Abstract
Clear evidence has linked obesity to a high risk of incidence as well as poor clinical outcome of breast cancer. It has been proven that changes in the levels of adipokines caused by obesity are associated with the initiation and progression of breast cancer. Resistin is a novel adipokine that is upregulated in breast cancer patients and promotes breast cancer cell growth, invasion, and migration. The aim of the study was to investigate whether resistin affected the efficacy of doxorubicin (Dox), one of the most effective anthracycline chemotherapeutic agents in the treatment of breast cancer. Treatment with resistin significantly attenuated Dox-induced apoptosis in a dose- and time-dependent manner, resulting in an increase in breast cancer cells survival. Moreover, resistin significantly induced autophagy flux and inhibition of autophagy abrogated the pro-survival effect of resistin in doxorubicin-treated cells. Furthermore, the AMPK/mTOR/ULK1 and JNK signaling pathways were activated by resistin treatment. Inhibition of these two pathways markedly reduced the ratio of LC3B-II/LC3B-I and increased cell apoptosis induced by Dox. For the first time, our findings indicate that resistin confers resistance to doxorubicin-induced apoptosis through autophagy induction and that this process involves the activation of AMPK/mTOR/ULK1 and JNK signaling pathways. Our findings suggest that resistin antagonism may be a novel strategy to overcome resistance to doxorubicin-based chemotherapy in breast cancer patients.
Keywords: Resistin, doxorubicin, apoptosis, breast cancer, autophagy, drug resistance
Introduction
Breast cancer is the most frequently diagnosed cancer in women worldwide [1]. With the improvement in the combination of surgery, chemotherapy, radiotherapy, and hormonal therapy, the mortality rate of breast cancer has decreased by 34% since 1990 [2]. Doxorubicin (Dox) is a US Food and Drug Administration (FDA)-approved chemotherapeutic drug that has been routinely used to treat hematological cancers and several types of solid tumors. In breast cancer, Dox-containing adjuvant chemotherapy has been recommended as the first-line treatment in the 2016 NCCN’s breast cancer guidelines [3]. Dox induces DNA damage and results in an increase in the mitochondrial membrane permeability, which causes the release of cytochrome c from mitochondria to the cytosol, where it activates the caspase family of proteases, leading to cancer cell apoptosis [4]. However, resistance to Dox remains a major obstacle to a better treatment outcome for many patients. Identification of factors that contribute to resistance to Dox may open a new pathway to improving the treatment efficacy of Dox-containing adjuvant chemotherapy.
Obesity not only increases the risk for metabolic, cardiovascular and chronic inflammatory diseases, but also is associated with increased risk of most cancers and with poor outcome of breast cancer [5,6]. Increasing studies have indicated that adipokines secreted by adipose tissue may be responsible for this association [7-12]. Resistin, a novel adipokine secreted by adipocytes, macrophages, and bone marrow cells, has been originally implicated in the pathogenesis of obesity-mediated insulin resistance. Recent studies have found that resistin may be a potential mediator in many cancers including breast, colorectal, lung, and prostate cancers [13-17]. A higher level of serum resistin was observed in patients with breast cancer than in normal controls [13,18]. In addition, high level of resistin in breast cancer was found to be associated with increased tumor stage, size, lymph node metastasis and poor prognosis [19]. Furthermore, serum resistin level significantly increased following systemic treatment in breast cancer patients [20], suggesting that resistin may affect the efficacy of chemotherapy. Several studies have reported that resistin plays a crucial role in the growth, invasion and migration of breast cancer cell [19,21,22]. However, there is no previous study investigating a causal relationship between resistin and chemoresistance.
Autophagy is an evolutionarily conserved self-degradation process by which cells degrade and renew cellular molecules and organelles. During autophagy, parts of the cytoplasm and cellular organelles are engulfed within a double-membrane vesicle known as autophagosome. The autophagosomes then fuse with lysosomes and their contents are degraded by lysosomal proteases [23]. Autophagy is considered as a protective mechanism by which cells eliminate unwanted or damaged materials to prevent carcinogenesis. However, tumor cells can utilize autophagy to survive cellular stress, such as hypoxia, nutrition deficiency, and chemotherapy [24,25]. Recent studies have shown that induction of autophagy facilitates cancer cells resistance to drug-induced apoptosis [26,27]. In the present study, we aim to understand the role of resistin in the resistance to Dox in breast cancer cells. We show that treatment with resistin protects breast cancer cells from Dox-induced apoptosis through autophagy induction. Furthermore, we demonstrate that activation of AMPK/mTOR and JNK signaling pathways are involved in autophagy induction. Our findings provide novel insights into how resistin contributes to Dox resistance and also suggest that resistin antagonism could be a strategy to overcome Dox resistance in breast cancer patients.
