Abstract
Background:
The estimated incidence and prevalence of tuberculosis in India are 2.1 and 2.6 million cases respectively. Immunotherapy may shorten tuberculosis treatments and improve the immunity of individuals as well. Hence we study the efficacy of levamisole (LVM) (immunomodulator) as an adjuvant to chemotherapy of pulmonary tuberculosis patients.
Materials and Methods:
A randomized, double-blind, placebo-controlled clinical trial was conducted for 21 months in newly diagnosed sputum positive pulmonary tuberculosis patients. Patients were subjected initially to clinical examination, sputum acid-fast bacilli smear and culture, tuberculin skin test and weight record. During follow-up, above investigations were repeated. Sixty-five patients were randomly assigned into two groups to receive either tab LVM 100 mg once in a day or matching placebo, orally as a single dose, thrice a week, for 2 months with short-course antituberculosis chemotherapy.
Results:
Sputum negativity at 1 week was observed in 11 (44%) patients in LVM group whereas only 3 (12%) in placebo group. All the patients 25 (100%) in LVM group were sputum negative compared to 14 (56%) in placebo group by the end of 3 weeks. In LVM group, 24 (96%) and 11 (44%) patients in placebo group show radiological improvement at 2 months. A direct correlation existed between quantum of immune response and weight gain with LVM. LVM rendered all anergic patients to positive tuberculin reactors. In LVM group, patients with initial Mantoux ≥20 mm and advanced cavitary disease, there was decrease in tuberculin reaction size.
Conclusion:
Adjuvant immunomodulation with levamisole has the potential of shortening the total duration of antitubercular therapy.
Keywords: Immune response, immunomodulator, mantoux test, tuberculosis
Introduction
Tuberculosis continues to be a major health problem despite the availability of effective chemotherapy.[1] There are estimated 88 million new cases every year corresponding to 52 thousand deaths per week or more than 7000 deaths per day.[2] Mathematical modeling suggested that 3.2% (or 273,000) of the world's estimated new tuberculosis cases (95% CI: 185,000, 414,000) were drug-resistant cases in the year 2000.[3]
Due to tubercle bacilli (TB) infection, there is immune suppression or dysregulation, where TH1 type response offers protective immunity while mixed TH1-TH2 response is detrimental.[4,5]
The tuberculous infection has suppressive effects on cellular immunity. In majority of tuberculosis patients, the cellular immune response may be depressed and manifested by reduced dermal hypersensitivity to tuberculin.[6]
Delayed hypersensitivity skin test has a great value in the overall assessment of immunocompetences and epidemiologic surveys.[7] As the antituberculous therapy has a longer duration, it can lead to noncompliance of the patient and which may contribute to the emergence of drug resistance of the organism.[8,9]
Immunomodulators are known to improve the management outcome by offering early cure and control of disease. Immunotherapy is used with expectation to enhance a specific type of immune response or restore a deficient immune system. Levamisole (LVM) has been used as an immunomodulator in autoimmune diseases, carcinomas, primary immunodeficient diseases, and immune impairments in infectious diseases.[10]
The combination of an immunopotentiating agent to augment the efficacy of anti-tubercular therapy has been considered previously.[11,12] Hence, this trial was planned to find the efficacy of LVM as an adjunct to short-course chemotherapy (SCC) in newly diagnosed sputum-positive tuberculosis patients.
