Abstract
Abstract
Three new drimane sesquiterpenoids (1–3) together with the known 2α-hydroxyisodrimeninol (4), and a new isochromone derivative (5), were obtained from the solid cultures of fungal strain Pestalotiopsis sp. M-23, an endophytic fungus isolated from the leaves of Leucosceptrum canum (Labiatae). Their structures were determined by comprehensive 1D and 2D NMR, and MS analyses. The metabolites were evaluated for their antibacterial activities, and compound 3 showed weak inhibitory activity against Bacillus subtilis.
Graphical Abstract
Electronic supplementary material
The online version of this article (doi:10.1007/s13659-016-0094-6) contains supplementary material, which is available to authorized users.
Keywords: Endophytic fungi, Pestalotiopsis sp. M-23, Leucosceptrum canum, Drimane sesquiterpenoids, Antibacterial activity
Introduction
Fungal endophytes are ubiquitous fungi that inhabit healthy plant tissues without causing apparent symptoms of disease, and their colonizations have been found in almost all kinds of plants, from algae to vascular plants [1]. Endophytic fungi have gained prominence as important sources of a variety of new biologically active natural products [2]. For example, the famous anticancer drug paclitaxel can be produced by the endophytic fungus Taxomyces andreance from Pacific yew [3]. An endophytic fungus Thielavia subthermophila [4] from the medicinal herb Hypericum perforatum has been reported to produce the antidepressant napthodianthrone derivative hypericin [5].
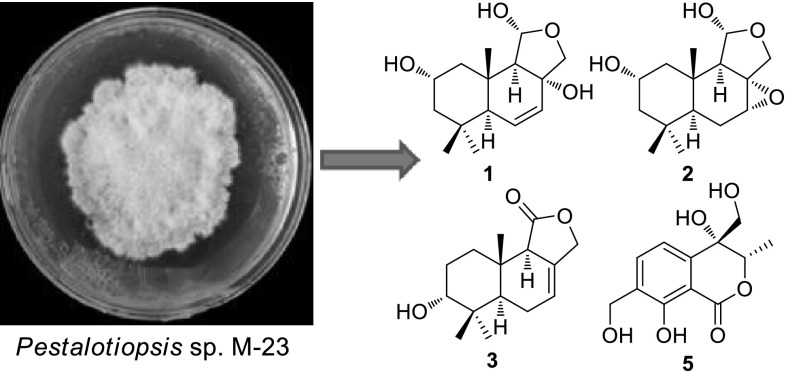
The fungal genus Pestalotiopsis, representing one of the largest biomasses of any plant-associated endophytic fungus in the world, produces a large variety of secondary metabolites, including terpenoids, alkaloids, chromone derivatives and phenolics [6], with cytotoxic [7], antibacterial and antifungal activities [8, 9]. In this study, an endophytic fungal strain, Pestalotiopsis sp. M-23, was isolated from the leaves of Leucosceptrum canum, a woody Labiatae (=Lamiaceae) plant with unique dark-brown colored nectar that has been proved to be caused by a novel bird attractant proline-benzoquinone, and with a unique class of sesterterpenoids named leucosceptroids that have been shown to function as defense against attack by insect herbivores and pathogens [10–14]. In this study, a phytochemical investigation on the solid culture of the endophytic fungus Pestalotiopsis sp. M-23 was carried out, which led to the isolation and identification of five compounds (1–5), including three new drimane sesquiterpenoids (1–3) and a new isochromone derivative (5). In addition, the antibacterial activities of these compounds were also evaluated.
Results and Discussion
More than one hundred endophytic fungal strains were isolated from the leaves of L. canum. Among them, M-23 was identified as Pestalotiopsis sp. using molecular biological techniques [15]. To carry out phytochemical investigation, the mycelia and solid culture media of Pestalotiopsis sp. M-23 were extracted with acetone, and the crude extract was fractionated by column chromatography on silica gel. Further purification was performed by repeated normal-phase, Sephadex LH-20, ODS column chromatographies, and reverse-phase semi-preparative HPLC to yield five compounds (1–5).
