Abstract
Purpose
To evaluate the analgesic efficacy of a diclofenac transdermal patch with diclofenac intra muscular injection in the immediate postoperative period in patients undergoing major oral surgical procedures.
Materials and Methods
Subjects who underwent bijaw surgeries for surgical correction of various dentofacial deformities were included. Sixty such patients who belonged to the above entity were randomly categorized into two groups from Jan 2012–Aug 2015. Group A (study group) received a single dose of 100 mg transdermal diclofenac patch, Group B (control group) received 75 mg intramuscular diclofenac and tramadol HCl 2 mg/kg body wt was used as rescue analgesic in the immediate post operative phase. The analgesic efficacy of the drugs are evaluated on periodic patient’s perception of pain in the immediate postoperative phase of 2nd, 6th, 12th, 24th and 48 hours.
Results
The mean VAS score in Group A was 2, mean duration of analgesia was 16 h 9 min, and in Group B the mean VAS score was 4, duration of analgesia was 8 h and 4 min. Tramadol HCl was given as rescue analgesia in 22 % (6) of patients belonging to Group A. None of the patients from both the groups reported local complications.
Conclusion
A noninvasive application of a single dose of 100 mg transdermal diclofenac patch is more effective than intramuscular diclofenac (75 mg) in the immediate post operative phase, without any significant side-effects is a novel ingress into the field of oral and maxillofacial surgery for post operative pain management.
Keywords: Diclofenac transdermal patch, Diclofenac intramuscular injection, Post operative pain management
Introduction
Management of post operative pain remains an arena for never ending research with better formulations and modalities constantly replacing the obsolete ones. Pain following surgery is one of the most important factors causing morbidity and mortality, two causes prolonging the hospital stay [1]. Post operative pain following surgical intervention in the maxillofacial region has often been a nemesis for the operator and the patient alike due to the considerable amount of inflammatory response involved. Peripheral tissue injury provokes two kinds of modifications in the responsiveness of the nervous system [2]. This results in an overall hypersensitivity state in the postoperative period. Prevention and establishment of this hypersensitivity state could lead to reduced postoperative pain [3]. This forms the basis of pre-emptive analgesia.
Non-steroidal anti-inflammatory drugs (NSAIDs) exert anti-inflammatory and analgesic effects through the inhibition of prostaglandin synthesis, by blocking the activity of cyclo-oxygenase [4]. Diclofenac sodium is the commonly used non-selective NSAID, and is available in various forms to treat pain [5]. The diclofenac transdermal patch (TP) is a newly introduced delivery system for postoperative pain management. The purpose of this paper is to evaluate the analgesic efficacy of diclofenac transdermal with intramuscular (IM) injection in the immediate post operative phase.
Materials and Methods
This study was undertaken in 60 subjects belonging to the age group 18–30 years. After obtaining institutional ethical committee clearance and informed consent from the subjects, they were randomly allocated into 2 groups of 30 each who underwent surgical intervention for correction of dentofacial deformities. Group A (study group) received a single dose of transdermal diclofenac patch 100 mg (SANDOR, DICLO-TOUCH) as shown in Fig. 1 and Group B (control group) received intramuscular diclofenac 75 mg (DIKLOFLAME).Patients undergoing similar type of surgical procedure for correction of dentofacial deformities were included in the study. Patients with history of renal pathology, bronchial asthma, active peptic ulceration, cardiac surgeries, bleeding disorders, allergic reactions induced by NSAIDs were excluded. Both the subgroups did not receive any parenterally administered analgesic or sedative prior to the surgery or during the surgery. All the 60 patients underwent Lefort I osteotomy and mandibular advancement under general anesthesia.
Fig. 1.
Diclofenac transdermal patch
Anesthesia Protocol
- PRE MEDICATION
- Inj.Glycopyrrolate 0.2 mg
- Inj.Midazolam 1 mg
- Inj.Nalbuphine 10 mg
- Inj.Zofer 4 mg
- Inj.Pantocid 40 mg
INDUCTION—Inj Propofol 100 mg
INTUBATION—Inj atracurium
MAINTAINENCE DOSE—Nitrous oxide 50 %, Sevoflurane, Vercuronium bromide-4 mg
ANALGESIA—Inj Nalbuphine hydrochloride 10 mg at the time of intubation.