Materials and methods
Cell lines and cell culture
Human breast cancer cell lines MCF-7 and MDA-MB-231 were obtained from the American Type Culture Collection (ATCC). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum and maintained at 37°C in a humidified atmosphere of 5% CO2.
Antibodies and reagents
Recombinant human resistin was purchased from the PeproTech (Rocky Hill, NJ, USA). The antibodies against cytochrome c, caspase-9, poly (ADP-ribose) polymerase (PARP), BECN1, SQSTM1, LC3B I/II, LAMP1, Atg5, phosphorylated (p)-ULK1 (Ser757), p-AMPK, p-mTOR, p-JNK (Thr183/Tyr185), and p-Bcl-2 were purchased from the Cell Signaling Technology (Danvers, MA, USA); the antibody against β-actin, Dox, Compound C and SP600125 from Sigma-Aldrich Corp (St. Louis, MO, USA); and the Alexa Fluor-488-conjugated anti-rabbit IgG antibody from the Molecular Probes.
Lentiviral infection of breast cancer cells with short-hairpin RNA (shRNA)
MCF-7 and MDA-MB-231 cells were infected with recombinant lentivirus containing non-target shRNA or human Atg5 (Sigma-Aldrich) according to the manufacturer’s protocol. Puromycin was used to select stably expressed cells.
Flow cytometry analysis of cell apoptosis
MCF-7 and MDA-MB-231 cells were treated with Dox (1, 2.5, 5 μM), with or without resistin (10-50 ng/mL), for indicated time. Apoptosis of treated cells was detected by annexin V-FITC/propidium iodide (PI) staining according to the manufacturer’s instructions. After 20 minutes of incubation at room temperature, cells were analyzed using a BD flow cytometer. The percentage of apoptotic cells was the sum of the percentage of annexin V-positive/PI-negative and both annexin V and PI-positive cells.
Western blot analyses
The treated cells were washed with PBS, lysed with the RIPA buffer (Sigma-Aldrich Corp) and protease inhibitor cocktail at 4°C for 20 min. After centrifuging at 13,000 rpm for 15 min at 4°C, the supernatant was collected as cell lysates. Protein concentrations were measured using a Pierce BCA Protein Assay kit (Thermo-Fisher Scientific Inc.). After mixed with 4 × loading buffer, cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and blocked with 5% milk for 1 h at room temperature. The membranes were then incubated overnight at 4°C with specific primary antibodies. After washing with TBS-T, the membranes were incubated with HRP-conjugated appropriate secondary antibodies at room temperature for 1 h. The membranes were developed using the ECL Western blotting system (Thermo Fisher Scientific Inc.) according to the manufacturer’s instruction.
Immunofluorescence
MCF-7 and MDA-MB-231 cells were seeded on glass coverslips and cultured in the presence of resistin for indicated time. The cells were fixed with 4% paraformaldehyde for 15 minutes at room temperature. Cells were rinsed with PBS twice and then permeabilized with 0.1% Triton X-100 in PBS for 20 minutes and blocked with 1% BSA in PBS for 1 h. Cells were incubated with the primary antibody (anti-LC3) diluted in 1% BSA overnight at 4°C. After rinsing in PBS three times, cells were incubated with Alexa Fluor-488-conjugated secondary anti-rabbit IgG antibody for 1 h at room temperature, then incubated with DAPI for 5 min. Slides were rinsed and mounted. Images were acquired using a fluorescence microscope.
Statistical analyses
All data are shown as means ± standard deviation for at least three independent experiments performed in triplicate. The Student t-test was used to compare experimental groups. A P value < 0.05 was considered statistically significant.
