 2014;9:e109026. doi: 10.1371/journal.pone.0109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mori R, Ishiguro H, Kuwabara Y, Kimura M, Mitsui A, Tomoda K, Mori Y, Ogawa R, Katada T, Harata K, Fujii Y. Targeting beta1 integrin restores sensitivity to docetaxel of esophageal squamous cell carcinoma. Oncol Rep. 2008;20:1345–1351. [PubMed] [Google Scholar]
- 14.Shimada Y, Imamura M, Wagata T, Yamaguchi N, Tobe T. Characterization of 21 newly established esophageal cancer cell lines. Cancer. 1992;69:277–284. doi: 10.1002/1097-0142(19920115)69:2<277::aid-cncr2820690202>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Ma G, Jing C, Li L, Huang F, Ding F, Wang B, Lin D, Luo A, Liu Z. MicroRNA-92b represses invasion-metastasis cascade of esophageal squamous cell carcinoma. Oncotarget. 2016;7:20209–20222. doi: 10.18632/oncotarget.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Ma G, Jing C, Liu Z. Guanylate-binding protein 1 (GBP1) promotes lymph node metastasis in human esophageal squamous cell carcinoma. Discov Med. 2015;20:369–378. [PubMed] [Google Scholar]
- 17.Liu QZ, Gao XH, Chang WJ, Gong HF, Fu CG, Zhang W, Cao GW. Expression of ITGB1 predicts prognosis in colorectal cancer: a large prospective study based on tissue microarray. Int J Clin Exp Pathol. 2015;8:12802–12810. [PMC free article] [PubMed] [Google Scholar]
- 18.Tanabe K, Shinsato Y, Furukawa T, Kita Y, Hatanaka K, Minami K, Kawahara K, Yamamoto M, Baba K, Mori S, Uchikado Y, Maemura K, Tanimoto A, Natsugoe S. Filamin C promotes lymphatic invasion and lymphatic metastasis and increases cell motility by regulating Rho GTPase in esophageal squamous cell carcinoma. Oncotarget. 2017;8:6353–6363. doi: 10.18632/oncotarget.14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen MB, Lamar JM, Li R, Hynes RO, Kamm RD. Elucidation of the roles of tumor integrin beta1 in the extravasation stage of the metastasis cascade. Cancer Res. 2016;76:2513–2524. doi: 10.1158/0008-5472.CAN-15-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14:159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 21.Han S, Li Z, Master LM, Master ZW, Wu A. Exogenous IGFBP-2 promotes proliferation, invasion, and chemoresistance to temozolomide in glioma cells via the integrin beta1-ERK pathway. Br J Cancer. 2014;111:1400–1409. doi: 10.1038/bjc.2014.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, Li Y, Dang YZ, Gao HX, Jiang JL, Chen ZN. HAb18G/CD147 promotes radioresistance in hepatocellular carcinoma cells: a potential role for integrin beta1 signaling. Mol Cancer Ther. 2015;14:553–563. doi: 10.1158/1535-7163.MCT-14-0618. [DOI] [PubMed] [Google Scholar]
- 23.Eke I, Zscheppang K, Dickreuter E, Hickmann L, Mazzeo E, Unger K, Krause M, Cordes N. Simultaneous beta1 integrin-EGFR targeting and radiosensitization of human head and neck cancer. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/dju419. [DOI] [PubMed] [Google Scholar]
- 24.Li LY, Jiang H, Xie YM, Liao LD, Cao HH, Xu XE, Chen B, Zeng FM, Zhang YL, Du ZP, Chen H, Huang W, Jia W, Zheng W, Xie JJ, Li EM, Xu LY. Macrolide analog F806 suppresses esophageal squamous cell carcinoma (ESCC) by blocking beta1 integrin activation. Oncotarget. 2015;6:15940–15952. doi: 10.18632/oncotarget.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]