HYPOTENSION—Inj Nitroglycerine infusion
REVERSAL—Inj.Neostigmine—2.5 mg, Inj Glycopyrrolate—0.5 mg
In Group A (study group), a transdermal diclofenac patch containing 100 mg (DICLO-TOUCH) was applied to the subjects at the beginning of the surgical intervention on non hair bearing area like deltoid region, chest for females, abdomen for males as shown in Fig. 2.
Fig. 2.
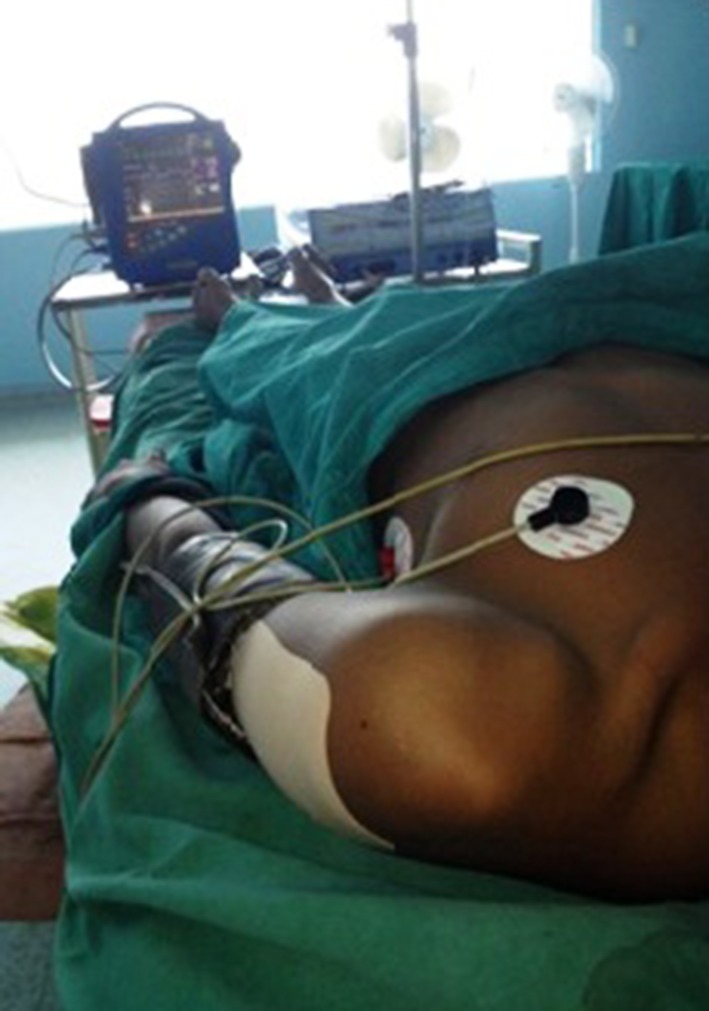
Diclofenac transdermal patch applied at the deltoid region
In Group B (control group) 75 mg of diclofenac sodium injection was given intramuscularly half an hour before the end of the surgical procedure. Subjective and objective parameters were evaluated periodically. Subjective parameters included pain which was assessed postoperatively at 2, 6, 12, 24, 48 h respectively using a Visual Analogue Scale (VAS). In the immediate post operative phase whenever the VAS score was greater than 5, tramadol HCl 2 mg/kg body wt was administered intramuscularly as rescue analgesic and time was noted. Objective parameters included duration of surgery (from the beginning of the surgical procedure till the end of the procedure), duration of analgesia (from the time of administration of the analgesic till the rescue analgesic given), necessity for rescue analgesia, renal parameters which were measured from serum urea and creatinine in the pre and immediate post operatively phase after 48 h, and hemodynamic changes. In the study group, allergic reactions at site of transdermal patch application was checked while in the control group patients were evaluated for nausea and vomiting, gastritis in the immediate postoperative phase.