Results
Resistin protects human breast cancer cells against Dox-induced apoptosis
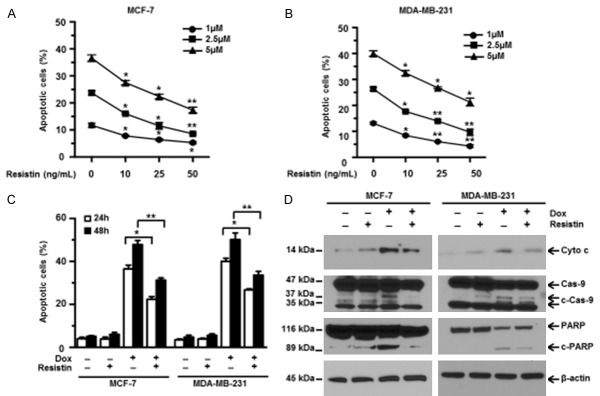
Dox has been known to exert its anticancer effects by inducing apoptosis. In order to study the effects of resistin on cells’ response to chemotherapy, human breast cancer MCF-7 and MDA-MB-231 cell lines were incubated in media containing different concentrations of Dox in the presence or absence of recombinant resistin for 24 h. As shown in Figure 1A and 1B, Dox effectively induced the apoptosis of both MCF-7 and MDA-MB-231 cells, which is consistent with previous reports [28,29]. Moreover, we found that the addition of resistin significantly decreased Dox-induced apoptosis of breast cancer cells in a dose-dependent manner (Figure 1A and 1B). Furthermore, we treated MCF-7 and MDA-MB-231 cells with 5 µM Dox, with or without 25 ng/mL resistin, for 24 and 48 h. Our results showed that resistin dramatically decreased apoptosis induced by Dox in a time-dependent manner (Figure 1C). It is well known that caspase cascades are the functional regulators and executioners of apoptosis [30]. Therefore, the treated cells were then harvested and subjected to western blot analyses of key modulators of apoptosis. As expected, MCF-7 and MDA-MB-231 cells treated with Dox alone had significantly higher levels of cytochrome c, cleaved caspase-9, and cleaved PARP than untreated cells, while addition of resistin significantly decreased the levels of these proteins in the presence of Dox (Figure 1D). These data demonstrate that resistin protects human breast cancer cells against Dox-induced apoptosis.
Figure 1.
Resistin protects breast cancer cells from doxorubicin-induced apoptosis in a dose- and time-dependent manner. Human breast cancer cell lines MCF-7 (A) and MDA-MB-231 (B) cells were treated with 1, 2.5, 5 μM doxorubicin (Dox) plus resistin (0, 10, 25 or 50 ng/mL) for 24 h. Apoptosis in the treated cells was determined by using an annexin V-binding assay. Shown are the percentages of apoptotic cells in both cell lines. (C) MCF-7 and MDA-MB-231 cells were cultured in media containing Dox (5 μM) with or without resistin (25 ng/mL) for 24 or 48 h. Cells cultured without Dox or resistin were used as controls. Percentages of apoptotic cells were shown. (D) MCF-7 and MDA-MB-231 cells were treated with Dox (5 μM) and/or resistin (25 ng/mL) for 24 h. Western blot analyses showed the expression levels of cytochrome c (Cyto c), cleaved caspase 9 (c-Cas-9) and PARP (c-PARP) in the cells. Cells cultured without treatments were used as controls. β-actin was used as a protein loading control. The sizes of protein bands are indicated on the left. Data are presented as mean ± SD from three independent experiments. *P < 0.05; **P < 0.01.
Resistin activates autophagy in human breast cancer cells
Previous findings indicate that autophagy activation inhibits caspase cleavage to induce chemotherapy resistance in cancer cells. To determine whether resistin affects autophagy in human breast cancer cells, we first detected the accumulation of LC3, a hallmark of mammalian autophagy, by immunofluorescence. Addition of resistin resulted in a remarkable increase in LC3 dots in MCF-7 and MDA-MB-231 cells (Figure 2A and 2B). Autophagy is orchestrated by a series of autophagy-related genes (ATGs) such as BECN1 (a critical autophagy-regulating protein), SQSTM1 (also known as p62, LC3-binding adaptor protein), and LAMP1 (lysosomal-associated membrane protein 1). The induction of autophagy is associated with up-regulation of LC3B-II, increased the ratio of LC3B-II to LC3B-I, and down-regulation of SQSTM1 [31-33]. These ATGs are commonly used to evaluate autophagy activity [23]. Thus, the effects of resistin on the expression levels of these ATGs were detected by western blot analyses. As shown in Figure 2C and 2D, addition of resistin dramatically increased the expression of LC3B-II, BECN1, LAMP1, and the ratio of LC3B-II to LC3B-I, and decreased the expression of SQSTM1 in a dose-dependent manner in MCF-7 and MDA-MB-231 cells. These results suggest that resistin activates autophagy in breast cancer cells.
Figure 2.