Materials and Methods
This was a randomized, double-blind, placebo-controlled clinical trial conducted after approval from the Institutional Ethics Committee. Patients were recruited from those attending TB Outpatient Department, Department of Respiratory Medicine of a tertiary care teaching hospital. The inclusion criteria were newly diagnosed sputum-positive tuberculosis patients above the age of 15 years of either gender. Clinically, the symptom cough as an indicator of better response but as the bacteriological test is an objective parameter for the assessment of outcome of chemotherapy as per the World Health Organization (WHO) guidelines,[13] also there were supporting studies quoted by various authors,[14] hence the bacteriological test (sputum acid-fast bacilli [AFB] smear and culture) was considered. The exclusion criteria were extra-pulmonary tuberculosis, human immunodeficiency virus, diabetes mellitus, hypertension, pregnant, and lactating women and patients on long-term corticosteroid therapy. Patients meeting the inclusion criteria were explained about the trial, and provided with detailed information sheet in vernacular language understood by them. All their queries were answered. Informed consent was taken from those who agreed to participate. All the participants (n = 65) were given the following antitubercular treatment: Intensive phase (2 months treatment-orally) 300 mg isoniazid, 450 mg rifampicin, 1500 mg pyrazinamide and 800 mg ethambutol (daily) followed by continuation phase (4 months) isoniazid 300 mg and rifampicin 450 mg daily. All the patients fulfilling the inclusion criteria for the study were weighing <50 kg and the standard rifampicin dose for such patients was 450 mg in SCC as per the WHO guidelines.[13]
All 65 participants were randomly allocated to two groups.
Group 1 (LVM group): Tablet LVM 100 mg once in a day (OD) orally on alternate days under supervision during intensive phase of chemotherapy. Tablet LVM was purchased from a local pharmacy.
Group 2 (placebo group): Matching placebo was administered as a single dose orally on alternate days under supervision during intensive phase of chemotherapy. The identical placebo tablets were provided by a local pharmaceutical company. The antituberculosis drugs were supplied from the institutional pharmacy. Sputum smear for AFB, X-ray chest, tuberculin test, and body weight were recorded at the baseline. Bacteriological response (sputum negativity) was measured by microscopic examination of AFB bacilli; radiological response with chest X-ray and immunological response were measured by tuberculin skin test (TST).
In the follow-up period, bacteriological response as sputum negativity was determined every week for initial 2 months. Radiological response was tested at the end of 2 months while body weight gain was noted at 2 and 6 months. Adverse drug reactions (ADRs), if any, were recorded at every visit.
After decoding it was found that there were 32 patients in the LVM group and 33 in the placebo group. Seven patients from LVM group and eight from placebo group dropped out during the trial as they were lost to follow-up. Independent physician and radiologist evaluated the radiological changes. Response to Mantoux (Mx) test (delayed hypersensitivity) in the form of indurations measured in millimeters and development of tissue necrosis were used for the assessment of immune-competence status of participants.[7]
Statistical analysis
Comparison of rate of sputum negativity, radiological improvement, and cavity closure between the two groups was done by using Fisher's exact test whereas unpaired t-test was used to compare weight gain. Graphpad Prism version 5.0 was used for statistical analysis. The value P < 0.05 was considered statistically significant.
Results
Twenty-five patients from each group who completed the entire study were considered for analysis. Baseline characteristics were identical in the two study groups [Table 1]. Figure 1 shows the bacteriological response in LVM and placebo groups. Earliest sputum negativity within 1 week was observed in 11 (44%) patients in LVM group as compared to 3 (12%) in the placebo group. By 3rd week, all 25 (100%) patients in LVM group were sputum negative compared to 14 (56%) in placebo group. In LVM-treated group, there was no unfavorable bacteriological response such as treatment failure or relapse, while it was observed in two patients of the placebo group. The number of sputum-negative patients was statistically significantly more in the LVM group than in the placebo group at 1 week (P = 0.0255) and at 3 weeks (P = 0.0002).
Table 1.
Baseline characteristics of study population
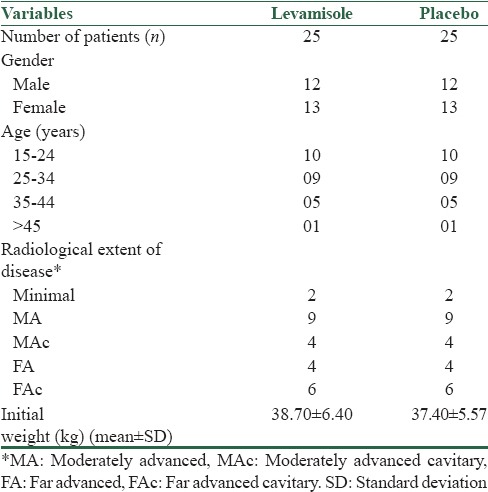
Figure 1.