Results
Descriptive analysis has been carried out with mean and standard deviation being compared. SPSS version 16 software was used and comparison of categorical values was done using Chi square test and continuous variables by independent sample t test and Mann–Whitney test.
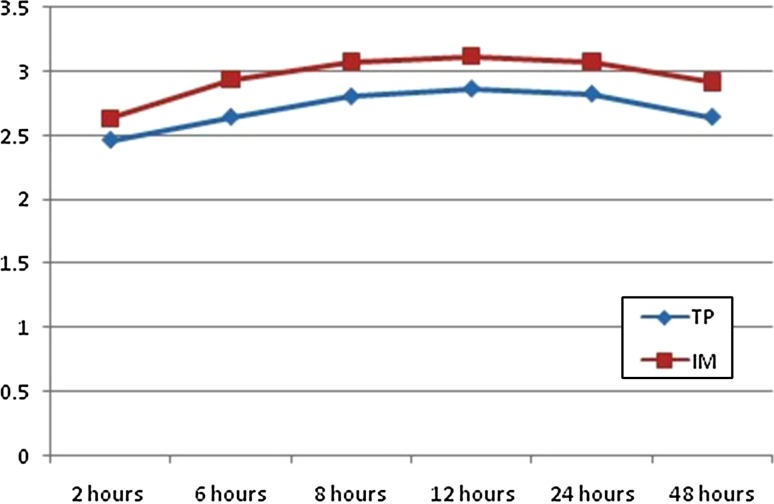
The mean age of the patients was 25.38 years. Pain was assessed postoperatively using VAS at 2, 6, 12, 24, 48 h as shown in Fig. 3.
Fig. 3.
VAS scores at 2, 6, 12, 24, 48 h
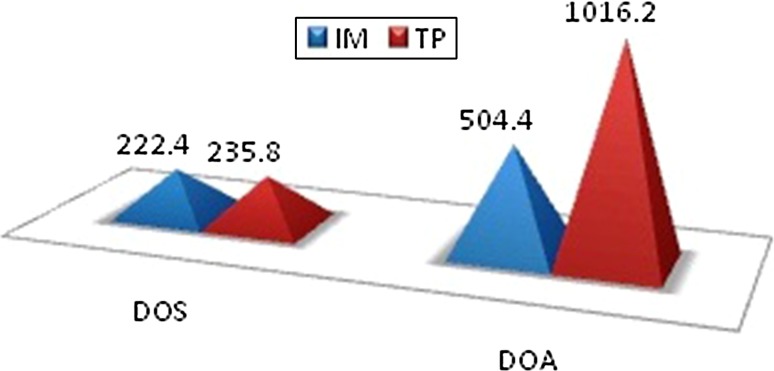
If VAS score was <5, a further assessment was carried out till 24 h. If the patient had a score of ≥5, rescue analgesia was administered and further evaluation was performed. In (Group A) control group, at 2 h postoperatively, 23 (76 %) patients had no pain, 7 (24 %) had VAS score greater than 5. At the end of 12 h post operatively 6 (22 %) patients had a VAS score 5 or greater. The mean duration of surgery in the control group was 222.40 min and in the study group it was 235.80 min which was not significant as shown in Fig. 4. The mean time at which rescue analgesia was administered in the control group was 8 h and 4 min. Our study reveals that the duration of analgesia provided by intramuscular diclofenac is short. In study group, at 2 h post operatively, no patients had pain. At the end of 12 h post operatively, 24 (79 %) patients had VAS score of 2 and 6 (19 %) had VAS score of 5 where rescue analgesic was given. The mean time at which rescue analgesia was administered in study group was 16 h and 9 min. At 2 h postoperatively, the VAS was comparable between the groups, and was not statistically significant (p value = 1). At 12 h postoperatively, 3 (11 %) patients in the study group had a score of 5. The mean time at which rescue analgesia was given in the control group was 8 h and 4 min and in the study group it was 16 h and 9 min and were found to be significant (p value < 0.001) as shown in Fig. 4.