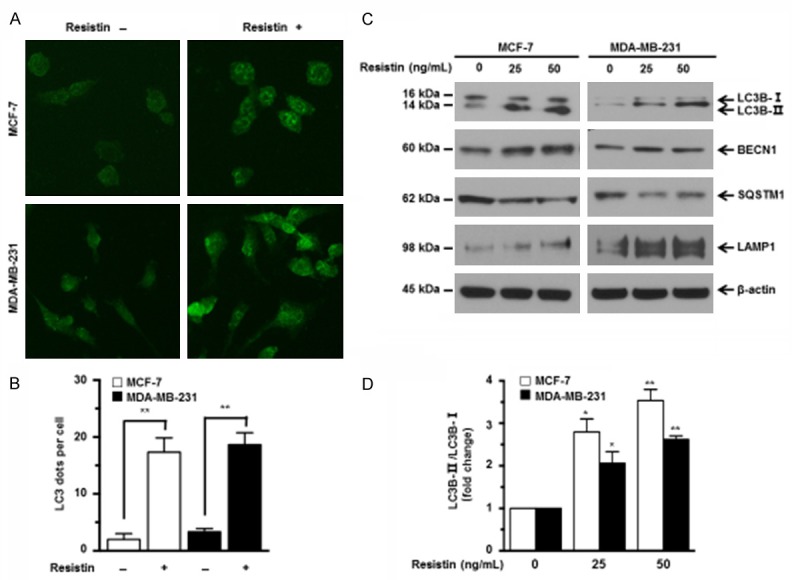
Resistin activates autophagy in breast cancer cells. (A) MCF-7 and MDA-MB-231 cells were treated without or with resistin (25 ng/mL) for 24 h, then fixed, permeabilized and stained for LC3 expression using a LC3-specific antibody. The LC3 dots were visualized using a fluorescence microscope. Representative images were shown. (B) Quantification of average LC3 dots per cell in (A) from three independent experiments. (C) MCF-7 and MDA-MB-231 cells were cultured in media containing various concentrations of resistin (0, 25 or 50 ng/mL) for 24 h. The expression levels of autophagy-related proteins (BECN1, SQSTM1, LC3B-I/II, LAMP1) were detected by western blot analyses. (D) Quantitative analysis of LC3B-II/LC3B-I ratio in (C). β-actin was used as a protein loading control. The sizes of protein bands are indicated on the left. Data are presented as mean ± SD from three independent experiments. *P < 0.05; **P < 0.01. Data are presented as mean ± SD. *P < 0.05; **P < 0.01.
Autophagy induced by resistin confers Dox resistance in breast cancer cells
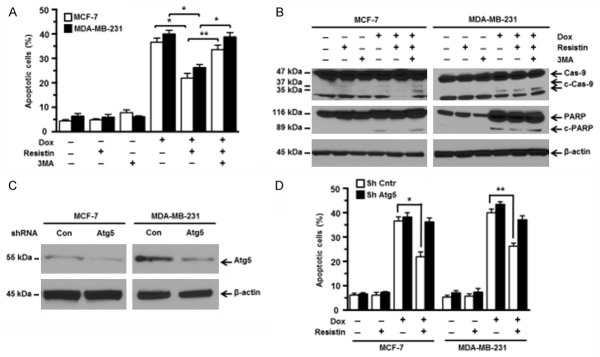
To further confirm that resistin-induced resistance to Dox was mediated through activated autophagy, we added 3-methyladenine (3-MA), a specific autophagy inhibitor, to the cell culture containing Dox and/or resistin. As shown in Figure 3A, 3-MA at 2 mM had no cytotoxic effects on MCF-7 and MDA-MB-231 cells according to the flow cytometry results. As expected, cells treated with 3-MA significantly increased the percentages of apoptotic cells compared with those treated with Dox and resistin (Figure 3A). Consistent with the flow cytometry results, western blot analyses also showed that addition of 3-MA increased the expression levels of cleaved caspase-9 and cleaved PARP proteins in Dox-treated MCF-7 and MDA-MB-231 cells, even in the presence of resistin (Figure 3B). We further knocked down the expression of Atg5 using a lentiviral vector containing shRNA of Atg5 in MCF-7 and MDA-MB-231 cells, as monitored by western blot analysis (Figure 3C). Then, wild type or Atg5 knocked down cells were treated with Dox and/or resistin. As shown in Figure 3D, knockdown of Atg5 abrogated the protective effect of resistin in Dox-treated cells. These results strongly suggest that resistin induces pro-survival autophagy to inhibit caspase activation and attenuate Dox-induced cell apoptosis.
Figure 3.
Autophagy induced by resistin confers doxorubicin resistance in breast cancer cells. MCF-7 and MDA-MB-231 cells were pretreated with autophagy inhibitor 3-methyladenine (3-MA; 2 mM) for 1 h and then cultured in media containing Dox (5 μM) with or without resistin (25 ng/mL) for 24 h. A. The percentages of apoptotic cells were determined by annexin V-binding assays. B. The expression levels of cleaved caspase-9 (c-Cas-9) and PARP (c-PARP) were detected by western blot analyses. MCF-7 and MDA-MB-231 cells were infected by lentivirus containing Atg5 shRNA or nontargeting control shRNA. Subsequently, cells were treated with Dox (5 μM) and/or resistin (25 ng/mL) for 24 h. C. The efficiency of Atg5 knockdown was analyzed by western blot analyses. D. Cell apoptosis was analyzed by annexin V-binding assays. β-actin was used as a protein loading control. The sizes of protein bands are indicated on the left Data are presented as mean ± SD. Results shown are representative of three independent experiments. *P < 0.05; **P < 0.01.