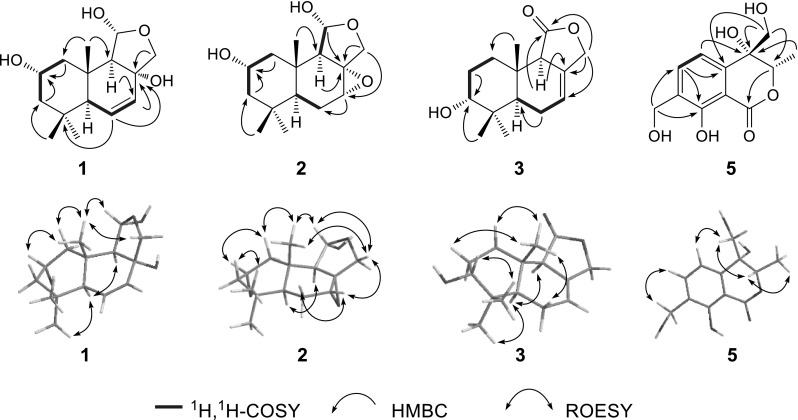
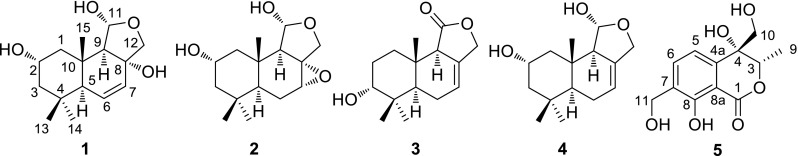
Compound 1 was isolated as colorless oil. Its molecular formula was established as C15H24O4 by its HR-ESI-MS (m/z 291.1570, [M + Na]+) and 13C NMR spectroscopic data. The IR spectrum showed typical absorptions at 1630 and 3425 cm−1 for double bond and hydroxyl groups, respectively. In the 1H NMR spectrum (Table 1), three tertiary methyl resonances at δ H 1.01, 0.94, and 0.88 (each 3H, s) were clearly shown. A pair of AB doublets was observed at δ H 4.08 (d, J = 9.4 Hz) and 3.70 (d, J = 9.4 Hz), indicating the presence of an oxygenated methylene group. An AMX system was observed at δ H 5.88 (dd, J = 9.8, 1.7 Hz), 5.78 (dd, J = 9.8, 2.8 Hz) and 1.93 (dd, J = 2.8, 1.7 Hz), implying the presence of an endo-double bond. The 13C NMR spectrum (Table 1) showed the presence of 15 carbon resonances which were classified using DEPT experiments into three methyls (δ C at 33.4, 23.0, and 15.8), three methylenes (including one oxygenated methylene at δ C 80.9), six methines (including a double bond group at δ C 130.4 and 129.4; an oxymethine δ C 64.8; and a hemiacetal methine at δ C 101.7), and three quaternary carbons (including an oxygen-bearing carbon at δ C 79.0). These data suggested that 1 was a drimane sesquiterpenoid [16, 17]. Comparing the NMR spectra (Table 1) of 1 with those of 2α-hydroxyisodrimeninol (4) [17, 18], a drimane sesquiterpenoid also isolated from this fungus, revealed that the two compounds were similar. Compound 1 showed the typical hemiacetal and oxymethine resonances as those in 4. The major difference between these two compounds was that the tri-substituted double bond in 4 was replaced by a di-substituted double bond located between C-6 and C-7 in 1, which was confirmed by the 1H-1H COSY correlations of H-6 (δ H 5.88) with H-5 (δ H 1.93) and H-7 (δ H 5.78). An oxygenated quaternary carbon was found to occur at C-8 (δ C 79.0) in 1 due to the HMBC correlations from H-7 (δ H 5.78), H-9 (δ H 2.07), and H2-12 (δ H 3.70 and 4.08) to C-8 (Fig. 2). The relative configuration of 1 was deduced from the results of ROESY experiment and comparison with the data in literature [16, 17]. The ROESY correlations of Me-15 (δ H 0.88) with H-2 (δ H 3.91), H-11 (δ H 5.37) and Ha-12 (δ H 3.70) revealed that these protons were β-oriented, and the ROESY correlation between H-5 (δ H 1.93) and H-9 (δ H 2.07) suggested their α-oriented configuration (Fig. 2). Consequently, the structure of 1 was determined as 2α,8α-dihydroxy-6,7-en-isodrimeninol (Fig. 1).