Fig. 4.
Duration of surgery duration of analgesia
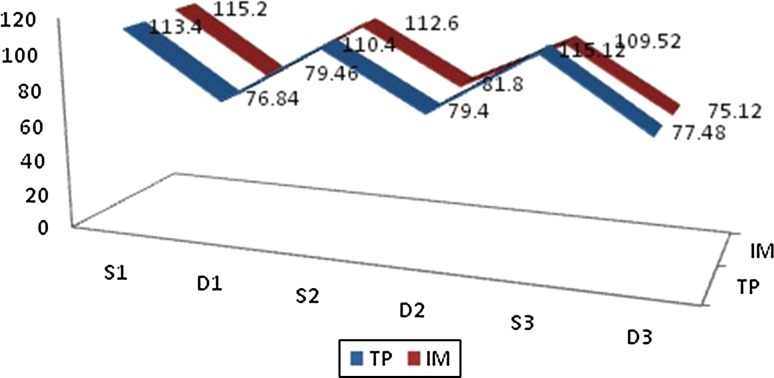
Side effects in the control group included nausea and gastritis in 5 (18 %) of the patients, pain at the site of injection in 7 (24 %) patients while there were no noticeable side effects in the study group. Renal clearance was achieved faster in the study group (0.026) which was significant. Hemodynamic changes in either groups measured perioperatively were insignificant as shown in Fig. 5.
Fig. 5.
Haemodynamic variables
Discussion
Topical administration of non-steroidal anti-inflammatory drugs (NSAIDs) offers the advantage of local, enhanced drug delivery to affected tissues with a lower incidence of systemic adverse effects due to reduced plasma concentrations [6]. On applying the patch to the skin, a drug concentration gradient is developed and the drug starts to move down the gradient into the skin and is absorbed into the local capillary vasculature and is then transported into the systemic circulation [7].
The efficacy of NSAIDs in reducing the post operative pain depends on their ability to inhibit cyclo-oxygenases (COX-1, COX-2) which are key in prostaglandin synthesis [8]. Transdermal drug administration bypasses first-pass metabolism in the liver, and overcomes concerns regarding drugs that are poorly absorbed in the gastrointestinal tract [9]. It offers several advantages as it avoids the need for intravenous or intramuscular drug administration, prolonged duration of onset and offset, typically 12–24 h, patient compliance, shorter analgesic consumption and hospital stay, reduced systemic adverse effects due to lower plasma concentrations [1] thus carving out a niche for themselves as therapeutic analgesic modalities with established benefits. The mode of onset of action of transdermal drug delivery systems depend on the manufacturer’s choice. The mode of onset of action of the diclofenac patch which was used in the study was 4 h. The drug would reach its peak plasma concentration within 10–12 h and duration of action was 24 h. Hence, the study was designed for the transdermal patch to be applied before the intubation. The action would start by the time surgical procedure and extubation would have completed and the patient would experience pain, thus acting as pre emptive analgesic.
Parenteral drug delivery with intravenous, subcutaneous, or intramuscular injection, can gain easy access to systemic circulation with rapid drug absorption. However, this rapid drug absorption is also accompanied by a rapid decline in the drug levels in the systemic circulation [10].
Currently, regimens consisting of a combination of analgesics (multimodal analgesia) are recommended for the management of postoperative pain. Adjunctive techniques, such as wound infiltration with local anaesthetics, the use of NSAIDs or corticosteroids have been recommended to treat postoperative pain [11]. Of these, the NSAIDs have gained increasing popularity in treating postoperative pain. NSAIDs are excellent analgesics with no clinically important difference in efficacy among specific drugs.