Resistin induces autophagy via AMPK/mTOR and JNK signaling
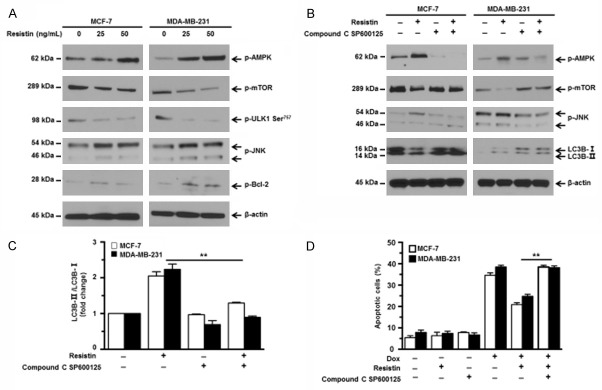
To elucidate mechanisms involved in the induction of autophagy, we focused on AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) and c-Jun N-terminal kinase (JNK) signaling pathways, both of which are of great importance in regulating autophagy [31,32]. AMPK is a negative regulator of mTOR which has been well documented as a major suppressor of autophagy. mTOR kinase prevents the autophagy-initiating kinase ULK1 activation by phosphorylating ULK1 at Ser757 to inhibit autophagy activation [31]. Western blot analyses showed that addition of resistin significantly upregulated the expression of p-AMPK and downregulated the expression of p-mTOR and p-ULK1 Ser757 in a dose-dependent manner (Figure 4A). It is well known that activated JNK can phosphorylate Bcl-2 to promote Bcl2-BECN1 complex dissociation, thereby releasing BECN1 and subsequently inducing autophagy [34]. Resistin was found to upregulate the expression of p-JNK and p-Bcl-2 in a dose dependent manner (Figure 4A). We further investigated whether the AMPK/mTOR/ULK1 and JNK activation accounted for the induction of autophagy. Compound C and SP600125 were used to inhibit AMPK and JNK activation, respectively. As shown in Figure 4B, these two inhibitors decreased resistin-induced AMPK and JNK activation and increased mTOR activation. Meanwhile, autophagy induced by resistin was abrogated by inhibitors, indicated by the expression levels of LC3B, as well as the ratio of LC3B-II/LC3B-I (Figure 4C). Furthermore, addition of inhibitors led to increased Dox-induced apoptosis in MCF-7 and MDA-MB-231 cells co-treated with resistin (Figure 4D). These data indicate that activation of AMPK/mTOR/ULK1 and JNK signalings are responsible for pro-survival autophagy induced by resistin.
Figure 4.
Resistin activates AMPK/mTOR and JNK signaling pathways. (A) MCF-7 and MDA-MB-231 cells were treated with various concentrations of resistin (0, 25 or 50 ng/mL) for 12 h. The expression levels of phosphorylated (p)-AMPK, p-mTOR, p-ULK1 Ser757, p-JNK (Thr183/Tyr185) and p-Bcl-2 were detected by western blot analyses, using specific antibodies. (B) MCF-7 and MDA-MB-231 cells were pretreated with Compound C (5 μM) and SP600125 (10 μM) for 1 h and then cultured with or without resistin (25 ng/mL) for 12 h. The expression levels of p-m AMPK, p-mTOR, p-JNK (Thr183/Tyr185), and LC3B-I/II were determined by western blot analysis. (C) Quantitative analysis of LC3B-II/LC3B-I ratio in (B) was shown. (D) MCF-7 and MDA-MB-231 cells were pretreated with Compound C (5 μM) and SP600125 (10 μM) for 1 h and then cultured in media containing Dox (5 μM) with or without resistin (25 ng/mL) for 24 h. Cell apoptosis was evaluated by an annexin V-binding assay. β-actin was used as a protein loading control. The sizes of protein bands are indicated on the left. Data are presented as mean ± SD. *P < 0.05; **P < 0.01.
Discussion
Association of resistin with breast cancer has been reported previously. Resistin is shown to promote cell growth and invasiveness through increased STAT3 expression and phosphorylation [21]. It is also found to induce invasion and migration through p-c-Src [22]. Moreover, an inverse correlation of resistin expression with disease-free and survival rates has been demonstrated in breast cancer patients [19]. However, the effect of resistin on cell’s response to chemotherapy-induced apoptosis remains unknown. For the first time, our study has uncovered a new role of resistin in promoting the chemoresistance in breast cancer cells.
One largely studied factor promoting cancer cell resistance against chemotherapy is the development of aberrant apoptosis [35]. Dox, a widely used drug to treat breast cancer, functions as an apoptosis inducer [36]. We experimentally showed that resistin inhibited Dox-induced apoptosis of MCF-7 and MDA-MB-231 cells in a dose- and time-dependent manner through flow cytometry and western blot analyses (Figure 1).