Table 1.
1H and 13C NMR data of 1–4 [δ in ppm; J in Hz]
| Position | 1 a | 2 a | 3 b | 4 b | ||||
|---|---|---|---|---|---|---|---|---|
| δ cH | δ dC | δ cH | δ dC | δ cH | δ eC | δ cH | δ eC | |
| 1α | 1.13 m | 48.2 t | 1.03 m | 49.8 t | 1.91 m | 31.3 t | 1.17 m | 49.9 t |
| 1β | 2.08 m | 2.02 m | 2.09 m | 2.13 overlap | ||||
| 2α | 3.91 m | 64.8 d | 3.74 m | 64.7 d | 1.57 m | 25.8 t | 3.84 m | 64.0 d |
| 2β | 1.94 m | |||||||
| 3α | 1.17 m | 51.0 t | 1.12 m | 51.9 t | 75.5 d | 1.17 m | 52.4 t | |
| 3β | 1.80 m | 1.71 overlap | 3.44 br s | 1.76 m | ||||
| 4 | – | 34.6 s | – | 35.1 s | – | 37.9 s | – | 35.0 s |
| 5 | 1.93 dd (2.8, 1.7) | 52.7 d | 0.93 overlap | 44.9 d | 1.89 m | 43.6 d | 1.28 m | 50.2 d |
| 6 | 5.88 dd (9.8, 1.7) | 130.4 d | 1.71 overlap | 23.7 d | 2.07 m | 23.5 t | 1.91 m | 24.2 t |
| 2.05 m | 2.11 m | 2.13 overlap | ||||||
| 7 | 5.78 dd (9.8, 2.8) | 129.4 d | 3.38 br s | 59.6 d | 5.79 br s | 121.3 d | 5.51 br s | 116.8 d |
| 8 | – | 79.0 s | – | 65.2 s | – | 131.6 s | – | 138.4 s |
| 9 | 2.07 br s | 70.2 d | 1.70 d (1.9) | 62.3 d | 2.94 s | 54.0 d | 2.23 d (3.0) | 62.5 d |
| 10 | – | 40.9 s | – | 36.0 s | – | 34.5 s | – | 35.6 s |
| 11 | 5.37 br s | 101.7 d | 5.32 d (1.9) | 100.1 d | – | 175.5 s | 5.22 d (3.0) | 99.5 d |
| 12a | 3.70 d (9.4) | 80.9 t | 3.75 d (10.0) | 68.0 t | 4.63 d (11.7) | 70.2 t | 4.06 d (11.3) | 68.5 t |
| 12b | 4.08 d (9.4) | 4.00 d (10.0) | 4.70 d (11.7) | 4.35 d (11.3) | ||||
| 13 | 0.94 s (3H) | 23.0 q | 0.97 s (3H) | 23.4 q | 0.93 s (3H) | 22.1 q | 0.91 s (3H) | 22.7 q |
| 14 | 1.01 s (3H) | 33.4 q | 0.92 s (3H) | 33.3 q | 0.96 s (3H) | 28.7 q | 0.94 s (3H) | 33.5 q |
| 15 | 0.88 s (3H) | 15.8 q | 0.92 s (3H) | 16.2 q | 0.86 s (3H) | 14.3 q | 0.85 s (3H) | 15.2 q |
| 3-OH | 3.52 br s | |||||||
aRecorded in CD3OD
bRecorded in acetone-d 6
cRecorded at 400 MHz
dRecorded at 150 MHz
eRecorded at 100 MHz
Fig. 2.