Neadal et al. [4] demonstrated that because of low systemic concentrations, topical NSAIDs have a reduced risk of upper gastrointestinal complications, such as gastric and peptic ulcers, and gastrointestinal nuisance symptoms, such as dyspepsia [4]. The results of our study suggest that subjects receiving the diclofenac patch had statistically and clinically significant reductions in pain scores and were free of pain significantly earlier than patients in the control group. Krishna et al. [9] reported that intraoperative application of a single dose of 100 mg transdermal diclofenac patch is as effective as a single dose of intramuscular diclofenac (75 mg) for acute postoperative pain, without any significant side-effects which was in agreement to the present study. The mean duration of surgery was comparable between the two groups. The duration of surgery has a direct bearing on the postoperative analgesic requirement, as prolonged duration of tissue handling increases the local production of inflammatory substances and oedema, hence increasing the requirement for analgesics [10]. Thus, a single dose intra operative application of diclo patch just before the beginning of the surgical intervention would reduce additional analgesic requirement in the post operative phase as the drug contained in the patch enters the skin and eventually diffuses into the capillaries by the time patient complains of pain, thus acting as pre emptive analgesic. Alessandri et al. [11] administered transdermal diclofenac sodium and placebo into the incision area in postoperative period and observed that there was significantly less analgesic consumption and a shorter duration of hospital discharge. Bruhlmann et al. [12] evaluated effectiveness of transdermal diclofenac sodium on 103 patients with knee osteoarthritis and reported that transdermal diclofenac sodium application to the placebo group is an effective and reliable method, all of which were in agreement to the present study. In terms of safety profile, pre operative and immediate post operative serum urea, creatinine levels after 48 h were measured for all the subjects enrolled in the study. The safety profile, tolerability of both intramuscular and topical formulations were evaluated in both the groups which were comparable but not statistically significant in both the groups. In terms of safety, the patch was well tolerated and did not cause any local or systemic adverse effects whereas patients in the control group reported nausea, gastritis (18 %), pain at the site of injection (24 %). Pradel et al. [6] used a diclofenac patch for acute traumatic blunt soft tissue injuries, and they found that the diclofenac patch was effective and well tolerated. Mason et al. [13] studied the safety profile of topical NSAIDS and concluded that topical NSAIDs were effective and safe in treating chronic musculoskeletal conditions for 2 weeks.
Bhasker et al. [14] compared the efficacy of transdermal with oral diclofenac following extraction and stated that transdermal patch was more comfortable due to once-a-day application with lesser systemic adverse effects. Agarwal et al. [15] reported the occurrence of erythematous rash at the site of application of transdermal patch. It is contrary to the present study perhaps due to the fact that each successive application of diclofenac patch was done at a different site. We did not encounter any problems pertaining to the adhesivenss of the patch as it was applied on non hair bearing area like deltoid region and chest for females and abdomen for males. Haematological and biochemical variables revealed no statistical or clinically significant differences between groups during the course of the study. Likewise, no clinically relevant changes in vital signs were noted during the study correlating to the study conducted by Pradel et al. [6]. The results of our study suggest that a single dose of application of diclofenac transdermal patch is more effective than intramuscular injection in prolonging the requirement of postoperative analgesic.
Conclusion
The transdermal patch forms delivers slow release of drug into the body over time resulting in longterm effectiveness and added convenience. Thus, it seems to be a promising modality carving a niche for the management of pain in the immediate post operative phase. Preference of diclofenac sodium in patch forms will increase patient’s comfort by eliminating a needle prick, reducing side effect profile by decreasing additional analgesic requirement. Hence, intraoperative application of a single dose of 100 mg transdermal diclofenac patch is effective than intramuscular diclofenac (75 mg) in the immediate postoperative phase, without any significant side-effects. However, further long term clinical trials have to be carried out to evaluate the efficacy of these drugs.
Acknowledgments
The authors would like to thank Dr. Pranavi and Dr. Sasank for their general support and assistance.