Autophagy has been implied as a mechanism that reduces the sensitivity of cancer cells towards chemotherapy by reducing drug’s apoptosis potential [26,37,38]. Recent studies showed that induction of autophagy contributes to chemoresistance against cisplatin, Dox, and many other drugs [26,27]. Moreover, recent study showed that adipocytes-secreted adipokines such as leptin and adipsin suppressed chemotherapy-induced apoptosis in myeloma cells through autophagy activation [39]. In our study, we clearly observed that resistin induced autophagy activation evidenced by an increase dots of LC3 fluorescence in breast cancer cells (Figure 2A and 2B). We further confirmed the induction of autophagy by resistin using western blot analysis. Western blot result demonstrated the conversion of the molecular form of LC3B-I to LC3B-II, the upregulation of BECN1 and LAMP1 expression, and the downregulation of SQSTM1 expression, which are commonly used markers for detecting autophagy (Figure 2C and 2D). To further investigate what role autophagy plays in Dox-induced apoptosis, we used 3-MA to inhibit autophagy. Flow cytometry and western blot analyses revealed that cell apoptosis was increased in Dox+resistin+3-MA group compared with that in the Dox+resistin group, revealing that induction of autophagy by resistin protected breast cancer cells from Dox-induced apoptosis (Figure 3A and 3B). Moreover, the shRNA approach was used to further confirm the effect of autophagy. Atg 5 (a key autophagy regulator) was knocked down (Figure 3C). We observed that knockdown of Atg5 abolished the protective effect of resistin against Dox-induced apoptosis compared with the shRNA control group (Figure 3D). Taken together, these data indicate that autophagy induced by resistin contributes to Dox resistance in breast cancer cells.
We further studied the molecular mechanism underlying the induction of autophagy by resistin in breast cancer. AMPK/mTOR signaling pathway plays a crucial role in activating autophagy. mTOR is one of the most important regulator which prevents autophagy-initiating kinase ULK1 activation by phosphorylating ULK1 at Ser757 to inhibit autophagy activation [31]. AMPK, a major metabolic energy sensor, is a negative regulator of mTOR. Activated AMPK inhibits mTOR to relieve Ser757 phosphorylation, leading to ULK1-AMPK interaction to stimulate autophagy [31,33]. Our results showed that resistin activated AMPK/mTOR/ULK1 signaling pathway in breast cancer cells, as evidenced by the downregulation of p-mTOR and p-ULK1 expression, and upregulation of p-AMPK expression (Figure 4A). Another important signaling molecule involved in the regulation of autophagy is JNK kinase. Previous findings suggest that JNK kinase can regulate the anti-autophagy activity of Bcl-2 via phosphorylation, leading to Bcl-2 dissociation from BECN1 [34,40]. BECN1-associated class III PI3K activity then stimulates autophagy. Similarly, we found that resistin activated JNK and JNK-mediated phosphorylation of Bcl-2 (Figure 4A). In addition, the AMPK inhibitor compound C and JNK inhibitor SP600125 abolished resistin-induced autophagy, as shown by the decreased ratio of LC3B-II/LC3B-I (Figure 4B and 4C). Moreover, these two inhibitors attenuated the protection of resistin against Dox-induced apoptosis (Figure 4D). These findings suggested that autophagy induction by resistin was due to activation of AMPK/mTOR/ULK1 and JNK signaling pathway.
Although we have demonstrated the functional role of resistin in Dox resistance through the aforementioned experiments, there is still work that needs to be done in future studies. We have shown the protective role of resistin in breast cancer cell lines, but we did not determine which resistin receptor is involved. Our experiments were all performed in an in vitro setting and we will need to investigate the effect of resistin in in vivo animal models.
In conclusion, we have shown that adipokine resistin is able to attenuate Dox-induced apoptosis through autophagy induction. This finding suggests that upregulated levels of resistin in breast cancer patients could facilitate the chemoresistance of cancer cells. Thus, our study reveals a new role of resistin in breast cancer, and also provides a potential molecular target to overcome chemoresistance in the treatment of breast cancer.
Acknowledgements
This study was supported in part by grants from the National Natural Science Foundation of China (Grant No. 30872194 and 81041098), National High Technology Research and Development Program of China (863 Program, Grant No. [2000]139) and Bethune Program B of Jilin University (Grant No. 2012217). D.X.L.’s research programme is supported by the New Zealand Breast Cancer Foundation and the Breast Cancer Cure charities.
Disclosure of conflict of interest
None.