Major correlations in 2D NMR spectra of compounds 1–3 and 5
Fig. 1.
Chemical structures of compounds 1–5
Compound 2 was obtained as colorless oil. It gave a molecular formula of C15H24O4 according to 13C NMR spectroscopic and HR-EI-MS data (m/z 268.1677 [M]+), with 4° of unsaturation. The 1H and 13C NMR spectroscopic data (Table 1) were similar to those of 4, suggesting that 2 was also a drimane sesquiterpenoid with a hemiacetal moiety and a hydroxyl group located at C-11 and C-2, respectively. Major difference between these two compounds was that the resonances of double bond between C-7 and C-8 in 4 were missing, instead an oxymethine at δ C 59.6 and an oxygen-bearing quaternary carbon at δ C 65.2 appeared in 2. The HMBC correlations from H2-12 (δ H 3.75 and 4.00) to C-7 (δ C 59.6) and C-8 (δ C 65.2), and from H-7 (δ H 3.38) to C-6 (δ C 23.7) in 2, suggested the presence of an epoxide group between C-7 and C-8 in 2. The ROESY spectrum revealed that the relative configuration of 2 was similar to that of 4, and the ROESY correlation of H-7 (δ H 3.38) with H-12 (δ H 3.75) indicated that H-7 and H-12 were at β-position, and thus the epoxide ring occupied α-position (Fig. 2). Therefore, the structure of 2 was concluded as 2α-hydroxy-7α,8α-epoxy-isodrimeninol (Fig. 1).
Compound 3 exhibited an [M + Na]+ ion peak at m/z 273.1462 in the HR-ESI-MS, corresponding to the molecular formula C15H22O3 with 5° of unsaturation, suggesting that 3 was also a sesquiterpenoid. Comparing its 1D NMR spectra data (Table 1) with those of dendocarbin B [17] indicated that 3 had the similar drimane skeleton with an ester carbonyl group located at C-11 and a double bond between C-7 and C-8, which were further confirmed by analyzing the HMBC and HSQC spectra of 3. The oxymethine at δ C 75.5 in 3 obviously appeared at lower field than the one in dendocarbin B (δ C 63.6) [17], indicating that the position of the hydroxyl group shifted in 3. In the HMBC spectrum of 3, the correlations from Me-13 (δ H 0.93) and Me-14 (δ H 0.96) to the oxymethine carbon at δ C 75.5 indicated that the hydroxyl group was assignable to C-3. The coupling pattern of H-3 (δ H 3.44, br s) indicated an α-oriented configuration of 3-OH [19] (Fig. 2), which was supported by the ROESY cross-peak between H-3 and Me-13, and by the upfield shifted C-14 due to a γ-gauch effect from 3α-OH. Thus, the structure of 3 was established as 11-dehydro-3α-hydroxyisodrimeninol (Fig. 1).