Compliance with Ethical Standards
Conflict of interest
None.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Dobbs FF, Kumar V, Alexander JI, Hull MG. Pain after laparoscopy related to posture and ring versus clip sterilization. Br J Obstet Gyneacol. 1987;94:262–266. doi: 10.1111/j.1471-0528.1987.tb02365.x. [DOI] [PubMed] [Google Scholar]
- 2.Hepner DL. Preemptive analgesia: what does it really mean? Anesthesiology. 2000;93:1368. doi: 10.1097/00000542-200011000-00050. [DOI] [PubMed] [Google Scholar]
- 3.Ong CKS, Lirk P, Saymour R. The efficacy of preemptive analgesia for acute postoperative pain management: a metanalysis. Anesth Analg. 2005;100:573–575. doi: 10.1213/01.ANE.0000144428.98767.0E. [DOI] [PubMed] [Google Scholar]
- 4.Naedal J, Brown K. NSAID-associated adverse effects and acid control aids to prevent them: a review of current treatment options. Drug Saf. 2006;29:119–132. doi: 10.2165/00002018-200629020-00002. [DOI] [PubMed] [Google Scholar]
- 5.Shang AB, Gan TJ. Optimising postoperative pain management in the ambulatory patient. Drugs. 2003;63:855–867. doi: 10.2165/00003495-200363090-00002. [DOI] [PubMed] [Google Scholar]
- 6.Predel HG, Koll R, Pabst H, Dieter R, Gallacchi G, Giannetti B, Bulitta M, Heidecker JL, Mueller EA. Diclofenac patch for topical treatment of acute impact injuries: a randomised, double blind, placebo controlled, multicentre study. Br J Sports Med. 2004;38:318–323. doi: 10.1136/bjsm.2003.005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Margetts Lyn, Sawyer Richard. Transdermal drug delivery: principles and opioid therapy. Br J Anaesth. 2007;7:171–176. [Google Scholar]
- 8.Heyneman CA, Lawless-Liday C, Wall GC. Oral vs topical NSAIDS in rheumatic diseases: a comparison. Drugs. 2000;60:555–574. doi: 10.2165/00003495-200060030-00004. [DOI] [PubMed] [Google Scholar]
- 9.Krishna Nataraj. Efficacy of a single dose of a transdermal diclofenac patch as pre-emptive postoperative analgesia: a comparison with intramuscular diclofenac. S Afr J Anaesth Analg. 2012;18:194–197. [Google Scholar]
- 10.Vaile JH, Davis P. Topical NSAIDs for musculoskeletal conditions. A review of the literature. Drugs. 1998;56:783–799. doi: 10.2165/00003495-199856050-00004. [DOI] [PubMed] [Google Scholar]
- 11.Alessandri F, Lijoi D, Mistrangelo E, Nicoletti A, Crosa M, Ragni N. Topical diclofenac patch for postoperative wound pain in laparoscopic gynecologic surgery: a randomized study. J Minim Invasive Gynecol. 2006;13:195–200. doi: 10.1016/j.jmig.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Brühlmann P, Michel BA. Topical diclofenac patch in patients with knee osteoarthritis: a randomized, double-blind, controlled clinical trial. Clin Exp Rheumatol. 2003;21:193–198. [PubMed] [Google Scholar]
- 13.Mason L, Moore RA, Edwards JE, Derry S. McQuay HJ Topical NSAIDs for chronic musculoskeletal pain: systematic review and meta-analysis. BMC Musculoskelet Disord. 2004;19:28. doi: 10.1186/1471-2474-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhasker H, Kapoor P. Comparison of transdermal diclofenac patch with oral diclofenac as an analgesic modality following multiple premolar extractions in orthodontic patients: a cross over efficacy trial. Contemp Clin Dent. 2010;1:158–163. doi: 10.4103/0976-237X.72783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal A, Dhiraaj S, Kumar A, Singhal V, Singh U. Evaluation of a diclofenac transdermal patch for the attenuation of venous cannulation pain: a prospective, randomized, double blind placebo controlled study. Anesthesia. 2006;61:360–362. doi: 10.1111/j.1365-2044.2006.04538.x. [DOI] [PubMed] [Google Scholar]