Authors’ contribution
Conceived and designed the experiments: ZL, AS, DS, DXL, ZF. Performed the experiments: ZL, LM, AS. Analyzed the data: ZL, LM, AS, BH, ZZ. Wrote the paper: ZL, DXL, ZF.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 3.Gradishar W, Salerno KE. NCCN guidelines update: breast cancer. J Natl Compr Canc Netw. 2016;14:641–644. doi: 10.6004/jnccn.2016.0181. [DOI] [PubMed] [Google Scholar]
- 4.Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65:157–170. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U. S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.Dal Maso L, Zucchetto A, Talamini R, Serraino D, Stocco CF, Vercelli M, Falcini F, Franceschi S Prospective Analysis of Case-control studies on Environmental factors and health (PACE) study group. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123:2188–2194. doi: 10.1002/ijc.23747. [DOI] [PubMed] [Google Scholar]
- 7.Lorincz AM, Sukumar S. Molecular links between obesity and breast cancer. Endocr Relat Cancer. 2006;13:279–292. doi: 10.1677/erc.1.00729. [DOI] [PubMed] [Google Scholar]
- 8.Perera CN, Chin HG, Duru N, Camarillo IG. Leptin-regulated gene expression in MCF-7 breast cancer cells: mechanistic insights into leptin-regulated mammary tumor growth and progression. J Endocrinol. 2008;199:221–233. doi: 10.1677/JOE-08-0215. [DOI] [PubMed] [Google Scholar]
- 9.Pfeiler GH, Buechler C, Neumeier M, Schaffler A, Schmitz G, Ortmann O, Treeck O. Adiponectin effects on human breast cancer cells are dependent on 17-beta estradiol. Oncol Rep. 2008;19:787–793. [PubMed] [Google Scholar]
- 10.Nkhata KJ, Ray A, Schuster TF, Grossmann ME, Cleary MP. Effects of adiponectin and leptin co-treatment on human breast cancer cell growth. Oncol Rep. 2009;21:1611–1619. doi: 10.3892/or_00000395. [DOI] [PubMed] [Google Scholar]
- 11.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J. Clin. Oncol. 2010;28:4058–4065. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fasshauer M, Bluher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015;36:461–470. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Kang JH, Yu BY, Youn DS. Relationship of serum adiponectin and resistin levels with breast cancer risk. J Korean Med Sci. 2007;22:117–121. doi: 10.3346/jkms.2007.22.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiaka EK, Manolakis AC, Kapsoritakis AN, Potamianos SP. The implication of adiponectin and resistin in gastrointestinal diseases. Cytokine Growth Factor Rev. 2011;22:109–119. doi: 10.1016/j.cytogfr.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Kuo CH, Chen KF, Chou SH, Huang YF, Wu CY, Cheng DE, Chen YW, Yang CJ, Hung JY, Huang MS. Lung tumor-associated dendritic cell-derived resistin promoted cancer progression by increasing Wolf-Hirschhorn syndrome candidate 1/Twist pathway. Carcinogenesis. 2013;34:2600–2609. doi: 10.1093/carcin/bgt281. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Lee YS, Won EH, Chang IH, Kim TH, Park ES, Kim MK, Kim W, Myung SC. Expression of resistin in the prostate and its stimulatory effect on prostate cancer cell proliferation. BJU Int. 2011;108:E77–83. doi: 10.1111/j.1464-410X.2010.09813.x. [DOI] [PubMed] [Google Scholar]
- 17.Fain JN, Cheema PS, Bahouth SW, Lloyd Hiler M. Resistin release by human adipose tissue explants in primary culture. Biochem Biophys Res Commun. 2003;300:674–678. doi: 10.1016/s0006-291x(02)02864-4. [DOI] [PubMed] [Google Scholar]
- 18.Sun CA, Wu MH, Chu CH, Chou YC, Hsu GC, Yang T, Chou WY, Yu CP, Yu JC. Adipocytokine resistin and breast cancer risk. Breast Cancer Res Treat. 2010;123:869–876. doi: 10.1007/s10549-010-0792-4. [DOI] [PubMed] [Google Scholar]
- 19.Lee YC, Chen YJ, Wu CC, Lo S, Hou MF, Yuan SS. Resistin expression in breast cancer tissue as a marker of prognosis and hormone therapy stratification. Gynecol Oncol. 2012;125:742–750. doi: 10.1016/j.ygyno.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 20.Coskun T, Kosova F, Ari Z, Sakarya A, Kaya Y. Effect of oncological treatment on serum adipocytokine levels in patients with stage II-III breast cancer. Mol Clin Oncol. 2016;4:893–897. doi: 10.3892/mco.2016.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deshmukh SK, Srivastava SK, Bhardwaj A, Singh AP, Tyagi N, Marimuthu S, Dyess DL, Dal Zotto V, Carter JE, Singh S. Resistin and interleukin-6 exhibit racially-disparate expression in breast cancer patients, display molecular association and promote growth and aggressiveness of tumor cells through STAT3 activation. Oncotarget. 