Compound 5 was obtained as colorless oil and has a molecular formula of C12H14O6 as determined from HR-ESI-MS (m/z 249.1105 [M + Na]+), with 6° of unsaturation. In the 1H NMR spectrum (Table 2), a secondary methyl resonance at δ H 1.57 (d, J = 6.7 Hz, 3H) and an oxymethine group at δ H 5.46 (q, J = 6.7 Hz, 1H) were evident, indicating the existence of an AB3 system in 5. A singlet at δ H 5.17 (2H) and a pair of AB doublets at δ H 4.35 (d, J = 11.3 Hz, 1H) and 4.10 (d, J = 11.3 Hz, 1H) were clearly shown, revealing the presence of two oxygenated methylene groups. In the low-field region, two ortho-coupled aromatic doublets at δ H 7.65 (d, J = 7.6 Hz) and 8.19 (d, J = 7.6 Hz) were observed, indicative of a 1,2,3,4-tetra-substituted phenyl ring. The 13C NMR and DEPT spectra (Table 2) of 5 displayed the signals of one methyl group at δ C 15.3, two oxygenated methylene groups at δ C 58.7 and 67.3, two olefinic methine groups at δ C 116.6 and 134.6, and six quaternary carbons (including one ester carbonyl group at δ C 169.7; four olefinic carbons at δ C 107.5, 159.0, 142.0, and 131.0; and an oxygen-bearing carbon at δ C 72.6). These NMR data suggested the presence of an isochromenone derivative similar to gamahorin [20], which was isolated from the fungus Epichloe typhina. The HMBC correlations from H-5 (δ H 7.65) to the oxygen-bearing quaternary carbon (δ C 72.6), and from the AB doublets at δ H 4.35 and 4.10 to C-3 (δ C 79.2), C-4a (δ C 142.0), and the oxygen-bearing quaternary carbon (δ C 72.6), indicated the presence of hydroxyl groups at C-4 and C-10. In the ROESY spectrum, the correlations of H2-10 (δ H 4.35 and 4.10) with H-3 (δ H 5.46) indicated β-orientation of H2-10 and H-3 (Fig. 2). Therefore, the structure of 5 was determined as 4,10-dihydroxy-gamahorin (Fig. 1).
Table 2.
1H and 13C NMR data of 5 (in pyridine-d 5, at 600 and 150 MHz, resp.) [δ in ppm, J in Hz]
| Position | δ H | δ C | Position | δ H | δ C |
|---|---|---|---|---|---|
| 1 | – | 169.7 s | 8 | – | 159.0 s |
| 3 | 5.46 q (6.7) | 79.2 d | 8a | – | 107.5 s |
| 4 | – | 72.6 s | 9 | 1.57 d (6.7, 3H) | 15.3 q |
| 4a | – | 142.0 s | 10 | 4.35 d (11.3) | 67.3 t |
| 5 | 7.65 d (7.6) | 116.6 d | 4.10 d (11.3) | ||
| 6 | 8.19 d (7.6) | 134.6 d | 11 | 5.17 s (2H) | 58.7 t |
| 7 | – | 131.0 s |
A known drimane sesquiterpenoid was also isolated and identified as 2α-hydroxyisodrimeninol (4) [17, 18], by comparing its NMR data with those previously reported in the literature.
Dramine sesquiterpenoids have been shown to possess extensive biologically activities, such as antifeedant, anti-inflammatory, cytotoxic, antioxidant and α-amylase inhibitory activities [21–23]. Antibacterial activity of 1–5 against Staphylococcus aureus, Bacillus subtilis and Micrococcus luteus were evaluated in this study. Compound 3 showed weak inhibitory effect on B. subtilis with IC50 value of 280.27 μM. However, none of these compounds showed obvious activity against S. aureus and M. luteus. Despite, our results indicated that Pestalotiopsis sp. M-23 as a prolific resource of characteristic dramine sesquiterpenoids, is an interesting endophyte worthy of further in-depth investigation.
Experiments
General Experimental Procedures
Column chromatographies were performed on 200–300 mesh silica gel (Qingdao Marine Chemical Factory, P. R. China), Sephadex LH-20 (25–100 μm, GE Healthcare), and ODS (75 μm, YMC gel). Optical rotations were measured on a Horiba-SEAP-300 spectropolarimeter. UV spectral data were obtained on a Shimadzu-210A double-beam spectrophotometer. IR spectra were recorded on a Bruker-Tensor-27 spectrometer with KBr pellets. NMR experiments were carried out on either a Bruker AM-400 or an Avance-600 spectrometer with TMS as internal standard. ESI-MS and HR-ESI-MS were recorded on an API-QSTAR-TOF. EI-MS and HR-EI-MS were recorded on an Autospec Premier P776 mass spectrometer. Semi-preparative HPLC analyses were performed on an Agilent 1200 series instrument equipped with a quaternary pump, a vacuum degasser, an autosampler, a thermostated column compartment and a diode array detector.