Bacteriological response (sputum negativity) in levamisole and placebo groups (n = 25 in each group) *P < 0.05, ***P < 0.001 when compared to placebo. The number of sputum negative patients was statistically significantly more in the levamisole group than in the placebo group at 1 week (P = 0.0255) and at 3 weeks (P = 0.0002)
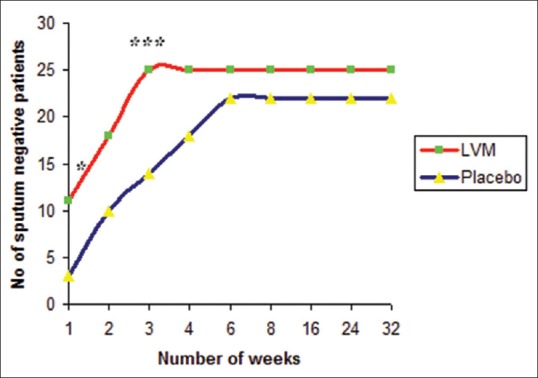
Radiological response in LVM and placebo groups at 2 months is shown in Figure 2. Twenty-four (96%) patients in LVM group and 11 (44%) patients in placebo group showed radiological improvement. The difference is statistically significantly higher in LVM treated group with P = 0.0001. Of 10 patients with cavitation, cavity closure was observed in 7 (70%) in LVM group compared to nil in placebo group, which is statistically significant with P = 0.0031.
Figure 2.
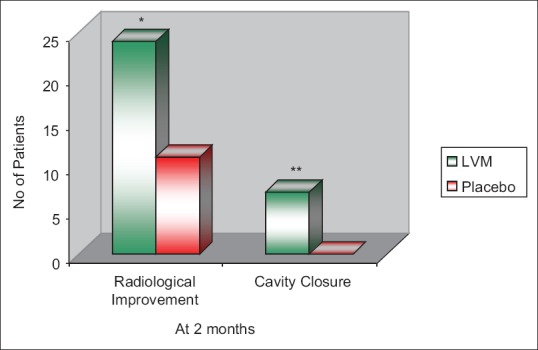
Radiological response of patients in levamisole and placebo groups at 2 months (n = 25 in each group) *P = 0.0001, **P = 0.0031 when compared to placebo. The radiological improvement and cavity closure was significantly higher in levamisole-treated group with P = 0.0001 and 0.0031, respectively, when compared to placebo group
Table 2a shows an immunological response in LVM-treated group in terms of positive Mx change, i.e., increase in the size of skin induration of at least 5 mm after 2 months. All the 10 anergic patients regained normal protective immune response in LVM group. Three patients having Mx ≥ 20 mm, i.e., with dysregulated immune response also reverted to normal protective immune response whereas remaining patient showed boosting of normal immune response.
Table 2a.
Immunological response in levamisole group (n=25)
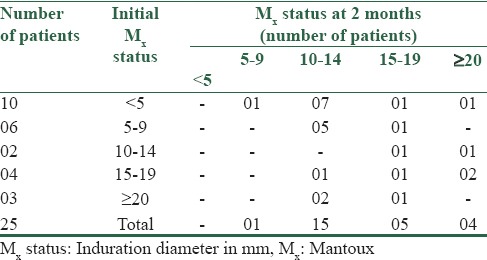
Table 2b shows immunological response in placebo group. Among three anergic patients, only one patient gained positive immune response. In three patients with immune dysregulation, only one restored to protective immune response, whereas other patients either showed no change in immune status or boosting of immune response.
Table 2b.