2015;6:11231–11241. doi: 10.18632/oncotarget.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JO, Kim N, Lee HJ, Lee YW, Kim SJ, Park SH, Kim HS. Resistin, a fat-derived secretory factor, promotes metastasis of MDA-MB-231 human breast cancer cells through ERM activation. Sci Rep. 2016;6:18923. doi: 10.1038/srep18923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Li G, Zheng Y, Shen HM, Hu X, Ming QL, Huang C, Li P, Gao N. A novel autophagy/mitophagy inhibitor liensinine sensitizes breast cancer cells to chemotherapy through DNM1L-mediated mitochondrial fission. Autophagy. 2015;11:1259–1279. doi: 10.1080/15548627.2015.1056970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Notte A, Ninane N, Arnould T, Michiels C. Hypoxia counteracts taxol-induced apoptosis in MDA-MB-231 breast cancer cells: role of autophagy and JNK activation. Cell Death Dis. 2013;4:e638. doi: 10.1038/cddis.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanno S, Yomogida S, Tomizawa A, Yamazaki H, Ukai K, Mangindaan RE, Namikoshi M, Ishikawa M. Papuamine causes autophagy following the reduction of cell survival through mitochondrial damage and JNK activation in MCF-7 human breast cancer cells. Int J Oncol. 2013;43:1413–1419. doi: 10.3892/ijo.2013.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su YC, Davuluri GV, Chen CH, Shiau DC, Chen CC, Chen CL, Lin YS, Chang CP. Galectin-1-induced autophagy facilitates cisplatin resistance of hepatocellular carcinoma. PLoS One. 2016;11:e0148408. doi: 10.1371/journal.pone.0148408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fong MY, Jin S, Rane M, Singh RK, Gupta R, Kakar SS. Withaferin A synergizes the therapeutic effect of doxorubicin through ROS-mediated autophagy in ovarian cancer. PLoS One. 2012;7:e42265. doi: 10.1371/journal.pone.0042265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buranrat B, Connor JR. Cytoprotective effects of ferritin on doxorubicin-induced breast cancer cell death. Oncol Rep. 2015;34:2790–2796. doi: 10.3892/or.2015.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Lu C, Li Q, Xie J, Chen T, Tan Y, Wu C, Jiang J. The role of Kif4A in doxorubicin-induced apoptosis in breast cancer cells. Mol Cells. 2014;37:812–818. doi: 10.14348/molcells.2014.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding AX, Sun G, Argaw YG, Wong JO, Easwaran S, Montell DJ. CasExpress reveals widespread and diverse patterns of cell survival of caspase-3 activation during development in vivo. Elife. 2016:5. doi: 10.7554/eLife.10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang S, Yang ZJ, Yu C, Sinicrope FA. Inhibition of mTOR kinase by AZD8055 can antagonize chemotherapy-induced cell death through autophagy induction and down-regulation of p62/sequestosome 1. J Biol Chem. 2011;286:40002–40012. doi: 10.1074/jbc.M111.297432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karanasios E, Walker SA, Okkenhaug H, Manifava M, Hummel E, Zimmermann H, Ahmed Q, Domart MC, Collinson L, Ktistakis NT. Autophagy initiation by ULK complex assembly on ER tubulovesicular regions marked by ATG9 vesicles. Nat Commun. 2016;7:12420. doi: 10.1038/ncomms12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramos P, Bentires-Alj M. Mechanism-based cancer therapy: resistance to therapy, therapy for resistance. Oncogene. 2015;34:3617–3626. doi: 10.1038/onc.2014.314. [DOI] [PubMed] [Google Scholar]
- 36.Pilco-Ferreto N, Calaf GM. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int J Oncol. 2016;49:753–762. doi: 10.3892/ijo.2016.3558. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Yu H, Qin H, Kang J, Yu C, Zhong J, Su J, Li H, Sun L. Inhibition of autophagy enhances cisplatin cytotoxicity through endoplasmic reticulum stress in human cervical cancer cells. Cancer Lett. 2012;314:232–243. doi: 10.1016/j.canlet.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 38.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z, Xu J, He J, Liu H, Lin P, Wan X, Navone NM, Tong Q, Kwak LW, Orlowski RZ, Yang J. Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget. 2015;6:34329–34341. doi: 10.18632/oncotarget.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei Y, Sinha S, Levine B. Dual role of JNK1-mediated phosphorylation of Bcl-2 in autophagy and apoptosis regulation. Autophagy. 2008;4:949–951. doi: 10.4161/auto.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]