Fungal Strain Isolation and Identification
Healthy, asymptomatic leaves of L. canum were harvested at Kunming Botanical Garden, P. R. China, in March 2014, and disinfected with 75 % EtOH for 10 s, and then rinsed with sterile distilled water for 5 times. The surface disinfected leaves were aseptically cut into 2 cm × 2 cm pieces, and cultivated in potato dextrose agar (PDA) plates containing 30 μg/mL streptomycin to inhibit the bacterial growth at 28 °C. A pure strain coded as M-23 was obtained by transferring monosporic isolates to fresh PDA gradually. M-23 was incubated in potato dextrose broth (PDB) at 28 °C in 100 mL Erlenmeyer flask for 3 days and shaking at 220 rpm.
Genomic DNA of M-23 was extracted from fungal mycelia (20 mg) grown in PBD using fungal DNA isolation mini kit (Sangon, Shanghai, China). The PCR reaction was performed using isolated genomic DNA as template, ITS5/ITS4 as primer pairs. The amplified DNA fragment was purified and sequenced using the same primer pairs by BGI Inc. The obtained DNA sequence data were searched for similar sequences in GenBank.
M-23 strain, whose ITS sequence data were found to be identical to those of Pestalotiopsis clavata MFLUCC 12-0268 (NR120182), P. neglecta Q13DW (EF055210) and P. lespedezae SY16E (EF055205) using BLAST search in NCBI, was finally identified as Pestalotiopsis sp.. The obtained sequence data were submitted to and deposited at GenBank (Accession No. KT372852).
Fermentation and Isolation
The Pestalotiopsis sp. M-23 was cultivated on autoclaved rice media (300 g) at room temperature for 40 days. Then the fermented materials were extracted 5 times with acetone (600 mL for each time) and concentrated to give a crude extract (44.7 g). The crude extract was chromatographed using silica gel column with CHCl3/acetone (from 10:0 to 0:10, v/v) to give seven fractions, Fr. A-G. Fr. C (2.9 g) was subjected to silica gel column chromatography using petroleum ether (PE)/acetone (8:1, v/v) as eluent to afford nine subfractions, Fr. C1–C9. Fr. C6 (20 mg) was further chromatographed on a Sephadex LH-20 column eluting with CHCl3/MeOH (1:1, v/v) and purified by reversed-phase semi-preparative HPLC (80 % MeOH in H2O) to yield 3 (4 mg). Fr. E (1.1 g) was subjected to ODS column chromatography eluting with MeOH/H2O (from 1:1 to 10:0) to afford seven subfractions, E1–E7. Fr. E2 (30 mg) was purified by reversed-phase semi-preparative HPLC (75 % MeOH in H2O) to yield 5 (5 mg). Fr. E3 (48 mg) was subjected to silica gel column chromatography using PE/acetone (2:1, v/v) as eluent to yield 1 (6 mg). In the same way, Fr. E4 (21 mg) and Fr. E6 (17 mg) were subjected to silica gel column chromatographies to yield 4 (PE/acetone 3:1, 3 mg) and 2 (PE/acetone 5:2, 9 mg), respectively.
2α,8α-Dihydroxy-6,7-en-isodrimeninol (1)
Colorless oil; [α]26.1D −101.1 (c 0.3, MeOH); UV (MeOH) λ max (log ε) 201 (3.57) nm; IR(KBr) ν max 3425, 2955, 2925, 1630, 1580, 1461, 1033 cm−1; 1H and 13C NMR: Table 1. ESI-MS m/z 291 [M + Na]+; HR-ESI-MS: 291.1570 (calcd for 291.1567).
2α-Hydroxy-7α,8α-epoxy-isodrimeninol (2)
Colorless oil; [α]25.3D −27.6 (c 0.3, MeOH); UV (MeOH) λ max (log ε) 201 (3.02), 215 (2.94) nm; IR(KBr) ν max 3430, 2958, 2816; 1H and 13C NMR see Table 1; HR-EI-MS m/z 268.1677 [M]+ (calcd for 268.1675).