Immunological response in placebo group (n=25)
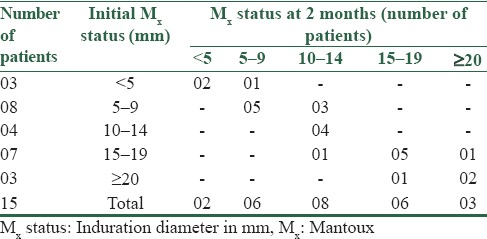
Figure 3 depicts the mean weight gain in LVM and placebo groups. The initial mean weight in LVM group was 38.7 ± 6.40 kg whereas in the placebo group it was 37.4 ± 5.35 kg. Mean weight gain at the end of 2 months and 6 months in LVM group was significantly higher than the placebo group with P < 0.0001.
Figure 3.
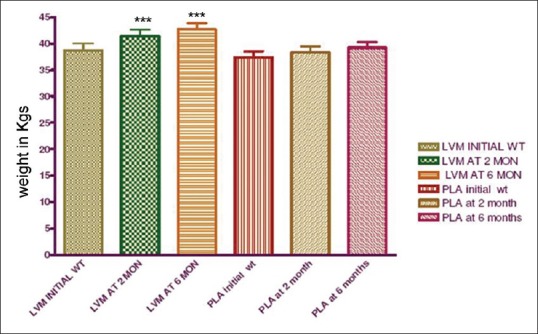
Mean weight gain in levamisole and placebo group at 2 and 6 months (n = 25 in each group) ***P < 0.0001, LVM: Levamisole, PLA: Placebo, WT: Weight in kilograms. MON - months. The weight gain was significantly higher than the placebo group at 2 and 6 months
Three patients in LVM group and four in the placebo group complained of gastritis which did not warrant discontinuation of therapy and could be controlled with antacids. No other significant ADRs associated with LVM were observed. Thus, LVM was found to be safe in a dose of 100 mg OD, every alternate day.
Discussion
Heterogenecity to cell-mediated immune response against Mycobacterium tuberculosis (MTB) infection exists among individuals, which has been attributed to individuals’ genetic constitution, mental health status, and bacillary load.[15]
We assessed the immunocompetence status of the patients with TST and observed that 26% patients with moderate or advanced disease were anergic (negative to TST), which is in agreement with earlier reports others.[16,17,18] Three patients with far advanced cavitary disease and one patient with moderate cavitary disease showed strongly positive Mx response, which is similar to earlier published studies.[13,19]
The striking increase in production of IL4 by CD4+ and CD8+ T cells in patients with active tuberculosis related to the presence of pulmonary cavities has been reported.[20] It has been suggested that TH2 or mixed TH1 and TH2 response is the cause of extensive tissue necrosis seen in progressive primary tuberculosis.[21] These reports provide evidence that progressive disease with or without cavitations is associated with Type 2 cytokine response, as a consequence of disease.
It was proved that large indurated, necrotizing response of TST as seen by objective technique of laser Doppler velocimetry, reflects Type-2 cytokine response.[22] Hence, patients having cavitary disease with large indurated tuberculin response, i.e., ≥18 mm can be presumed to be manifestation of Type-2 cytokine immune response.
Our observations of early sputum negativity by LVM suggest improvement or restoration of immune competence. Early sputum negativity implies early cessation of multiplication and rapid killing of drug-sensitive bacilli. A rapid reduction of the bacterial population in the lesions averts deterioration of the disease and subsequent death as well as the further transmission of infection.[23]
Studies in tubercular immunology have shown that MTB induces very early events dependent on the MTB bacillary load which in turn leads to immunosuppression or dysregulation.[24,25] Therefore, along with early normalization of the immune response, it is important to kill mycobacteria rapidly.
It was observed that the degree of radiological improvement was significantly better in LVM-treated group compared to the group receiving the only antitubercular drug at the end of 2 months. Yet another study reported earlier cavity closure in LVM group than usual.[14,26]
The clinical improvement with LVM treatment was observed in 93.75% as compared to 50% observed by others.[14] The better response in our study could be explained on the basis that along with immunostimulant therapy, early killing of bacilli was achieved with effective SCC.
The increase in Mx size after chemotherapy is known to be a good prognostic sign.[27] In LVM group increase in Mx size, more than 5 mm is definitely attributed to immunostimulatory action of LVM. However, three patients of LVM group with initial Mx > 20 mm and one with 18 mm showed reversion in Mx size. Out of these, two had advanced cavitary disease. These patients probably represent as having type-2 cytokine immune response against MTB. In these patients, immunomodulatory effect of LVM may have shifted the balance from TH2 or mixed TH1 and TH2 toward TH1 type response. Had it been an unfavorable response, patients would not have shown positive response in bacteriological, radiological responses, and weight gain. The patients became sputum negative within 7–14 days, cavitation disappeared and in patients with far advanced cavitary disease, weight gain was more than 4 kg at the end of 2 months. This indicates that LVM shifts the balance from TH2 type cytokine response to TH1 type cytokine response. An animal study showed that LVM acts by resetting the immune balance toward type-1 response via induction of IL-18,[28] which we clinically observed.
The observed weight gain of 7.26% in LVM group suggests better clinical response which might be due to decreased level of tumor necrosis factor (TNF) or decrease in susceptibility of normal tissue to TNF brought about by immunomodulatory action of LVM through mechanisms unknown. Our observation that higher the Mx change higher the weight gain favors the hypothesis that more the immune response as induced by LVM, more is the reduction in TNF reflected as increase in weight gain. It has been documented that TNF is responsible for loss of weight due to susceptibility of normal tissue to the destructive action of TNF.[29]
In this study, no severe adverse events were noted. Only a few patients had reported gastrointestinal upset which was managed conservatively, did not warrant withhold of medication.
However, there are reports of various side effects with use of LVM, but the incidence and severity of side effects are limited. Patients may complain of nausea, vomiting, headache, fever, shivers, and dizziness.[30] Furthermore, some reported allergy in atopic patients.[31]
Agranulocytosis and blood dyscrasia were reported in patients receiving LVM for the treatment of rheumatoid arthritis, although agranulocytosis is reversible on discontinuation of LVM.[32]
Regardless of the modalities of immunomodulation, whether drug (LVM or β-sitosterol)[33] or organism (Mycobacterium vaccae),[34] immunotherapy along with chemotherapy plays a profound role in early alleviation of the suffering. With combination of immunotherapy as an adjunct to SCC of pulmonary tuberculosis, it may be possible to cut short the existing duration of chemotherapy. This would lead to improvement in patient compliance, early clinical cure, less development of multidrug-resistant tuberculosis, and increase in productive life of the patient.
Limitation
During the study period, a total of 65 patients were enrolled fulfilling the inclusion criteria. Out of these, 50 could complete the study protocol.
Immune response was assessed by Mountax test and indirectly by weight gain. Other immunological parameters of immune response such as CD4, CD8 counts, serum Interleukins gamma interferons, TNF could not be assessed due to financial constraints.
Possible difference in severity of disease among two groups is other limitation of the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge the kind help of Dr. M. P. Shrivastava, professor and head, Department of Pharmacology; Indira Gandhi Medical College, Nagpur. We would also like to thank Dr. R. P. Munje, associate professor, Department of Respiratory Medicine, Government Medical College, Nagpur, for her invaluable help in conduct of this trial. We also thank Dr. Smita Sontakke, associate professor, and Dr. Avinash Turankar, associate professor, Government Medical College, Nagpur, for their contribution.