11-Dehydro-3α-hydroxyisodrimeninol (3)
Colorless oil; [α]25.8D −14.7 (c 0.2, MeOH); UV (MeOH) λ max (log ε) 213 (3.61) nm; IR(KBr) ν max 3439, 2957, 2930, 2874, 1758, 1688, 1385, 1014; 1H and 13C NMR see Table 1; ESI-MS m/z 273 [M + Na]+; HR-ESI-MS 273.1462 (calcd for 273.1461).
4,10-Dihydroxy-gamahorin (5)
Colorless oil; [α]25.9D +16.0 (c 0.2, MeOH); UV (MeOH) λ max (log ε) 211 (4.39), 244 (3.70), 318 (3.57) nm; IR(KBr) ν max 3426, 2927, 1667, 1621, 1430, 1384, 1122, 1058; 1H and 13C NMR see Table 2. ESIMS 254 [M + Na]+; HR-ESI-MS 249.1105 (calcd for 249.1097).
Antibacterial Tests
Antibacterial activity of 1–5 against Staphylococcus aureus, Bacillus subtilis and Micrococcus luteus were evaluated using broth dilution method [24] with modification. Briefly, test compound was dissolved in proper solvent to obtain the highest concentration of 10.24 mg/mL. Serial dilutions of mother solution were performed with final concentration ranging from 512, 256, 128, 64, 32, 16 and 0 μg/mL. Ampicillin was used as positive control. All assays were performed in triplicate. The results were expressed as the minimum concentration inhibiting 50 % of bacterial growth (IC50) [25]. The IC50 values were calculated after 24 h of growth at 37 °C.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported financially by the National Natural Science Foundation of China (Nos. 31525005, U1202263, 31570362), the Youth Innovation Promotion association of Chinese Academy of Sciences (awarded to Shi-Hong Luo), the ‘‘Western Light’’ Program of Chinese Academy of Sciences (awarded to Shi-Hong Luo), the “Hundred Talents Program” of the Chinese Academy of Sciences (awarded to Sheng-Hong Li).
Compliance with Ethical Standards
Conflict of Interest
All authors declare no conflict of interest.
Contributor Information
Shi-Hong Luo, Phone: +86-0871-65223035, Email: luoshihong@mail.kib.ac.cn.
Sheng-Hong Li, Phone: +86-0871-65223035, Email: shli@mail.kib.ac.cn.
References
- 1.Arnold AE, Maynard Z, Gilbert GS, Coley PD, Kursar TA. Ecol. Lett. 2000;3:267–274. doi: 10.1046/j.1461-0248.2000.00159.x. [DOI] [Google Scholar]
- 2.De Souza JJ, Curcino IJ, Rodrigues Filho E, Braz Filho R. Molecules. 2011;16:10604–10618. doi: 10.3390/molecules161210604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stierle A, Strobel G, Stierle D. Science. 1993;260:214–216. doi: 10.1126/science.8097061. [DOI] [PubMed] [Google Scholar]
- 4.Kusari S, Zühlke S, Kosuth J, Cellárová E, Spiteller M. J. Nat. Prod. 2009;72:1825–1835. doi: 10.1021/np9002977. [DOI] [PubMed] [Google Scholar]
- 5.Kusari S, Lamshoeft M, Zuehlke S, Spiteller M. J. Nat. Prod. 2008;71:159–162. doi: 10.1021/np070669k. [DOI] [PubMed] [Google Scholar]
- 6.Yang XL, Zhang JZ, Luo DQ. Nat. Prod. Rep. 2012;29:622–641. doi: 10.1039/c2np00073c. [DOI] [PubMed] [Google Scholar]