References
- 1.Cox HS, Orozco JD, Male R, Ruesch-Gerdes S, Falzon D, Small I, et al. Multidrug-resistant tuberculosis in central Asia. Emerg Infect Dis. 2004;10:865–72. doi: 10.3201/eid1005.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Report on TB. Geneva: The World Health Organization; 2003. [Google Scholar]
- 3.Espinal MA. The global situation of MDR-TB. Tuberculosis (Edinb) 2003;83:44–51. doi: 10.1016/s1472-9792(02)00058-6. [DOI] [PubMed] [Google Scholar]
- 4.Seah GT, Scott GM, Rook GA. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J Infect Dis. 2000;181:385–9. doi: 10.1086/315200. [DOI] [PubMed] [Google Scholar]
- 5.Sirenko IA, Nastas PN. Use of levamisole electrophoresis in chemical prophylaxis in adolescents with a change in tuberculin reaction. Probl Tuberk. 1993;3:30–3. [PubMed] [Google Scholar]
- 6.Youmans GP. Development of delayed (tuberculin) hypersensitivity in tuberculosis. In: Yomans GP, Paterson PY, Sommers HM, editors. The Biological and Clinical Basis of Infectious Disease. 3rd ed. Philadelphia: WB Saunders Company; 1985. pp. 355–8. [Google Scholar]
- 7.Stites DP, Holds JD, Schmitz J. Clinical laboratory method for detection of cellular immunity. In: Stites DP, Terr AI, Parslow TG, editors. Medical Immunology. 9th ed. Appleton and Lange: A Simon and Schuster Company; 1997. p. 255. [Google Scholar]
- 8.Addington WW. Patient compliance: The most serious remaining problem in the control of tuberculosis in the United States. Chest. 1979;76(6 Suppl):741–3. doi: 10.1378/chest.76.6_supplement.741. [DOI] [PubMed] [Google Scholar]
- 9.Second East African/British Medical Research Council Report. Controlled clinical trial of four short-course (6 months) regimens of chemotherapy for treatment of pulmonary tuberculosis. Lancet. 1974;1:1100–8. [PubMed] [Google Scholar]
- 10.Renoux G. The general immunopharmacology of levamisole. Drugs. 1980;20:89–99. doi: 10.2165/00003495-198020020-00001. [DOI] [PubMed] [Google Scholar]
- 11.Stanford JL, Bahr GM, Byass P, Corrah T, Dowlati Y, Lucas S, et al. A modern approach to the immunotherapy of tuberculosis. Bull Int Union Tuberc Lung Dis. 1990;65:27–9. [PubMed] [Google Scholar]
- 12.Etemadi A, Farid R, Stanford JL. Immunotherapy for drug-resistant tuberculosis. Lancet. 1992;340:1360–1. doi: 10.1016/0140-6736(92)92551-p. [DOI] [PubMed] [Google Scholar]
- 13.Maher D, Chaulet P, Spinaci S, Harries A. Treatment of Tuberculosis: Guidelines for National Programmes. 2nd ed. Geneva: World Health Organisation; 1997. WHO/TB/97. 220:59. [Google Scholar]
- 14.Singh MM, Kumar P, Malaviya AN, Kumar R. Levamisole as an adjunct in the treatment of pulmonary tuberculosis. Am Rev Respir Dis. 1981;123:277–9. doi: 10.1164/arrd.1981.123.3.277. [DOI] [PubMed] [Google Scholar]
- 15.Wilsher ML, Hagan C, Prestidge R, Wells AU, Murison G. Human in vitro immune responses to Mycobacterium tuberculosis. Tuber Lung Dis. 1999;79:371–7. doi: 10.1054/tuld.1999.0223. [DOI] [PubMed] [Google Scholar]
- 16.Ellner JJ. Regulation of the human cellular immune response to Mycobacterium tuberculosis. The mechanism of selective depression of the response to PPD. Bull Int Union Tuberc Lung Dis. 1991;66:129–32. [PubMed] [Google Scholar]
- 17.Takahashi S, Setoguchi Y, Nukiwa T, Kira S. Soluble interleukin-2 receptor in sera of patients with pulmonary tuberculosis. Chest. 1991;99:310–4. doi: 10.1378/chest.99.2.310. [DOI] [PubMed] [Google Scholar]
- 18.Sodhi A, Gong J, Silva C, Qian D, Barnes PF. Clinical correlates of interferon gamma production in patients with tuberculosis. Clin Infect Dis. 1997;25:617–20. doi: 10.1086/513769. [DOI] [PubMed] [Google Scholar]
- 19.Huygen K, Van Vooren JP, Turneer M, Bosmans R, Dierckx P, De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988;27:187–94. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 20.Van Crevel R, Karyadi E, Preyers F, Leenders M, Kullberg BJ, Nelwan RH, et al. Increased production of interleukin 4 by CD4+and CD8+T cells from patients with tuberculosis is related to the presence of pulmonary cavities. J Infect Dis. 2000;181:1194–7. doi: 10.1086/315325. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Pando R, Rook GA. The role of TNF-alpha in T-cell-mediated inflammation depends on the Th1/Th2 cytokine balance. Immunology. 1994;82:591–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Grange JM. The microanatomical nature of the tuberculin reaction. In: Narang P, Mendiratta DK, editors. Proceedings of International CME on Tuberculosis. Sevagram, India: MGIMS; 1996. pp. 11–19. [Google Scholar]
- 23.Toman K. Tuberculosis case finding and chemotherapy. Geneva: WHO; 1979. p. 151. [Google Scholar]
- 24.Vanham G, Toossi Z, Hirsch CS, Wallis RS, Schwander SK, Rich EA, et al. Examining a paradox in the pathogenesis of human pulmonary tuberculosis: Immune activation and suppression/anergy. Tuber Lung Dis. 1999;79:371–7. doi: 10.1016/s0962-8479(97)90021-6. [DOI] [PubMed] [Google Scholar]
- 25.Santucci MB, Amicosante M, Ciccani R, Montesano C, Casarini M, et al. Mycobacterium tuberculosis induced apoptosis in monocytes/macrophages: Early membrane modification and intracellular mycobacterial viability. J Infect Dis. 2000;181:1506–9. doi: 10.1086/315371. [DOI] [PubMed] [Google Scholar]
- 26.Ganiev KG, Vavilova TA. Use of differentiated pathogenetic therapy in the treatment of newly detected pulmonary tuberculosis in adolescents and young persons. Probl Tuberk. 1990;9:35–6. [PubMed] [Google Scholar]
- 27.Youmans GP. Tuberculosis. 1st ed. Philadelphia: WB Saunders Company; 1979. pp. 202–9. [Google Scholar]
- 28.Szeto C, Gillespie KM, Mathieson PW. Levamisole induces interleukin-18 and shifts type 1/type 2 cytokine balance. Immunology. 2000;100:217–24. doi: 10.1046/j.1365-2567.2000.00042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wynn TA, Cheever AW, Jankovic D, Poindexter RW, Caspar P, Lewis FA, et al. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature. 1995;376:594–6. doi: 10.1038/376594a0. [DOI] [PubMed] [Google Scholar]
- 30.Renoux G. Modulation of immunity by levamisole. J Pharmacol Ther. 1978:2:288–96. [Google Scholar]
- 31.Turk JL, Parker D. Sensitization of guinea pigs to levamisole. Int Arch Allergy Appl Immunol. 1979;58:237–40. doi: 10.1159/000232198. [DOI] [PubMed] [Google Scholar]
- 32.Mielants H, Veys EM. A study of the hematological side effects of levamisole in rheumatoid arthritis with recommendations. J Rheumatol. 1978;4:77–83. [PubMed] [Google Scholar]
- 33.Donald PR, Lamprecht JH, Freestone M, Albrecht CF, Bouic PJ, Kotze D, et al. A randomised placebo-controlled trial of the efficacy of beta – Sitosterol and its glucoside as adjuvants in the treatment of pulmonary tuberculosis. Int J Tuberc Lung Dis. 1997;1:518–22. [PubMed] [Google Scholar]
- 34.Corlan E, Marica C, Macavei C, Stanford JL, Stanford CA. Immunotherapy with Mycobacterium vaccae in the treatment of tuberculosis in Romania. Newly-diagnosed pulmonary disease. Respir Med. 1997;91:13–9. doi: 10.1016/s0954-6111(97)90132-3. [DOI] [PubMed] [Google Scholar]