- 7.Liu SC, Guo LD, Che YS, Liu L. Fitoterapia. 2013;85:114–118. doi: 10.1016/j.fitote.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Ortega HE, Shen YY, Tendyke K, Rios N, Cubilla-Rios L. Tetrahedron Lett. 2014;55:2642–2645. doi: 10.1016/j.tetlet.2014.03.012. [DOI] [Google Scholar]
- 9.Liu SC, Liu XY, Guo LD, Che YS, Liu L. Chem. Biodivers. 2013;10:2007–2013. doi: 10.1002/cbdv.201200361. [DOI] [PubMed] [Google Scholar]
- 10.Luo SH, Luo Q, Niu XM, Xie MJ, Zhao X, Schneider B, Gershenzon J, Li SH. Angew. Chem. Int. Ed. 2010;49:4471–4475. doi: 10.1002/anie.201000449. [DOI] [PubMed] [Google Scholar]
- 11.Luo SH, Liu Y, Hua J, Niu XM, Jing SX, Zhao X, Schneider B, Gershenzon J, Li SH. Org. Lett. 2012;14:4146–4149. doi: 10.1021/ol3017879. [DOI] [PubMed] [Google Scholar]
- 12.Luo SH, Weng LH, Xie MJ, Li XN, Hua J, Zhao X, Li SH. Org. Lett. 2011;13:1864–1867. doi: 10.1021/ol200380v. [DOI] [PubMed] [Google Scholar]
- 13.Luo SH, Hua J, Li CH, Liu Y, Li XN, Zhao X, Li SH. Tetrahedron Lett. 2013;54:235–237. doi: 10.1016/j.tetlet.2012.11.010. [DOI] [Google Scholar]
- 14.Luo SH, Hugelshofer CL, Hua J, Jing SX, Li CH, Liu Y, Li XN, Zhao X, Magauer T, Li SH. Org. Lett. 2014;16:6416–6419. doi: 10.1021/ol503230s. [DOI] [PubMed] [Google Scholar]
- 15.Phongpaichit S, Rungjindamai N, Rukachaisirikul V, Sakayaroj J. FEMS Immunol. Med. Microbiol. 2006;48:367–372. doi: 10.1111/j.1574-695X.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 16.Ren R, Chen CJ, Hu SS, Ge HM, Zhu WY, Tan RX, Jiao RH. Chem. Biodivers. 2015;12:371–379. doi: 10.1002/cbdv.201400119. [DOI] [PubMed] [Google Scholar]
- 17.Sakio Y, Hirano YJ, Hayashi M, Komiyama K, Ishibashi M. J. Nat. Prod. 2001;64:726–731. doi: 10.1021/np000639g. [DOI] [PubMed] [Google Scholar]
- 18.Pulici M, Sugawara F, Koshino H, Uzawa J, Yoshida S, Lobkovsky E, Clardy J. J. Nat. Prod. 1996;59:47–48. doi: 10.1021/np9600053. [DOI] [Google Scholar]
- 19.Yang XY, Feng T, Ding JH, Li ZH, Li Y, Fan QY, Liu JK. Nat. Prod. Bioprospect. 2013;3:154–157. doi: 10.1007/s13659-013-0030-y. [DOI] [Google Scholar]
- 20.Koshino H, Yoshihara T, Okuno M, Sakamura S, Tajimi A, Shimanuki T. Biosci. Biotechnol. Biochem. 1992;56:1096–1099. doi: 10.1271/bbb.56.1096. [DOI] [PubMed] [Google Scholar]
- 21.Felix S, Sandjo LP, Opatz T, Erkel G. Bioorgan. Med. Chem. 2014;22:2912–2918. doi: 10.1016/j.bmc.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Zhang JW, Wen GL, Zhang L, Duan DM, Ren ZH. Bangl. J. Pharmacol. 2015;10:844–853. doi: 10.3329/bjp.v10i4.23708. [DOI] [Google Scholar]
- 23.Keskes H, Litaudon M, Cherif A, Belhadj S, Hamdi B, El Feki A, Dumontet V, Ben Salah A, Damak M, Allouche N. J. Asian Nat. Prod. Res. 2014;16:1132–1138. doi: 10.1080/10286020.2014.938646. [DOI] [PubMed] [Google Scholar]
- 24.Wiegand I, Hilpert K, Hancock REW. Nat. Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 25.Reed LJ, Muench H. Am. J. Epidemiol. 1938;27:493–497. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.